Sustainable Benzoxazine Copolymers with Enhanced Thermal Stability, Flame Resistance, and Dielectric Tunability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
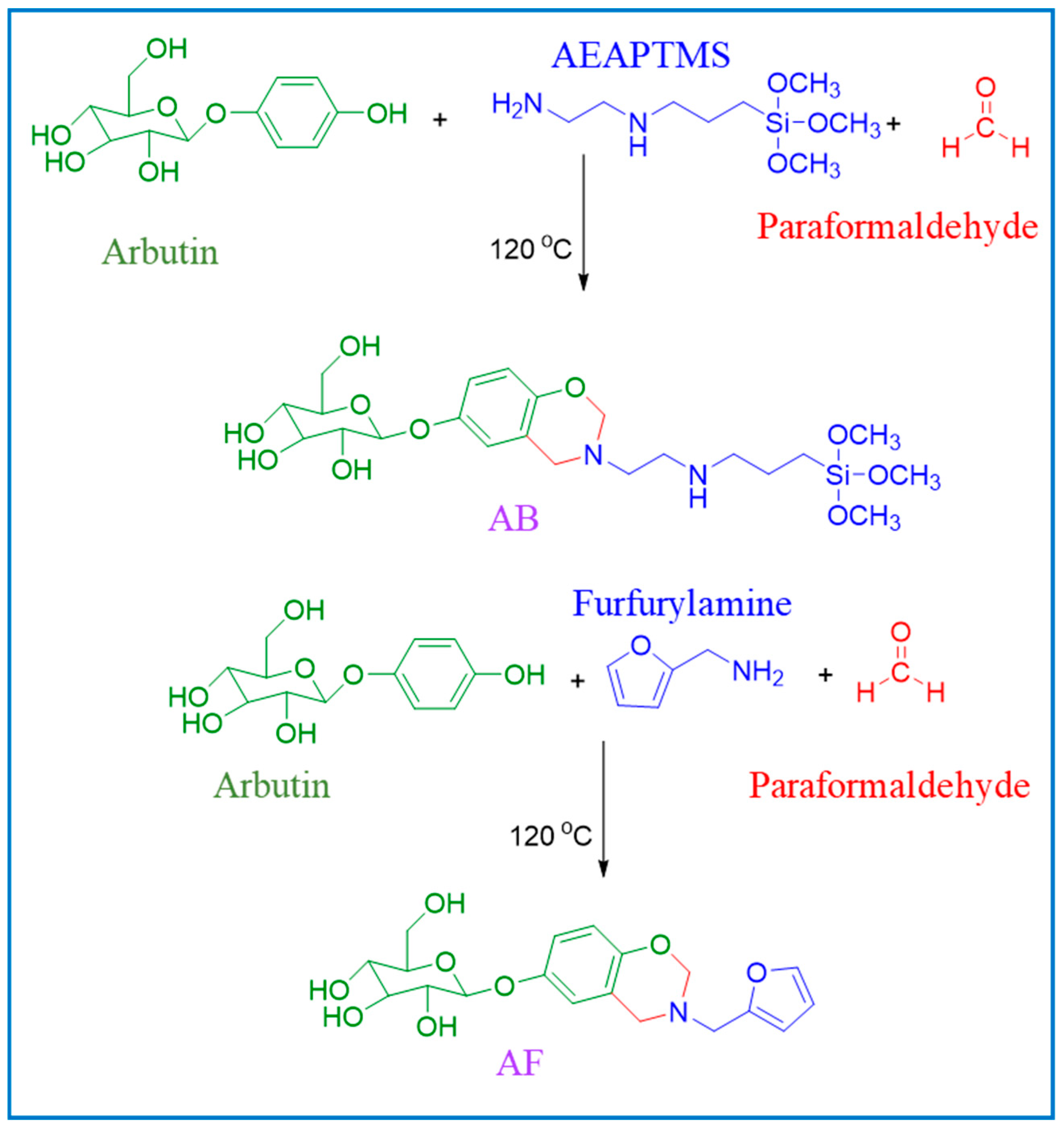
2.2.1. Synthesis of Bio-Based Arbutin-Derived Benzoxazine (AB) Monomer
2.2.2. Synthesis of Bio-Based Arbutin–Furfurylamine Benzoxazine (AF) Monomer
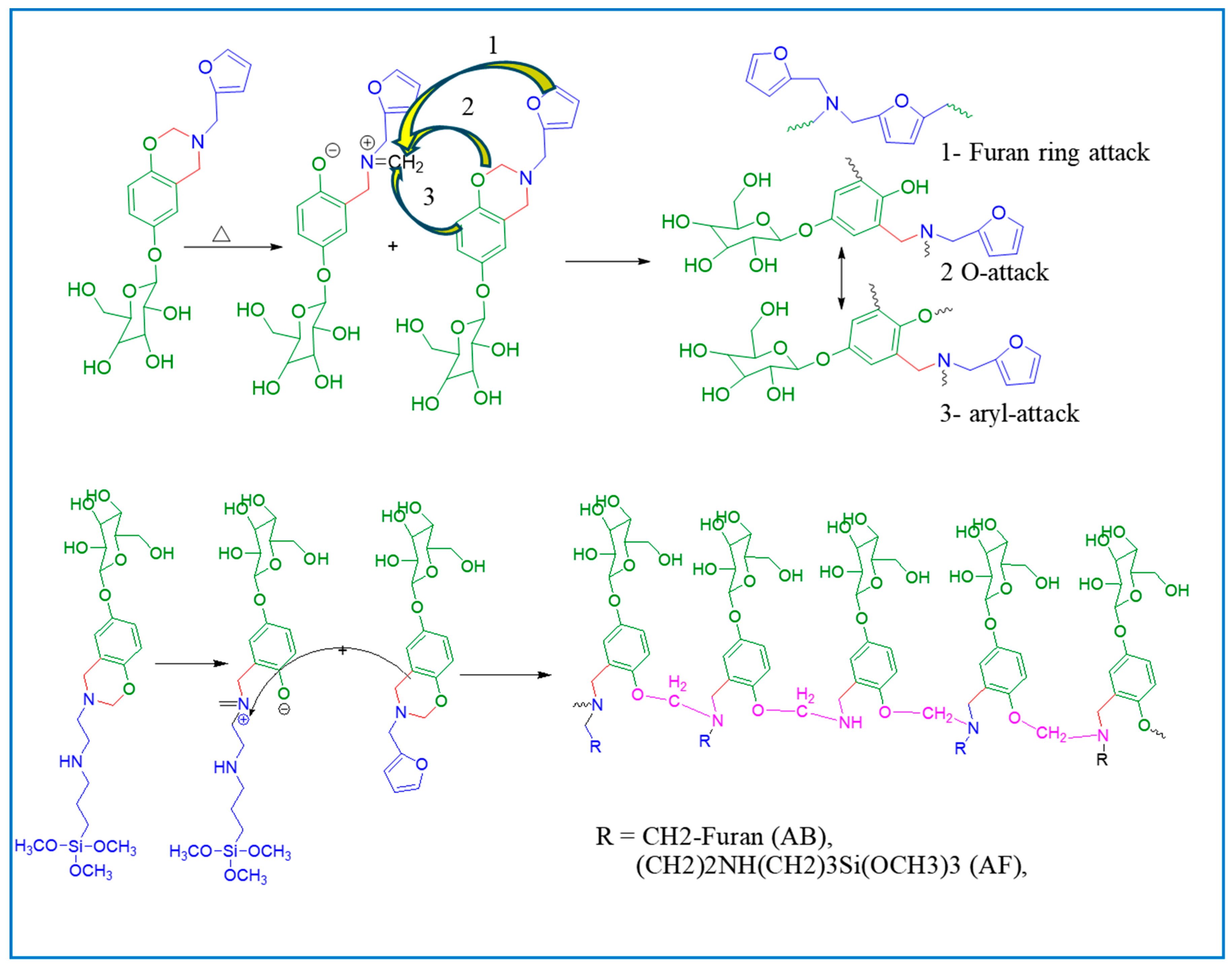
2.2.3. Copolymerization of AB and AF
2.3. Characterization
3. Results
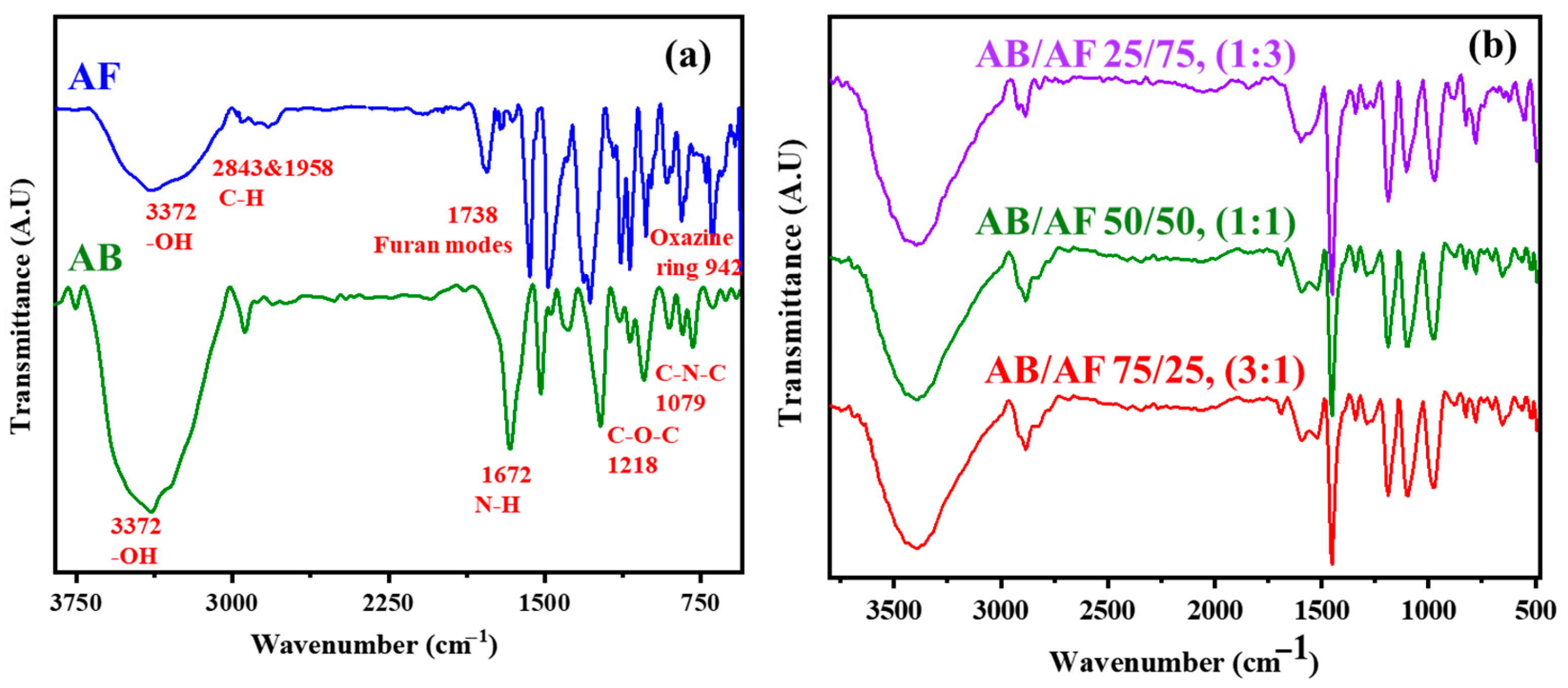
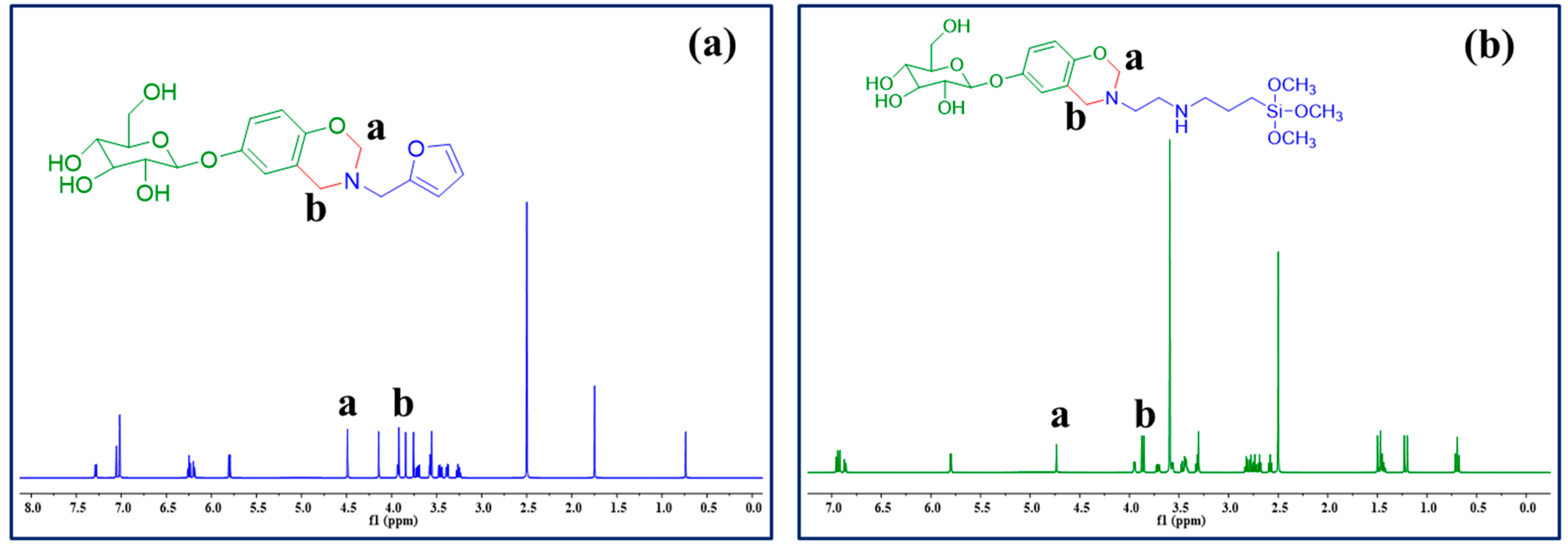
3.1. Structural Confirmation of AB and AF Benzoxazine Monomers
3.2. Curing Behavior of Benzoxazine Monomers and Their Copolymers
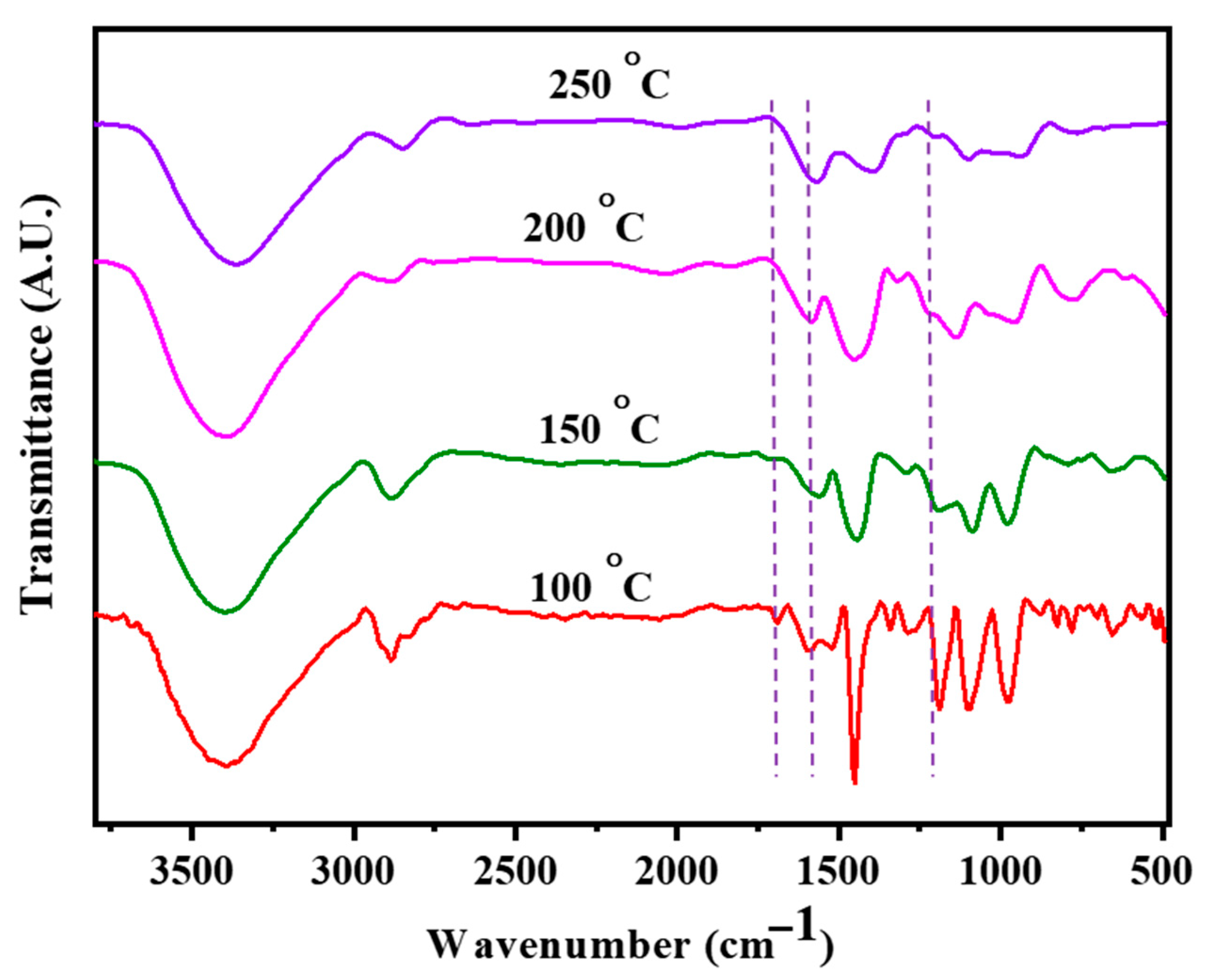
3.3. FT-IR Analysis of the Curing Process in AB/AF (1:1) Copolymer
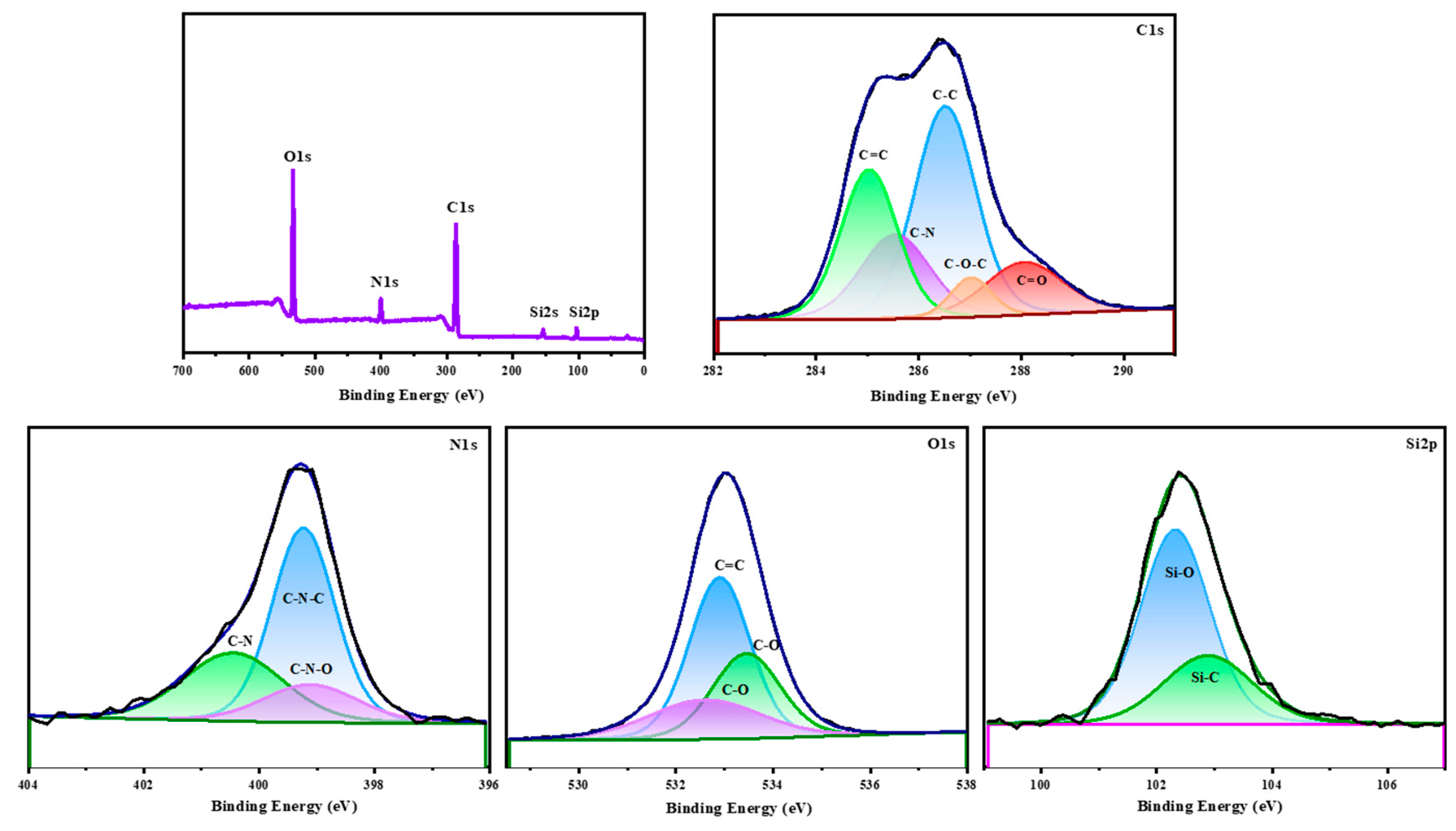
3.4. XPS Analysis of AB/AF (1:1) Copolymer
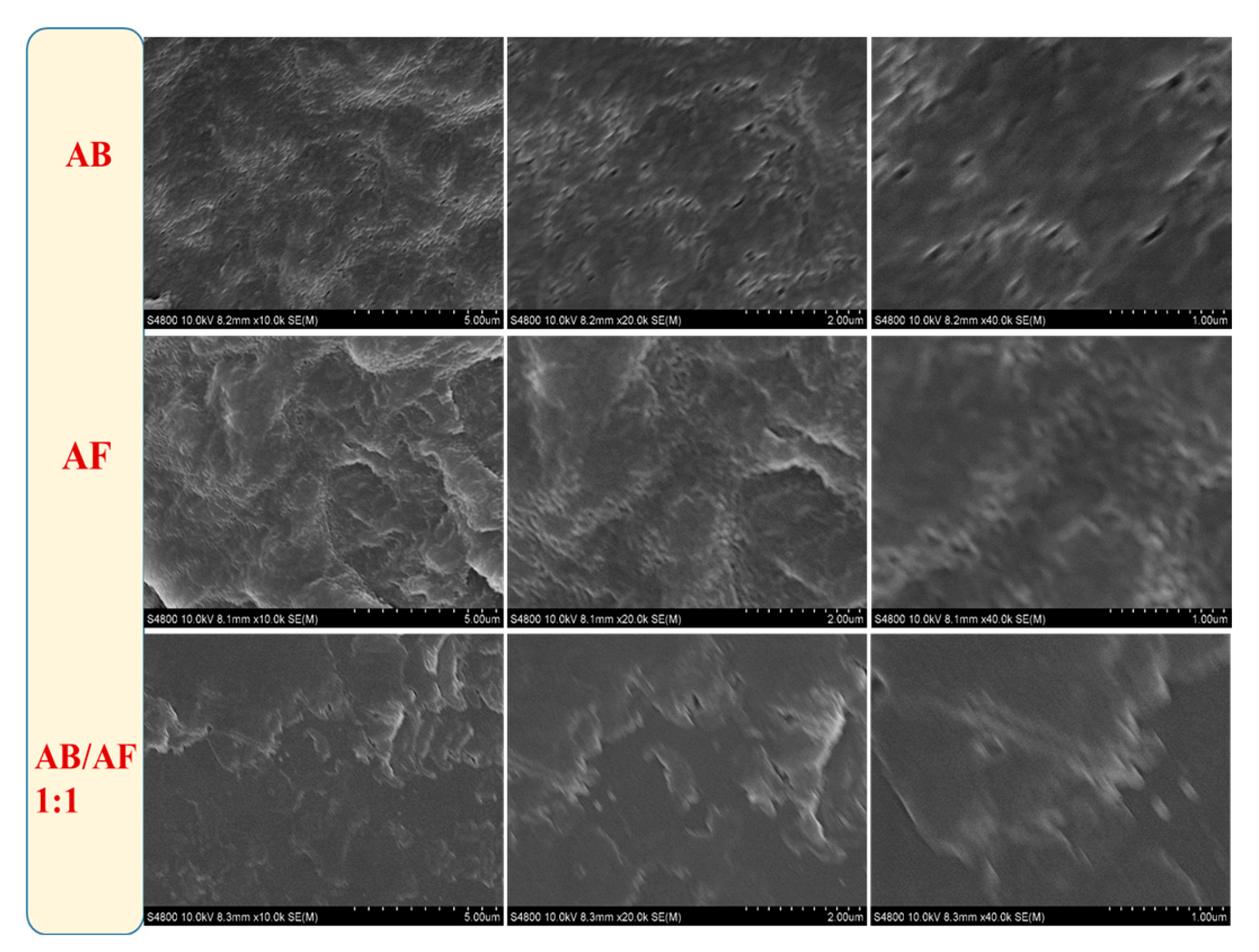
3.5. SEM Analysis of AB, AF, and AB/AF (1:1) Copolymers
3.6. Dielectric Properties of Poly(AB), Poly(AF), and AB/AF Copolymers
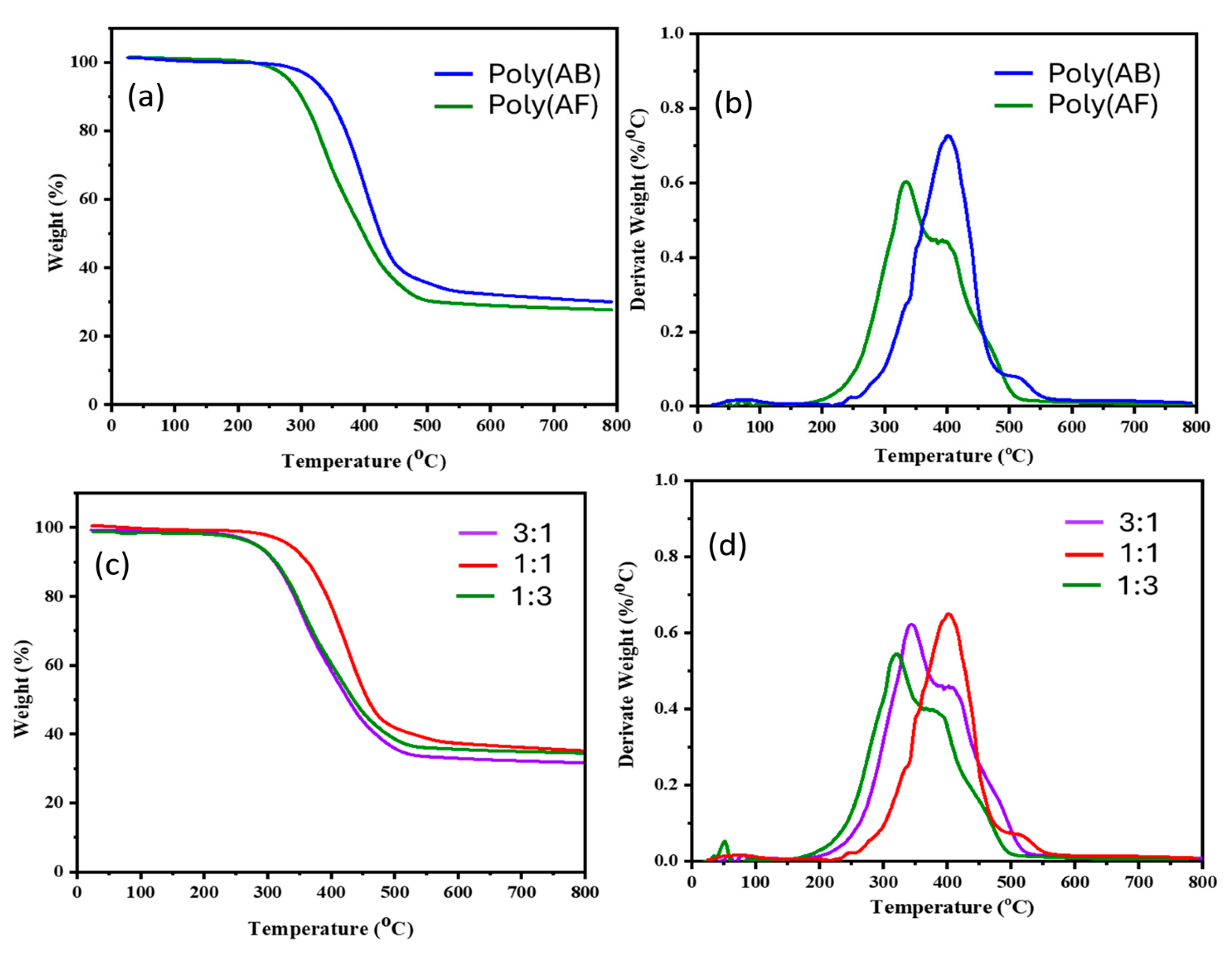
3.7. Thermal Stability Assessment of Poly(AB), Poly(AF), and AB/AF Copolymers
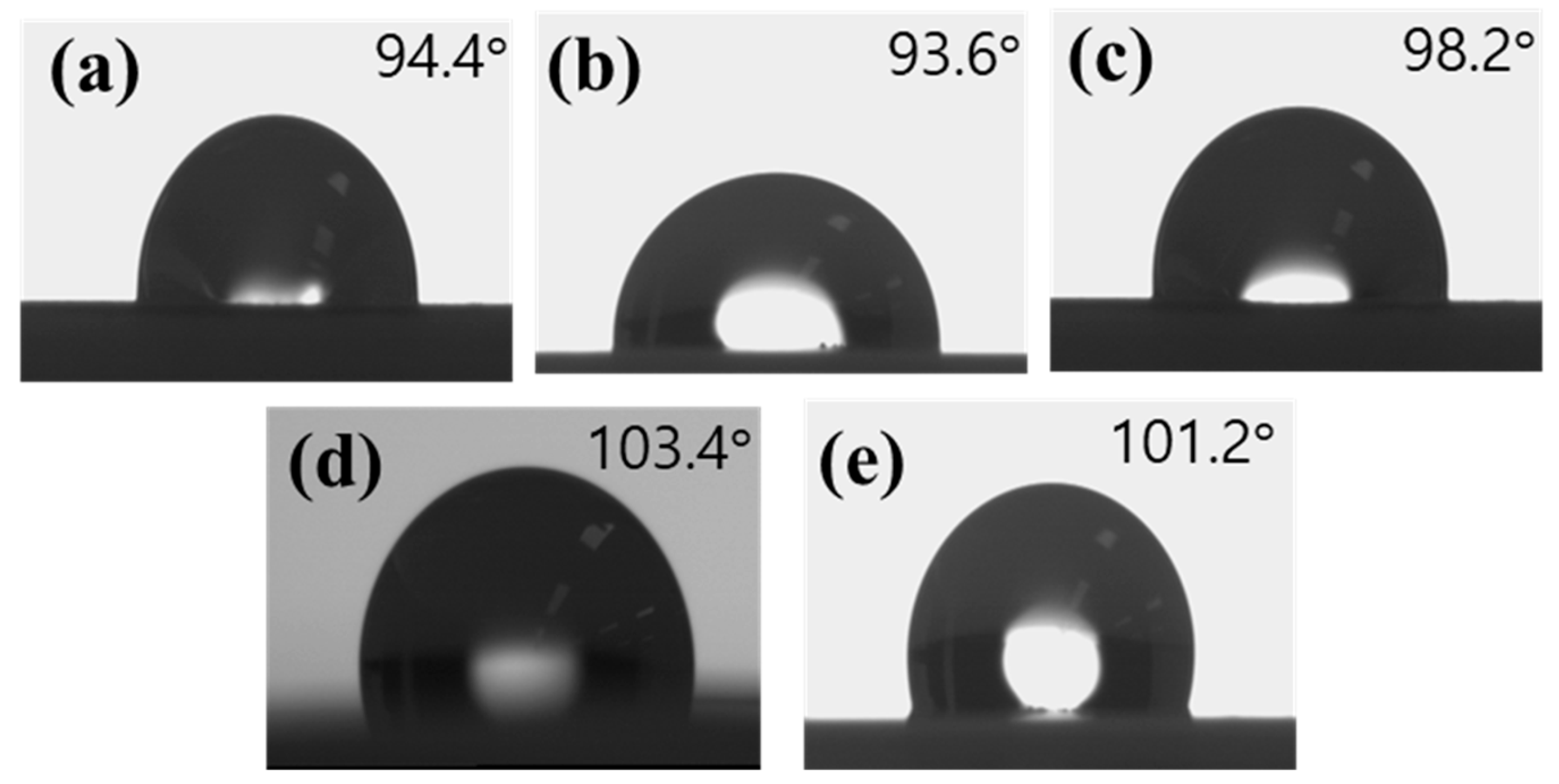
3.8. Water Contact Angle Analysis of Poly(AB), Poly(AF), and AB/AF Copolymers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, R.; Zhang, K. Design and Synthesis of Flavonoid-Based Mono-, Bis-, and Tri-Benzoxazines: Toward Elucidating Roles of Oxazine Ring Number and Hydrogen Bonding on Their Polymerization Mechanisms and Thermal Properties. Macromolecules 2025, 58, 616–626. [Google Scholar] [CrossRef]
- Xu, M.; He, L.; Zhang, J.; Fan, Z.; Li, B. Study on the Curing Behaviors of Benzoxazine Nitrile-Based Resin Featuring Fluorene Structures and the Excellent Properties of Their Glass Fiber-Reinforced Laminates. Materials 2024, 17, 6167. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Y.; Song, J.; Liang, H.; Yan, H.; Zhang, C.; Wang, Z. Structures and Performance of a Polybenzoxazine with High Heat Resistance and High-Frequency Low-Dielectric Properties. Polymer 2024, 313, 127742. [Google Scholar] [CrossRef]
- Zhang, A.P.; Zhao, W.; Li, X.K.; Bian, J.; Ni, K.Y.; Yang, K.C.; Lin, H.L.; Chen, D.Q. Design, Synthesis, and Evaluation of Benzoxazine Copolymers for Anti-Corrosion Coating Applications Based on Improved High Crosslinking Density and Hydrophobicity. J. Appl. Polym. Sci. 2025, 142, e56576. [Google Scholar] [CrossRef]
- Machado, I.; Shaer, C.; Hurdle, K.; Calado, V.; Ishida, H. Towards the Development of Green Flame Retardancy by Polybenzoxazines. Prog. Polym. Sci. 2021, 121, 101435. [Google Scholar] [CrossRef]
- Lyu, Y.; Zhang, Y.; Ishida, H. Intrinsically Noncombustible Polymers Without Flame Retardant Additives: Sulfur-Containing and Bio-Based Benzoxazines. Eur. Polym. J. 2020, 133, 109770. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Z.; Guo, Z.; Liu, T.; Yuan, Z.; Derradji, M.; Liu, W.; Wang, J. Synthesis of Triphenol-Diamine-Type Hyperbranched Benzoxazine and Improvement of Thermal Properties and Toughness of Typical Benzoxazines. J. Appl. Polym. Sci. 2024, 141, e55873. [Google Scholar] [CrossRef]
- Faiza, B.; Hardacre, B.; Scott, C.; Winroth, S.; Ishida, H. Synthesis of Bio-Based and Intrinsically Flame-Retardant Benzoxazine Containing Dynamic Ester Bond That Quantitatively Satisfies All Twelve Principles of Green Chemistry. ACS Sustain. Chem. Eng. 2024, 12, 13525–13534. [Google Scholar] [CrossRef]
- Chen, D.; Liu, B.; Wang, X.; Li, X.; Xu, X.; He, J.; Yang, R. High Flame Retardant and Heat-Resistance, Low Dielectric Benzoxazine Resin with Phthalimide Structure. Polym. Degrad. Stab. 2022, 205, 110150. [Google Scholar] [CrossRef]
- Appavoo, D.; Amarnath, N.; Lochab, B. Cardanol and Eugenol Sourced Sustainable Non-Halogen Flame Retardants for Enhanced Stability of Renewable Polybenzoxazines. Front. Chem. 2020, 8, 711. [Google Scholar] [CrossRef]
- Chen, C.; Lin, C.; Hon, J.; Wang, M.; Juang, T. First Halogen- and Phosphorus-Free, Flame-Retardant Benzoxazine Thermosets Derived from Main-Chain Type Bishydroxydeoxybenzoin-Based Benzoxazine Polymers. Polymer 2018, 154, 35–41. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Y.; Wang, J.; Jing, Y.; Zhang, K. Development of Intrinsically Flame-Retardant Bio-Thermosets with Further Enhanced Thermal Stability Through a Photothermal Dual Polymerization Strategy. Polym. Degrad. Stab. 2024, 229, 110948. [Google Scholar] [CrossRef]
- Zeng, M.; Zhu, W.; Feng, Z.; Chen, J.; Huang, Y.; Xu, Q.; Wang, J. Two Novel Halogen-Free, Phosphorus-Free, and Intrinsically Flame-Retardant Benzoxazine Thermosets Containing Electron-Withdrawing Bridge Groups. J. Appl. Polym. Sci. 2020, 137, 49300. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Chaitany, J.R.; Rajesh, H.; Shakila Parveen, A.; Kim, S.-C. Development of arbutin based sustainable polybenzoxazine resin for antifouling and anticorrosion of low carbon steel. Prog. Org. Coat. 2022, 170, 106968. [Google Scholar]
- Zhou, X.; Li, R.; Fu, F.; Shen, M.; Li, Q.; Liu, H.; Xu, X.; Song, Z. A Novel Efficient Flame-Retardant Curing Agent for Epoxy Resin Based on P–N Synergistic Effect: Bio-Based Benzoxazine Phosphate Ester. Chem. Eng. J. 2024, 499, 156441. [Google Scholar] [CrossRef]
- Combeau, M.; Batistella, M.; Breuillac, A.; Naess, M.K.; Perrin, D.; Lopez-Cuesta, J. POSS in Intumescent Flame-Retardant Systems to Improve Fire Behaviour of Virgin and Recycled High-Density Polyethylene. Polym. Degrad. Stab. 2025, 233, 111177. [Google Scholar] [CrossRef]
- Kopsidas, S.; Hamerton, I. Examining the Thermal Behaviour of Novel Aromatic Polybenzoxazine Blends Containing an Organophosphorous Compound and Polyhedral Oligomeric Silsesquioxane Reagents. Polym. Int. 2016, 65, 1015–1023. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H.; Guo, X.; Yang, R.; Li, X. Effects of an Organic–Inorganic Hybrid Containing Allyl Benzoxazine and POSS on Thermal Properties and Flame Retardancy of Epoxy Resin. Polymers 2019, 11, 770. [Google Scholar] [CrossRef]
- Selvi, M.; Devaraju, S.; Alagar, M. Cyclotriphosphazene Nanofiber-Reinforced Polybenzoxazine/Epoxy Nanocomposites for Low Dielectric and Flame-Retardant Applications. Polym. Bull. 2019, 76, 3785–3801. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Qi, X.; Wang, D. Recent Progress on Metal–Organic Framework and Its Derivatives as Novel Fire Retardants to Polymeric Materials. Nano-Micro Lett. 2020, 12, 173. [Google Scholar] [CrossRef]
- Zhao, C.; Li, P.; He, D.; Li, Y.; Lei, F.; Sue, H. Flame Retardation Behavior of Polybenzoxazine/α-ZrP Nanocomposites. RSC Adv. 2016, 6, 73485–73495. [Google Scholar] [CrossRef]
- Yu, M.; Gao, Z.; Xie, W.; Shi, X.; Zhang, O.; Shen, Z.; Fang, L.; Ren, M.; Sun, J. A Novel Strategy Utilizing Oxidation States of Phosphorus for Designing Efficient Phosphorus-Containing Flame Retardants and Its Performance in Epoxy Resins. Polym. Degrad. Stab. 2024, 230, 111016. [Google Scholar] [CrossRef]
- Yan, H.; Sun, C.; Fang, Z.; Liu, X.; Zhu, J.; Wang, H. Synthesis of an Intrinsically Flame Retardant Bio-Based Benzoxazine Resin. Polymer 2016, 97, 418–427. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Zhao, W.; Zhang, K. Bio-Benzoxazine Structural Design Strategy Toward Highly Thermally Stable and Intrinsically Flame-Retardant Thermosets. Chem. Eng. J. 2023, 457, 141232. [Google Scholar] [CrossRef]
- Cen, X.; Cao, Z.; Wang, Z. Flame Retardancy, Thermal Behavior and Mechanical Properties of an Epoxy Resin and Cyanate Ester Resin Copolymer Containing a Bio-Phytate-Based Flame Retardant. Polymer 2024, 291, 126610. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila Parveen, A.; Sarojadevi, M. Replacing bisphenol-A with bisguaiacol-F to synthesize polybenzoxazines for a pollution-free environment. New J. Chem. 2015, 39, 1691–1702. [Google Scholar]
- Cao, J.; Duan, H.; Zou, J.; Zhang, J.; Wan, C.; Zhang, C.; Ma, H. Bio-Based Phosphorus-Containing Benzoxazine Towards High Fire Safety, Heat Resistance and Mechanical Properties of Anhydride-Cured Epoxy Resin. Polym. Degrad. Stab. 2022, 198, 109878. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila Parveen, A.; Sarojadevi, M. Synthesis and copolymerization of fully biobased benzoxazines from renewable resources. ACS Sustain. Chem. Eng. 2014, 2, 2790–2801. [Google Scholar] [CrossRef]
- Bornosuz, N.V.; Korotkov, R.F.; Kolenchenko, A.A.; Shapagin, A.V.; Orlov, A.V.; Gorbunova, I.Y.; Kireev, V.V.; Sirotin, I.S. The Influence of Substituents in Phosphazene Catalyst-Flame Retardant on the Thermochemistry of Benzoxazine Curing. Polymers 2021, 13, 3111. [Google Scholar] [CrossRef]
- Liang, H.; Liu, H.; Chen, Y.; Cui, Y.; Bu, T.; Wang, Z. Enhancing the Toughness of Polybenzoxazines Through Multiple Sacrificial Bonds for Improved Overall Performance. J. Mater. Res. Technol. 2024, 31, 659–667. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Ranjith Kumar, M.; Shakila Parveen, A.; Atchudan, R.; Kim, S.-C. Sustainability and antimicrobial assessments of apigenin based polybenzoxazine film. Polymer 2019, 172, 100–109. [Google Scholar] [CrossRef]
- Liu, J.; Safronava, N.; Lyon, R.E.; Maia, J.; Ishida, H. Enhanced Thermal Property and Flame Retardancy via Intramolecular 5-Membered Ring Hydrogen Bond-Forming Amide Functional Benzoxazine Resins. Macromolecules 2018, 51, 9982–9991. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila Parveen, A.; Sarojadevi, M. Synthesis and characterization of novel bio-based benzoxazines from eugenol. RSC Adv. 2014, 4, 7959–7966. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, L.; Liang, G.; Gu, A. Developing Intrinsic Halogen-Free and Phosphorus-Free Flame Retardant Biobased Benzoxazine Resins with Superior Thermal Stability and High Strength. Eur. Polym. J. 2022, 180, 111581. [Google Scholar] [CrossRef]
- Qian, Z.; Li, Q.; Wang, L.; Fu, F.; Liu, X. The Chemical Effect of Furfuryl Amide on the Enhanced Performance of the Diphenolic Acid Derived Bio-Polybenzoxazine Resin. J. Polym. Sci. 2021, 59, 2057–2068. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Kim, S.; Lee, J. Synergistic Effect of PBz/Epoxy/PCLA Composite Films with Improved Thermal Properties. Sustainability 2024, 16, 3991. [Google Scholar] [CrossRef]
- Rao, W.; Zhao, P.; Yu, C.; Zhao, H.; Wang, Y. High Strength, Low Flammability, and Smoke Suppression for Epoxy Thermoset Enabled by a Low-Loading Phosphorus-Nitrogen-Silicon Compound. Compos. Part B Eng. 2021, 211, 108640. [Google Scholar] [CrossRef]
- Ran, Q.; Gu, Y. Concerted Reactions of Aldehyde Groups During Polymerization of an Aldehyde-Functional Benzoxazine. J. Polym. Sci. A Polym. Chem. 2011, 49, 1671–1677. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, A.P.; Li, X.K.; Bian, J.; Yang, S.K.; Ni, K.Y.; Yang, K.C.; Lin, H.L.; Chen, D.Q. Novel Flexible Polyether Segment Polybenzoxazine Shape Memory Nanocomposites Reinforced by Incorporating Functionalized Carbon Nanotubes. Compos. Part B 2025, 292, 112104. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila Parveen, A.; Atchudan, R.; Kim, S.-C. Sustainability and antimicrobial assessments of bio based polybenzoxazine film. Eur. Polym. J. 2018, 109, 248–256. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, S.; Yusuf, A.; Sun, J.; Zhai, Z.; Zhao, J.; Yin, G.-Z. Recent Advances in Flame Retardant and Mechanical Properties of Polylactic Acid: A Review. Int. J. Biol. Macromol. 2023, 243, 125050. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Pei, L.; Liu, H.; Wang, M.; Li, S.; Wang, Z. Modified Polybenzoxazine and Carbon Fiber Surface with Improved Mechanical Properties by Introducing Hydrogen Bonds. Eur. Polym. J. 2023, 182, 111717. [Google Scholar] [CrossRef]
- Wang, J.; Su, X.; Mao, Z. The Flame Retardancy and Thermal Property of Poly(Ethylene Terephthalate)/Cyclotriphosphazene Modified by Montmorillonite System. Polym. Degrad. Stab. 2014, 109, 154–161. [Google Scholar] [CrossRef]
- Qian, Z.; Xiao, Y.; Zhang, X.; Li, Q.; Wang, L.; Fu, F.; Diao, H.; Liu, X. Bio-Based Epoxy Resins Derived from Diphenolic Acid via Amidation Showing Enhanced Performance and Unexpected Autocatalytic Effect on Curing. Chem. Eng. J. 2022, 435, 135022. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, Q.; Gu, Y. Preparation and Properties of Benzoxazine Blends with Intumescent Flame Retardancy. Polym. Degrad. Stab. 2019, 163, 15–24. [Google Scholar] [CrossRef]
- Bao, Y.; Zhao, H.; Guo, R.; Gao, L.; Li, R.; Zhang, W.; Liu, C.; Wei, P. Enhanced Flame Retardancy and Smoke Suppression of Thermoplastic Polyurethane Membrane via “Two-Phase” Synergistic Effect. Polym. Degrad. Stab. 2025, 235, 111262. [Google Scholar] [CrossRef]
- Andreu, R.; Reina, I.A.; Ronda, J.C. Studies on the Thermal Polymerization of Substituted Benzoxazine Monomers: Electronic Effects. J. Polym. Sci. A Polym. Chem. 2008, 46, 3353–3366. [Google Scholar] [CrossRef]
- Xiang, H.; Ling, H.; Wang, J.; Song, L.; Gu, Y. A Novel High Performance RTM Resin Based on Benzoxazine. Polym. Compos. 2005, 26, 563–571. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Some Basic Aspects of Flame Resistance of Polymeric Materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]
- Allen, D.J.; Ishida, H. Effect of Phenol Substitution on the Network Structure and Properties of Linear Aliphatic Diamine-Based Benzoxazines. Polymer 2009, 50, 613–626. [Google Scholar] [CrossRef]
- Allen, D.J.; Ishida, H. Physical and Mechanical Properties of Flexible Polybenzoxazine Resins: Effect of Aliphatic Diamine Chain Length. J. Appl. Polym. Sci. 2006, 101, 2798–2809. [Google Scholar] [CrossRef]
- Chang, S.L.; Lin, C.H. Facile, One-Pot Synthesis of Aromatic Diamine-Based Benzoxazines and Their Advantages Over Diamines as Epoxy Hardeners. J. Polym. Sci. A Polym. Chem. 2010, 48, 2430–2437. [Google Scholar] [CrossRef]
- Hemvichian, K.; Ishida, H. Thermal Decomposition Processes in Aromatic Amine-Based Polybenzoxazines Investigated by TGA and GC-MS. Polymer 2002, 43, 4391–4402. [Google Scholar] [CrossRef]
- Hsieh, C.; Su, W.; Wu, C.; Lin, L.; Hsu, K.; Liu, Y. Benzoxazine-Containing Branched Polysiloxanes: Highly Efficient Reactive-Type Flame Retardants and Property Enhancement Agents for Polymers. Polymer 2013, 54, 2945–2951. [Google Scholar] [CrossRef]
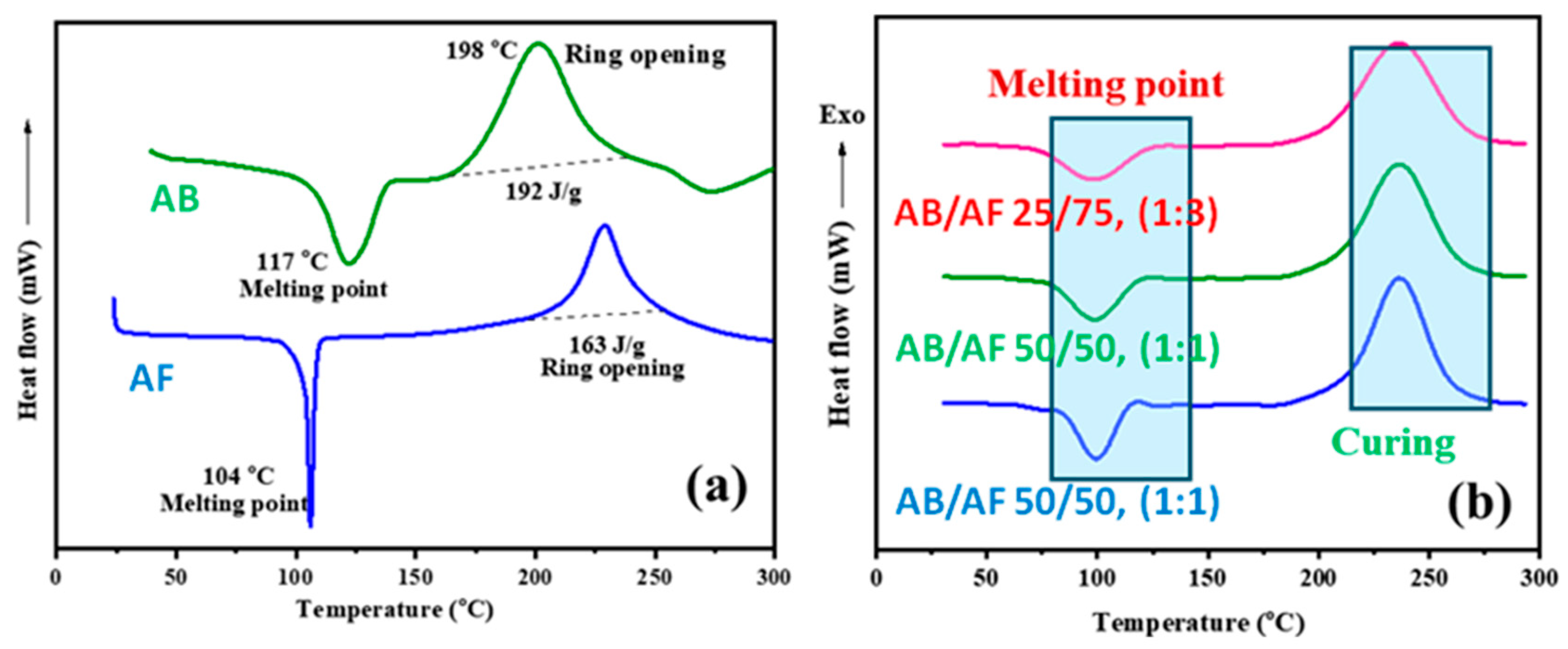


| Sample | Tm (℃) | Tonset (℃) | Tmax (℃) | Tfinal (℃) | PW (℃) |
|---|---|---|---|---|---|
| AB | 117 | 162 | 198 | 237 | 45 |
| AF | 104 | 190 | 227 | 256 | 86 |
| AB/AF (75/25) | 104 | 187 | 238 | 266 | 83 |
| AB/AF (50/50) | 103 | 186 | 240 | 265 | 83 |
| AB/AF (25/75) | 102 | 184 | 242 | 263 | 82 |
| Elements | Theoretical (%) | From XPS (%) | ||
|---|---|---|---|---|
| AB | AF | AB/AF (1:1) | AB/AF (1:1) | |
| C | 59.4 | 69.0 | 63.9 | 64.7 |
| N | 31.3 | 27.6 | 29.5 | 28.9 |
| O | 6.3 | 3.5 | 4.9 | 4.6 |
| Si | 3.1 | 0 | 1.6 | 1.8 |
| Sample | Ti (℃) | T10 (℃) | T50 (℃) | CY (%) |
|---|---|---|---|---|
| Poly(AB) | 250 | 302 | 350 | 32 |
| Poly(AF) | 296 | 350 | 394 | 38 |
| Poly(AB/AF) (75/25) | 253 | 310 | 356 | 35 |
| Poly(AB/AF) (50/50) | 305 | 348 | 396 | 39 |
| Poly(AB/AF) (25/75) | 252 | 309 | 354 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periyasamy, T.; Asrafali, S.P.; Lee, J. Sustainable Benzoxazine Copolymers with Enhanced Thermal Stability, Flame Resistance, and Dielectric Tunability. Polymers 2025, 17, 2092. https://doi.org/10.3390/polym17152092
Periyasamy T, Asrafali SP, Lee J. Sustainable Benzoxazine Copolymers with Enhanced Thermal Stability, Flame Resistance, and Dielectric Tunability. Polymers. 2025; 17(15):2092. https://doi.org/10.3390/polym17152092
Chicago/Turabian StylePeriyasamy, Thirukumaran, Shakila Parveen Asrafali, and Jaewoong Lee. 2025. "Sustainable Benzoxazine Copolymers with Enhanced Thermal Stability, Flame Resistance, and Dielectric Tunability" Polymers 17, no. 15: 2092. https://doi.org/10.3390/polym17152092
APA StylePeriyasamy, T., Asrafali, S. P., & Lee, J. (2025). Sustainable Benzoxazine Copolymers with Enhanced Thermal Stability, Flame Resistance, and Dielectric Tunability. Polymers, 17(15), 2092. https://doi.org/10.3390/polym17152092






