Reprocessing of Simulated Industrial PLA Waste for Food Contact Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing and Reprocessing of PLA
2.2.1. PLA Filament Production
2.2.2. PLA Film Production
2.3. Characterization of the Materials
2.3.1. Pellet Characterization
- Melt Flow Index
- Viscosity average molecular weight
- Volatile, semi-volatile and non-volatile compounds
- Specific Migration of Metals
2.3.2. Film Characterization
- Viscosity average molecular weight
- Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy
- Scanning electron microscopy
- Thermogravimetric analysis
- Differential Scanning Calorimetry
- Mechanical properties
- Static Water Contact Angle Measurements (WCA)
- Water Vapor Transmission Rate (WVTR)
- Oxygen Transmission Rate (OTR)
- Global Migration
- Disintegration Tests Under Composting Conditions
2.3.3. Statistical Analysis
3. Results and Discussion
3.1. PLA and PLA-RP Pellet Characterization
3.1.1. Melt Flow Index
3.1.2. Viscosity Average Molecular Weight
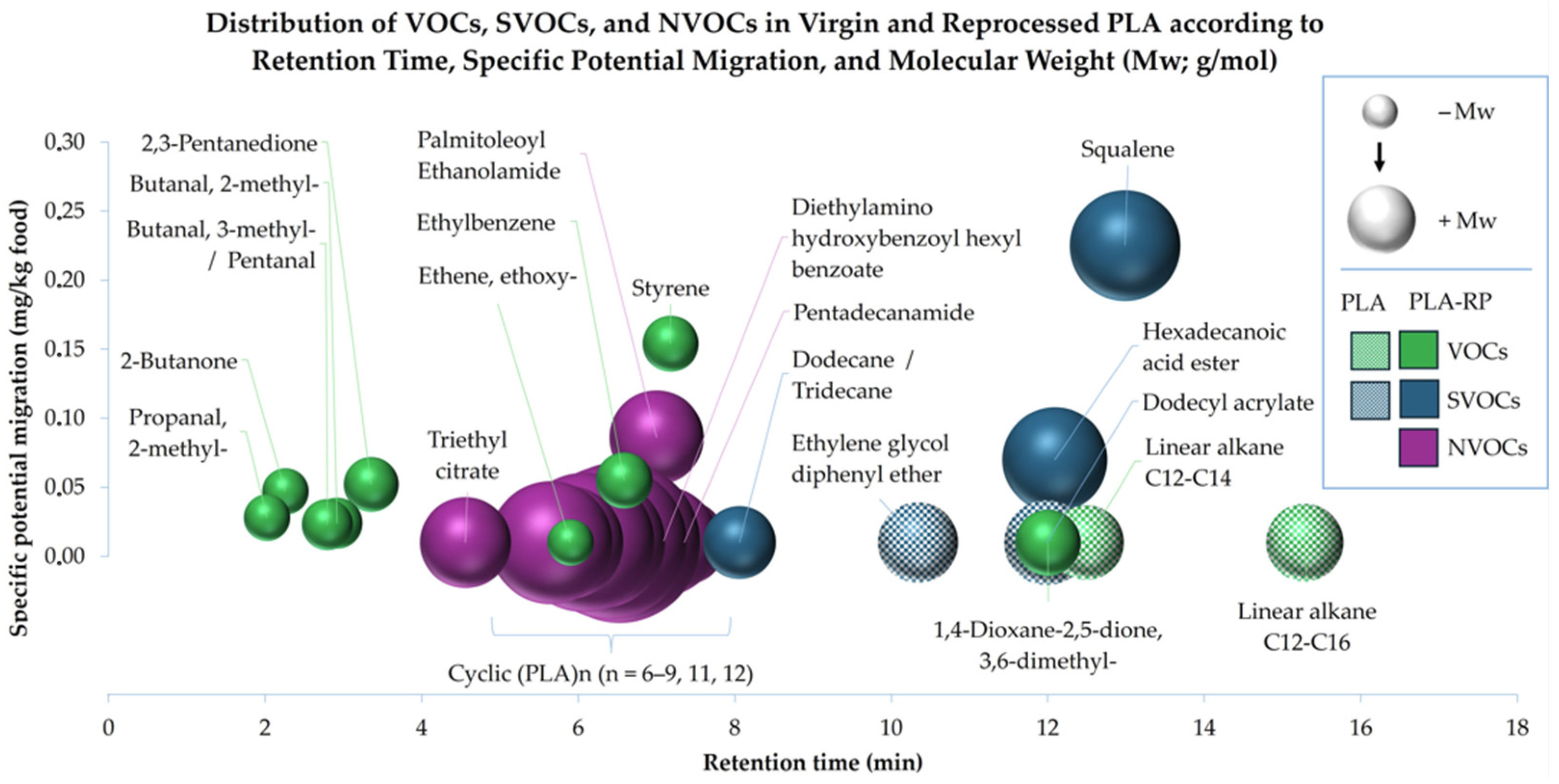
3.1.3. Volatile, Semi-Volatile and Non-Volatile Compounds
- (a)
- Analysis of volatile organic compounds (VOCs)
- (b)
- Analysis of semi-volatile organic compounds (SVOCs)
- (c)
- Analysis of non-volatile organic compounds (NVOCs)
3.1.4. Specific Migration of Metals
3.2. Neat PLA and PLA-RP Film Characterization
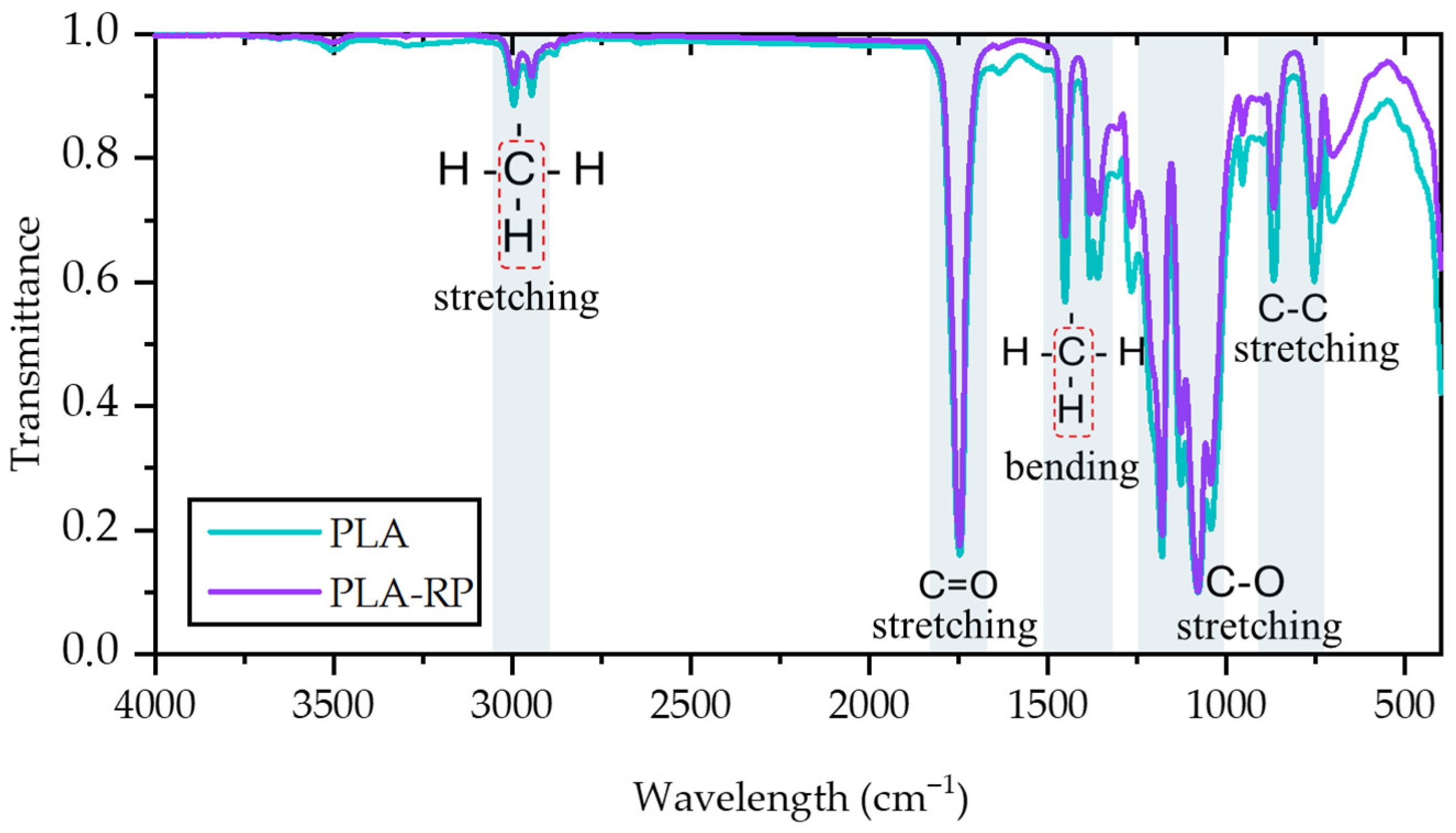
3.2.1. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
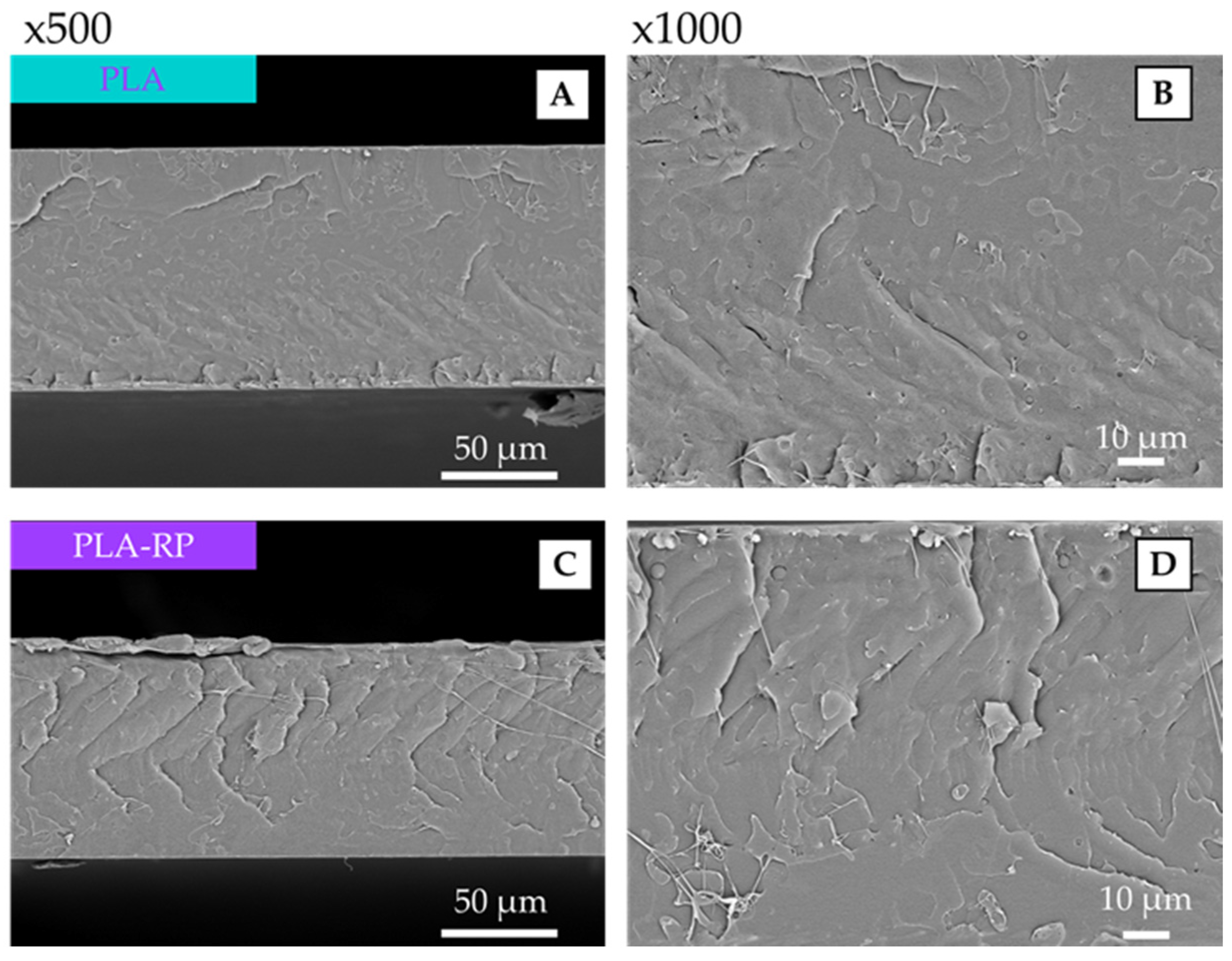
3.2.2. Scanning Electron Microscopy
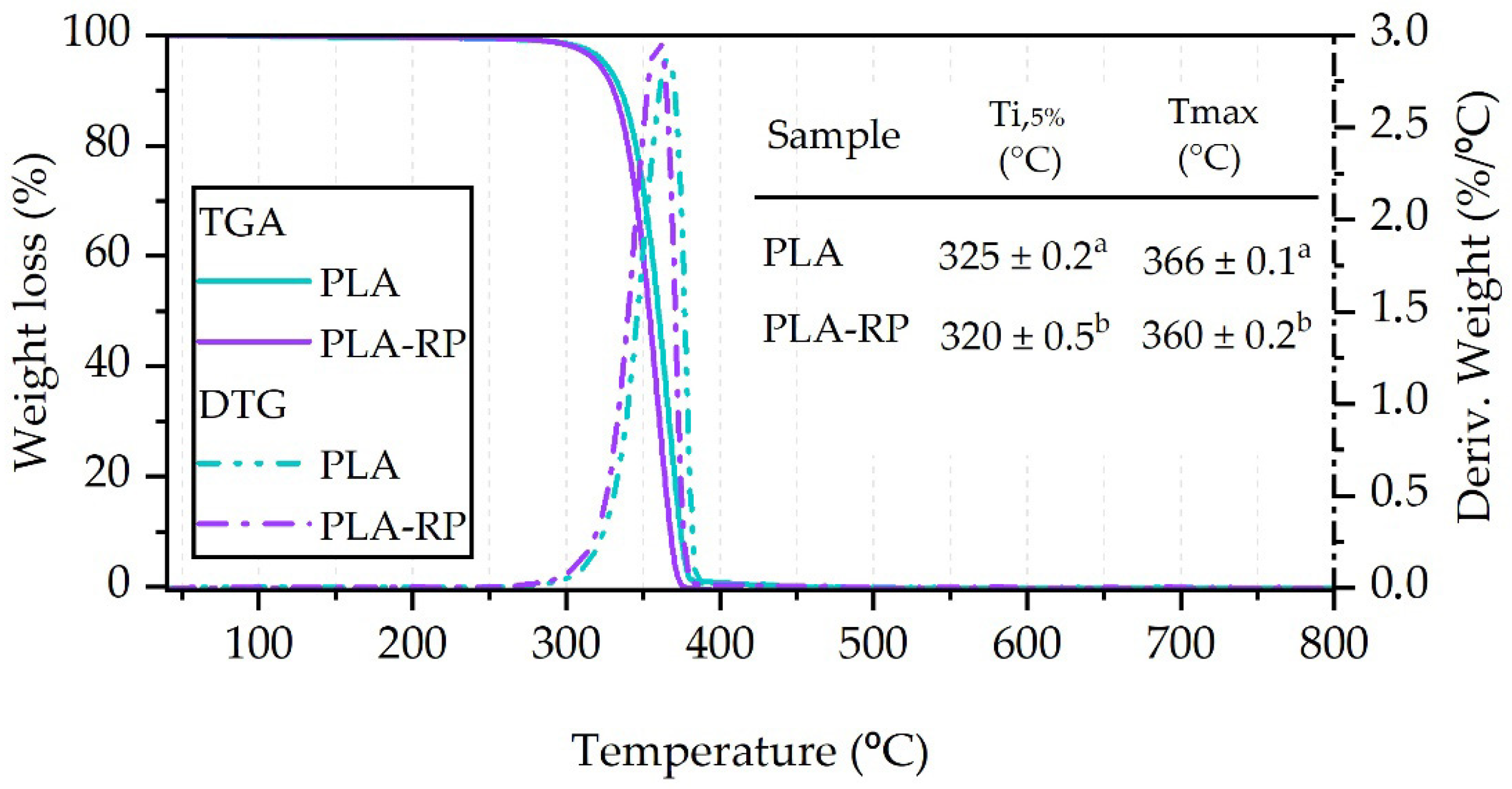
3.2.3. Thermogravimetric Analysis
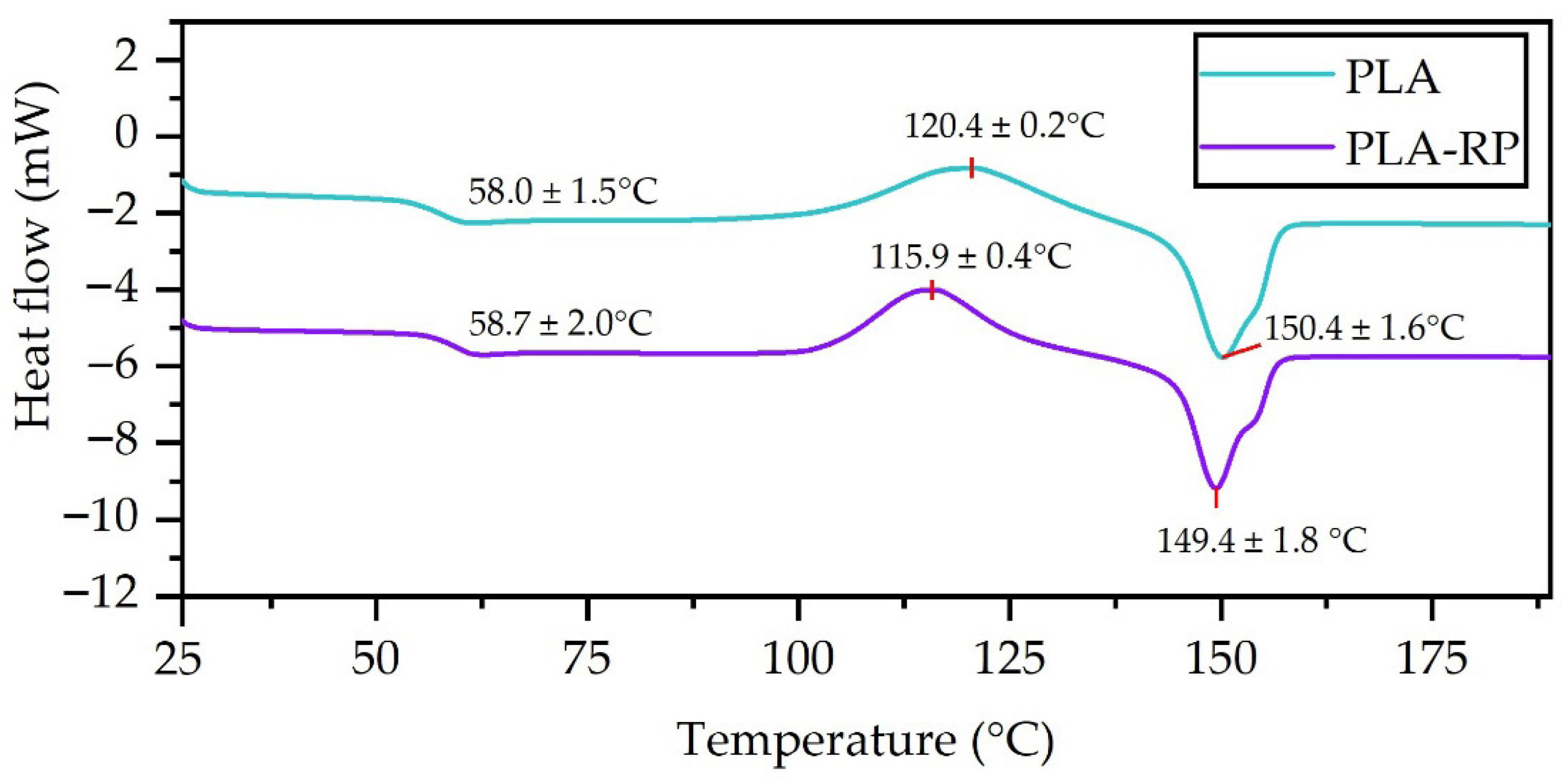
3.2.4. Differential Scanning Calorimetry
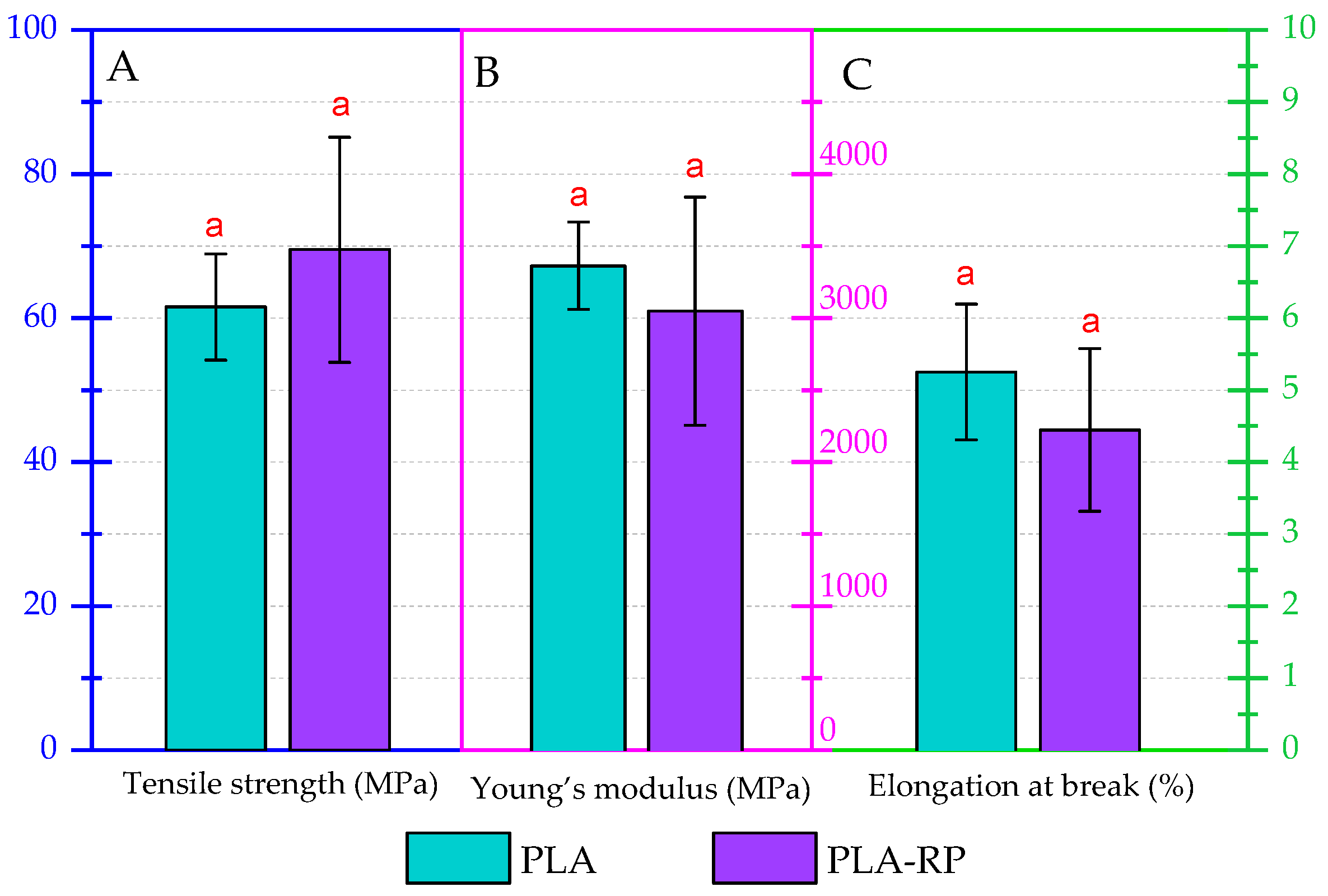
3.2.5. Mechanical Properties
3.2.6. Static Water Contact Angle Measurements (WCA)
3.2.7. Water Vapor Transmission Rate (WVTR)
3.2.8. Oxygen Transmission Rate (OTR)
3.2.9. Global Migration Test
3.2.10. Disintegration Under Composting Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auras, R.A.; Singh, S.P.; Singh, J.J. Evaluation of Oriented Poly (Lactide) Polymers vs. Existing PET and Oriented PS for Fresh Food Service Containers. Packag. Technol. Sci. Int. J. 2005, 18, 207–216. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and Ageing Study of Poly (Lactic Acid) Films Plasticized with Oligomeric Lactic Acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; López, J.; Jiménez, A. Combined Effect of Poly(Hydroxybutyrate) and Plasticizers on Polylactic Acid Properties for Film Intended for Food Packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Hidalgo-Carvajal, D.; Muñoz, Á.H.; Garrido-González, J.J.; Carrasco-Gallego, R.; Alcázar Montero, V. Recycled PLA for 3D Printing: A Comparison of Recycled PLA Filaments from Waste of Different Origins after Repeated Cycles of Extrusion. Polymers 2023, 15, 3651. [Google Scholar] [CrossRef]
- Agüero, A.; Morcillo, M.D.C.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Lascano, D.; Torres-Giner, S.; Fenollar, O. Study of the Influence of the Reprocessing Cycles on the Final Properties of Polylactide Pieces Obtained by Injection Molding. Polymers 2019, 11, 1908. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. Material Valorisation of Amorphous Polylactide. Influence of Thermo-Mechanical Degradation on the Morphology, Segmental Dynamics, Thermal and Mechanical Performance. Polym. Degrad. Stab. 2012, 97, 670–678. [Google Scholar] [CrossRef]
- Agüero, Á.; Corral Perianes, E.; de las Muelas, S.S.; Lascano, D.; de la Fuente García-Soto, M.D.M.; Peltzer, M.A.; Balart, R.; Arrieta, M.P. Plasticized Mechanical Recycled PLA Films Reinforced with Microbial Cellulose Particles Obtained from Kombucha Fermented in Yerba Mate Waste. Polymers 2023, 15, 285. [Google Scholar] [CrossRef]
- Badia, J.D.; Ribes-Greus, A. Mechanical Recycling of Polylactide, Upgrading Trends and Combination of Valorization Techniques. Eur. Polym. J. 2016, 84, 22–39. [Google Scholar] [CrossRef]
- Silva, T.; Rodríguez-Mercado, F.; Bruna, J.E.; Torres, A.; Arrieta, M.P.; Faba, S.; Galotto, M.J.; Guarda, A.; Romero, J. Characterization of Simulated Postconsumer Recycled Poly (Lactic Acid)(PLA): Evaluation of Reprocessing Cycles on Its Physicochemical Properties. J. Polym. Sci. 2025, 63, 2043–2054. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EU) 2025/40 of the European Parliament and of the Council of 19 December 2024 on Packaging and Packaging Waste; Official Journal of the European Union: European Union; European Parliament and Council of the European Union: Strasbourg, France, 2025; pp. 1–20. [Google Scholar]
- Sepúlveda-Carter, J.; Moreno De Castro, J.L.; Marín, L.; Baños, P.; Rodríguez, M.S.; Arrieta, M.P. Regulatory Frameworks and State-of-the-Art Decontamination Technologies for Recycled Polystyrene for Food Contact Applications. Polymers 2025, 17, 658. [Google Scholar] [CrossRef]
- Samper Madrigal, M.D.; Arrieta, M.P.; Ferrándiz Bou, S.; López Martínez, J. Influence of Biodegradable Materials in the Recycled Polystyrene. J. Appl. Polym. Sci. 2014, 131, 41161–41168. [Google Scholar] [CrossRef]
- Aldas, M.; Pavon, C.; De La Rosa-Ramírez, H.; Ferri, J.M.; Bertomeu, D.; Samper, M.D.; López-Martínez, J. The Impact of Biodegradable Plastics in the Properties of Recycled Polyethylene Terephthalate. J. Polym. Environ. 2021, 29, 2686–2700. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2022/1616 of 15 September 2022 on Recycled Plastic Materials and Articles Intended to Come into Contact with Foods; L 243; European Commission: Brussels, Belgium, 2022; pp. 3–46. [Google Scholar]
- European Food Safety Authority (EFSA). Guidelines on Submission of a Dossier for Safety Evaluation by the EFSA of a Recycling Process to Produce Recycled Plastics Intended to Be Used for Manufacture of Materials and Articles in Contact with Food-Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC). EFSA J. 2008, 6, 717. [Google Scholar]
- NatureWorks LLC. PLA 2003D Safety Data Sheets; NatureWorks LLC: Plymouth, MN, USA, 2022; Available online: https://www.natureworksllc.com/~/media/files/natureworks/technical-documents/safety-data-sheets/sds-polymers-na-eng/2003d%20us%20sds_pdf.pdf?la=en (accessed on 20 June 2025).
- UNE-EN 13130-1:2005; Materials and Articles in Contact with Foodstuffs—Plastics Substances Subject to Limitation—Part 1: Guide to Test Methods for the Specific Migration of Substances from Plastics to Foods and Food Simulants and the Determination of Substances in Plastics and the Selection of Conditions of Exposure to Food Simulants. Asociación Española de Normalización (UNE): Madrid, Spain, 2005.
- UNE-EN ISO 527-1:2020; Plastics—Determination of Tensile Properties—Part 1: General Principles (ISO 527-1:2019). Asociación Española de Normalización (UNE): Madrid, Spain, 2020.
- UNE-EN ISO 527-2:2025; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics (ISO 527-2:2025). Asociación Española de Normalización (UNE): Madrid, Spain, 2025.
- UNE 53097:2002; Sheet Materials—Determination of Water Vapour Transmission Rate—Gravimetric (dish) Method. Asociación Española de Normalización (UNE): Madrid, Spain, 2002.
- UNE-EN 1186-3:2023; Materials and Articles in Contact with Foodstuffs—Plastics—Part 3: Test Methods for Overall Migration in Evaporable Simulants. Asociación Español de Normalización: Madrid, Spain, 2023.
- UNE-EN ISO 20200:2024; Plastics—Determination of the Degree of Disintegration of Plastic Materials Under Composting Conditions in a Laboratory-Scale Test (ISO 20200:2023). Asociación Española de Normalización (UNE): Madrid, Spain, 2024.
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under Composting Conditions of Plasticized PLA–PHB Blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef]
- Aznar, M.; Ubeda, S.; Dreolin, N.; Nerín, C. Determination of Non-Volatile Components of a Biodegradable Food Packaging Material Based on Polyester and Polylactic Acid (PLA) and Its Migration to Food Simulants. J. Chromatogr. A 2019, 1583, 1–8. [Google Scholar] [CrossRef]
- Tejada-Oliveros, R.; Balart, R.; Ivorra-Martinez, J.; Gomez-Caturla, J.; Montanes, N.; Quiles-Carrillo, L. Improvement of Impact Strength of Polylactide Blends with a Thermoplastic Elastomer Compatibilized with Biobased Maleinized Linseed Oil for Applications in Rigid Packaging. Molecules 2021, 26, 240. [Google Scholar] [CrossRef]
- European Commission. Consolidated Text: Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food; L 12; European Commission: Brussels, Belgium, 2021; pp. 1–89. [Google Scholar]
- Roosen, M.; Van Laere, T.; Decottignies, V.; Morel, L.; Schnitzler, J.-L.; Schneider, J.; Schlummer, M.; Lase, I.S.; Dumoulin, A.; De Meester, S. Tracing the Origin of VOCs in Post-Consumer Plastic Film Bales. Chemosphere 2023, 324, 138281. [Google Scholar] [CrossRef] [PubMed]
- Palkopoulou, S.; Joly, C.; Feigenbaum, A.; Papaspyrides, C.D.; Dole, P. Critical Review on Challenge Tests to Demonstrate Decontamination of Polyolefins Intended for Food Contact Applications. Trends Food Sci. Technol. 2016, 49, 110–120. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.; Muncke, J. Food Packaging in the Circular Economy: Overview of Chemical Safety Aspects for Commonly Used Materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Plasteurope. Styrenics Circular Solutions: Application to EFSA for RPS as Food Contact Material/Successful Trials; Styrenics Circular Solutions: Brussels, Belgium, 2021. [Google Scholar]
- Kosior, E. Further Analysis of Decontaminated Recycled Polypropylene (RPP); Research Report Project IMT003-105; Waste and Resources Action Programme (WRAP): Banbury, UK, 2013; Available online: https://www.wrap.ngo/sites/default/files/2020-10/WRAP-Further%20analysis%20of%20decon%20rPP.pdf (accessed on 16 March 2025).
- Dvorak, R.; Kosior, E.; Moody, L. Development of a Food-Grade Recycling Process for Post-Consumer Polypropylene; Research Report Project MDP039; Waste and Resources Action Programme (WRAP): Banbury, UK, 2011; Available online: https://www.wrap.ngo/sites/default/files/2020-10/WRAP-Phase%202%20-%20Food%20Contact%20PP%20Report.pdf (accessed on 16 March 2025).
- Nextek NEXTLOOPP: Closing the Loop on Food Grade PP. NEXTLOOPP. 2020. Available online: https://www.nextloopp.com/project/nextloopp-mission-vision-and-benefits/ (accessed on 17 March 2025).
- EFSA CEF Panel. Scientific Opinion on the Safety Assessment of the Processes ‘Biffa Polymers’ and ‘CLRrHDPE’ Used to Recycle High-density Polyethylene Bottles for Use as Food Contact Material. EFSA J. 2015, 13, 4016. [Google Scholar] [CrossRef]
- Welle, F. Develop a Food Grade HDPE Recycling Process; WRAP Research Report; The Waste & Resources Action Programme; Fraunhofer Institute for Process Engineering and Packaging (IVV): Banbury, UK, 2005; ISBN 1-84405-225-7. [Google Scholar]
- Singh, S.; Pereira, J.; Brandão, T.; Oliveira, A.L.; Poças, F. Recycling of Polypropylene by Supercritical Carbon Dioxide for Extraction of Contaminants from Beverage Cups. A Comparison with Polyethylene Terephthalate and Polylactic Acid. J. Sci. Food Agric. 2023, 103, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Welle, F. Recycling of Post-Consumer Polystyrene Packaging Waste into New Food Packaging Applications—Part 2: Co-Extruded Functional Barriers. Recycling 2023, 8, 39. [Google Scholar]
- Welle, F. Twenty Years of PET Bottle to Bottle Recycling—An Overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Dutra, C.; Pezo, D.; Freire, M.T.D.A.; Nerín, C.; Reyes, F.G.R. Determination of Volatile Organic Compounds in Recycled Polyethylene Terephthalate and High-Density Polyethylene by Headspace Solid Phase Microextraction Gas Chromatography Mass Spectrometry to Evaluate the Efficiency of Recycling Processes. J. Chromatogr. A 2011, 1218, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, F.; Ribes-Greus, A.; Karlsson, S. Chromatographic Pattern in Recycled High-Impact Polystyrene (HIPS)–Occurrence of Low Molecular Weight Compounds during the Life Cycle. Polym. Degrad. Stab. 2010, 95, 172–186. [Google Scholar] [CrossRef]
- Cabanes, A.; Valdés, F.J.; Fullana, A. A Review on VOCs from Recycled Plastics. Sustain. Mater. Technol. 2020, 25, e00179. [Google Scholar] [CrossRef]
- Fabris, S.; Freire, M.T.D.A.; Wagner, R.; Reyes, F.G. A Method to Determine Volatile Contaminants in Polyethylene Terephthalate (PET) Packages by HDC-GC-FID and Its Application to Post-Consumer Materials. Ciência E Tecnol. De Aliment. 2010, 30, 1046–1055. [Google Scholar] [CrossRef]
- NatureWorks. Available online: https://www.natureworksllc.com/~/media/files/natureworks/technical-documents/properties-documents/ingeo-grades-made-to-stock-specifications_2-series_pdf.pdf?la=e (accessed on 20 June 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 8023, Ethyl Vinyl Ether; National Library of Medicine: Bethesda, MD, USA, 2025.
- Snow, N.H.; Rana, H. Does High Polarity Mean High Retention on Stationary Phases in Gas Chromatography? LCGC N. Am. 2023, 41, 18–22. [Google Scholar] [CrossRef]
- Salazar, R.; Domenek, S.; Plessis, C.; Ducruet, V. Quantitative Determination of Volatile Organic Compounds Formed During Polylactide Processing by MHS-SPME. Polym. Degrad. Stab. 2017, 136, 80–88. [Google Scholar] [CrossRef]
- EFSA FEEDAP Panel. Scientific Opinion on the Safety and Efficacy of Branched-Chain Primary Aliphatic Alcohols/Aldehydes/Acids, Acetals and Esters with Esters Containing Branched-Chain Alcohols and Acetals Containing Branched-Chain Aldehydes (Chemical Group 2) When Used as Flavourings for All Animal Species. EFSA J. 2012, 10, 2927. [Google Scholar] [CrossRef]
- Bradley, E.L. Distribution: 1. E L J Potter (FSA) (X1 + .Pdf Version) 2; Fera Library: Monroe, FL, USA, 2010. [Google Scholar]
- Holland Bioplastics. Plant Sugars PLA Products Hydrolysis; Holland Bioplastics: Haaksbergen, The Netherlands, 2025; Available online: https://www.natureworksllc.com/~/media/files/natureworks/news-and-events/news%20posts/holland-bioplastics-news-post/2024_06_24_petopla_microplastics-executive-brief_pdf.pdf (accessed on 28 July 2025).
- Gao, Y.; Xue, Y.; Sun, C.; She, L.; Peng, Y. Emission Characteristics of Volatile Organic Compounds from Material Extrusion Printers Using Acrylonitrile–Butadiene–Styrene and Polylactic Acid Filaments in Printing Environments and Their Toxicological Concerns. Toxics 2025, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- EFSA CEP Panel. Re-Assessment of the Risks to Public Health Related to the Genotoxicity of Styrene Present in Plastic Food Contact Materials. EFSA J. 2025, 23, e9473. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Vázquez-Loureiro, P.; Sendón, R.; Bustos, J.; Santillana, M.I.; Losada, P.P.; de Quirós, A.R.B. Characterization of Polyester Coatings Intended for Food Contact by Different Analytical Techniques and Migration Testing by LC-MSn. Polymers 2022, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Demets, R.; Roosen, M.; Vandermeersch, L.; Ragaert, K.; Walgraeve, C.; De Meester, S. Development and Application of an Analytical Method to Quantify Odour Removal in Plastic Waste Recycling Processes. Resour. Conserv. Recycl. 2020, 161, 104907. [Google Scholar] [CrossRef]
- Wojnowski, W.; Kalinowska, K.; Majchrzak, T.; Zabiegała, B. Real-Time Monitoring of the Emission of Volatile Organic Compounds from Polylactide 3D Printing Filaments. Sci. Total Environ. 2022, 805, 150181. [Google Scholar] [CrossRef]
- Cooper, T.A. Developments in Plastic Materials and Recycling Systems for Packaging Food, Beverages and Other Fast-Moving Consumer Goods. In Trends in Packaging of Food, Beverages and Other Fast-Moving Consumer Goods (FMCG); Farmer, N., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 58–107. ISBN 9780857095039. [Google Scholar] [CrossRef]
- Eckardt, M.; Kubicova, M.; Simat, T.J. Universal Response Quantification Approach Using a Corona Charged Aerosol Detector (CAD)—Application on Linear and Cyclic Oligomers Extractable from Polycondensate Plastics Polyesters, Polyamides and Polyarylsulfones. J. Chromatogr. A 2018, 1572, 187–202. [Google Scholar] [CrossRef]
- Zhong, H.N.; Su, Q.Z.; Chen, S.; Li, D.; Sui, H.; Zhu, L.; Dong, B.; Wu, S.; Wang, X. Revealing Contaminants in China’s Recycled PET: Enabling Safe Food Contact Applications. Resour. Conserv. Recycl. 2025, 212, 107947. [Google Scholar] [CrossRef]
- Bronczyk, K.; Dabrowska, A.; Bielicka-Daszkiewicz, K.; Milczewska, K. Safety of New Food Contact Materials: Migration and Sorption Studies Based on Tenax, Powdered Milk, Baby Cereal and Oat Flakes. Food Chem. 2025, 483, 144148. [Google Scholar] [CrossRef]
- Ruellan, A.; Guinault, A.; Sollogoub, C.; Ducruet, V.; Domenek, S. Solubility Factors as Screening Tools of Biodegradable Toughening Agents of Polylactide. J. Appl. Polym. Sci. 2015, 132, 1–12. [Google Scholar] [CrossRef]
- García Ibarra, V.; Rodríguez Bernaldo de Quirós, A.; Paseiro Losada, P.; Sendón, R. Non-Target Analysis of Intentionally and Non Intentionally Added Substances from Plastic Packaging Materials and Their Migration into Food Simulants. Food Packag. Shelf Life 2019, 21, 100325. [Google Scholar] [CrossRef]
- Emery Oleochemicals 2-EH-Palmitate Technical-Data-Sheet. Available online: https://www.emeryoleo.com/sites/default/files/2020-09/EMERY-E-6216%282-EH-palmitate%29Technical-Data-Sheet.pdf (accessed on 3 July 2025).
- European Food Safety Authority (EFSA). Flavouring Group Evaluation 25, (FGE.25)—Aliphatic and Aromatic Hydrocarbons from Chemical Group 31—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food. EFSA J. 2008, 6, 918. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Chemical Hazards Database (OpenFoodTox). Available online: https://www.efsa.europa.eu/en/data-report/chemical-hazards-database-openfoodtox (accessed on 16 June 2025).
- Su, Q.Z.; Vera, P.; Salafranca, J.; Nerín, C. Decontamination Efficiencies of Post-Consumer High-Density Polyethylene Milk Bottles and Prioritization of High Concern Volatile Migrants. Resour. Conserv. Recycl. 2021, 171, 105640. [Google Scholar] [CrossRef]
- Estremera, C.; Paiva, R.; Aznar, M.; Nerín, C.; Domeño, C. Identification of Volatile and Non-Volatile Migrants Released During Sous Vide Cooking by UPLC-IMS-QTOF and DI-SPME-GC-MS Using a Design of Experiments Approach. Food Packag. Shelf Life 2024, 43, 101297. [Google Scholar] [CrossRef]
- Ye, F.; Chen, W.; Deng, Z.; Chen, S.-L.; Dong, Z.; Dang, L.; Li, M.-D. Ultrafast Excited-State Energy Dissipation Pathway of Diethylamino Hydroxybenzoyl Hexyl Benzoate (DHHB) via the Nanoparticles. Photochem. Photobiol. Sci. 2023, 22, 2133–2142. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, M.; Li, Y.; Xiong, Y.; Wu, C. Recycling and Degradation of Polyamides. Molecules 2024, 29, 1742. [Google Scholar] [CrossRef]
- Dhaini, A.; Hardouin-Duparc, V.; Alaaeddine, A.; Carpentier, J.-F.; Guillaume, S.M. Recent Advances in Polyhydroxyalkanoates Degradation and Chemical Recycling. Prog. Polym. Sci. 2024, 149, 101781. [Google Scholar] [CrossRef]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-Sourced Polymers as Alternatives to Conventional Food Packaging Materials: A Review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Kameno, N.; Yamada, S.; Amimoto, T.; Amimoto, K.; Ikeda, H.; Koga, N. Thermal Degradation of Poly(Lactic Acid) Oligomer: Reaction Mechanism and Multistep Kinetic Behavior. Polym. Degrad. Stab. 2016, 134, 284–295. [Google Scholar] [CrossRef]
- Rung, C.; Welle, F.; Gruner, A.; Springer, A.; Steinmetz, Z.; Munoz, K. Identification and Evaluation of (Non-)Intentionally Added Substances in Post-Consumer Recyclates and Their Toxicological Classification. Recycling 2023, 8, 24. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Scientific Opinion on Exploring Options for Providing Advice about Possible Human Health Risks Based on the Concept of Threshold of Toxicological Concern (TTC). EFSA J. 2012, 10, 2750. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.; Zhang, Y.; Zhang, W.; Pang, X.; Zhang, S.; Lu, J.; Lv, J. Determination and Risk Assessment of Flavor Components in Flavored Milk. Foods 2023, 12, 2151. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aquilina, G.; Castle, L.; Degen, G.; Engel, K.-H.; Fowler, P.J.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Scientific Opinion on the Renewal of the Authorisation of SmokEz C-10 (SF-005) as a Smoke Flavouring Primary Product. EFSA Panel name on Food Additives and Flavourings (FAF). EFSA J. 2023, 21, e08367. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.; Degen, G.; Dubakiene, R.; Jazwiec-Kanyion, B.; Kapoulas, V.; Krutmann, J.; Lidén, C.; Marty, J.-P.; Platzek, T.; Rastogi, S.C.; et al. Scientific Committee on Consumer Products SCCP OPINION ON Diethylamino Hydroxybenzoyl Hexyl Benzoate COLIPA N° S83; European Commission: Brussels, Belgium, 2008; Available online: https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_130.pdf (accessed on 16 June 2025).
- Chibwe, L.; De Silva, A.O.; Spencer, C.; Teixera, C.F.; Williamson, M.; Wang, X.; Muir, D.C.G. Target and Nontarget Screening of Organic Chemicals and Metals in Recycled Plastic Materials. Environ. Sci. Technol. 2023, 57, 3380–3390. [Google Scholar] [CrossRef]
- Klingenberg, P.; Schirmeister, C.G.; Kappeler, M.; Calean, A.; Biester, H.; Licht, E.; Rapp, B. Quantification of Regulated Metals in Recycled Post-Consumer Polypropylene through Comparative ICP-MS, AAS and LIBS Analyses. Polym. Test. 2024, 136, 108480. [Google Scholar] [CrossRef]
- Sepulveda, J.; Villegas, C.; Torres, A.; Vargas, E.; Rodriguez, F.; Baltazar, S.; Prada, A.; Rojas, A.; Romero, J.; Faba, S.; et al. Effect of Functionalized Silica Nanoparticles on the Mass Transfer Process in Active PLA Nanocomposite Films Obtained by Supercritical Impregnation for Sustainable Food Packaging. J. Supercrit. Fluids 2020, 161, 104844. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Rocculi, P.; Rosa, M.D. Poly(Lactic Acid)-modified Films for Food Packaging Application: Physical, Mechanical, and Barrier Behavior. J. Appl. Polym. Sci. 2012, 125, E390–E401. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Blending Polylactic Acid with Polyhydroxybutyrate: The Effect on Thermal, Mechanical, and Biodegradation Properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Hu, Y.; Sato, H.; Zhang, J.; Noda, I.; Ozaki, Y. Crystallization Behavior of Poly(l-Lactic Acid) Affected by the Addition of a Small Amount of Poly(3-Hydroxybutyrate). Polymer 2008, 49, 4204–4210. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Arrieta, M.P.; Gaspar, G.; de la Orden, M.U.; Martínez Urreaga, J. Effect of Iignocellulosic Nanoparticles Extracted from Yerba Mate (Ilex Paraguariensis) on the Structural, Thermal, Optical and Barrier Properties of Mechanically Recycled Poly(Lactic Acid). Polymers 2020, 12, 1690. [Google Scholar] [CrossRef]
- Shabani Samghabady, M. Characterization of Mechanically Recycled Polylactic Acid (PLA) Filament for 3D-Printing by Evaluating Mechanical, Thermal, and Chemical Properties and Process Performance. Master’s Thesis, Clemson University, Clemson, SC, USA, 2023. [Google Scholar]
- Agüero, A.; Lascano, D.; Ivorra-Martinez, J.; Gómez-Caturla, J.; Arrieta, M.P.; Balart, R. Use of Bacterial Cellulose Obtained from Kombucha Fermentation in Spent Coffee Grounds for Active Composites Based on PLA and Maleinized Linseed Oil. Ind. Crops Prod. 2023, 202, 116971. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Iannoni, A.; Barbale, M.; Zaccheo, S.; Scavone, M.; Visai, L.; Kenny, J.M. New Multifunctional Poly(Lactide Acid) Composites: Mechanical, Antibacterial, and Degradation Properties. J. Appl. Polym. Sci. 2012, 124, 87–98. [Google Scholar] [CrossRef]
- Nomadolo, N.; Mtibe, A.; Ofosu, O.; Mekoa, C.; Letwaba, J.; Muniyasamy, S. The Effect of Mechanical Recycling on the Thermal, Mechanical, and Chemical Properties of Poly (Butylene Adipate-Co-Terephthalate) (PBAT), Poly (Butylene Succinate) (PBS), Poly (Lactic Acid) (PLA), PBAT-PBS Blend and PBAT-TPS Biocomposite. J. Polym. Environ. 2024, 32, 2644–2659. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of Poly(Lactic Acid): Characterization of Chemical Structure, Thermal Stability and Mechanical Properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Müller, A.J.; Ávila, M.; Saenz, G.; Salazar, J. Crystallization of PLA-Based Materials. In Poly(Lactic Acid) Science and Technology: Processing, Properties, Additives and Applications; Chemistry, R.S., Jiménez, A., Peltzer, M., Ruseckaite, R., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2014; pp. 66–98. ISBN 978-1-84973-879-8. [Google Scholar]
- Gálvez, J.; Correa Aguirre, J.P.; Hidalgo Salazar, M.A.; Vera Mondragón, B.; Wagner, E.; Caicedo, C. Effect of Extrusion Screw Speed and Plasticizer Proportions on the Rheological, Thermal, Mechanical, Morphological and Superficial Properties of PLA. Polymers 2020, 12, 2111. [Google Scholar] [CrossRef]
- Hambleton, A.; Fabra, M.-J.; Debeaufort, F.; Dury-Brun, C.; Voilley, A. Interface and Aroma Barrier Properties of Iota-Carrageenan Emulsion–Based Films Used for Encapsulation of Active Food Compounds. J. Food Eng. 2009, 93, 80–88. [Google Scholar] [CrossRef]
- Sahoo, B.L.; Mohanty, S.; Nayak, S.K. Synergistic Effects of Nucleating Agent, Chain Extender and Nanocrystalline Cellulose on PLA and PHB Blend Films: Evaluation of Morphological, Mechanical and Functional Attributes. Ind. Crops Prod. 2024, 215, 118614. [Google Scholar] [CrossRef]
- Tee, Y.B.; Rosnita, A.T.; Khalina, A.; Chin, N.L.; Roseliza, K.B.; Khairul Faezah, M.Y. Reinforcing Mechanical, Water Absorption and Barrier Properties of Poly (Lactic Acid) Composites with Kenaf-Derived Cellulose of Thermally-Grafted Aminosilane. Pertanika J. Trop. Agric. Sci. 2015, 38, 563–573. [Google Scholar]
- ASTM F1249; Standard Test Method for Water Vapor Transmission Rate Through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM International: West Conshohocken, PA, USA, 2020.
- Jung, B.N.; Jung, H.W.; Kang, D.H.; Kim, G.H.; Lee, M.; Shim, J.K.; Hwang, S.W. The Fabrication of Flexible and Oxygen Barrier Cellulose Nanofiber/Polylactic Acid Nanocomposites Using Cosolvent System. J. Appl. Polym. Sci. 2020, 137, 49536. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and Characterization of Plasticized PLA/PHB Blends for Biodegradable Multiphase Systems. Express Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Torre, L.; Jiménez, A.; Kenny, J.M. Effects of Modified Cellulose Nanocrystals on the Barrier and Migration Properties of PLA Nano-Biocomposites. Carbohydr. Polym. 2012, 90, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Afshar, S.V.; Boldrin, A.; Christensen, T.H.; Corami, F.; Daugaard, A.E.; Rosso, B.; Hartmann, N.B. Disintegration of Commercial Biodegradable Plastic Products under Simulated Industrial Composting Conditions. Sci. Rep. 2025, 15, 8569. [Google Scholar] [CrossRef]
- Feijoo, P.; Marín, A.; Samaniego-Aguilar, K.; Sánchez-Safont, E.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Effect of the Presence of Lignin from Woodflour on the Compostability of PHA-Based Biocomposites: Disintegration, Biodegradation and Microbial Dynamics. Polymers 2023, 15, 2481. [Google Scholar] [CrossRef] [PubMed]


| Sample | Mv (g/mol) | MFI (g/10 min) |
|---|---|---|
| PLA pellet | 113,200 ± 900 | 6.5 ± 0.1 |
| PLA-RP pellet | 107,000 ± 3500 | 9.9 ± 0.4 |
| PLA film | 106,000 ± 4000 | - |
| PLA-RP film | 99,800 ± 4000 | - |
| Sample | Tg (°C) | Tcc (°C) | Tm (°C) | ΔHcc (J/g) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|---|---|
| PLA | 58.5 ± 1.5 a | 120.4 ± 0.2 a | 150.4 ± 1.6 a | 29.6 ± 0.3 a | 29.7 ± 0.2 a | 0.11 ± 0.04 a |
| PLA-RP | 58.7 ± 2.0 a | 115.9 ± 0.4 b | 149.4 ± 1.8 a | 26.5 ± 0.5 b | 27.1 ± 1.4 a | 0.64 ± 0.06 b |
| Sample | WCA (°) | WVTR (g/m2·Day) | OTR.e (cm3·mm/m2·Day) |
|---|---|---|---|
| PLA | 74.6 ± 1.4 a | 31.2 ± 1.1 a | 19.6 ± 1.3 a |
| PLA-RP | 74.7 ± 1.3 a | 32.7 ± 1.9 a | 22.3 ± 1.1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepúlveda-Carter, J.; Faba, S.; Rodríguez, M.S.; Arrieta, M.P. Reprocessing of Simulated Industrial PLA Waste for Food Contact Applications. Polymers 2025, 17, 2439. https://doi.org/10.3390/polym17182439
Sepúlveda-Carter J, Faba S, Rodríguez MS, Arrieta MP. Reprocessing of Simulated Industrial PLA Waste for Food Contact Applications. Polymers. 2025; 17(18):2439. https://doi.org/10.3390/polym17182439
Chicago/Turabian StyleSepúlveda-Carter, Javiera, Simón Faba, Marcos Sánchez Rodríguez, and Marina P. Arrieta. 2025. "Reprocessing of Simulated Industrial PLA Waste for Food Contact Applications" Polymers 17, no. 18: 2439. https://doi.org/10.3390/polym17182439
APA StyleSepúlveda-Carter, J., Faba, S., Rodríguez, M. S., & Arrieta, M. P. (2025). Reprocessing of Simulated Industrial PLA Waste for Food Contact Applications. Polymers, 17(18), 2439. https://doi.org/10.3390/polym17182439










