Advancing Thermoset Technology: 4R Materials with Unchanged Mechanical Properties and Enhanced Sustainability Through Repellency, Recyclability, Reprocessability, and Repairability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blending Protocol
2.3. Characterization Techniques
3. Results and Discussion
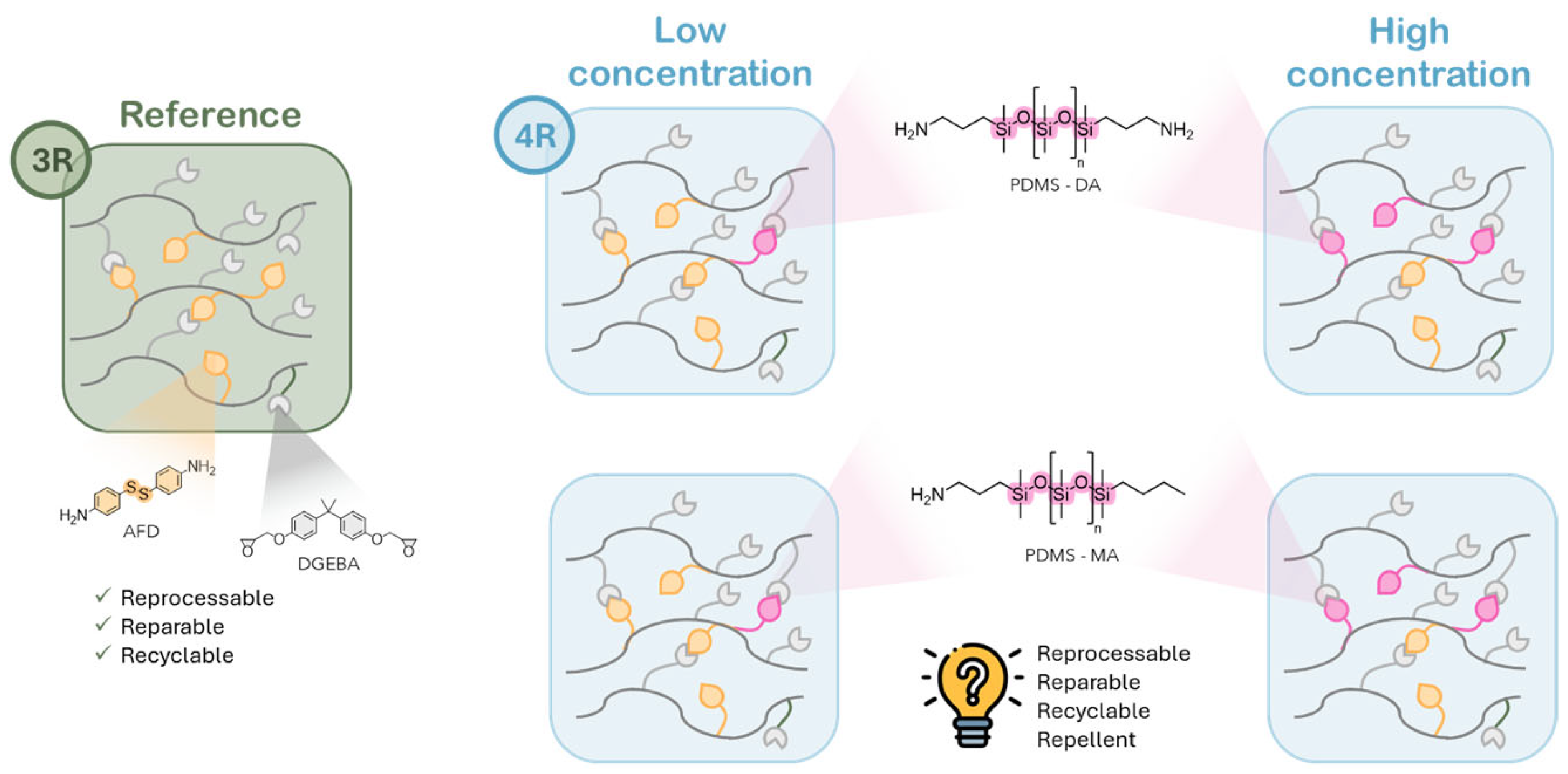
3.1. Context and Purpose
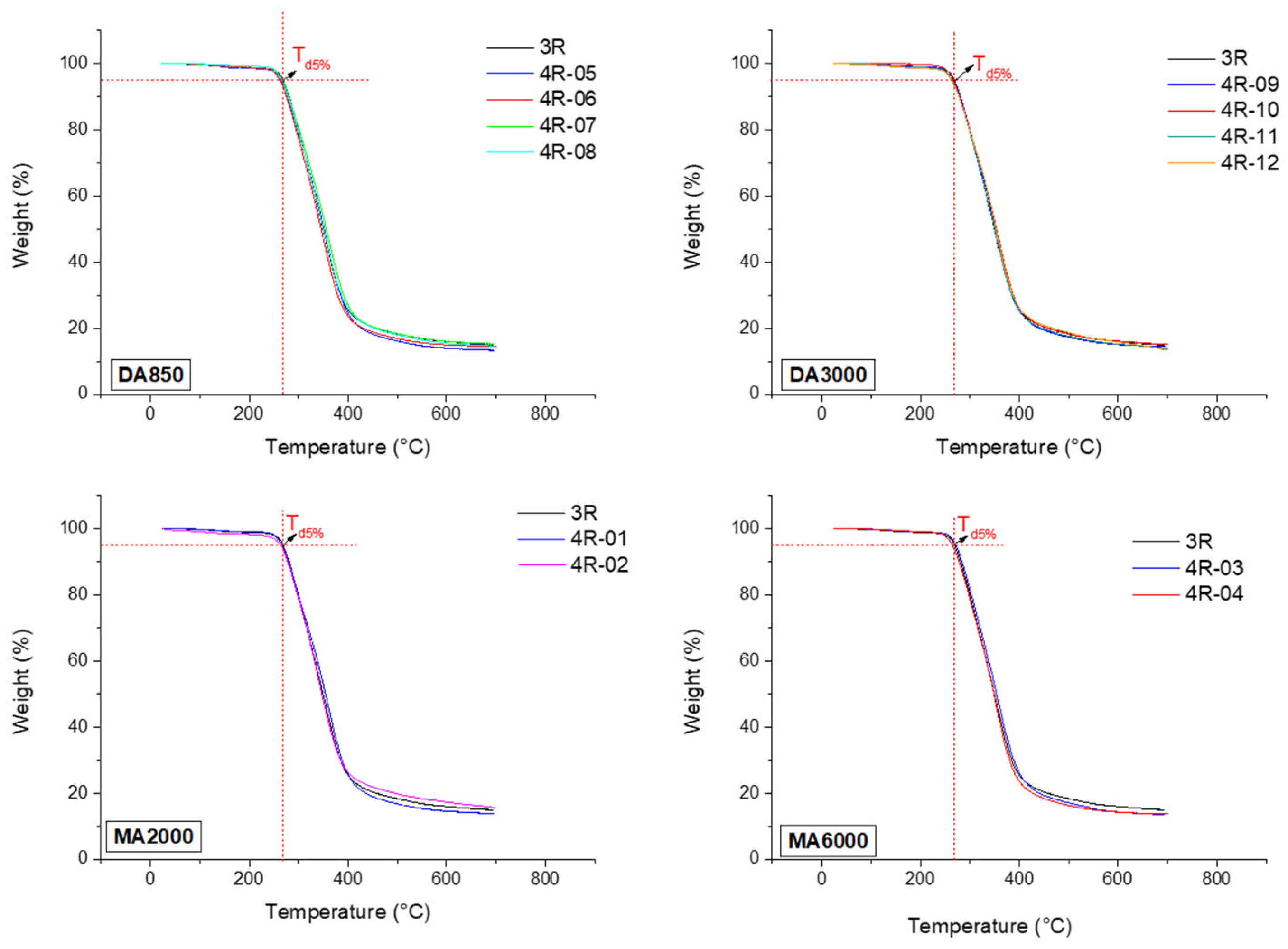
3.2. Characterization of the Thermal Properties
3.3. Surface Properties
3.3.1. Static Contact Angle Measurements
3.3.2. Dynamic Contact Angle Measurements
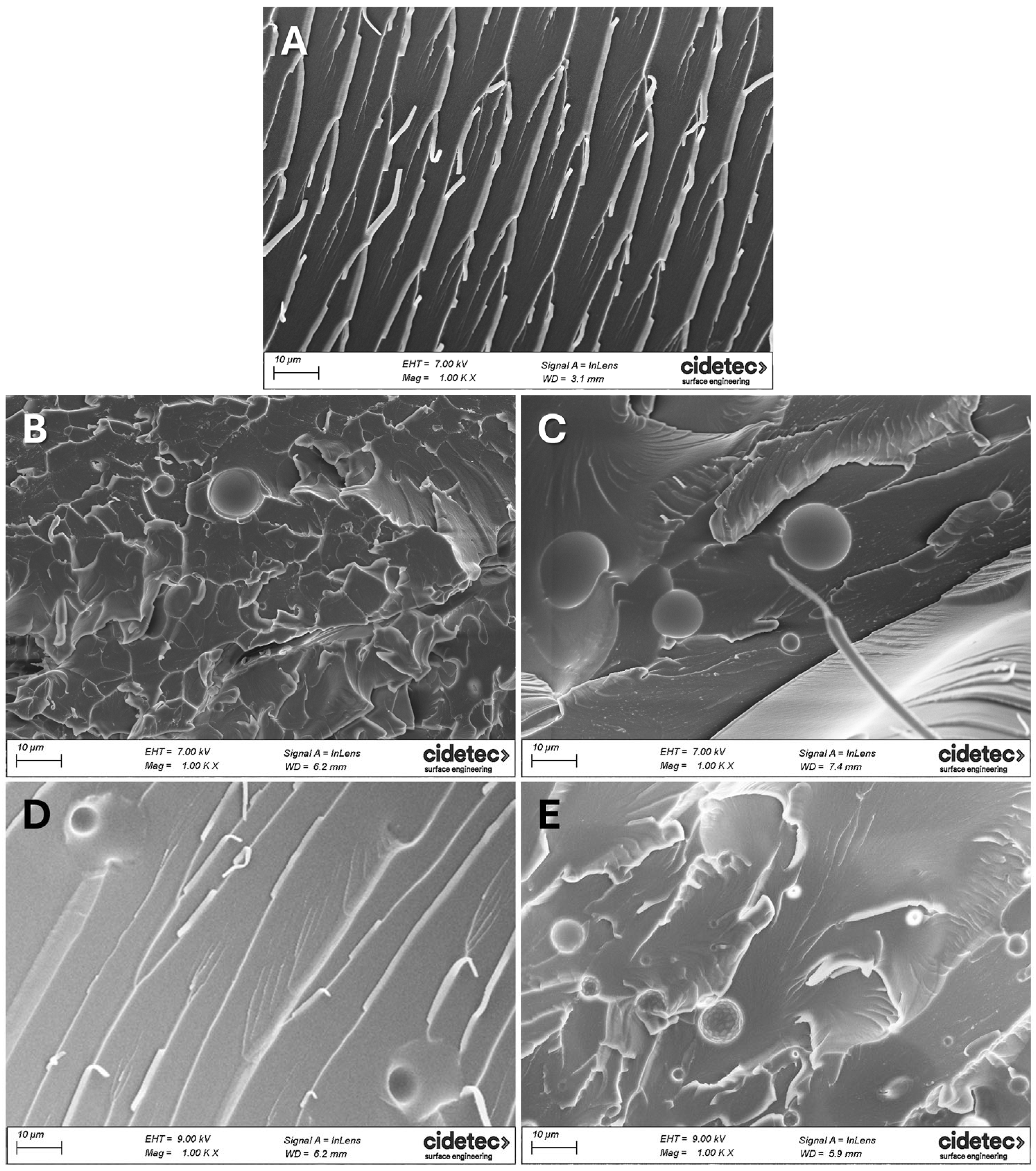
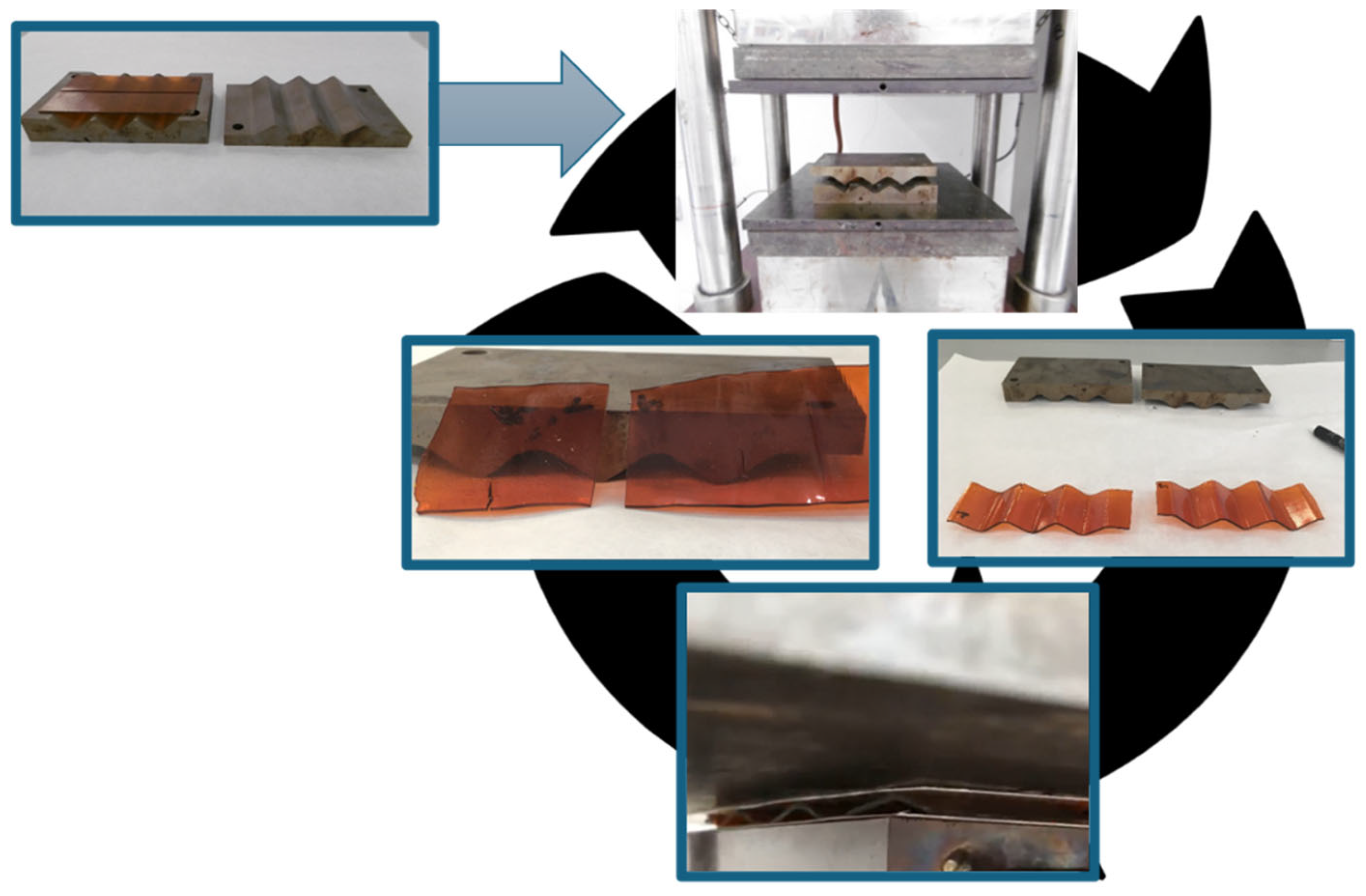
3.4. Morphological Analysis
3.5. Mechanical Properties
3.6. Dynamic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vozzola, E.; Overcash, M.; Twomey, J.; Gring, E.; Asmatulu, E. Thermoset composite recycling—Driving forces, development, and evolution of new opportunities. J. Compos. Mater. 2017, 52, 1033–1043. [Google Scholar]
- Kloxin, C.J.; Bowman, C.N. Covalent adaptable networks: Smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef] [PubMed]
- Azcune, I.; Elorza, E.; Ruiz de Luzuriaga, A.; Huegun, A.; Rekondo, A.; Grande, H.J. Analysis of the Effect of Network Structure and Disulfide Concentration on Vitrimer Properties. Polymers 2023, 15, 4123. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Luzuriaga, A.; Markaide, N.; Salaberria, A.M.; Azcune, I.; Rekondo, A.; Grande, H.J. Aero Grade Epoxy Vitrimer towards Commercialization. Polymers 2022, 14, 3180. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, C.B.; Gayraud, V.; Rodriguez, M.E.; Costa, J.; Salaberria, M.A.; Ruiz de Luzuriaga, A.; Markaide, N.; Keeryadath, P.D.; Zapatería, D.C. Study into the Mechanical Properties of a New Aeronautic-Grade Epoxy-Based Carbon-Fiber-Reinforced Vitrimer. Polymers 2022, 14, 1223. [Google Scholar] [CrossRef]
- Tripathi, S.; Supriya, H.; Bose, H. Covalent adaptable network offers “sustainable” closed-loop circularity in epoxy vitrimers. SPE Polym. 2024, 5, 95–111. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef]
- Addicoat, M.A.; Tsotsalas, M. Covalently linked organic networks. Front. Mater. 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Alabiso, W.; Schlögl, S. The Impact of Vitrimers on the Industry of the Future: Chemistry, Properties and Sustainable Forward-Looking Applications. Polymers 2020, 12, 1660. [Google Scholar] [CrossRef]
- Hayashi, M. Implantation of Recyclability and Healability into Cross-Linked Commercial Polymers by Applying the Vitrimer Concept. Polymers 2020, 12, 1322. [Google Scholar] [CrossRef]
- Khan, A.; Ahmed, N.; Rabnawaz, M. Covalent Adaptable Network and Self-Healing Materials: Current Trends and Future Prospects in Sustainability. Polymers 2020, 12, 2027. [Google Scholar] [CrossRef]
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: From Old Chemistry to Modern Day Innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef]
- Ciarella, S.; Ellenbroek, W.G. Swap-Driven Self-Adhesion and Healing of Vitrimers. Coatings 2019, 9, 114. [Google Scholar] [CrossRef]
- De Luzuriaga, A.R.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horiz. 2016, 3, 241–247, Correction in Mater. Horiz. 2020, 7, 2460–2461. [Google Scholar] [CrossRef]
- Verma, S.; Mohanty, S.; Nayak, S.K. Preparation of hydrophobic epoxy–polydimethylsiloxane–graphene oxide nanocomposite coatings for antifouling application. Soft Matter 2020, 16, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, S.; Lei, L.; Zhao, J.; Tong, Z. Toughening, synergistic fire retardation and water resistance of polydimethylsiloxane grafted graphene oxide to epoxy nanocomposites with trace phosphorus. Compos. Part A 2017, 100, 275–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, F.; Liu, Y. A superhydrophobic EP/PDMS nanocomposite coating with high gamma radiation stability. Appl. Surf. Sci. 2018, 436, 405–410. [Google Scholar] [CrossRef]
- Chang, J.F.; Liang, G.Z.; Gu, A.J.; Cai, S.D.; Yuan, L. The production of carbon nanotube/epoxy composites with a very high dielectric constant and low dielectric loss by microwave curing. Carbon 2012, 50, 689–698. [Google Scholar] [CrossRef]
- Hu, J.H.; Shan, J.Y.; Zhao, J.Q.; Tong, Z. Water resistance and curing kinetics of epoxy resins with a novel curing agent of biphenyl-containing amine synthesized by one-pot method. Thermochim. Acta 2015, 606, 58–65. [Google Scholar] [CrossRef]
- Qin, Y.R.; Xue, J.J.; Wang, S.P.; Fan, Y.; Wang, L.; Xu, J.N.; Zhao, J. Capsaicin-based silicone antifouling coating with enhanced interlocking adhesion via SIPN. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132346. [Google Scholar] [CrossRef]
- Pan, W.H.; Wang, Q.L.; Ma, J.; Xu, W.; Sun, J.; Liu, X.; Song, J.L. Solid-like slippery coating with highly comprehensive performance. Adv. Funct. Mater. 2023, 33, 2302311. [Google Scholar] [CrossRef]
- Bao, Y.; Yang, H.; Gao, L.; Zheng, X.; Shi, X.J.; Zhang, W.B.; Liu, C. Fabrication of anti-icing/de-icing superhydrophobic composite coating based on hydrangea-like ZnO@CuS. Sol. Energy Mater. Sol. Cells 2022, 245, 111838. [Google Scholar] [CrossRef]
- Tan, W.; Guo, Y.; Xiang, Y.; Meng, X.; Wang, C.; Ren, Q. Optimization of the performance of epoxy intumescent fire-retardant coatings utilizing amino-polysiloxanes with varying compositions. React. Funct. Polym. 2025, 210, 106191. [Google Scholar] [CrossRef]
- Tu, K.; Wang, X.; Kong, H.; Guan, H. Facile preparation of mechanically durable, self-healing and multifunctional superhydrophobic surfaces on solid wood. Mater. Des. 2018, 140, 30–36. [Google Scholar] [CrossRef]
- Boscher, N.D.; Vaché, V.; Carminati, P.; Grysan, P.; Choquet, P. A simple and scalable approach towards the preparation of superhydrophobic surfaces–importance of the surface roughness skewness. J. Mater. Chem. 2014, 2, 5744–5750. [Google Scholar] [CrossRef]
- Chang, H.; Tu, K.; Wang, X.; Liu, J. Fabrication of mechanically durable superhydrophobic wood surfaces using polydimethylsiloxane and silica nanoparticle. RSC Adv. 2015, 39, 30647–30653. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Wang, X.; Lin, T. Fluoroalkyl silane modified silicone rubber/nanoparticle composite: A super durable, robust superhydrophobic fabric coating. Adv. Mater. 2012, 24, 2409–2412. [Google Scholar] [CrossRef] [PubMed]
- Pakzad, H.; Liravi, M.; Moosavi, A.; Nouri-Borujerdi, A.; Najafkhani, H. Fabrication of durable superhydrophobic surfaces using PDMS and beeswax for drag reduction of internal turbulent flow. Appl. Surf. Sci. 2020, 513, 145754. [Google Scholar] [CrossRef]
- Chen, D.; Chen, F.; Hu, X.; Zhang, H.; Yin, X.; Zhou, Y. Thermal stability, mechanical and optical properties of novel addition cured PDMS composites with nano-silica sol and MQ silicone resin. Compos. Sci. Technol. 2015, 117, 307–314. [Google Scholar] [CrossRef]
- Meshkov, I.B.; Kalinina, A.A.; Gorodov, V.V.; Bakirov, A.V.; Krasheninnikov, S.V.; Chvalun, S.N.; Muzafarov, A.M. New Principles of Polymer Composite Preparation. MQ Copolymers as an Active Molecular Filler for Polydimethylsiloxane Rubbers. Polymers 2021, 13, 2848. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Polyakova, M.Y.; Kulichikhin, V.G. Phase behavior and rheology of miscible and immiscible blends of linear and hyperbranched siloxane macromolecules. Mater. Today Commun. 2020, 22, 100833. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Y.; Zhang, P.; Tian, L.M.; Xu, J.N.; Niu, S.C.; Ren, L.Q.; Zhao, J.; Ming, W.H. Dynamically oleophobic epoxy coating with surface enriched in silicone. Prog. Org. Coat. 2021, 154, 106170. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.Y.; Jia, X.H.; Li, Y.; Song, H.J. Fabrication of robust and transparent slippery coating with hot water repellency, antifouling property, and corrosion resistance. ACS Appl. Mater. Interfaces 2020, 12, 28645–28654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Yang, J.; Sun, C.C.; Zhang, X.; Wei, Q.J.; Ni, J.; Han, N.; Jia, X.H.; Song, H.J. Diving beetle-inspired durable omniphobic slippery coatings with pit-type non-smooth topography. Prog. Org. Coat. 2023, 185, 107898. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kulichikhin, V.G. Rheology and miscibility of linear/hyperbranched polydimethylsiloxane blends and an abnormal decrease in their viscosity. Macromolecules 2023, 56, 6818–6833. [Google Scholar] [CrossRef]
- Wang, K.Y.; Wang, C.Y.; Dong, Q.; Wang, K.L.; Qian, J.H.; Liu, Z.Z.; Huang, F.R. Enhancing corrosion and abrasion resistances simultaneously of epoxy resin coatings by novel hyperbranched amino-polysiloxanes. Prog. Org. Coat. 2023, 196, 108680. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, Y.; Yuan, X.; Zhou, X.; Luo, J.; Zhou, X.; Liu, C.; Qu, J. Sorbitol-based epoxy anti-smudge coatings with liquid repellency for various applications. J. Appl. Polym. Sci. 2023, 140, e54593. [Google Scholar] [CrossRef]
- Boumezgane, O.; Suriano, R.; Fedel, M.; Tonelli, C.; Deflorian, F.; Turri, S. Self-healing epoxy coatings with microencapsulated ionic PDMS oligomers for corrosion protection based on supramolecular acid-base interactions. Prog. Org. Coat. 2022, 162, 106558. [Google Scholar] [CrossRef]
- Mirchandani, G.; Basutkar, S.; Raghavendra, V.B.; Shyamroy, S.; Singha, N.K. Omniphobic coatings based on functional acrylic polymer. Prog. Org. Coat. 2023, 183, 107764. [Google Scholar] [CrossRef]
- Sobhani, S.; Jannesari, A.; Bastani, S. Effect of Molecular Weight and Content of PDMS on Morphology and Properties of Silicone-Modified Epoxy Resin. J. Appl. Polym. Sci. 2012, 123, 162–178. [Google Scholar] [CrossRef]
- Bok, G.; Lim, G.; Kwak, M.; Kim, Y. Super-Toughened Fumed-Silica-Reinforced Thiol-Epoxy Composites Containing Epoxide-Terminated Polydimethylsiloxanes. Int. J. Mol. Sci 2021, 22, 8097. [Google Scholar] [CrossRef]
- Putnam-Neeb, A.; Stafford, A.; Babu, S.; Chapman, S.J.; Hemmingsen, C.M.; Islam, S.; Roy, A.K.; Kalow, J.A.; Varshney, V.; Nepal, D.; et al. Oligosiloxane-Based Epoxy Vitrimers: Adaptable Thermoestting Networks with Dual Dynamic Bonds. ACS Appl. Polym. Mater. 2024, 6, 14229–14234. [Google Scholar] [CrossRef]
- Du, Y.; Wang, D. One-Pot Preparation of Double-Network Epoxy Vitrimers with High Performance, Recyclability, and Two-Stage Stress Relaxation. ACS Appl. Mater. Interfaces 2024, 16, 41551–41561. [Google Scholar] [CrossRef] [PubMed]
- Fanlo, P.; Konuray, O.; Ochoteco, O.; Ximenis, M.; Rekondo, A.; Grande, H.J.; Fernández-Francos, X.; Sardon, H.; Ruiz de Luzuriaga, A. Dynamic by design: Unlocking full relaxation in disulfide epoxy networks. Polym. Chem. 2025, 16, 2701–2717. [Google Scholar] [CrossRef]
- Law, K.Y. Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: Getting the basics right. J. Phys. Chem. Lett. 2014, 4, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Beitollahpoor, M.; Farzam, M.; Pesika, N.S. Determination of the sliding angle of water drops on surfaces from friction force measurements. Langmuir 2022, 38, 2132–2136. [Google Scholar] [CrossRef]
- Law, K.Y. Contact angle hysteresis on smooth/flat and rough surfaces. Interpretation, mechanism, and origin. Acc. Mater. Res. 2022, 3, 1–7. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Tuteja, A. The design and applications of superomniphobic surfaces. NPG Asia Mater. 2014, 6, e109–e124. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. A review on special wettability textiles: Theoretical models, fabrication technologies and multifunctional applications. J. Mater. Chem. A 2017, 5, 31–55. [Google Scholar] [CrossRef]
- Wooh, S.; Vollmer, D. Silicone brushes: Omniphobic Surfaces with Low Sliding Angle. Angew. Chem. Int. Ed. 2016, 55, 6822–6824. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. (Eds.) Polymer Handbook, 4th ed.; John Wiley and Sons: New York, NY, USA, 1999; pp. VII/499–VII/524. [Google Scholar]
- Verma, S.; Das, S.; Mohanty, S.A.; Kumar, S. A facile preparation of epoxy-polydimethylsiloxane (EP-PDMS) polymer coatings for marine applications. J. Mater. Res. 2019, 34, 2881–2894. [Google Scholar] [CrossRef]
- Wong, T.S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired Self-Repairing Slippery Surfaces with Pressure-Stable Omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Rico, M.; López, J.; Montero, B.; Bouza, R.; Díez, F.J. Influence of the molecular weight of a modifier on the phase separation in an epoxy thermoset modified with a thermoplastic. Eur. Polym. J. 2014, 58, 125–134. [Google Scholar] [CrossRef]
- Rico, M.; López, J.; Montero, B.; Ramírez, C.; Bouza, R. Thermodynamic analysis of polymerization-induced phase separation of a polystyrene in epoxy/monoamine-diamine systems. Effect of monoamine-diamine proportion on the phase diagram. Eur. Polym. J. 2011, 47, 1676–1685. [Google Scholar] [CrossRef]
- Rico, M.; López, J.; Díez, F.J.; Bellas, R. Simulation of molecular fractionation and species distributions in phase separation of polystyrene-modified epoxy/monoamine-diamine blends. Eur. Polym. J. 2011, 47, 2432–2441. [Google Scholar] [CrossRef]
- Rico, M.; López, J.; Montero, B.; Bellas, R. Phase separation and morphology development in a thermoplastic-modified toughened epoxy. Eur. Polym. J. 2012, 48, 1660–1673. [Google Scholar] [CrossRef]
- ISO 527-2-2012; Plastics–Determination of Tensile Properties. Part 2: Test Conditions for Moulding and Extrusion Plastics. International Organization for Standardization (ISO): Geneva, Switzerland, 2012.
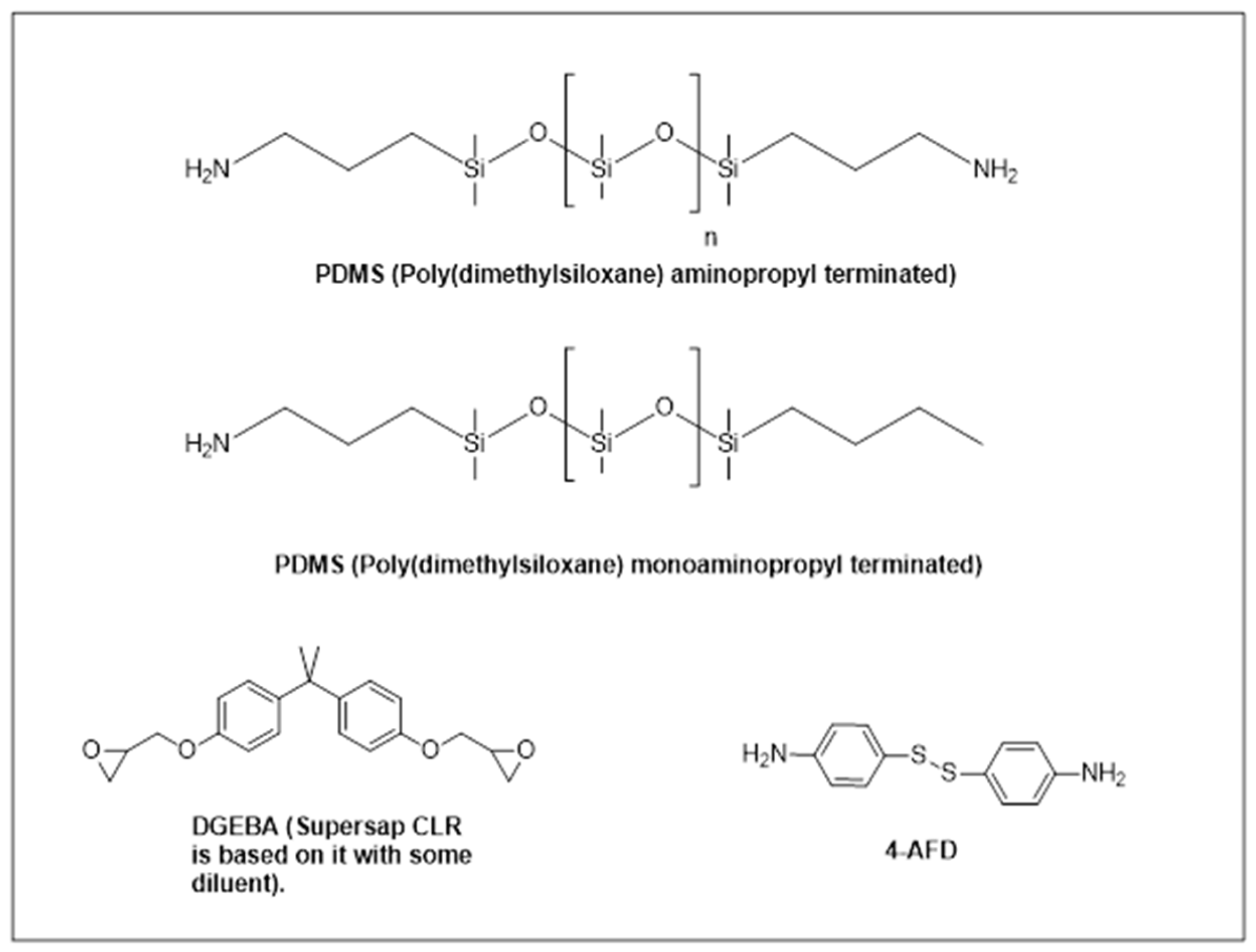
| Code Name | Terminal Structure | Mw (g·mol−1) | Amino Equivalent Weight (g·eq−1) |
|---|---|---|---|
| MA2000 | Monoamine | 2000 | 1000 |
| MA6000 | Monoamine | 6000 | 3000 |
| DA850 | Diamine | 850–900 | 212.5 |
| DA3000 | Diamine | 3000 | 750 |
| Sample | PDMS | Tg (°C) | |
|---|---|---|---|
| Molecule | %wt | ||
| 3R-REF | - | - | 123 |
| 4R-01 | MA2000 | 0.2 | 120 |
| 4R-02 | 0.8 | 113 | |
| 4R-03 | MA6000 | 0.2 | 118 |
| 4R-04 | 0.8 | 115 | |
| 4R-05 | DA850 | 0.2 | 120 |
| 4R-06 | 0.8 | 114 | |
| 4R-07 | 1.6 | 114 | |
| 4R-08 | 3.2 | 112 | |
| 4R-09 | DA3000 | 0.2 | 120 |
| 4R-10 | 0.8 | 118 | |
| 4R-11 | 1.6 | 117 | |
| 4R-12 | 3.2 | 117 | |
| Sample | PDMS | Surface Properties | ||||
|---|---|---|---|---|---|---|
| Molecule | %wt | WCA (°) | HCA (°) | WSA (°) | HSA (°) | |
| 3R-REF | - | - | 97 ± 3 | 35 ± 2 | DNS | DNS |
| 4R-01 | MA2000 | 0.2 | 104 ± 1 | 29 ± 1 | 70 ± 5 | DNS |
| 4R-02 | 0.8 | 104 ± 3 | 18 ± 5 | 45 ± 5 | DNS | |
| 4R-03 | MA6000 | 0.2 | 103 ± 2 | 30 ± 1 | 49 ± 6 | DNS |
| 4R-04 | 0.8 | 100 ± 1 | 31 ± 1 | 41 ± 2 | 7 ± 1 | |
| 4R-05 | DA850 | 0.2 | 103 ± 3 | 44 ± 1 | DNS | DNS |
| 4R-06 | 0.8 | 109 ± 5 | 42 ± 1 | DNS | DNS | |
| 4R-07 | 1.6 | 104 ± 2 | 40 ± 1 | DNS | DNS | |
| 4R-08 | 3.2 | 105 ± 2 | 41 ± 1 | DNS | DNS | |
| 4R-09 | DA3000 | 0.2 | 103 ± 2 | 39 ± 1 | DNS | 21 ± 1 |
| 4R-10 | 0.8 | 109 ± 4 | 38 ± 1 | DNS | 15 ± 1 | |
| 4R-11 | 1.6 | 111 ± 2 | 42 ± 1 | DNS | 19 ± 4 | |
| 4R-12 | 3.2 | 110 ± 1 | 45 ± 1 | DNS | 11 ± 5 | |
| Sample | PDMS | Surface Properties | |||
|---|---|---|---|---|---|
| Molecule | %wt | a-WCA (°) | r-WCA (°) | h-WCA (°) | |
| 3R-REF | - | - | DNS | DNS | DNS |
| 4R-01 | MA2000 | 0.2 | 117 ± 4 | 86 ± 2 | 31 |
| 4R-02 | 0.8 | 113 ± 1 | 83 ± 1 | 30 | |
| 4R-03 | MA6000 | 0.2 | 111 ± 3 | 87 ± 2 | 24 |
| 4R-04 | 0.8 | 112 ± 2 | 90 ± 1 | 22 | |
| Sample | PDMS | Tensile Properties | Crosslinking Density Tg + 50 °C | |||
|---|---|---|---|---|---|---|
| Molecule | %wt | Fracture Strength (σf) [MPa] | Strain at Fracture Point (εf) [%] | Young’s Modulus (E) [MPa] | Crosslinking Density (νXL) (mol·cm−3) | |
| 3R-REF | - | - | 82 ± 3 | 5.9 ± 0.8 | 2952 ± 278 | 1.7 × 10−3 |
| 4R-02 | MA2000 | 0.8 | 80 ± 5 | 5.7 ± 0.9 | 3070 ± 393 | 1.6 × 10−3 |
| 4R-04 | MA6000 | 0.8 | 82 ± 2 | 5.7 ± 0.5 | 3097 ± 553 | 1.9 × 10−3 |
| 4R-08 | DA850 | 3.2 | 77 ± 2 | 5.6 ± 0.7 | 2690 ± 272 | 1.8 × 10−3 |
| 4R-12 | DA3000 | 3.2 | 75 ± 5 | 4.9 ± 0.9 | 2686 ± 117 | 1.7 × 10−3 |
| Sample | PDMS | Relaxation Time (s) | |
|---|---|---|---|
| Molecule | %wt | Tg + 50 °C | |
| 3R-REF | - | - | 66 s (at 175 °C) |
| 4R-02 | MA2000 | 0.8 | 116 s (at 165 °C) |
| 4R-04 | MA6000 | 0.8 | 168 s (at 165 °C) |
| 4R-08 | DA850 | 3.2 | 140 s (at 165 °C) |
| 4R-12 | DA3000 | 3.2 | 140 s (at 165 °C) |
| Sample | Surface Properties | |||
|---|---|---|---|---|
| WCA (°) | HCA (°) | WSA (°) | HSA (°) | |
| 3R-REF | 97 ± 3 | 35 ± 2 | DNS | DNS |
| 4R-04 | 100 ± 1 | 31 ± 1 | 41 ± 2 | 7 ± 1 |
| WAFFLED 3R | 97 ± 2 | 22 ± 3 | DNS | DNS |
| WAFFLED 4R | 98 ± 1 | 17 ± 2 | 12 ± 1 | DNS |
| FLATTENED 3R | 96 ± 3 | 17 ± 1 | DNS | DNS |
| FLATTENED 4R | 98 ± 2 | 13 ± 4 | 17 ± 3 | DNS |
| Sample | Roughness Properties | |
|---|---|---|
| Average Surface Roughness (Ra, µm) | Roughness Depth (Rz, µm) | |
| 3R-REF | 0.53 ± 0.11 | 3.73 ± 0.76 |
| 4R-04 | 0.39 ± 0.01 | 2.51 ± 0.17 |
| WAFFLED 3R | 0.43 ± 0.04 | 3.18 ± 0.28 |
| WAFFLED 4R | 0.33 ± 0.06 | 1.54 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genua, A.; Indakoetxea, N.; Elorza, E.; Iturri, J.; Fanlo, P.; Jubete, E.; Grande, H.-J.; Garcia, I. Advancing Thermoset Technology: 4R Materials with Unchanged Mechanical Properties and Enhanced Sustainability Through Repellency, Recyclability, Reprocessability, and Repairability. Polymers 2025, 17, 3147. https://doi.org/10.3390/polym17233147
Genua A, Indakoetxea N, Elorza E, Iturri J, Fanlo P, Jubete E, Grande H-J, Garcia I. Advancing Thermoset Technology: 4R Materials with Unchanged Mechanical Properties and Enhanced Sustainability Through Repellency, Recyclability, Reprocessability, and Repairability. Polymers. 2025; 17(23):3147. https://doi.org/10.3390/polym17233147
Chicago/Turabian StyleGenua, Aratz, Nagore Indakoetxea, Edurne Elorza, Jagoba Iturri, Paula Fanlo, Elena Jubete, Hans-J. Grande, and Ignacio Garcia. 2025. "Advancing Thermoset Technology: 4R Materials with Unchanged Mechanical Properties and Enhanced Sustainability Through Repellency, Recyclability, Reprocessability, and Repairability" Polymers 17, no. 23: 3147. https://doi.org/10.3390/polym17233147
APA StyleGenua, A., Indakoetxea, N., Elorza, E., Iturri, J., Fanlo, P., Jubete, E., Grande, H.-J., & Garcia, I. (2025). Advancing Thermoset Technology: 4R Materials with Unchanged Mechanical Properties and Enhanced Sustainability Through Repellency, Recyclability, Reprocessability, and Repairability. Polymers, 17(23), 3147. https://doi.org/10.3390/polym17233147









