Preparation and Characterization of Thermo-Compressed Guar Gum/Microcrystalline Cellulose Composites for Applications in Sustainable Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
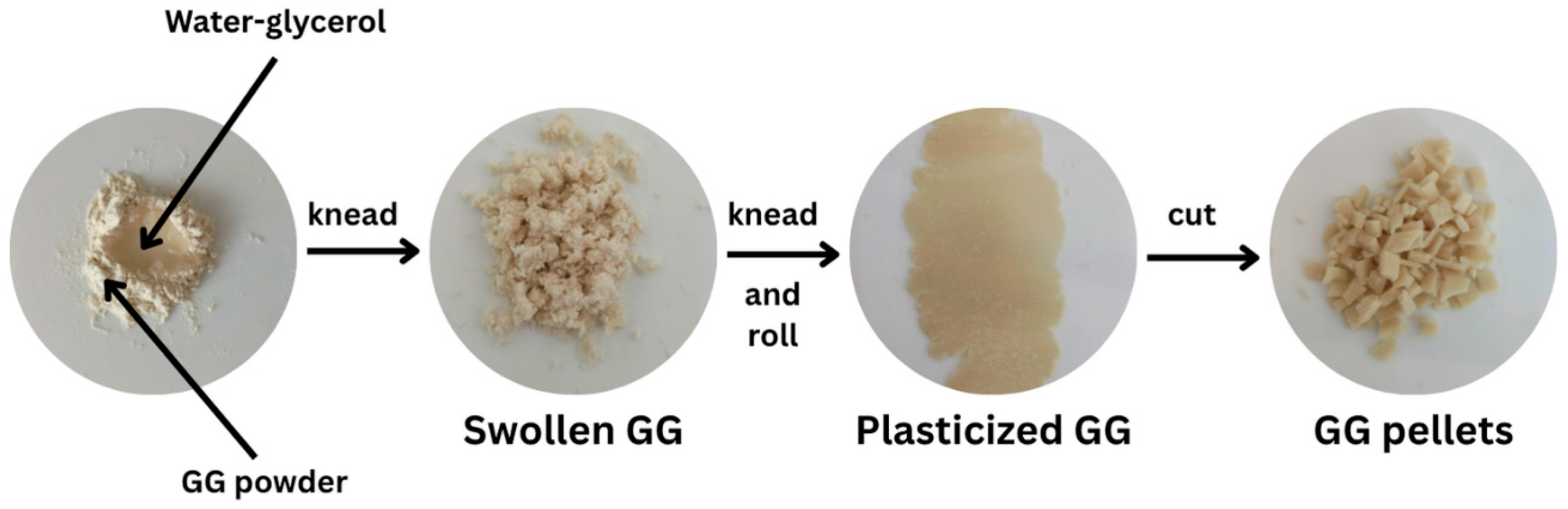
2.2. Preparation of Thermo-Compressed GG-Based Films
2.3. Characterization of GG Powder, MCC Powder, and Thermo-Compressed GG Films
2.3.1. FTIR Analysis
2.3.2. Thermal Stability
2.3.3. Crystalline Structures
2.3.4. Mechanical Testing
2.3.5. Morphology Analysis
2.3.6. Moisture Content
2.3.7. Film Opacity
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of Glycerol on Properties of GG Films
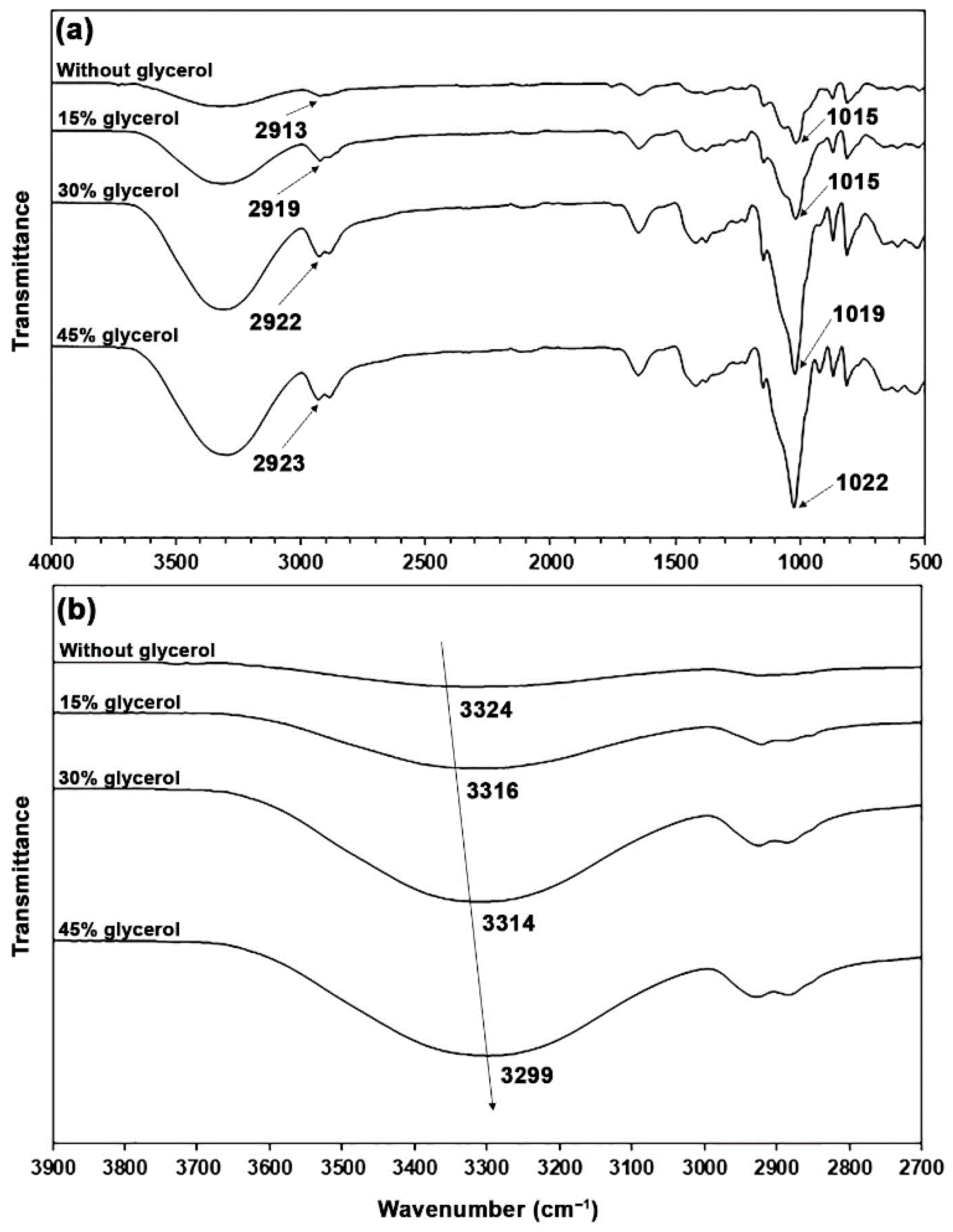
3.1.1. FTIR Analysis
3.1.2. TGA
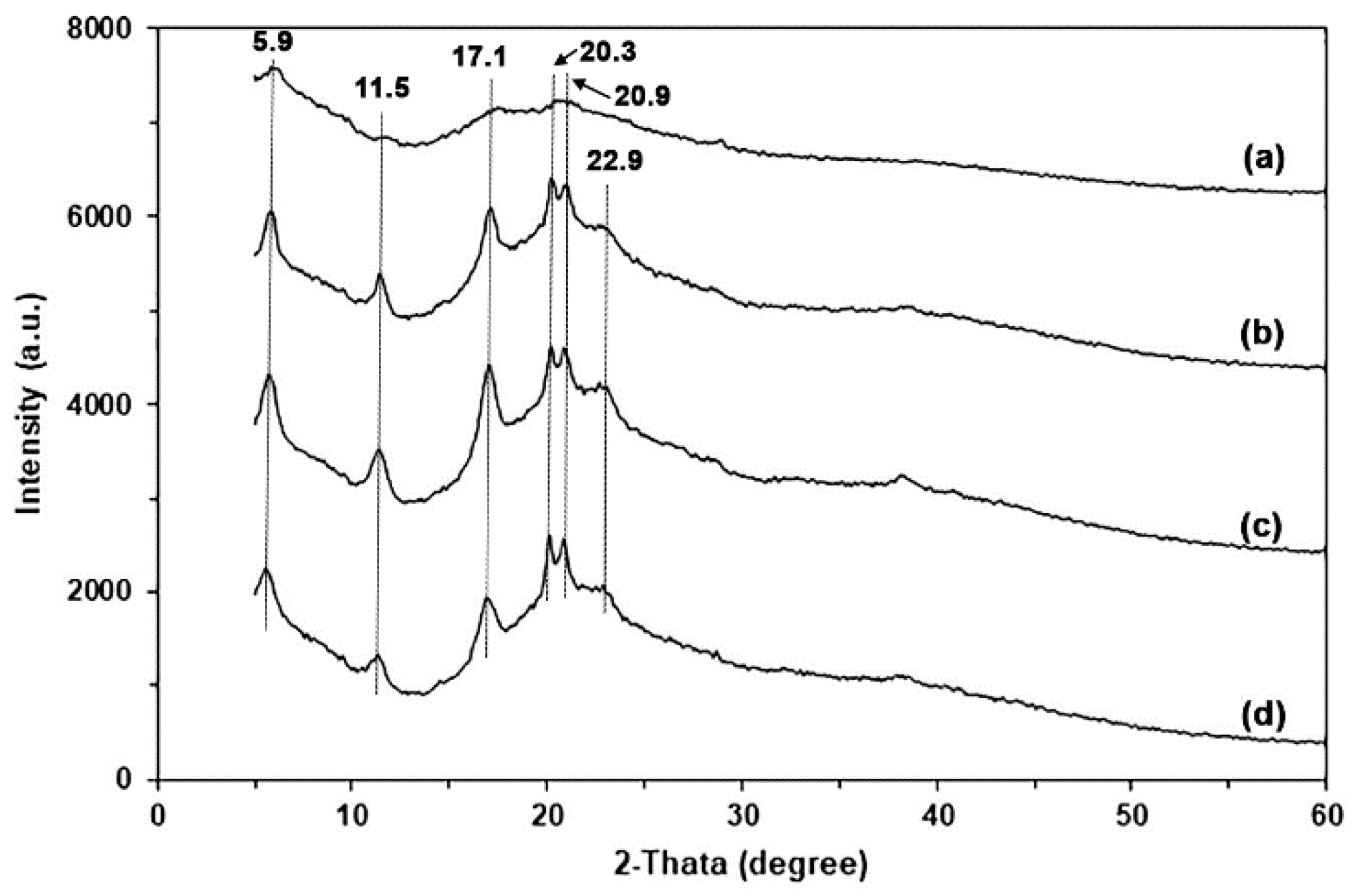
3.1.3. XRD
3.1.4. Tensile Properties
3.1.5. SEM
3.1.6. Moisture Content and Film Opacity
3.2. Effect of MCC on Properties of Plasticized GG Films
3.2.1. FTIR Analysis
3.2.2. TGA
3.2.3. XRD
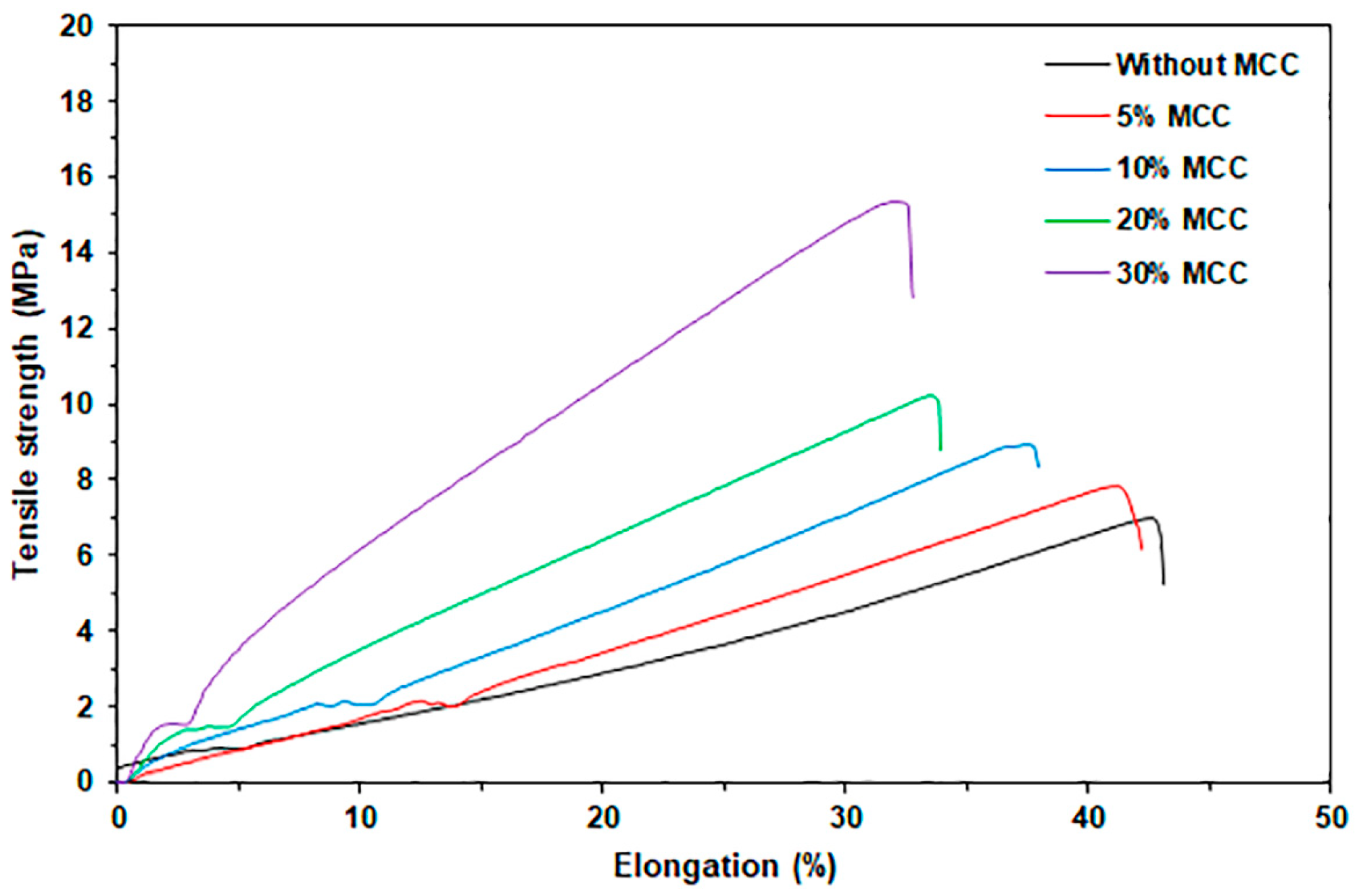
3.2.4. Tensile Properties
3.2.5. SEM
3.2.6. Moisture Content and Film Opacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathi, N.; Misra, M.; Mohanty, A.K. Durable polylactic acid (PLA)-based sustainable engineered blends and biocomposites: Recent developments, challenges, and opportunities. ACS Eng. Au 2021, 1, 7–38. [Google Scholar] [CrossRef]
- Andreeßen, C.; Steinbüchel, A. Recent developments in non-biodegradable biopolymers: Precursors, production processes, and future perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 143–157. [Google Scholar] [CrossRef]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V. Biodegradation of polymers: Stages, measurement, standards and prospects. Macromol 2023, 3, 371–399. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in research and development of bioplastic for food packaging. J. Sci. Food Agric. 2020, 100, 5032–5045. [Google Scholar] [CrossRef]
- Kakadellis, S.; Harris, Z.M. Don’t scrap the waste: The need for broader system boundaries in bioplastic food packaging life cycle assessment-A critical review. J. Clean Prod. 2020, 274, 122831. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Narrowing the gap for bioplastic use in food packaging: An update. Environ. Sci. Technol. 2020, 54, 4712–4732. [Google Scholar] [CrossRef]
- Flores, Y.; Pelegrín, C.J.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Chapter 9—Use of herbs and their bioactive compounds in active food packaging. In Aromatic Herbs in Food; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2021; pp. 323–365. [Google Scholar]
- Cheng, J.; Gao, R.; Zhu, Y.; Lin, Q. Applications of biodegradable materials in food packaging: A review. Alex. Eng. J. 2024, 91, 70–83. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Kumar, A.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Mola, G.T.; Stadler, F.J. Guar gum and its composites as potential materials for diverse applications: A review. Carbohydr. Polym. 2018, 199, 534–545. [Google Scholar] [CrossRef]
- Sharahi, M.; Bahrami, S.H.; Karimi, A. A comprehensive review on guar gum and its modified biopolymers: Their potential applications in tissue engineering. Carbohydr. Polym. 2025, 347, 122739. [Google Scholar] [CrossRef] [PubMed]
- Saya, L.; Malik, V.; Singh, A.; Singh, S.; Gambhir, G.; Singh, W.R.; Chandra, R.; Hooda, S. Guar gum based nanocomposites: Role in water purification through efficient removal of dyes and metal ions. Carbohydr. Polym. 2021, 261, 117851. [Google Scholar] [CrossRef]
- Hasan, A.M.A.; Abdel-Raouf, M.E. Applications of guar gum and its derivatives in petroleum industry: A review. Egypt J. Pet. 2018, 27, 1043–1050. [Google Scholar] [CrossRef]
- Maurizzi, E.; Bigi, F.; Volpelli, L.A.; Pulvirenti, A. Improving the post-harvest quality of fruits during storage through edible packaging based on guar gum and hydroxypropyl methylcellulose. Food Packag. Shelf Life 2023, 40, 101178. [Google Scholar] [CrossRef]
- Singha, T.; Tanwara, M.; Gupta, R.K. Carboxymethyl guar gum-based bioactive and biodegradable film for food packaging. Polym. Sci. Ser. A 2024, 66, 202–215. [Google Scholar] [CrossRef]
- Priyadarsini, P.; Biswal, M.; Gupta, S.; Mohanty, S.; Nayak, S.K. Development and characterization of ester modified endospermic guar gum/polyvinyl alcohol (PVA) blown film: Approach towards greener packaging. Ind. Crops Prod. 2022, 187, 115319. [Google Scholar] [CrossRef]
- Kirtil, E.; Aydogdu, A.; Svitova, T.; Radke, C.J. Assessment of the performance of several novel approaches to improve physical properties of guar gum based biopolymer films. Food Packag. Shelf Life 2021, 29, 100687. [Google Scholar] [CrossRef]
- Tripathi, J.; Ambolikar, R.S.; Gupta, S.; Variyar, P.S. Preparation and characterization of methylated guar gum based nano-composite films. Food Hydrocoll. 2022, 124, 107312. [Google Scholar] [CrossRef]
- Dehankar, H.B.; Mali, P.S.; Kumar, P. Edible composite films based on chitosan/guar gum with ZnONPs and roselle calyx extract for active food packaging. Appl. Food Res. 2023, 3, 100276. [Google Scholar] [CrossRef]
- Bal-Öztürk, A.; Torkay, G.; İdil, N.; Özkahraman, B.; Zehra Özbaş, Z. Gellan gum/guar gum films incorporated with honey as potential wound dressings. Polym. Bull. 2024, 81, 1211–1228. [Google Scholar] [CrossRef]
- Mondal, K.; Bhattacharjee, S.K.; Goud, V.V.; Katiyar, V. Effect of waste Dunaliella tertiolecta biomass ethanolic extract and turmeric essential oil on properties of guar gum-based active films. Food Hydrocoll. 2024, 146, 109199. [Google Scholar] [CrossRef]
- Karthik, K.; Velumayil, R.; Perumal, S.N.; Venkatesan, E.P.; Reddy, D.S.K.; Annakodi, V.A.; Alwetaishi, M.; Prabhakar, S. Synthesis and characterization of Borassus flabellifer flower waste-generated cellulose fillers reinforced PMC composites for lightweight applications. Sci. Rep. 2024, 14, 28389. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.S.; Yeu, Y.L.; Chung, P.P.; Soon, K.H. Extraction and characterization of microcrystalline cellulose (MCC) from durian rind for biocomposite application. J. Polym. Environ. 2024, 32, 6544–6575. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr. Polym. 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Wittaya, T. Microcomposites of rice starch film reinforced with microcrystalline cellulose from palm pressed fiber. Int. Food Res. J. 2009, 16, 493–500. [Google Scholar]
- Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R.; Montero, B. Processing and characterization of polyols plasticized-starch reinforced with microcrystalline cellulose. Carbohydr. Polym. 2016, 149, 83–93. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Tripathi, P.; Kumar, S.; Gaikwad, K.K. Enhanced heat sealability and barrier performance of guar gum/polyvinyl alcohol based on biocomposite film reinforced with micro-fibrillated cellulose for packaging application. Polym. Bull. 2025, 82, 3755–3783. [Google Scholar] [CrossRef]
- Schmid, M.; Reichert, K.; Hammann, F. Stabler, storage time-dependent alteration of molecular interaction-property relationships of whey protein isolate-based films and coating. J. Mater. Sci. 2025, 50, 4396–4404. [Google Scholar] [CrossRef]
- Gao, C.; Pollet, E.; Avérous, L. Properties of glycerol-plasticized alginate films obtained by thermo-mechanical mixing. Food Hydrocoll. 2017, 63, 414–420. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S.; Dehghannya, J. Development and characterization of biocomposite films made from kefiran, carboxymethyl cellulose and Satureja Khuzestanica essential oil. Food Chem. 2019, 289, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Fan, F.; Chu, Z.; Fan, C.; Qin, Y. Barrier properties and characterizations of poly(lactic acid)/ZnO nanocomposites. Molecules 2020, 25, 1310. [Google Scholar] [CrossRef] [PubMed]
- Nandal, K.; Vaid, V.; Rahul; Saini, P.; Devanshi; Sharma, R.K.; Joshi, V.; Jindal, R.; Mittal, H. Synthesis and characterization of κ-carrageenan and guar gum-based hydrogels for controlled release fertilizers: Optimization, release kinetics, and agricultural impact. Ind. Crops Prod. 2025, 225, 120587. [Google Scholar] [CrossRef]
- Ananthi, P.; Hemkumar, K.; Pius, A. Development of MOF and Cleome Gynandra infused guar gum film: A high-performance antibacterial and antioxidant packaging solution for fruits shelf life extension. Food Packag. Shelf Life 2025, 49, 101522. [Google Scholar] [CrossRef]
- Bhatia, S.; Shah, Y.A.; Al-Harrasi, A.; Alhadhrami, A.S.; Al Hashmi, D.S.H.; Jawad, M.; Dıblan, S.; Al Dawery, S.K.H.; Esatbeyoglu, T.; Anwer, M.K.; et al. Characterization of biodegradable films based on guar gum and calcium caseinate incorporated with clary sage oil: Rheological, physicochemical, antioxidant, and antimicrobial properties. J. Agric. Food Res. 2024, 15, 100948. [Google Scholar] [CrossRef]
- Madhu, K.; Dhal, M.K.; Banerjee, A.; Katiyar, V.; Kumar, A.A. Melt-processed cast films of calcite reinforced starch/guar-gum biopolymer composites for packaging applications. J. Mater. Sci. 2025, 60, 2689–2708. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Rheological and structural characterisation of film-forming solutions and biodegradable edible film made from kefiran as affected by various plasticizer types. Int. J. Biol. Macromol. 2011, 49, 814–821. [Google Scholar] [CrossRef]
- Khan, N.; Kumar, D.; Kumar, P. Silver nanoparticles embedded guar gum/gelatin nanocomposite: Green synthesis, characterization and antibacterial activity. Colloid Interfac. Sci. Comm. 2020, 35, 100242. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocoll. 2014, 41, 265–273. [Google Scholar] [CrossRef]
- Gao, C.; Pollet, E.; Avérous, L. Innovative plasticized alginate obtained by thermo-mechanical mixing: Effect of different biobased polyols systems. Carbohydr. Polym. 2017, 157, 669–676. [Google Scholar] [CrossRef]
- Palanichamy, P.; Venkatachalam, S.; Gupta, S. Tough, flexible and oil-resistant film from sonicated guar gum and cellulose nanofibers for food packaging. Food Packag. Shelf Life 2023, 40, 101189. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, S.; Liu, S.; Sun, Y.; Xu, H. A Contribution to improve barrier properties and reduce swelling ratio of κ-carrageenan film from the incorporation of guar gum or Locust bean gum. Polymers 2023, 15, 1751. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Bajpai, A. Synthesis of hydrophobically modified guar gum film for packaging materials. Mater. Today Proc. 2020, 29 Pt 4, 1132–1142. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef]
- Todica, M.; Nagy, E.M.; Niculaescu, C.; Stan, O.; Cioica, N.; Pop, C.V. XRD investigation of some thermal degraded starch-based materials. J. Spectrosc. 2016, 2016, 9605312. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Bergo, P.V.A.; Carvalho, R.A.; Sobral, P.J.A.; Santos, R.M.C.; Silva, F.B.R.; Prison, J.M.; Solorza-Feria, J.; Habitante, A.M.Q.M. Physical properties of edible films based on cassava starch as affected by the plasticizer concentration. Packag. Technol. Sci. 2002, 21, 85–89. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Noomhorm, A. Effect of plasticizers on mechanical and barrier properties of rice starch film. Starch 2004, 56, 348–356. [Google Scholar] [CrossRef]
- Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Film forming capacity of chemically modified corn starches. Carbohydr. Polym. 2008, 73, 573–581. [Google Scholar] [CrossRef]
- Talja, R.A.; Hele´n, H.; Ross, Y.H.; Jouppila, K. Effect of type and content of binary polyol mixtures on physical and mechanical properties of starch-based edible films. Carbohydr. Polym. 2008, 71, 269–276. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]
- Zakaria, N.H.; Muhammad, N.; Abdullah, M.M.A.B. Effect of glycerol content on mechanical, microstructure and physical properties of thermoplastic potato starch film. AIP Conf. Proc. 2018, 2030, 020230. [Google Scholar] [CrossRef]
- Rocha Plácido Moore, G.; Maria Martelli, S.; Gandolfo, C.; José do Amaral Sobral, P.; Borges Laurindo, J. Influence of the glycerol concentration on some physical properties of feather keratin films. Food Hydrocoll. 2006, 20, 975–982. [Google Scholar] [CrossRef]
- Saberi, B.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Water sorption isotherm of pea starch edible films and prediction models. Foods 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Saberi, B.; Thakur, R.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Optimization of physical and optical properties of biodegradable edible films based on pea starch and guar gum. Ind. Crops Prod. 2016, 86, 342–352. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (Arenga pinnata) starch for food packaging. J. Food Sci. Technol. 2016, 53, 326–336. [Google Scholar] [CrossRef]
- Dai, X.; Xiong, Z.; Na, H.; Zhu, J. How does epoxidized soybean oil improve the toughness of microcrystalline cellulose filled polylactide acid composites? Compos. Sci. Technol. 2014, 90, 9–15. [Google Scholar] [CrossRef]
- Lertprapaporn, T.; Manuspiya, H.; Laobuthee, A. Dielectric improvement from novel polymeric hybrid films derived by polylactic acid/nanosilver coated microcrystalline cellulose. Mater. Today Proc. 2018, 5 Pt 2, 9326–9335. [Google Scholar] [CrossRef]
- Othman, N.A.; Adam, F.; Yasin, N.H.M. Reinforced bioplastic film at different microcrystalline cellulose concentration. Mater. Today Proc. 2021, 41 Pt 1, 77–82. [Google Scholar] [CrossRef]
- Isıtan, A.; Pasquardini, L.; Bersani, M.; Gök, C.; Fioravanti, S.; Lunelli, L.; Çaglarer, E.; Koluman, A. Sustainable production of microcrystalline and nanocrystalline cellulose from textile waste using HCl and NaOH/urea treatment. Polymers 2025, 17, 48. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, K.; Zhou, M.; Wei, Y.J.; Cheng, F.; Lin, Y.; Zhu, P.X. High-performance starch films reinforced with microcrystalline cellulose made from Eucalyptus pulp via ball milling and mercerization. Starch 2019, 71, 1800218. [Google Scholar] [CrossRef]
- Rammak, T.; Boonsuk, P.; Kaewtatip, K. Mechanical and barrier properties of starch blend films enhanced with kaolin for application in food packaging. Int. J. Biol. Macromol. 2021, 192, 1013–1020. [Google Scholar] [CrossRef]
- Fouad, H.; Kian, L.K.; Jawaid, M.; Alotaibi, M.D.; Alothman, O.Y.; Hashem, M. Characterization of microcrystalline cellulose isolated from Conocarpus fiber. Polymers 2020, 12, 2926. [Google Scholar] [CrossRef]
- Asif, M.; Ahmed, D.; Ahmad, N.; Qamar, M.T.; Alruwaili, N.K.; Bukhari, S.N.A. Extraction and characterization of microcrystalline cellulose from Lagenaria siceraria fruit pedicles. Polymers 2022, 14, 1867. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Z.; Yang, S.; Ji, N.; Li, D. Extraction process research and characterization of microcrystalline cellulose derived from bamboo (Phyllostachys edulis (Carrière) J. Houz.) fibers. Polymers 2025, 17, 1143. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.-X.; Lian, Z.-X.; Wang, X.-X.; Zhou, J.; Ma, Z.-S. The effects of ultrasonic/microwave assisted treatment on the properties of soy protein isolate/microcrystalline wheat-bran cellulose film. J. Food Eng. 2013, 114, 183–191. [Google Scholar] [CrossRef]
- Hoque, M.B.; Solaiman; Alam, A.B.M.H.; Mahmud, H.; Nobi, A. Mechanical, degradation and water uptake properties of fabric reinforced polypropylene based composites: Effect of alkali on composites. Fibers 2018, 6, 94. [Google Scholar] [CrossRef]
- Kaushik, A.; Singh, M.; Verma, G. Green nanocomposites based on thermoplastic starch and steam exploded cellulose nanofibrils from wheat straw. Carbohydr. Polym. 2010, 82, 337–345. [Google Scholar] [CrossRef]
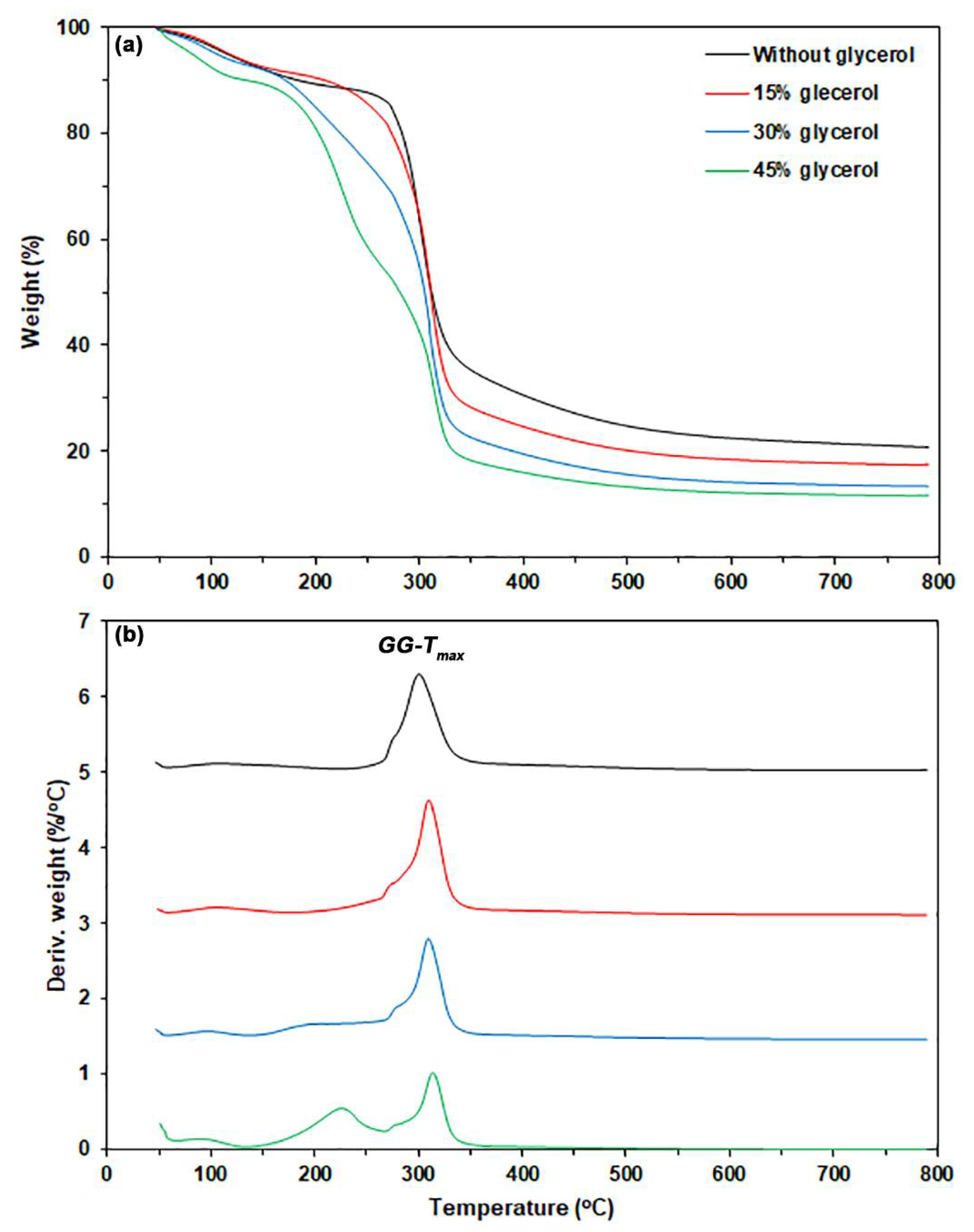
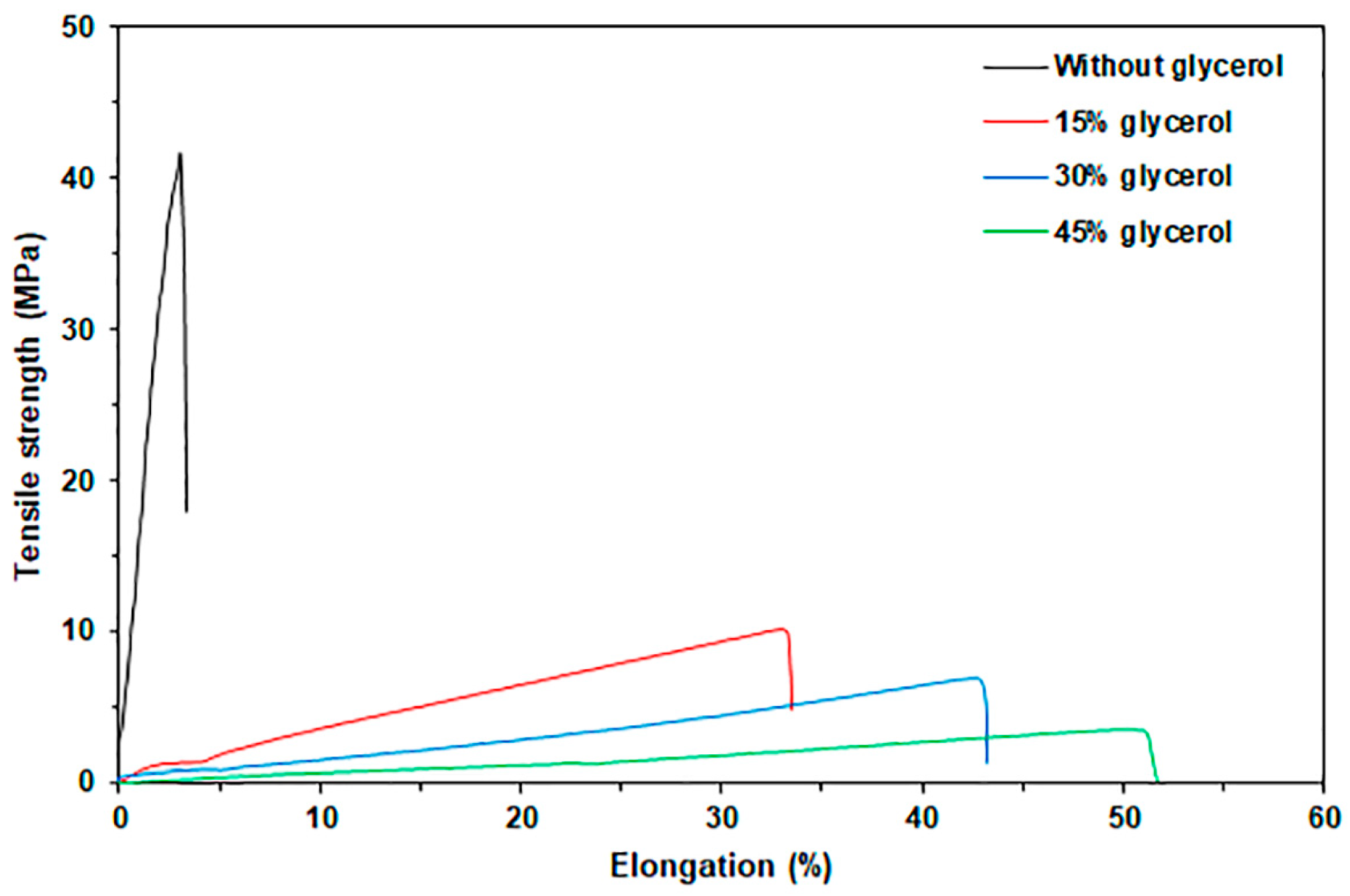
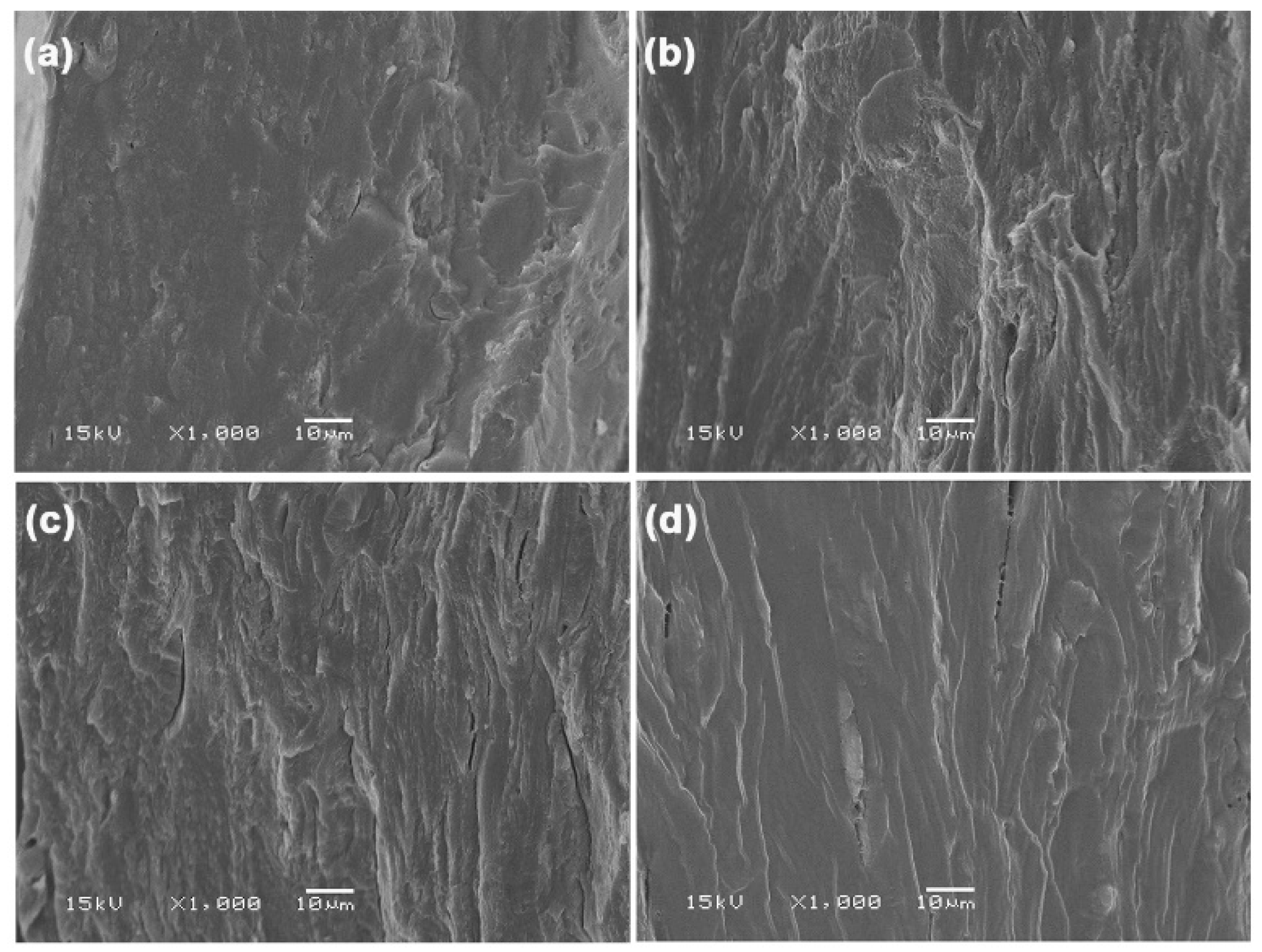


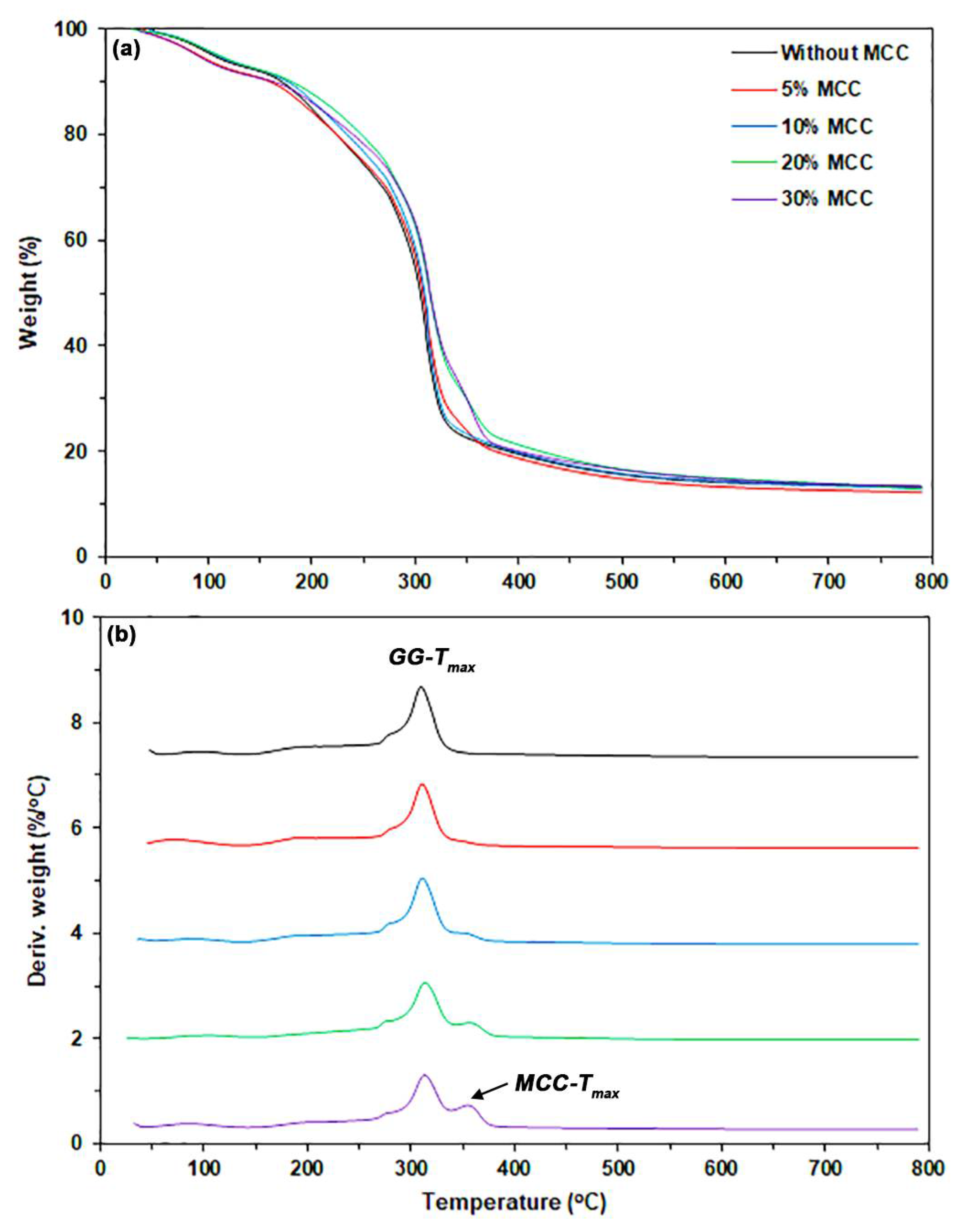
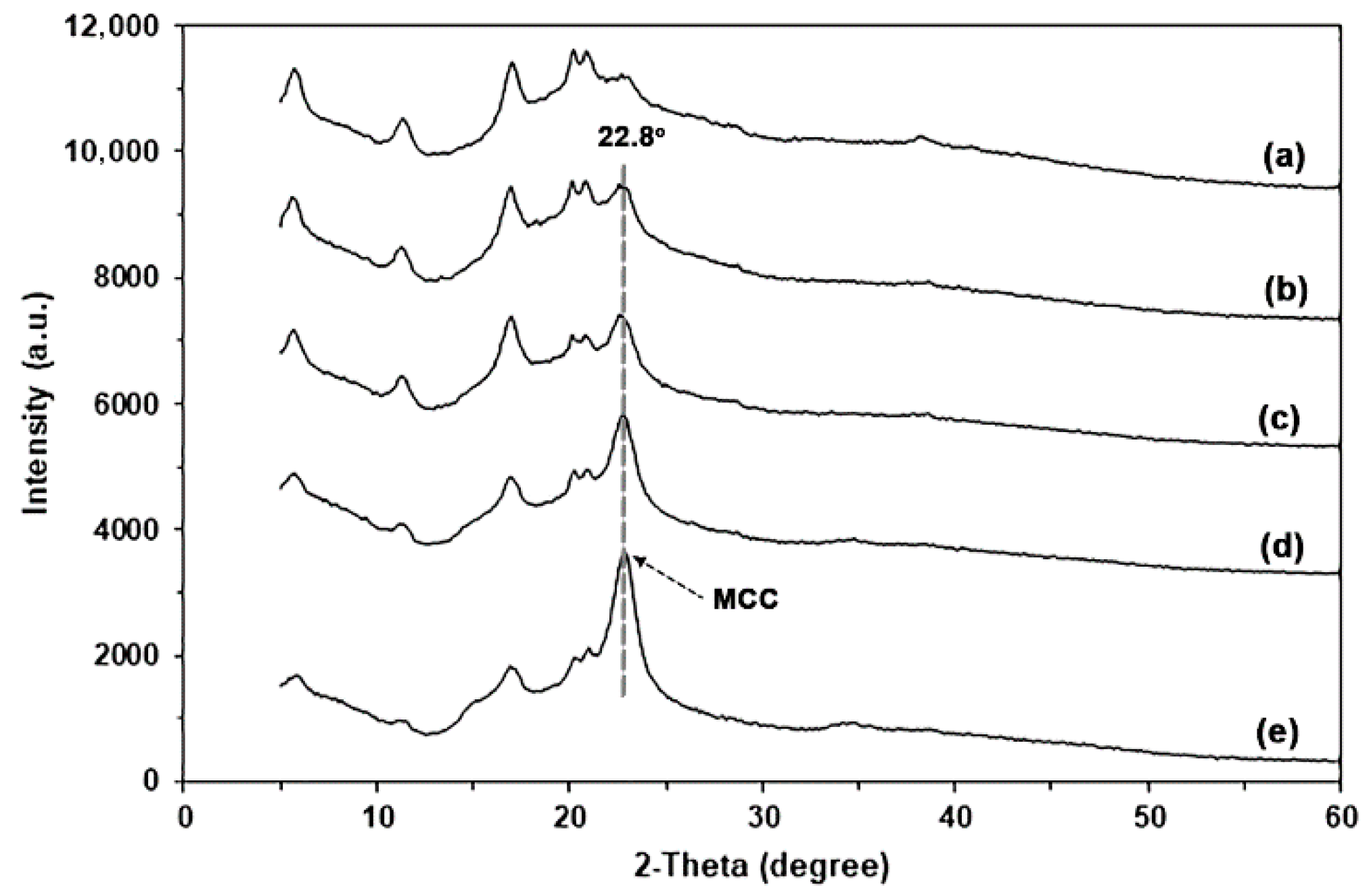


| Sample Code | Glycerol Content (wt%) | Weight Loss of Moisture (50–120 °C) (%) | Char Residue at 800 °C (%) | GG-Tmax (°C) |
|---|---|---|---|---|
| GG | - | 5.5 | 20.8 | 300 |
| GG/15Gly | 15 | 5.6 | 17.2 | 310 |
| GG/30Gly | 30 | 6.2 | 14.6 | 311 |
| GG/45Gly | 45 | 9.4 | 11.6 | 314 |
| Sample Code | Glycerol Content (wt%) | Maximum Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| GG | - | 41 ± 3 a | 3 ± 1 a | 988 ± 16 a |
| GG/15Gly | 15 | 11 ± 1 b | 35 ± 4 b | 30 ± 5 b |
| GG/30Gly | 30 | 8 ± 1 c | 42 ± 5 c | 9 ± 2 c |
| GG/45Gly | 45 | 4 ± 1 d | 57 ± 5 d | 8 ± 2 c |
| Sample Code | Glycerol Content (wt%) | Film Thickness (mm) | Moisture Content (%) | Film Opacity (mm−1) |
|---|---|---|---|---|
| GG | - | 0.19 ± 0.06 a | 10.2 ± 0.2 a | 1.67 ± 0.09 a |
| GG/15Gly | 15 | 0.20 ± 0.05 a | 13.1 ± 0.4 a | 1.71 ± 0.08 a |
| GG/30Gly | 30 | 0.21 ± 0.06 a | 26.1 ± 0.6 b | 1.70 ± 0.10 a |
| GG/45Gly | 45 | 0.19 ± 0.08 a | 41.8 ± 0.5 c | 1.68 ± 0.12 a |
| Sample Code | MCC Content (wt%) | Char Residue at 800 °C (%) | GG-Tmax (°C) | MCC-Tmax (°C) |
|---|---|---|---|---|
| GG/30Gly | - | 14.6 | 310 | - |
| GG/30Gly/5MCC | 5 | 12.9 | 311 | - |
| GG/30Gly/10MCC | 10 | 13.1 | 311 | 355 |
| GG/30Gly/20MCC | 20 | 13.3 | 314 | 358 |
| GG/30Gly/30MCC | 30 | 13.2 | 314 | 358 |
| Sample Code | MCC Content (wt%) | Maximum Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| GG/30Gly | - | 8 ± 1 a | 42 ± 5 c | 9 ± 2 a |
| GG/30Gly/5MCC | 5 | 9 ± 1 a,b | 42 ± 4 b,c | 17 ± 2 b |
| GG/30Gly/10MCC | 10 | 10 ± 1 b,c | 39 ± 3 b | 26 ± 3 c |
| GG/30Gly/20MCC | 20 | 11 ± 1 c | 33 ± 3 a | 30 ± 2 c |
| GG/30Gly/30MCC | 30 | 16 ± 2 d | 32 ± 2 a | 67 ± 5 d |
| Sample Code | MCC Content (wt%) | Film Thickness (mm) | Moisture Content (%) | Film Opacity (mm−1) |
|---|---|---|---|---|
| GG/30Gly | - | 0.21 ± 0.06 a | 26.1 ± 0.6 c | 1.70 ± 0.10 a |
| GG/30Gly/5MCC | 5 | 0.34 ± 0.11 ab | 11.5 ± 1.2 b | 2.58 ± 0.11 b |
| GG/30Gly/10MCC | 10 | 0.43 ± 0.08 b | 10.1 ± 0.8 b | 3.04 ± 0.08 c |
| GG/30Gly/20MCC | 20 | 0.62 ± 0.10 c | 8.4 ± 0.9 a | 4.40 ± 0.06 d |
| GG/30Gly/30MCC | 30 | 0.83 ± 0.12 d | 8.1 ± 0.8 a | 5.07 ± 0.10 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srihanam, P.; Jirum, J.; Noppawan, P.; Khotsaeng, N.; Baimark, Y. Preparation and Characterization of Thermo-Compressed Guar Gum/Microcrystalline Cellulose Composites for Applications in Sustainable Packaging. Polymers 2025, 17, 3124. https://doi.org/10.3390/polym17233124
Srihanam P, Jirum J, Noppawan P, Khotsaeng N, Baimark Y. Preparation and Characterization of Thermo-Compressed Guar Gum/Microcrystalline Cellulose Composites for Applications in Sustainable Packaging. Polymers. 2025; 17(23):3124. https://doi.org/10.3390/polym17233124
Chicago/Turabian StyleSrihanam, Prasong, Jenjira Jirum, Pakin Noppawan, Nuanchai Khotsaeng, and Yodthong Baimark. 2025. "Preparation and Characterization of Thermo-Compressed Guar Gum/Microcrystalline Cellulose Composites for Applications in Sustainable Packaging" Polymers 17, no. 23: 3124. https://doi.org/10.3390/polym17233124
APA StyleSrihanam, P., Jirum, J., Noppawan, P., Khotsaeng, N., & Baimark, Y. (2025). Preparation and Characterization of Thermo-Compressed Guar Gum/Microcrystalline Cellulose Composites for Applications in Sustainable Packaging. Polymers, 17(23), 3124. https://doi.org/10.3390/polym17233124








