Novel Bioactive Kefiran-Based Films Enriched with Grape Pomace Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Reagents
2.1.2. Plant Material
2.2. Obtaining of Grape Pomace Extract (GPE)
2.2.1. Preparation of Grape Pomace
2.2.2. Preparation of GPE
2.3. Evaluation of the Antioxidant and Antimicrobial Capacity of GPE
2.3.1. Antioxidant Activity and Total Phenolic Content of GPE
Antioxidant Activity by the ABTS Method
Antioxidant Capacity by the DPPH Method
Total Phenolic Content
2.3.2. Determination of Antimicrobial Activity
Preparation of Pathogenic Microorganism Inocula
Minimum Inhibitory and Bactericidal Concentrations
2.4. Kefiran Production
2.4.1. Kefir Grain Fermentation
2.4.2. Kefiran Extraction
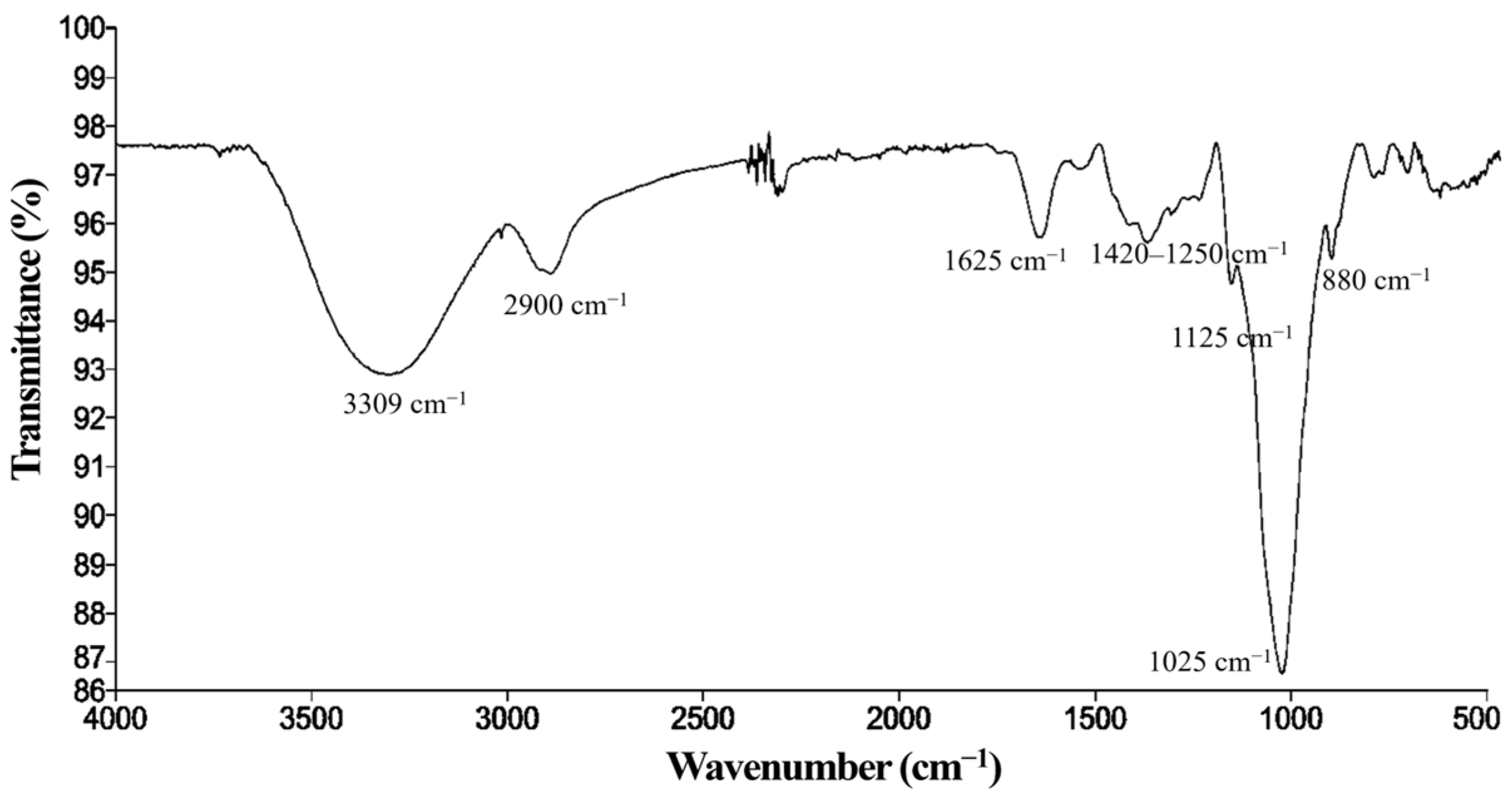
2.4.3. Kefiran Characterization by Fourier Transform Infrared (FT-IR) Spectroscopy
2.5. Development of Kefiran Films Incorporated with GPE
2.5.1. Preparation of Film-Forming Solutions
2.5.2. Film Preparation
2.6. Characterization of Kefiran/GPE Based Films
2.6.1. Evaluation of Physicochemical and Mechanical Properties
Color Analysis
UV-Vis Barrier Capacity
FT-IR Spectroscopy Analysis
Thickness and Mechanical Properties
Water Solubility Evaluation
Determination of Water Vapor Permeability (WVP)
2.6.2. Biological Activity of the Kefiran/GPE Films
Antioxidant Activity
Antimicrobial Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extraction of GPE
3.2. Antioxidant and Antimicrobial Activity of GPE
3.3. Kefiran Production by Fermentation
Kefiran Characterization by FT-IR
3.4. Development of Kefiran-Based Films with Grape Pomace Extract
3.5. Characterization of the Kefiran/GPE-Based Films
3.5.1. Physicochemical and Mechanical Properties
Color
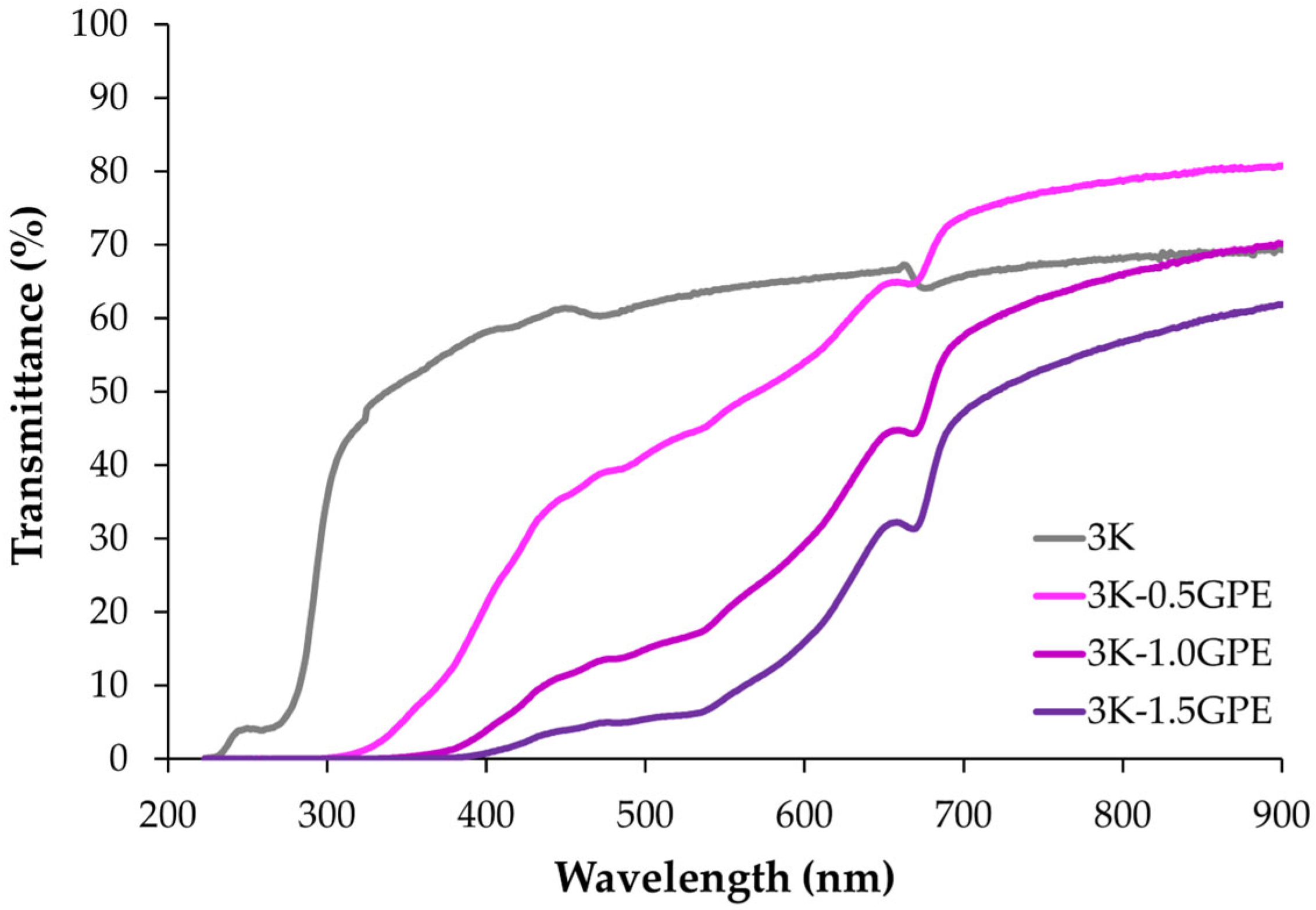
UV–Vis Protection
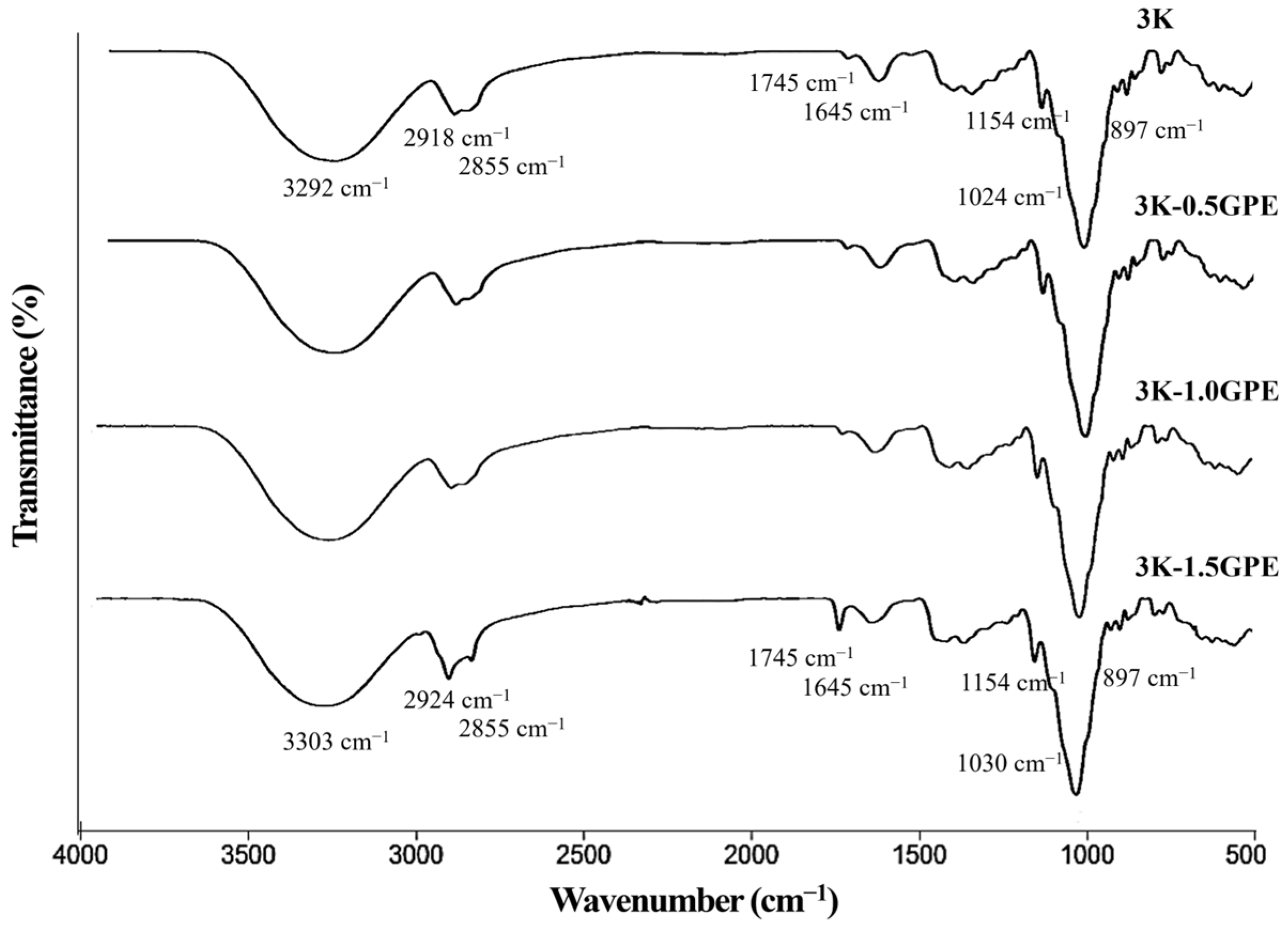
FT-IR Spectroscopy
Thickness, Tensile Strength, and Elongation
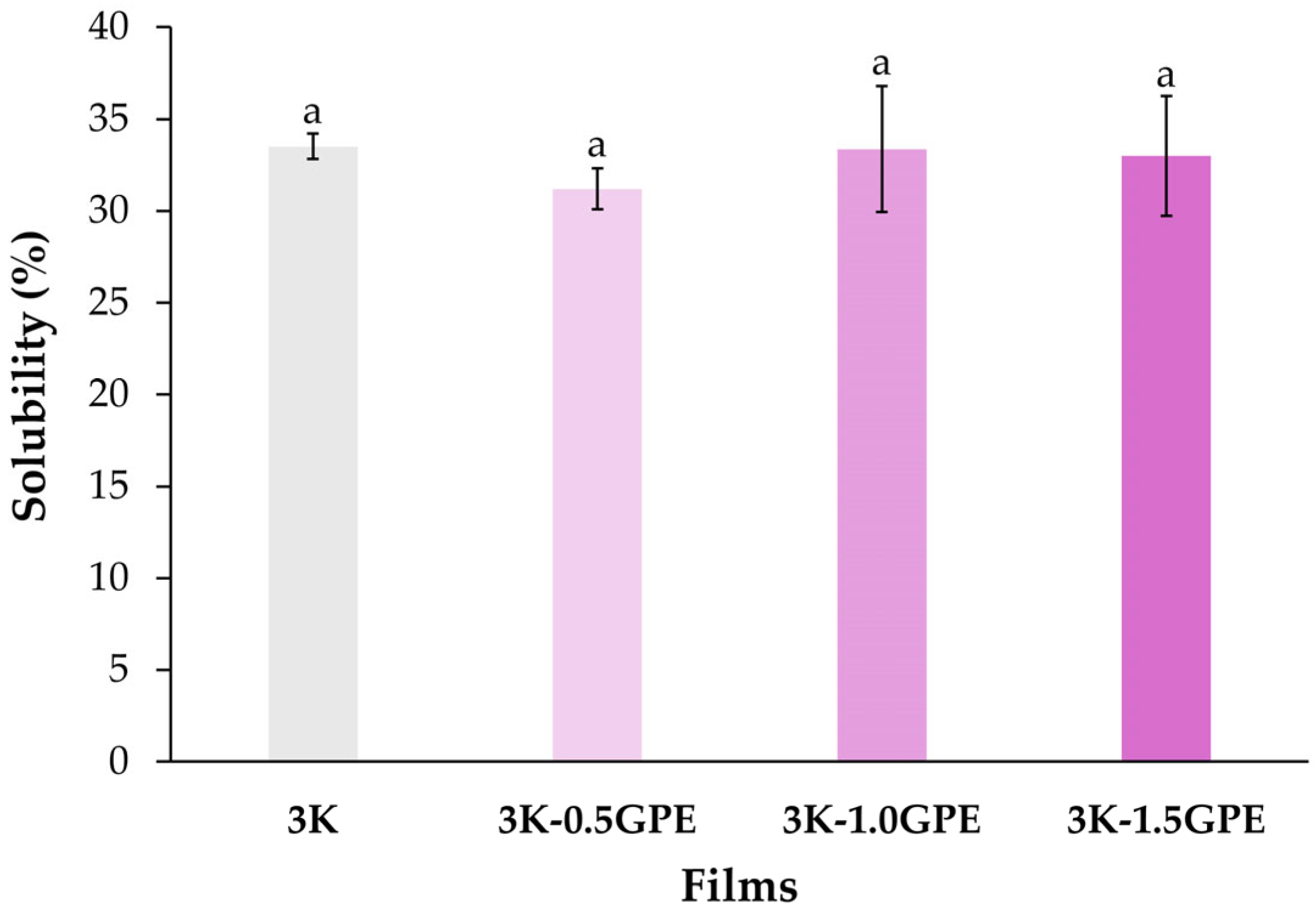
Water Solubility
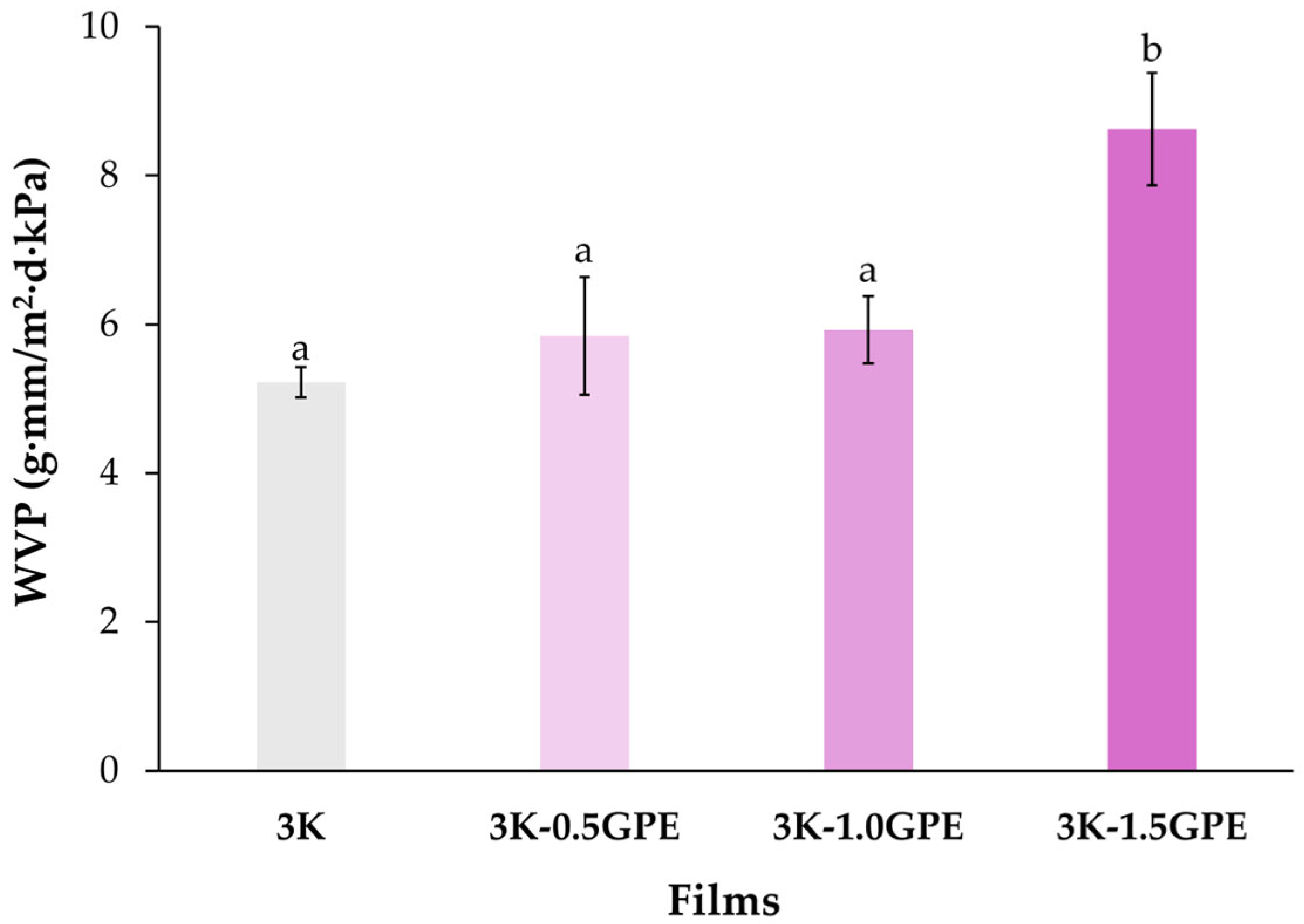
Water Vapor Permeability (WVP)
3.5.2. Bioactive Properties of the Kefiran/GPE-Based Films
Antioxidant Capacity of the Films
Antimicrobial Activity of the Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández-García, E.; Vargas, M.; González-Martínez, C.; Chiralt, A. Biodegradable Antimicrobial Films for Food Packaging: Effect of Antimicrobials on Degradation. Foods 2021, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pratibha; Prasad, J.; Yadav, A.; Upadhyay, A.; Neeraj; Shukla, S.; Petkoska, A.T.; Heena; Suri, S. Recent Trends in Edible Packaging for Food Applications—Perspective for the Future. Food Eng. Rev. 2023, 15, 718–747. [Google Scholar] [CrossRef]
- Dirpan, A.; Ainani, A.F.; Djalal, M. A Review on Biopolymer-Based Biodegradable Film for Food Packaging: Trends over the Last Decade and Future Research. Polymers 2023, 15, 2781. [Google Scholar] [CrossRef]
- Khan, N.; Sudhakar, K.; Mamat, R. Biodegradable Plastics from Marine Biomass: A Solution to Marine Plastic Pollution. J. Hazard. Mater. Adv. 2025, 17, 100559. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-Based Films and Coatings for Food Packaging: A Review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Khodaei, D.; Álvarez, C.; Mullen, A.M. Biodegradable Packaging Materials from Animal Processing Co-Products and Wastes: An Overview. Polymers 2021, 13, 2561. [Google Scholar] [CrossRef]
- Prakoso, F.A.H.; Indiarto, R.; Utama, G.L. Edible Film Casting Techniques and Materials and Their Utilization for Meat-Based Product Packaging. Polymers 2023, 15, 2800. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Hossain, A. Preservation of Aquatic Food Using Edible Films and Coatings Containing Essential Oils: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 66–105. [Google Scholar] [CrossRef]
- Matloob, A.; Ayub, H.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Khalid, W.; Nayik, G.A.; Ramniwas, S.; et al. A Review on Edible Coatings and Films: Advances, Composition, Production Methods, and Safety Concerns. ACS Omega 2023, 8, 28932–28944. [Google Scholar] [CrossRef]
- Ribeiro, I.S.; Maciel, G.M.; Bortolini, D.G.; Fernandes, I.A.A.; Maroldi, W.V.; Pedro, A.C.; Rubio, F.T.V.; Haminiuk, C.W.I. Sustainable Innovations in Edible Films and Coatings: An Overview. Trends Food Sci. Technol. 2024, 143, 104272. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Antonino, C.; Difonzo, G.; Faccia, M.; Caponio, F. Effect of Edible Coatings and Films Enriched with Plant Extracts and Essential Oils on the Preservation of Animal-Derived Foods. J. Food Sci. 2024, 89, 748–772. [Google Scholar] [CrossRef]
- de Carvalho, A.P.A.; Conte-Junior, C.A. Food-Derived Biopolymer Kefiran Composites, Nanocomposites and Nanofibers: Emerging Alternatives to Food Packaging and Potentials in Nanomedicine. Trends Food Sci. Technol. 2021, 116, 370–386. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Vieira, R.P.; Rodrigues Anjos, C.A. Kefiran-Based Films: Fundamental Concepts, Formulation Strategies and Properties. Carbohydr. Polym. 2020, 246, 116609. [Google Scholar] [CrossRef]
- Radhouani, H.; Gonçalves, C.; Maia, F.R.; Oliveira, J.M.; Reis, R.L. Kefiran Biopolymer: Evaluation of Its Physicochemical and Biological Properties. J. Bioact. Compat. Polym. 2018, 33, 461–478. [Google Scholar] [CrossRef]
- Tan, K.X.; Chamundeswari, V.N.; Loo, S.C.J. Prospects of Kefiran as a Food-Derived Biopolymer for Agri-Food and Biomedical Applications. RSC Adv. 2020, 10, 25339–25351. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, J. Kefir: The Champagne of Fermented Beverages. In Fermented Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2019; pp. 473–527. [Google Scholar] [CrossRef]
- Moradi, Z.; Kalanpour, N. Kefiran, a Branched Polysaccharide: Preparation, Properties and Applications: A Review. Carbohydr. Polym. 2019, 223, 115100. [Google Scholar] [CrossRef] [PubMed]
- Piermaria, J.A.; Pinotti, A.; Garcia, M.A.; Abraham, A.G. Films Based on Kefiran, an Exopolysaccharide Obtained from Kefir Grain: Development and Characterization. Food Hydrocoll. 2009, 23, 684–690. [Google Scholar] [CrossRef]
- Piermaria, J.; Bosch, A.; Pinotti, A.; Yantorno, O.; Garcia, M.A.; Abraham, A.G. Kefiran Films Plasticized with Sugars and Polyols: Water Vapor Barrier and Mechanical Properties in Relation to Their Microstructure Analyzed by ATR/FT-IR Spectroscopy. Food Hydrocoll. 2011, 25, 1261–1269. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Physical, Mechanical, Barrier, and Thermal Properties of Polyol-Plasticized Biodegradable Edible Film Made from Kefiran. Carbohydr. Polym. 2011, 84, 477–483. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A.; Yarmand, M.S. Development and Characterisation of a New Biodegradable Edible Film Made from Kefiran, an Exopolysaccharide Obtained from Kefir Grains. Food Chem. 2011, 127, 1496–1502. [Google Scholar] [CrossRef]
- Motedayen, A.A.; Khodaiyan, F.; Salehi, E.A. Development and Characterisation of Composite Films Made of Kefiran and Starch. Food Chem. 2013, 136, 1231–1238. [Google Scholar] [CrossRef]
- Sabaghi, M.; Maghsoudlou, Y.; Habibi, P. Enhancing Structural Properties and Antioxidant Activity of Kefiran Films by Chitosan Addition. Food Struct. 2015, 5, 66–71. [Google Scholar] [CrossRef]
- Hasheminya, S.M.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S.; Dehghannya, J. Development and Characterization of Biocomposite Films Made from Kefiran, Carboxymethyl Cellulose and Satureja khuzestanica Essential Oil. Food Chem. 2019, 289, 443–452. [Google Scholar] [CrossRef]
- Motedayen, A.A.; Khodaiyan, F.; Salehi, E.A.; Hosseini, S. Characterisation of Biocomposite Film Made of Kefiran and Carboxymethyl Cellulose (CMC). J. Food Bioprocess Eng. 2019, 2, 61–70. [Google Scholar]
- Salmanian, H.; Khodaiyan, F.; Hosseini, S.S. Biodegradable Kefiran-Chitosan-Nanocellulose Blend Film: Production and Physical, Barrier, Mechanical, Thermal, and Structural Properties. J. Food Bioprocess Eng. 2019, 2, 101–106. [Google Scholar]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. The Improvement of Characteristics of Biodegradable Films Made from Kefiran-Whey Protein by Nanoparticle Incorporation. Carbohydr. Polym. 2014, 109, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. Development and Characterization of the Kefiran-Whey Protein Isolate-TiO2 Nanocomposite Films. Int. J. Biol. Macromol. 2014, 65, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Hedayati Rad, F.; Sharifan, A.; Asadi, G. Physicochemical and Antimicrobial Properties of Kefiran/Waterborne Polyurethane Film Incorporated with Essential Oils on Refrigerated Ostrich Meat. LWT 2018, 97, 794–801. [Google Scholar] [CrossRef]
- Jourabian, F.; Nouri, M. Optimization of Formulated Kefiran/Malva Neglecta Film with Rice Bran Oil to Maintain Cauliflower Quality in Storage. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 697–703. [Google Scholar] [CrossRef]
- Pinto, T.; Pinto, A.; Vilela, A. Edible Coatings and Films for Preparation of Grapevine By-Product Infusions and in Freshly Processed Products. Coatings 2023, 13, 1350. [Google Scholar] [CrossRef]
- Benković, M.; Cigić, F.; Valinger, D.; Sokač Cvetnić, T.; Jurinjak Tušek, A.; Jurina, T.; Gajdoš Kljusurić, J.; Radojčić Redovniković, I. Towards Wine Waste Reduction: Up-Cycling Wine Pomace into Functional Fruit Bars. Processes 2024, 12, 2941. [Google Scholar] [CrossRef]
- Wang, C.; You, Y.; Huang, W.; Zhan, J. The High-Value and Sustainable Utilization of Grape Pomace: A Review. Food Chem. X 2024, 24, 101845. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape Pomace as a Source of Phenolic Compounds and Diverse Bioactive Properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef]
- Singh, A.K.; Kim, J.Y.; Lee, Y.S. Phenolic Compounds in Active Packaging and Edible Films/Coatings: Natural Bioactive Molecules and Novel Packaging Ingredients. Molecules 2022, 27, 7513. [Google Scholar] [CrossRef]
- Goiana, M.L.; de Freitas Rosa, M.; Mattos, A.L.A.; Fernandes, F.A.N. Development of Plasma-Treated Corn-Starch-Based Film Incorporated with Acerola and Grape Pomace Extract Possessing pH-Sensing Capability. Polymers 2025, 17, 938. [Google Scholar] [CrossRef]
- Xu, Y.; Scales, A.; Jordan, K.; Kim, C.; Sismour, E. Starch Nanocomposite Films Incorporating Grape Pomace Extract and Cellulose Nanocrystal. J. Appl. Polym. Sci. 2017, 134, 44438. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Nunes, C.; Castro, A.; Ferreira, P.; Coimbra, M.A. Influence of Grape Pomace Extract Incorporation on Chitosan Films Properties. Carbohydr. Polym. 2014, 113, 490–499. [Google Scholar] [CrossRef]
- Xu, Y.; Willis, S.; Jordan, K.; Sismour, E. Chitosan Nanocomposite Films Incorporating Cellulose Nanocrystals and Grape Pomace Extracts. Packag. Technol. Sci. 2018, 31, 631–638. [Google Scholar] [CrossRef]
- Gorrasi, G.; Viscusi, G.; Gerardi, C.; Lamberti, E.; Giovinazzo, G. Physicochemical and Antioxidant Properties of White (Fiano cv) and Red (Negroamaro cv) Grape Pomace Skin Based Films. J. Polym. Environ. 2022, 30, 3609–3621. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Marasciulo, C.; Maggi, F.; Caprioli, G.; Mustafa, A.M.; Fini, P.; De Vietro, N.; Aresta, A.M.; Cosma, P. Realizing Eco-Friendly Water-Resistant Sodium-Alginate-Based Films Blended with a Polyphenolic Aqueous Extract from Grape Pomace Waste for Potential Food Packaging Applications. Int. J. Mol. Sci. 2023, 24, 11462. [Google Scholar] [CrossRef]
- Deng, Q.; Zhao, Y. Physicochemical, Nutritional, and Antimicrobial Properties of Wine Grape (cv. Merlot) Pomace Extract-Based Films. J. Food Sci. 2011, 76, E309–E317. [Google Scholar] [CrossRef] [PubMed]
- Bruna, J.E.; Castillo, M.; López de Dicastillo, C.; Muñoz-Shugulí, C.; Lira, M.; Guarda, A.; Rodríguez-Mercado, F.J.; Galotto, M.J. Development of Active Biocomposite Films Based on Poly (lactic acid) and Wine by-Product: Effect of Grape Pomace Content and Extrusion Temperature. J. Appl. Polym. Sci. 2023, 140, 54425. [Google Scholar] [CrossRef]
- da Silva, D.J.; de Oliveira, M.M.; Wang, S.H.; Carastan, D.J.; Rosa, D.S. Designing Antimicrobial Polypropylene Films with Grape Pomace Extract for Food Packaging. Food Packag. Shelf Life 2022, 34, 100929. [Google Scholar] [CrossRef]
- Gaber Ahmed, G.H.; Fernández-González, A.; Díaz García, M.E. Nano-Encapsulation of Grape and Apple Pomace Phenolic Extract in Chitosan and Soy Protein via Nanoemulsification. Food Hydrocoll. 2020, 108, 105806. [Google Scholar] [CrossRef]
- Guzmán-Díaz, D.A.; Treviño-Garza, M.Z.; Rodríguez-Romero, B.A.; Gallardo-Rivera, C.T.; Amaya-Guerra, C.A.; Báez-González, J.G. Development and Characterization of Gelled Double Emulsions Based on Chia (Salvia hispanica L.) Mucilage Mixed with Different Biopolymers and Loaded with Green Tea Extract (Camellia sinensis). Foods 2019, 8, 677. [Google Scholar] [CrossRef] [PubMed]
- Treviño-Garza, M.Z.; Yañez-Echeverría, S.A.; García, S.; Mora-Zúñiga, A.E.; Arévalo-Niño, K. Physico-Mechanical, Barrier and Antimicrobial Properties of Linseed Mucilage Films Incorporated with H. Virginiana Extract. Rev. Mex. Ing. Quím. 2020, 19, 983–996. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A Comparative Study on Different Extraction Techniques to Recover Red Grape Pomace Polyphenols from Vinification Byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Montoille, L.; Morales Vicencio, C.; Fontalba, D.; Ortiz, J.A.; Moreno-Serna, V.; Peponi, L.; Matiacevich, S.; Zapata, P.A. Study of the Effect of the Addition of Plasticizers on the Physical Properties of Biodegradable Films Based on Kefiran for Potential Application as Food Packaging. Food Chem. 2021, 360, 129966. [Google Scholar] [CrossRef]
- Amadeu, C.A.A.; Silva, F.B.; Souza, C.J.F.; Koschevic, M.T.; Schoeninger, V.; Falcão, E.A.; Garcia, V.A.D.S.; Cardoso, C.A.L.; Martelli, S.M. Pectin Edible Films Filled with Ilex Paraguariensis Concentrate Extract and Its Characterization. Polymers 2024, 16, 3158. [Google Scholar] [CrossRef]
- Moges, T.G.; Kassa, H.S.; Woldemariam, H.W. Optimization and Characterization of Active Bio-Plastic Film from Tamarind (Tamarindus indica L.) Seed Starch Enriched with Red Grape Pomace Extract. Biomass Convers. Biorefin. 2024, 15, 14179–14197. [Google Scholar] [CrossRef]
- Knapp, M.A.; dos Santos, D.F.; Pilatti-Riccio, D.; Deon, V.G.; dos Santos, G.H.F.; Pinto, V.Z. Yerba Mate Extract in Active Starch Films: Mechanical and Antioxidant Properties. J. Food Process. Preserv. 2019, 43, e13897. [Google Scholar] [CrossRef]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic Compounds, Antioxidant, and Antibacterial Properties of Pomace Extracts from Four Virginia-Grown Grape Varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine By-Products: Phenolic Characterization and Antioxidant Activity Evaluation of Grapes and Grape Pomaces from Six Different French Grape Varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef] [PubMed]
- Ky, I.; Teissedre, P.L. Characterisation of Mediterranean Grape Pomace Seed and Skin Extracts: Polyphenolic Content and Antioxidant Activity. Molecules 2015, 20, 2190–2207. [Google Scholar] [CrossRef]
- Moutinho, J.; Gouvinhas, I.; Domínguez-Perles, R.; Barros, A. Optimization of the Extraction Methodology of Grape Pomace Polyphenols for Food Applications. Molecules 2023, 28, 3885. [Google Scholar] [CrossRef]
- Batista, D.; de Matuoka e Chiocchetti, G.; Macedo, J.A. Effect of Enzymatic Biotransformation on the Hypotensive Potential of Red Grape Pomace Extract. Foods 2023, 12, 4109. [Google Scholar] [CrossRef] [PubMed]
- Chengolova, Z.; Ivanov, Y.; Godjevargova, T. Comparison of Identification and Quantification of Polyphenolic Compounds in Skins and Seeds of Four Grape Varieties. Molecules 2023, 28, 4061. [Google Scholar] [CrossRef]
- Rasines-Perea, Z.; Ky, I.; Cros, G.; Crozier, A.; Teissedre, P.L. Grape Pomace: Antioxidant Activity, Potential Effect Against Hypertension and Metabolites Characterization after Intake. Diseases 2018, 6, 60. [Google Scholar] [CrossRef]
- Cheng, V.J.; Bekhit, A.E.-D.A.; McConnell, M.; Mros, S.; Zhao, J. Effect of Extraction Solvent, Waste Fraction and Grape Variety on the Antimicrobial and Antioxidant Activities of Extracts from Wine Residue from Cool Climate. Food Chem. 2012, 134, 474–482. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical Composition, Antioxidant and Antimicrobial Activity of Phenolic Compounds Extracted from Wine Industry by-Products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Grosu, A.C.; Diguță, F.C.; Pristavu, M.-C.; Popa, A.; Badea, F.; Dragoi Cudalbeanu, M.; Orțan, A.; Dopcea, I.; Băbeanu, N. Exploring the Phytochemical Profiles, and Antioxidant and Antimicrobial Activities of the Hydroethanolic Grape Pomace Extracts from Two Romanian Indigenous Varieties. Fermentation 2024, 10, 470. [Google Scholar] [CrossRef]
- Hassan, Y.I.; Kosir, V.; Yin, X.; Ross, K.; Diarra, M.S. Grape Pomace as a Promising Antimicrobial Alternative in Feed: A Critical Review. J. Agric. Food Chem. 2019, 67, 9705–9718. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.F.; de Moura, N.K.; de Moura, T.K.; de Araújo, T.V.; Machado, J.P.B.; Passador, F.R.; Esposito, E. Determination and Standardization of the Kefiran Extraction Protocol for Possible Pharmacological Applications. Carbohydr. Polym. Technol. Appl. 2022, 3, 100198. [Google Scholar] [CrossRef]
- Pop, C.R.; Salanță, L.C.; Rotar, A.M.; Semeniuc, C.A. Influence of Extraction Conditions on Characteristics of Microbial Polysaccharide Kefiran Isolated from Kefir Grains Biomass. J. Food Nutr. Res. 2016, 55, 121–130. [Google Scholar]
- Pop, C.R.; Coldea, T.E.; Salanţă, L.C.; Nistor, A.L.; Borşa, A.; Fărcaș, A.C.; Florian, V.C.; Rotar, A.M. The Effect of Extraction Conditions on the Barrier and Mechanical Properties of Kefiran Films. Coatings 2021, 11, 602. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Soares, I.H.B.T.; Soares, C.T.; Fakhouri, F.M.; de Oliveira, R.A. Development and Characterization of Arrowroot Starch Films Incorporated with Grape Pomace Extract. Polysaccharides 2022, 3, 250–263. [Google Scholar] [CrossRef]
- Etxabide, A.; Yang, Y.; Maté, J.I.; de la Caba, K.; Kilmartin, P.A. Developing Active and Intelligent Films through the Incorporation of Grape Skin and Seed Tannin Extracts into Gelatin. Food Packag. Shelf Life 2022, 33, 100896. [Google Scholar] [CrossRef]
- Mileti, O.; Baldino, N.; Filice, F.; Lupi, F.R.; Sinicropi, M.S.; Gabriele, D. Formulation Study on Edible Film from Waste Grape and Red Cabbage. Foods 2023, 12, 2804. [Google Scholar] [CrossRef]
- Pan, J.; Li, C.; Liu, J.; Jiao, Z.; Zhang, Q.; Lv, Z.; Yang, W.; Chen, D.; Liu, H. Polysaccharide-Based Packaging Coatings and Films with Phenolic Compounds in Preservation of Fruits and Vegetables—A Review. Foods 2024, 13, 3896. [Google Scholar] [CrossRef]
- Schmitz, L.; Moura, S. Grape By-Products for Smart Packaging Films: Antioxidant, pH-Sensitive, UV-Blocking and Antimicrobial Properties for Food Preservation. Packag. Technol. Sci. 2025, 38, 571–586. [Google Scholar] [CrossRef]
- Qiu, Z.; Niu, W.; Wang, S.; Yu, F.; Yu, Y.; Fan, J.; Zheng, L.; Wang, Y.; Xiao, Z.; Xie, Y. Multifunctional Composite Film Based on Biodegradable Grape Skin and Polyvinyl Alcohol. Cellulose 2021, 28, 6467–6479. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, S.M.; Rhim, J.W. Carboxymethyl Cellulose-Based Multifunctional Film Combined with Zinc Oxide Nanoparticles and Grape Seed Extract for the Preservation of High-Fat Meat Products. Sustain. Mater. Technol. 2021, 29, e00325. [Google Scholar] [CrossRef]
- Ji, X.; Xu, Z.; Xia, X.; Wei, Z.; Zhang, J.; Xia, G.; Ji, X. Cellulose/Grape-Seed-Extract Composite Films with High Transparency and Ultraviolet Shielding Performance Fabricated from Old Cotton Textiles. Polymers 2023, 15, 1451. [Google Scholar] [CrossRef] [PubMed]
- Botalo, A.; Inprasit, T.; Ummartyotin, S.; Chainok, K.; Vatthanakul, S.; Pisitsak, P. Smart and UV-Resistant Edible Coating and Films Based on Alginate, Whey Protein, and Curcumin. Polymers 2024, 16, 447. [Google Scholar] [CrossRef]
- Silva, J.T.d.P.; Borges, M.H.; de Souza, C.A.C.; Fávaro-Trindade, C.S.; Sobral, P.J.d.A.; de Oliveira, A.L.; Martelli-Tosi, M. Grape Pomace Rich-Phenolics and Anthocyanins Extract: Production by Pressurized Liquid Extraction in Intermittent Process and Encapsulation by Spray-Drying. Foods 2024, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; An, X.; Lu, Y.; Li, Z.; Gao, Z.; Tian, S. Biodegradable Active Packaging Material Containing Grape Seed Ethanol Extract and Corn Starch/κ-Carrageenan Composite Film. Polymers 2022, 14, 4857. [Google Scholar] [CrossRef]
- Kowalonek, J.; Łukomska, B.; Łukomska, O.; Stachowiak-Trojanowska, N. Alginate Films Enriched in Raspberry and/or Black Currant Seed Oils as Active Food Packaging. Molecules 2024, 29, 2012. [Google Scholar] [CrossRef]
- Shah, Y.A.; Bhatia, S.; Chinnam, S.; Al-Harrasi, A.; Tarahi, M.; Khan, T.S.; Alam, T.; Koca, E.; Aydemir, L.Y.; Philip, A.K.; et al. Myrrh Oleo-Gum Resin as a Functional Additive in Pectin and κ-Carrageenan Composite Films for Food Packaging. Food Sci. Nutr. 2024, 12, 10284–10295. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the Structural, Physical and Functional Properties of κ-Carrageenan Films Incorporated with Pomegranate Flesh and Peel Extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef]
- Wang, N.; Wei, J.; Wang, C.; Ren, J. Preparation, Physicochemical Properties, Biological Activity of a Multifunctional Composite Film Based on Zein/Citric Acid Loaded with Grape Seed Extract and Its Application in Solid Lipid Packaging. Foods 2025, 14, 1698. [Google Scholar] [CrossRef]
- Coma, M.E.; Peltzer, M.A.; Delgado, J.F.; Salvay, A.G. Water Kefir Grains as an Innovative Source of Materials: Study of Plasticiser Content on Film Properties. Eur. Polym. J. 2019, 120, 109234. [Google Scholar] [CrossRef]
- Yekta, R.; Dabbagh Moghaddam, A.; Hosseini, H.; Sharifan, A.; Hadi, S.; Hosseini-Shokouh, S.J. Effect of Using Biodegradable Film Constituting Red Grape Anthocyanins as a Novel Packaging on the Qualitative Attributes of Emergency Food Bars during Storage. Food Sci. Nutr. 2024, 12, 2702–2723. [Google Scholar] [CrossRef]
- Ramírez-Tapias, Y.A.; Rezzani, G.D.; Delgado, J.F.; Peltzer, M.A.; Salvay, A.G. New Materials from the Integral Milk Kefir Grain Biomass and the Purified Kefiran: The Role of Glycerol Content on the Film’s Properties. Polymers 2024, 16, 3106. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; Garcia, M.A.; Martino, M.N.; Zaritzky, N.E. Antiplasticizing effect of glycerol and sorbitol on the properties of cassava starch films. Braz. J. Food Technol. 2008, 11, 194–200. [Google Scholar]
- Kuan, Y.L.; Sivanasvaran, S.N.; Pui, L.P.; Yusof, Y.A.; Senphan, T. Physicochemical Properties of Sodium Alginate Edible Film Incorporated with Mulberry (Morus australis) Leaf Extract. Pertanika J. Trop. Agric. Sci. 2020, 43, 359–376. [Google Scholar]
- Choi, I.; Chang, Y.; Shin, S.H.; Joo, E.; Song, H.J.; Eom, H.; Han, J. Development of Biopolymer Composite Films Using a Microfluidization Technique for Carboxymethylcellulose and Apple Skin Particles. Int. J. Mol. Sci. 2017, 18, 1278. [Google Scholar] [CrossRef] [PubMed]
- Fitriani, F.; Aprilia, S.; Bilad, M.R.; Arahman, N.; Usman, A.; Huda, N.; Kobun, R. Optimization of Biocomposite Film Based on Whey Protein Isolate and Nanocrystalline Cellulose from Pineapple Crown Leaf Using Response Surface Methodology. Polymers 2022, 14, 3006. [Google Scholar] [CrossRef]
- Granados-Guzmán, G.; Salazar-Aranda, R.; Garza-Tapia, M.; Castro-Ríos, R.; Waksman De Torres, N. Optimization and Validation of Two High-Throughput Methods Indicating Antiradical Activity. Curr. Anal. Chem. 2017, 13, 499–507. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Marudova, M.; Sotirov, S.; Zhelyazkov, S.; Zsivanovits, G. Formulation and Characterization of Hydroxypropyl Methylcellulose Edible Films Containing Grape Seed Oil. Macromol. Symp. 2021, 395, 2000278. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Poças, F.; Pintado, M.; Morais, R.M.S.C.; Morais, A.M.M.B. Edible Films with Protein and Bioactive Compounds from Arthrospira sp. Biol. Life Sci. Forum 2024, 40, 6. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; de Oliveira Rios, A.; Alves, V.D.; Bandarra, N.; Moldão-Martins, M.; Hickmann Flôres, S. Biodegradable Films Based on Gelatin and Papaya Peel Microparticles with Antioxidant Properties. Food Bioprocess Technol. 2018, 11, 536–550. [Google Scholar] [CrossRef]
- Nguyen, T.A. Characterization of Chitosan-Based Active Film Incorporating with Gallic Acid. Int. J. Multidiscip. Res. Anal. 2023, 6, 3693–3698. [Google Scholar] [CrossRef]
- Mauro, M.; Pinto, P.; Settanni, L.; Puccio, V.; Vazzana, M.; Hornsby, B.L.; Fabbrizio, A.; Di Stefano, V.; Barone, G.; Arizza, V. Chitosan Film Functionalized with Grape Seed Oil—Preliminary Evaluation of Antimicrobial Activity. Sustainability 2022, 14, 5410. [Google Scholar] [CrossRef]
- Mugnaini, G.; Bonini, M.; Gentile, L.; Panza, O.; Del Nobile, M.A.; Conte, A.; Esposito, R.; D’Errico, G.; Moccia, F.; Panzella, L. Effect of design and molecular interactions on the food-preserving properties of alginate/pullulan edible films loaded with grape pomace extract. J. Food Eng. 2024, 361, 111716. [Google Scholar] [CrossRef]
- Xue, F.; Zhao, M.; Liu, X.; Chu, R.; Qiao, Z.; Li, C.; Adhikari, B. Physicochemical properties of chitosan/zein/essential oil emulsion-based active films functionalized by polyphenols. Future Foods 2021, 3, 100033. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Vargas, M.; Chiralt, A.; González-Martínez, C. Release of polyphenols from starch–chitosan based films containing thyme extract. Carbohydr. Polym. 2017, 175, 122–130. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Wang, Y.; Wen, X.; Peng, H.; Peng, R.; Shi, Q.; Xie, X.; Li, L. Outer Membrane Porins Contribute to Antimicrobial Resistance in Gram-Negative Bacteria. Microorganisms 2023, 11, 1690. [Google Scholar] [CrossRef] [PubMed]

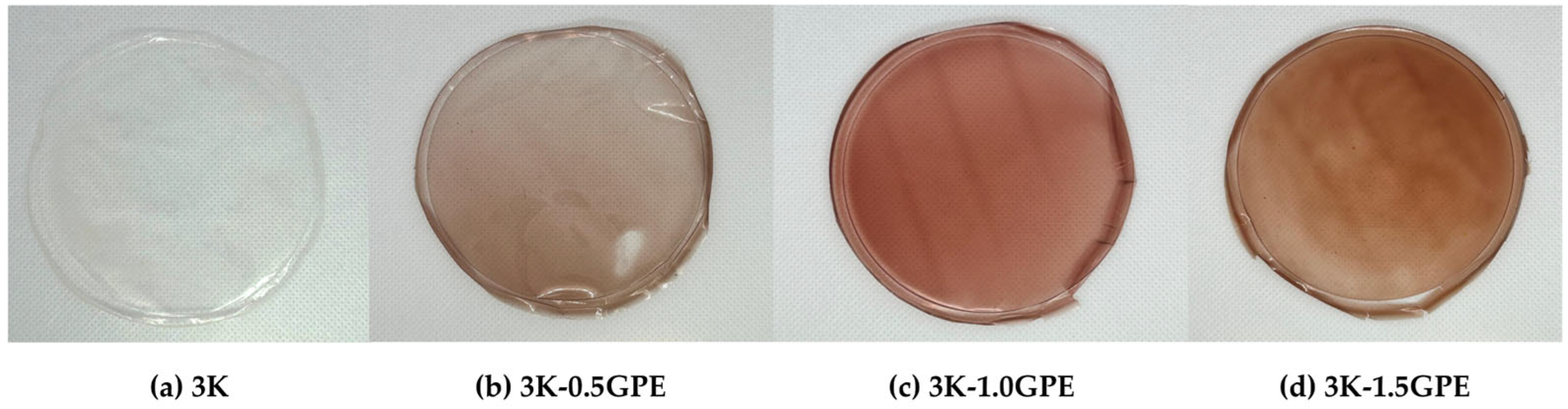
| Formulation | Kefiran (%) | Tween 80 (%) | Glycerol (%) | GPE (%) |
|---|---|---|---|---|
| 3K | 3.00 | 0.05 | 0.75 | 0.0 |
| 3K-0.5GPE | 3.00 | 0.05 | 0.75 | 0.5 |
| 3K-1.0GPE | 3.00 | 0.05 | 0.75 | 1.0 |
| 3K-1.5GPE | 3.00 | 0.05 | 0.75 | 1.5 |
| Antioxidant Activity and Total Phenolic Content | Antimicrobial Activity and Total Phenolic Content | |||||
|---|---|---|---|---|---|---|
| ABTS (µmol TE/g) | DPPH (µmol TE/g) | Total Phenolics (mg GAE/g) | E. coli | L. monocytogenes | ||
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |||
| 1081.11 ± 26.73 | 819.46 ± 10.57 | 21.65 ± 1.92 | 50.00 | 50.00 | 6.25 | 25.00 |
| Films | L* | a* | b* | ΔE |
|---|---|---|---|---|
| 3K | 90.09 ± 0.64 a | −0.66 ± 0.03 a | −4.22 ± 0.21 a | 0.00 ** |
| 3K-0.5GPE | 75.38 ± 0.91 b | 1.70 ± 0.09 b | −1.68 ± 0.09 b | 15.11 ± 0.92 a |
| 3K-1.0GPE | 62.50 ± 0.42 c | 4.47 ± 0.08 d | 0.22 ± 0.04 c | 28.42 ± 0.43 b |
| 3K-1.5GPE | 55.07 ± 1.67 d | 4.20 ± 0.18 c | 2.46 ± 0.15 d | 35.98 ± 1.71 c |
| Films | Thickness (mm) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| 3K | 0.052 ± 0.004 a | 9.48 ± 1.61 a | 58.03 ± 14.91 a |
| 3K-0.5GPE | 0.069 ± 0.006 b | 5.59 ± 1.14 b | 40.82 ± 8.72 a |
| 3K-1.0GPE | 0.089 ± 0.007 c | 5.29 ± 0.35 b | 95.02 ± 16.48 b |
| 3K-1.5GPE | 0.113 ± 0.010 d | 3.91 ± 0.69 c | 104.68 ± 14.13 b |
| Films | ABTS (µmol TE/g) | DPPH (µmol TE/g) | Total Phenolics (mg GAE/g) |
|---|---|---|---|
| 3K | 9.87 ± 1.07 a | 5.83 ± 0.89 a | 1.00 ± 0.06 a |
| 3K-0.5GPE | 51.72 ± 4.48 b | 34.56 ± 1.21 b | 6.33 ± 0.61 b |
| 3K-1.0GPE | 66.11 ± 2.37 c | 41.27 ± 1.60 c | 12.31 ± 0.82 c |
| 3K-1.5GPE | 89.15 ± 2.53 d | 56.67 ± 1.57 d | 16.62 ± 1.14 d |
| Inhibition Zone Diameter (mm) | ||
|---|---|---|
| Films | E. coli | L. monocytogenes |
| 3K | n.d.a | n.d.a |
| 3K-0.5GPE | n.d.a | 7.50 ± 0.50 b |
| 3K-1.0GPE | n.d.a | n.d.a |
| 3K-1.5GPE | n.d.a | n.d.a |
| Control (−) * | n.d.a | n.d.a |
| Control (+) ** | 17.50 ± 0.71 b | 33.00 ± 2.82 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islas-Enríquez, R.P.; Márquez-Reyes, J.M.; Báez-González, J.G.; Galindo-Rodríguez, S.A.; Gallardo-Rivera, C.T.; Viveros-Valdez, E.; Amaya-Guerra, C.A.; Bautista-Villarreal, M.; Treviño-Garza, M.Z. Novel Bioactive Kefiran-Based Films Enriched with Grape Pomace Extract. Polymers 2025, 17, 3108. https://doi.org/10.3390/polym17233108
Islas-Enríquez RP, Márquez-Reyes JM, Báez-González JG, Galindo-Rodríguez SA, Gallardo-Rivera CT, Viveros-Valdez E, Amaya-Guerra CA, Bautista-Villarreal M, Treviño-Garza MZ. Novel Bioactive Kefiran-Based Films Enriched with Grape Pomace Extract. Polymers. 2025; 17(23):3108. https://doi.org/10.3390/polym17233108
Chicago/Turabian StyleIslas-Enríquez, Rosalba Paola, Julia M. Márquez-Reyes, Juan G. Báez-González, Sergio A. Galindo-Rodríguez, Claudia T. Gallardo-Rivera, Ezequiel Viveros-Valdez, Carlos Abel Amaya-Guerra, Minerva Bautista-Villarreal, and Mayra Z. Treviño-Garza. 2025. "Novel Bioactive Kefiran-Based Films Enriched with Grape Pomace Extract" Polymers 17, no. 23: 3108. https://doi.org/10.3390/polym17233108
APA StyleIslas-Enríquez, R. P., Márquez-Reyes, J. M., Báez-González, J. G., Galindo-Rodríguez, S. A., Gallardo-Rivera, C. T., Viveros-Valdez, E., Amaya-Guerra, C. A., Bautista-Villarreal, M., & Treviño-Garza, M. Z. (2025). Novel Bioactive Kefiran-Based Films Enriched with Grape Pomace Extract. Polymers, 17(23), 3108. https://doi.org/10.3390/polym17233108









