Nanomaterials from Textile Waste for Purification and Environmental Applications
Abstract
1. Introduction
2. Methodology
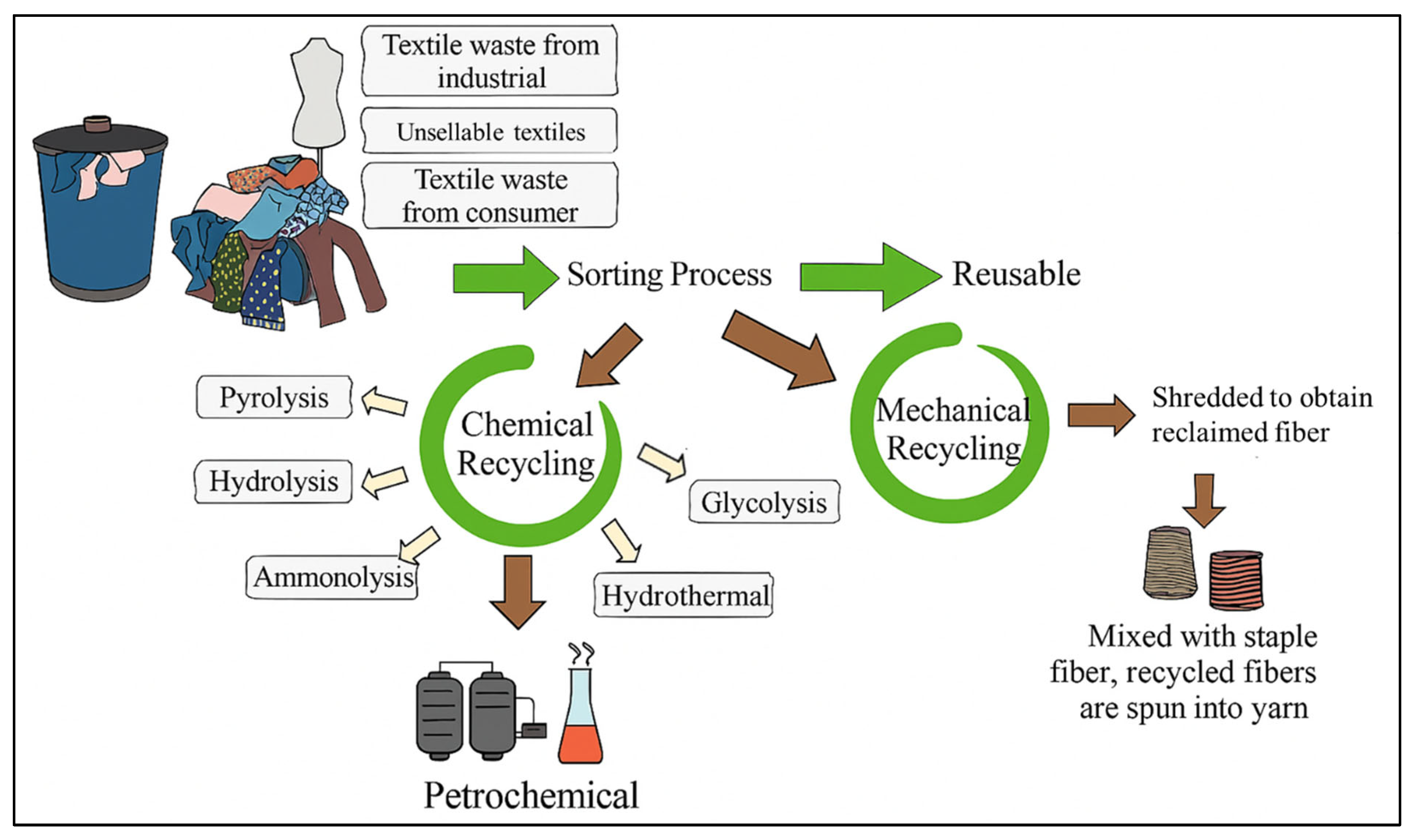
3. Recycling of Textile Waste
3.1. Mechanical Recycling
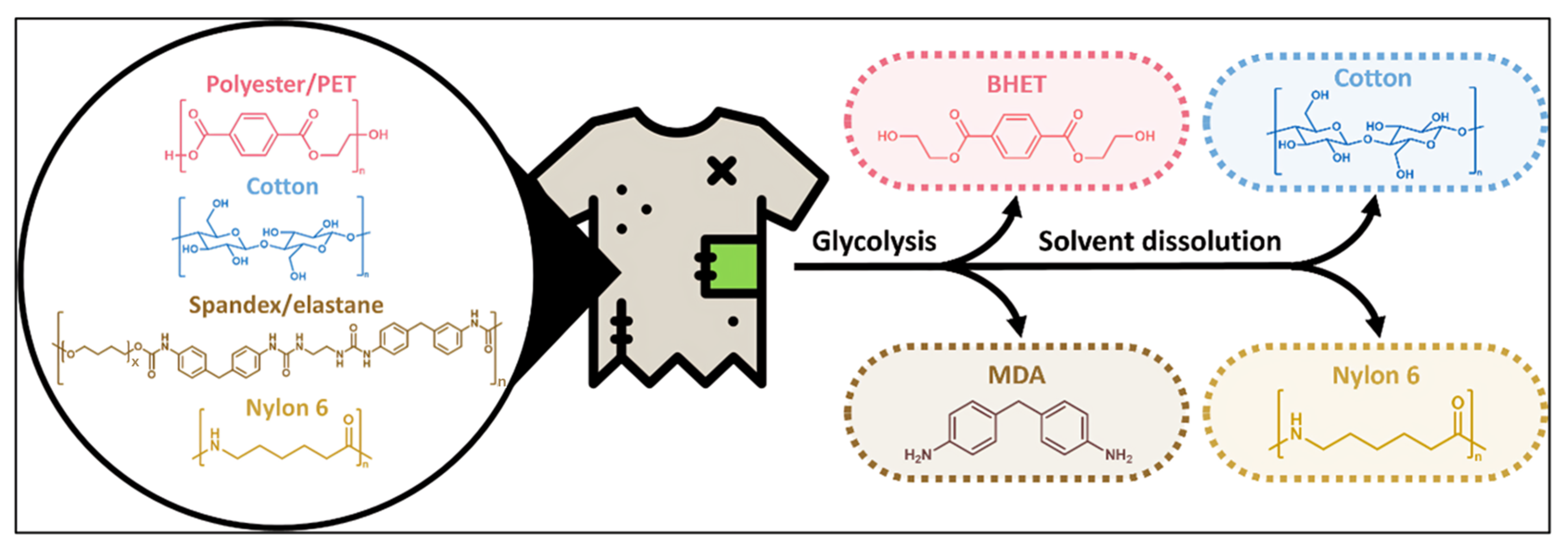
3.2. Chemical Recycling
3.3. Thermal Recycling
4. Textile Fibers to Micro- and Nano-Fibers and Particles
4.1. Cellulose Nanofibers
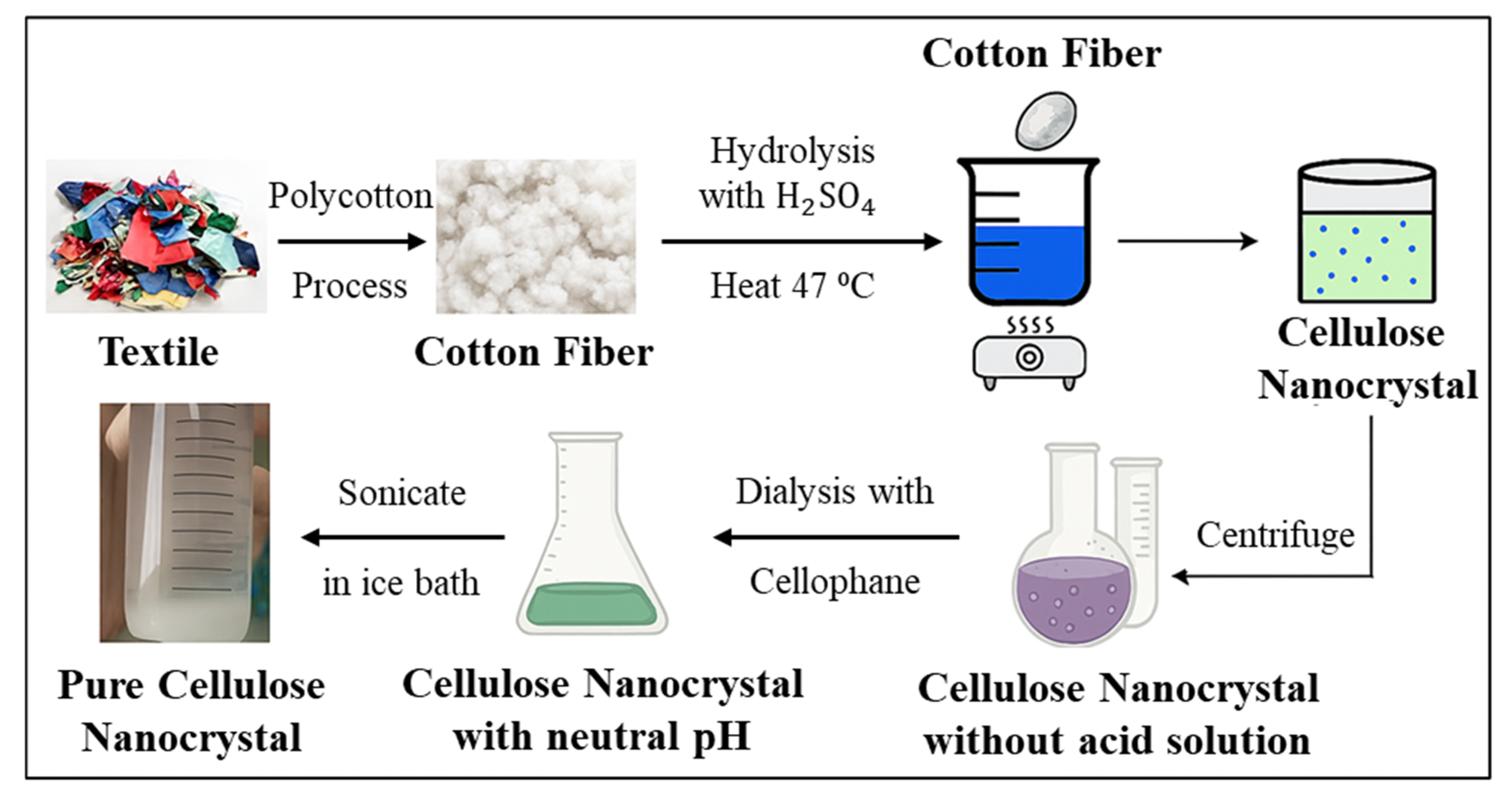
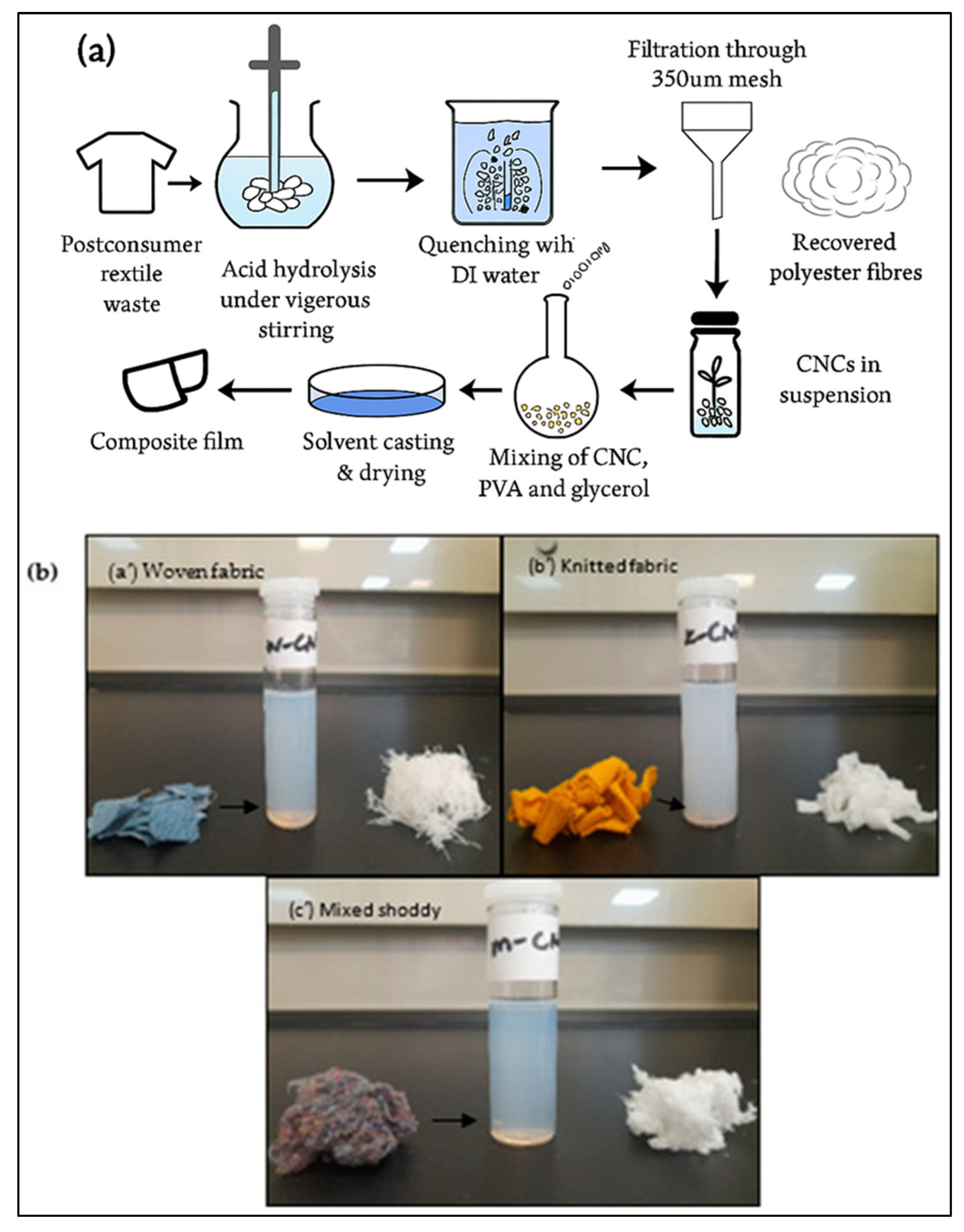
4.2. Cellulose Nanocrystal
4.3. Carbon Nanoparticles
5. Properties of Textile Waste Nanomaterials
5.1. Properties of Nanocellulose from Textile Waste
5.2. Properties of Carbon Nanoparticles from Textile Waste
6. Environmental Applications of Nanomaterials Derived from Textile Waste
7. Challenges and Future Research
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jayadevan, S.; Aliyana, A.K.; Stylios, G. An overview of advances and challenges in developing nanofiber yarns for wearable technology. Nano Energy 2024, 129, 110034. [Google Scholar] [CrossRef]
- Wu, Q.; Miao, W.-S.; Zhang, Y.-D.; Gao, H.-J.; Hui, D. Mechanical properties of nanomaterials: A review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Njuguna, J.; Ansari, F.; Sachse, S.; Zhu, H.; Rodriguez, V.M. Nanomaterials, nanofillers, and nanocomposites: Types and properties. In Health and Environmental Safety of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–37. [Google Scholar]
- Srichola, P.; Witthayolankowit, K.; Sukyai, P.; Sampoompuang, C.; Lobyam, K.; Kampakun, P.; Toomtong, R. Recycling of Nanocellulose from Polyester–Cotton Textile Waste for Modification of Film Composites. Polymers 2023, 15, 3324. [Google Scholar] [CrossRef]
- Yalcin-Enis, I.; Kucukali-Ozturk, M.; Sezgin, H. Risks and Management of Textile Waste. In Nanoscience and Biotechnology for Environmental Applications; Springer: Cham, Switzerland, 2019; pp. 29–53. [Google Scholar]
- Das, A.K.; Hossain, F.; Khan, B.U.; Rahman, M.; Asad, M.A.Z.; Akter, M. Circular economy: A sustainable model for waste reduction and wealth creation in the textile supply chain. SPE Polym. 2025, 6, e10171. [Google Scholar] [CrossRef]
- Juanga-Labayen, J.P.; Labayen, I.V.; Yuan, Q. A Review on Textile Recycling Practices and Challenges. Textiles 2022, 2, 174–188. [Google Scholar] [CrossRef]
- Kamble, Z.; Behera, B.K. Upcycling textile wastes: Challenges and innovations. Text. Prog. 2021, 53, 65–122. [Google Scholar] [CrossRef]
- Patti, A.; Cicala, G.; Acierno, D. Eco-Sustainability of the Textile Production: Waste Recovery and Current Recycling in the Composites World. Polymers 2021, 13, 134. [Google Scholar] [CrossRef]
- Pereira, A.V.; Barroca, J.; Dias, R.; Varela, M.; Galvão, R. Methodologies for Determining the Opportunity Cost of Capital: Financing in the Selection of Investments. Rev. Gestão RGSA 2024, 18, e07607. [Google Scholar] [CrossRef]
- Baloyi, R.B.; Gbadeyan, O.J.; Sithole, B.; Chunilall, V. Recent advances in recycling technologies for waste textile fabrics: A review. Text. Res. J. 2023, 94, 508–529. [Google Scholar] [CrossRef]
- Ghosh, J.; Repon, R.; Rupanty, N.S.; Asif, T.R.; Tamjid, M.I.; Reukov, V. Chemical Valorization of Textile Waste: Advancing Sustainable Recycling for a Circular Economy. ACS Omega 2025, 10, 11697–11722. [Google Scholar] [CrossRef]
- Sathasivam, T.; Sugiarto, S.; Yew, M.P.Y.; Oh, X.Y.; Chan, S.Y.; Chan, B.Q.Y.; Tim, M.J.; Kai, D. Transforming textile waste into nanocellulose for a circular future. Nanoscale 2024, 16, 14168–14194. [Google Scholar] [CrossRef]
- de Oliveira Neto, G.C.; Teixeira, M.M.; Souza, G.L.V.; Arns, V.D.; Tucci, H.N.P.; Amorim, M. Assessment of the Eco-Efficiency of the Circular Economy in the Recovery of Cellulose from the Shredding of Textile Waste. Polymers 2022, 14, 1317. [Google Scholar] [CrossRef]
- Patoary, M.K.; Islam, S.R.; Farooq, A.; Rashid, M.A.; Sarker, S.; Hossain, Y.; Rakib, M.A.N.; Amin, A.; Liu, L. Phosphorylation of nanocellulose: State of the art and prospects. Ind. Crop. Prod. 2023, 201, 116965. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, W.; Luan, S.; Wei, J.; Yang, Y.; Miao, J. Nanomaterials for smart wearable fibers and textiles: A critical review. iScience 2025, 28, 113126. [Google Scholar] [CrossRef]
- Raza, M.; Abu-Jdayil, B. Cellulose nanocrystals from lignocellulosic feedstock: A review of production technology and surface chemistry modification. Cellulose 2022, 29, 685–722. [Google Scholar] [CrossRef]
- Signori-Iamin, G.; Santos, A.F.; Corazza, M.L.; Aguado, R.; Tarrés, Q.; Delgado-Aguilar, M. Prediction of cellulose micro/nanofiber aspect ratio and yield of nanofibrillation using machine learning techniques. Cellulose 2022, 29, 9143–9162. [Google Scholar] [CrossRef]
- Lynd, L.R.; Beckham, G.T.; Guss, A.M.; Jayakody, L.N.; Karp, E.M.; Maranas, C.; McCormick, R.L.; Amador-Noguez, D.; Bomble, Y.J.; Davison, B.H.; et al. Toward low-cost biological and hybrid biological/catalytic conversion of cellulosic biomass to fuels. Energy Environ. Sci. 2022, 15, 938–990. [Google Scholar] [CrossRef]
- Baloyi, R.B.; Sithole, B.B.; Chunilall, V. Physicochemical Properties of Cellulose Nanocrystals Extracted from Postconsumer Polyester/Cotton-Blended Fabrics and Their Effects on PVA Composite Films. Polymers 2024, 16, 1495. [Google Scholar] [CrossRef]
- Ahmed, B.; Hossain, J.; Al Parvez, A.; Talukder, A.; Al-Amin, M.; Al Mahmud, A.; Islam, T. Recent Advancements of MXene/Nanocellulose-Based Hydrogel and Aerogel: A Review. Adv. Energy Sustain. Res. 2024, 5, 2300231. [Google Scholar] [CrossRef]
- Farooq, A.; Islam, S.R.; Amin, A.; Patoary, M.K.; Hossain, T.; Khawar, M.T.; Wang, Z.; Tian, M. From farm to function: Exploring new possibilities with jute nanocellulose applications. Carbohydr. Polym. 2024, 342, 122423. [Google Scholar] [CrossRef]
- Moseson, D.E.; Taylor, L.S. Crystallinity: A Complex Critical Quality Attribute of Amorphous Solid Dispersions. Mol. Pharm. 2023, 20, 4802–4825. [Google Scholar] [CrossRef]
- Alves, D.I.; Barreiros, M.; Fangueiro, R.; Ferreira, D.P. Valorization of textile waste: Non-woven structures and composites. Front. Environ. Sci. 2024, 12, 1365162. [Google Scholar] [CrossRef]
- Damayanti, D.; Wulandari, L.A.; Bagaskoro, A.; Rianjanu, A.; Wu, H.-S. Possibility Routes for Textile Recycling Technology. Polymers 2021, 13, 3834. [Google Scholar] [CrossRef] [PubMed]
- Ribul, M.; Lanot, A.; Pisapia, C.T.; Purnell, P.; McQueen-Mason, S.J.; Baurley, S. Mechanical, chemical, biological: Moving towards closed-loop bio-based recycling in a circular economy of sustainable textiles. J. Clean. Prod. 2021, 326, 129325. [Google Scholar] [CrossRef]
- Shamsuzzaman, M.; Islam, M.; Mamun, M.A.A.; Rayyaan, R.; Sowrov, K.; Islam, S.; Sayem, A.S.M. Fashion and textile waste management in the circular economy: A systematic review. Clean. Waste Syst. 2025, 11, 100268. [Google Scholar] [CrossRef]
- Harmsen, P.; Scheffer, M.; Bos, H. Textiles for Circular Fashion: The Logic behind Recycling Options. Sustainability 2021, 13, 9714. [Google Scholar] [CrossRef]
- Todor, M.P.; Rackov, M.; Kiss, I.; Cioata, V.G. Composite solutions with recycled textile wastes. J. Phys. Conf. Ser. 2022, 2212, 012030. [Google Scholar] [CrossRef]
- Tang, K.H.D. State of the Art in Textile Waste Management: A Review. Textiles 2023, 3, 454–467. [Google Scholar] [CrossRef]
- Arafat, Y.; Uddin, A.J. Recycled fibers from pre- and post-consumer textile waste as blend constituents in manufacturing 100% cotton yarns in ring spinning: A sustainable and eco-friendly approach. Heliyon 2022, 8, e11275. [Google Scholar] [CrossRef]
- Tripathi, M.; Sharma, M.; Bala, S.; Thakur, V.K.; Singh, A.; Dashora, K.; Hart, P.; Gupta, V.K. Recent technologies for transforming textile waste into value-added products: A review. Curr. Res. Biotechnol. 2024, 7, 100225. [Google Scholar] [CrossRef]
- Loo, S.-L.; Yu, E.; Hu, X. Tackling critical challenges in textile circularity: A review on strategies for recycling cellulose and polyester from blended fabrics. J. Environ. Chem. Eng. 2023, 11, 110482. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Tomme, B.; Goshayeshi, B.; Mynko, O.; Wang, Y.; Roy, S.; Kumar, R.; Baruah, B.; De Clerck, K.; De Meester, S.; et al. Advancing Textile Waste Recycling: Challenges and Opportunities Across Polymer and Non-Polymer Fiber Types. Polymers 2025, 17, 628. [Google Scholar] [CrossRef]
- Zhang, N.; Hou, X.; Cui, X.; Chai, L.; Li, H.; Zhang, H.; Wang, Y.; Deng, T. Amphiphilic catalyst for decomposition of unsaturated polyester resins to valuable chemicals with 100% atom utilization efficiency. J. Clean. Prod. 2021, 296, 126492. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Cui, Y.; Chen, N. Removal of Copper Ions from Wastewater: A Review. Int. J. Environ. Res. Public Health 2023, 20, 3885. [Google Scholar] [CrossRef]
- Ramírez, E.; Bringué, R.; Fité, C.; Iborra, M.; Tejero, J.; Cunill, F. Assessment of ion exchange resins as catalysts for the direct transformation of fructose into butyl levulinate. Appl. Catal. A Gen. 2021, 612, 117988. [Google Scholar] [CrossRef]
- Enking, J.; Becker, A.; Schu, G.; Gausmann, M.; Cucurachi, S.; Tukker, A.; Gries, T. Recycling processes of polyester-containing textile waste—A review. Resour. Conserv. Recycl. 2025, 219, 108256. [Google Scholar] [CrossRef]
- Mardenborough, K.; Florentino, M.; Haxhari, R.; Lin, Y.-C.; Rafailovich, M.; Halada, G.; Jung, H.J.; Kim, T. Influence of NaOH concentration on the decolorization of crystal violet dyed cotton fabric. Environ. Eng. Res. 2023, 28, 210643. [Google Scholar] [CrossRef]
- Mu, B.; Yu, X.; Shao, Y.; McBride, L.; Hidalgo, H.; Yang, Y. Profitable and environmentally responsible recycling of fibers and reactive dyes from afterlife cotton textiles via controlled dye hydrolysis, fiber swelling and dissolution. Chem. Eng. J. 2023, 475, 146150. [Google Scholar] [CrossRef]
- Barnard, E.; Arias, J.J.R.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Luo, H.; Tyrrell, H.; Bai, J.; Muazu, R.I.; Long, X. Fundamental, technical and environmental overviews of plastic chemical recycling. Green Chem. 2024, 26, 11444–11467. [Google Scholar] [CrossRef]
- Quicker, P.; Seitz, M.; Vogel, J. Chemical recycling: A critical assessment of potential process approaches. Waste Manag. Res. 2022, 40, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Pegoretti, A. Towards sustainable structural composites: A review on the recycling of continuous-fiber-reinforced thermoplastics. Adv. Ind. Eng. Polym. Res. 2021, 4, 105–115. [Google Scholar] [CrossRef]
- Rossignolo, G.; Malucelli, G.; Lorenzetti, A. Recycling of polyurethanes: Where we are and where we are going. Green Chem. 2024, 26, 1132–1152. [Google Scholar] [CrossRef]
- Andini, E.; Bhalode, P.; Gantert, E.; Sadula, S.; Vlachos, D.G. Chemical recycling of mixed textile waste. Sci. Adv. 2024, 10, eado6827. [Google Scholar] [CrossRef]
- Siva Jagadish Kumar, M.; Naidu, M.R.; Hayavadana, J. Effect of Alkaline Hydrolysis of Jute/Polyester Union Fabrics on Low-Stress Mechanical Properties. J. Fiber Sci. Technol. 2020, 76, 412–421. [Google Scholar] [CrossRef]
- El Darai, T.; Ter-Halle, A.; Blanzat, M.; Despras, G.; Sartor, V.; Bordeau, G.; Lattes, A.; Franceschi, S.; Cassel, S.; Chouini-Lalanne, N.; et al. Chemical recycling of polyester textile wastes: Shifting towards sustainability. Green Chem. 2024, 26, 6857–6885. [Google Scholar] [CrossRef]
- Mu, B.; Yu, X.; Shao, Y.; McBride, L.; Hidalgo, H.; Yang, Y. Complete recycling of polymers and dyes from polyester/cotton blended textiles via cost-effective and destruction-minimized dissolution, swelling, precipitation, and separation. Resour. Conserv. Recycl. 2023, 199, 107275. [Google Scholar] [CrossRef]
- Luo, L.-B.; Chen, R.; Lian, Y.-X.; Wu, W.-J.; Zhang, J.-H.; Fu, C.-X.; Sun, X.-L.; Xiao, L.-R. Recycled PET/PA6 Fibers from Waste Textile with Improved Hydrophilicity by In-Situ Reaction-Induced Capacity Enhancement. Polymers 2024, 16, 1052. [Google Scholar] [CrossRef] [PubMed]
- Pitak, I.; Sholokhova, A. Transforming textile waste into alternative fuel by thermochemical methods: A mini-review. Clean. Waste Syst. 2025, 10, 100199. [Google Scholar] [CrossRef]
- Jamaludin, S.I.S.; Zaini, M.A.A.; Sadikin, A.N.; Jani, W.N.F.A. Textile waste valorization as potential activated carbon precursor for the removal of water contaminants: Commentary. Mater. Today Proc. 2024, 96, 110–115. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Kliucininkas, L.; Lukošiūtė, S.-I.; Yan, L. Sustainable green technology for recovery of cotton fibers and polyester from textile waste. J. Clean. Prod. 2020, 254, 120078. [Google Scholar] [CrossRef]
- Randviir, E.P.; Kanou, O.; Liauw, C.M.; Miller, G.J.; Andrews, H.G.; Smith, G.C. The physicochemical investigation of hydrothermally reduced textile waste and application within carbon-based electrodes. RSC Adv. 2019, 9, 11239–11252. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.P.; Cai, Z.; Watanabe, F.; Agarwal, U.P.; Zhang, J. Thermal Conversion of Pine Wood Char to Carbon Nanomaterials in the Presence of Iron Nanoparticles. For. Prod. J. 2012, 62, 462–466. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, S.; Li, H.; Zhao, L.; Pan, Z.; Wu, Z.; Shi, C.; Zhen, Z.; Zhou, Z.; Zhang, H.; et al. Initial products analysis and mechanistic insights into polystyrene pyrolysis via in-situ photoionization mass spectrometry. Chem. Eng. J. 2025, 511, 162118. [Google Scholar] [CrossRef]
- dos Santos Batista, L.A.P.; de Melo Morgado, G.F.; Brazil, T.R.; Anjos, E.G.R.D.; Guimarães, A.; Rezende, M.C.; Passador, F.R. Comparative Analysis of Thermal Recycling Approaches for Carbon Fiber Recovery from CFRP Waste. ACS Sustain. Resour. Manag. 2024, 1, 2108–2118. [Google Scholar] [CrossRef]
- Ruiz-Caldas, M.-X.; Carlsson, J.; Sadiktsis, I.; Jaworski, A.; Nilsson, U.; Mathew, A.P. Cellulose Nanocrystals from Postconsumer Cotton and Blended Fabrics: A Study on Their Properties, Chemical Composition, and Process Efficiency. ACS Sustain. Chem. Eng. 2022, 10, 3787–3798. [Google Scholar] [CrossRef]
- Ruiz-Caldas, M.-X.; Apostolopoulou-Kalkavoura, V.; Mathew, A.P. Unlocking the potential of post-consumer garments as a source of nanocellulose. Cell Rep. Phys. Sci. 2024, 5, 101795. [Google Scholar] [CrossRef]
- Jacob, D. Process of Converting Textile or Plastic Solid Waste into Activated Carbon. WO2014162267A1, 1 April 2014. [Google Scholar]
- Wang, C.; Su, J.; Liu, T.; Ge, S.; Liew, R.K.; Zhang, H.; Naushad, M.; Lam, S.S.; Ng, H.S.; Sonne, C.; et al. A sustainable strategy to transform cotton waste into renewable cellulose fiber self-reinforcing composite paper. J. Clean. Prod. 2023, 429, 139567. [Google Scholar] [CrossRef]
- Maciel, M.M.Á.D.; de Carvalho Benini, K.C.C.; Voorwald, H.J.C.; Cioffi, M.O.H. Obtainment and characterization of nanocellulose from an unwoven industrial textile cotton waste: Effect of acid hydrolysis conditions. Int. J. Biol. Macromol. 2019, 126, 496–506. [Google Scholar] [CrossRef]
- Rizal, S.; Olaiya, F.G.; Saharudin, N.I.; Abdullah, C.K.; N. G., O.; Mohamad Haafiz, M.K.; Yahya, E.B.; Sabaruddin, F.A.; Ikramullah; Khalil H. P. S., A. Isolation of Textile Waste Cellulose Nanofibrillated Fibre Reinforced in Polylactic Acid-Chitin Biodegradable Composite for Green Packaging Application. Polymers 2021, 13, 325. [Google Scholar] [CrossRef]
- Khan, M.J.; Karim, Z.; Charnnok, B.; Poonsawat, T.; Posoknistakul, P.; Laosiripojana, N.; Wu, K.C.-W.; Sakdaronnarong, C. Fabrication and Characterization of Functional Biobased Membranes from Postconsumer Cotton Fabrics and Palm Waste for the Removal of Dyes. Int. J. Mol. Sci. 2023, 24, 6030. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Dhandapani, R.; Liang, D.; Wang, J.; Wolcott, M.P.; Van Fossen, D.; Liu, H. Nanocellulose from recycled indigo-dyed denim fabric and its application in composite films. Carbohydr. Polym. 2020, 240, 116283. [Google Scholar] [CrossRef]
- Wu, Q.; Ding, C.; Wang, B.; Rong, L.; Mao, Z.; Feng, X. Green, chemical-free, and high-yielding extraction of nanocellulose from waste cotton fabric enabled by electron beam irradiation. Int. J. Biol. Macromol. 2024, 267, 131461. [Google Scholar] [CrossRef]
- Huang, S.; Tao, R.; Ismail, A.; Wang, Y. Cellulose Nanocrystals Derived from Textile Waste through Acid Hydrolysis and Oxidation as Reinforcing Agent of Soy Protein Film. Polymers 2020, 12, 958. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Liu, C.; Xu, Y.; Li, G.; Zhang, H.; Huang, J.; Miao, J. Carbon fiber reinforced elastomeric thermal interface materials for spacecraft. Carbon 2022, 187, 432–438. [Google Scholar] [CrossRef]
- Huang, B.-T.; Zhu, J.-X.; Weng, K.-F.; Li, V.C.; Dai, J.-G. Ultra-high-strength engineered/strain-hardening cementitious composites (ECC/SHCC): Material design and effect of fiber hybridization. Cem. Concr. Compos. 2022, 129, 104464. [Google Scholar] [CrossRef]
- Ben Shalom, T.; Belsey, S.; Chasnitsky, M.; Shoseyov, O. Cellulose Nanocrystals and Corn Zein Oxygen and Water Vapor Barrier Biocomposite Films. Nanomaterials 2021, 11, 247. [Google Scholar] [CrossRef]
- Thambiraj, S.; Shankaran, D.R. Preparation and physicochemical characterization of cellulose nanocrystals from industrial waste cotton. Appl. Surf. Sci. 2017, 412, 405–416. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, Z.; Zhou, J.; Zhang, Y. Reuse of waste cotton cloth for the extraction of cellulose nanocrystals. Carbohydr. Polym. 2017, 157, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xia, Z.; Farooq, A.; Zhang, M.; Li, M.; Liu, L. Efficient recovery of the dyed cotton–polyester fabric: Cellulose nanocrystal extraction and its application in composite films. Cellulose 2021, 28, 3235–3248. [Google Scholar] [CrossRef]
- Pandi, N.; Sonawane, S.H.; Kishore, K.A. Synthesis of cellulose nanocrystals (CNCs) from cotton using ultrasound-assisted acid hydrolysis. Ultrason. Sonochem. 2021, 70, 105353. [Google Scholar] [CrossRef]
- Ye, S.; Yu, H.-Y.; Wang, D.; Zhu, J.; Gu, J. Green acid-free one-step hydrothermal ammonium persulfate oxidation of viscose fiber wastes to obtain carboxylated spherical cellulose nanocrystals for oil/water Pickering emulsion. Cellulose 2018, 25, 5139–5155. [Google Scholar] [CrossRef]
- Culsum, N.T.U.; Melinda, C.; Leman, I.; Wibowo, A.; Budhi, Y.W. Isolation and characterization of cellulose nanocrystals (CNCs) from industrial denim waste using ammonium persulfate. Mater. Today Commun. 2021, 26, 101817. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, Z.; Zhou, J.; He, M.; Jiang, Q.; Li, A.; Li, S.; Liu, M.; Luo, S.; Zhang, D. Improvement of polylactic acid film properties through the addition of cellulose nanocrystals isolated from waste cotton cloth. Int. J. Biol. Macromol. 2019, 129, 878–886. [Google Scholar] [CrossRef]
- Hemmati, F.; Jafari, S.M.; Taheri, R.A. Optimization of homogenization-sonication technique for the production of cellulose nanocrystals from cotton linter. Int. J. Biol. Macromol. 2019, 137, 374–381. [Google Scholar] [CrossRef]
- Jordan, J.H.; Easson, M.W.; Dien, B.; Thompson, S.; Condon, B.D. Extraction and characterization of nanocellulose crystals from cotton gin motes and cotton gin waste. Cellulose 2019, 26, 5959–5979. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Xia, Z.P.; Yang, A.P.; Li, J.X.; Wang, L.; Chen, H.; Zheng, X.; Liu, Y. Nanocellulose extracted from waste polyester/cotton fabric by chemical-mechanical separation technology. J. Phys. Conf. Ser. 2021, 1790, 012074. [Google Scholar] [CrossRef]
- Vanzetto, A.B.; Beltrami, L.V.R.; Zattera, A.J. Textile waste as precursors in nanocrystalline cellulose synthesis. Cellulose 2021, 28, 6967–6981. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ahuja, A.; Mahur, B.K.; Rastogi, V.K. Valorization of nylon and viscose-rayon textile yarn wastes for fabricating nanocomposite films. Express Polym. Lett. 2023, 17, 196–210. [Google Scholar] [CrossRef]
- Mari, R.P.; Sornas, J.J.; Bierhalz, A.C.K. Polysaccharide-based films reinforced with nanocellulose isolated from raw and bleached cotton. Cellulose 2023, 30, 1657–1668. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Pang, G.-X.; Shen, W.-H.; Tong, X.; Jia, M.-Y. Preparation and characterization of the ribbon-like cellulose nanocrystals by the cellulase enzymolysis of cotton pulp fibers. Carbohydr. Polym. 2019, 207, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Zhang, X.; Tian, D.; Zhou, Z.; Lu, C. Comparing microcrystalline with spherical nanocrystalline cellulose from waste cotton fabrics. Cellulose 2012, 19, 1189–1198. [Google Scholar] [CrossRef]
- Saleh, M.; Gul, A.; Nasir, A.; Moses, T.O.; Nural, Y.; Yabalak, E. Comprehensive review of Carbon-based nanostructures: Properties, synthesis, characterization, and cross-disciplinary applications. J. Ind. Eng. Chem. 2025, 146, 176–212. [Google Scholar] [CrossRef]
- Zhu, Y.; Miao, J.; Zhang, Y.; Li, C.; Wang, Y.; Cheng, Y.; Long, M.; Wang, J.; Wu, C. Carbon nanotubes production from real-world waste plastics and the pyrolysis behaviour. Waste Manag. 2023, 166, 141–151. [Google Scholar] [CrossRef]
- Yousef, S.; Kalpokaitė-Dičkuvienė, R.; Baltušnikas, A.; Pitak, I.; Lukošiūtė, S.I. A new strategy for functionalization of char derived from pyrolysis of textile waste and its application as hybrid fillers (CNTs/char and graphene/char) in cement industry. J. Clean. Prod. 2021, 314, 128058. [Google Scholar] [CrossRef]
- Xu, T.; Chen, L.; Chen, J.; Lei, Y.; Wang, X.; Yang, X.; Yang, Z. Characteristics of Pyrolysis Products of Tar-Rich Coal Under Cryogenic Pretreatment with Liquid Nitrogen. Processes 2025, 13, 1064. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Maafa, I.M. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef]
- Selvaraj, P.S.; Ettiyagounder, P.; Sabarish, K.; Periasamy, K.; Rengasamy, B.; Veeraswamy, D.; Karchiyappan, T.; Kathirvel, S. Hydrothermal carbonization approach for transforming biomass waste to value added hydrochar and its applications in water remediation. Desalination Water Treat. 2025, 322, 101199. [Google Scholar] [CrossRef]
- Parrilla-Lahoz, S.; Zambrano, M.C.; Pawlak, J.J.; Venditti, R.A.; Reina, T.R.; Odriozola, J.A.; Duyar, M.S. Textile microfibers valorization by catalytic hydrothermal carbonization toward high-tech carbonaceous materials. iScience 2024, 27, 111427. [Google Scholar] [CrossRef]
- Parrilla-Lahoz, S.; Jiménez-Páez, E.; Masteghin, M.G.; Pawlak, J.J.; Venditti, R.A.; Bird, R.; Servin, P.; Odriozola, J.A.; Reina, T.R.; Duyar, M.S. Upcycling textile derived microplastics waste collected from washer and dryers to carbonaceous products using hydrothermal carbonization. Waste Manag. 2025, 200, 114740. [Google Scholar] [CrossRef]
- Petrović, J.; Ercegović, M.; Simić, M.; Koprivica, M.; Dimitrijević, J.; Jovanović, A.; Pantić, J.J. Hydrothermal Carbonization of Waste Biomass: A Review of Hydrochar Preparation and Environmental Application. Processes 2024, 12, 207. [Google Scholar] [CrossRef]
- Ischia, G.; Fiori, L. Hydrothermal Carbonization of Organic Waste and Biomass: A Review on Process, Reactor, and Plant Modeling. Waste Biomass-Valorization 2021, 12, 2797–2824. [Google Scholar] [CrossRef]
- Mohamed, M.Z.; Mohamed, A.S.; Mohamed, M.G.; Mazrouaa, A.M.; Ismail, D.A. Applications of Solid Waste-Derived Carbon Nanomaterials in Fabrics and Fibers. Waste Deriv. Carbon Nanomater. 2025, 2, 37–54. [Google Scholar]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S. Pyrolysis and beyond: Sustainable valorization of plastic waste. Appl. Energy Combust. Sci. 2025, 21, 100311. [Google Scholar] [CrossRef]
- Feng, Z.; Adolfsson, K.H.; Xu, Y.; Fang, H.; Hakkarainen, M.; Wu, M. Carbon dot/polymer nanocomposites: From green synthesis to energy, environmental and biomedical applications. Sustain. Mater. Technol. 2021, 29, e00304. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef]
- Li, M.; Magdassi, S.; Gao, Y.; Long, Y. Hydrothermal Synthesis of VO2 Polymorphs: Advantages, Challenges and Prospects for the Application of Energy Efficient Smart Windows. Small 2017, 13, 1701147. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Qian, H.; Nie, Y.; Bai, X.; Xia, T.; Rehman, M.L.U.; Li, Q.; Ju, M. Upcycling and catalytic degradation of plastic wastes. Cell Rep. Phys. Sci. 2021, 2, 100514. [Google Scholar] [CrossRef]
- Qi, R.; Xu, Z.; Zhou, Y.; Zhang, D.; Sun, Z.; Chen, W.; Xiong, M. Clean solid fuel produced from cotton textiles waste through hydrothermal carbonization with FeCl3: Upgrading the fuel quality and combustion characteristics. Energy 2021, 214, 118926. [Google Scholar] [CrossRef]
- Ansari, M.; Heo, Y.; Do, K.; Ghosh, M.; Son, Y.-O. Nanocellulose derived from agricultural biowaste by-products–Sustainable synthesis, biocompatibility, biomedical applications, and future perspectives: A review. Carbohydr. Polym. Technol. Appl. 2024, 8, 100529. [Google Scholar] [CrossRef]
- Halim, A.F.M.F.; Poinern, G.E.J.; Fawcett, D.; Sharma, R.; Surendran, S.; R, R. Biomass-Derived Carbon Nanomaterials: Synthesis and Applications in Textile Wastewater Treatment, Sensors, Energy Storage, and Conversion Technologies. CleanMat 2025, 2, 4–58. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Mmuoegbulam, A.O.; Okezie, O.; Iya, N.I.D.; Mohammed, S.E.; James, P.H.; Muhammad, A.B.; Unimke, A.A.; Alim, S.A.; Yahaya, S.M.; et al. Recent progress in carbon-based nanomaterials: Critical review. J. Nanoparticle Res. 2024, 26, 106. [Google Scholar] [CrossRef]
- Pirozzi, A.; Rincon, E.; Espinosa, E.; Donsì, F.; Serrano, L. Nanocellulose-based aerogels for dyes removal. Chem. Eng. Trans. 2023, 101, 115–120. [Google Scholar]
- Ayach, J.; El Malti, W.; Duma, L.; Lalevée, J.; Al Ajami, M.; Hamad, H.; Hijazi, A. Comparing Conventional and Advanced Approaches for Heavy Metal Removal in Wastewater Treatment: An In-Depth Review Emphasizing Filter-Based Strategies. Polymers 2024, 16, 1959. [Google Scholar] [CrossRef]
- Amiralian, N.; Mustapic, M.; Hossain, S.A.; Wang, C.; Konarova, M.; Tang, J.; Na, J.; Khan, A.; Rowan, A. Magnetic nanocellulose: A potential material for removal of dye from water. J. Hazard. Mater. 2020, 394, 122571. [Google Scholar] [CrossRef]
- Bassyouni, M.; Zoromba, M.S.; Abdel-Aziz, M.H.; Mosly, I. Extraction of Nanocellulose for Eco-Friendly Biocomposite Adsorbent for Wastewater Treatment. Polymers 2022, 14, 1852. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Mallick, T.K. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Sujanani, R.; Landsman, M.R.; Jiao, S.; Moon, J.D.; Shell, M.S.; Lawler, D.F.; Katz, L.E.; Freeman, B.D. Designing Solute-Tailored Selectivity in Membranes: Perspectives for Water Reuse and Resource Recovery. ACS Macro Lett. 2020, 9, 1709–1717. [Google Scholar] [CrossRef]
- Liu, P.; Borrell, P.F.; Božič, M.; Kokol, V.; Oksman, K.; Mathew, A.P. Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. J. Hazard. Mater. 2015, 294, 177–185. [Google Scholar] [CrossRef]
- Li, M.; Messele, S.A.; Boluk, Y.; El-Din, M.G. Isolated cellulose nanofibers for Cu (II) and Zn (II) removal: Performance and mechanisms. Carbohydr. Polym. 2019, 221, 231–241. [Google Scholar] [CrossRef]
- Tang, J.; Song, Y.; Zhao, F.; Spinney, S.; Bernardes, J.d.S.; Tam, K.C. Compressible cellulose nanofibril (CNF) based aerogels produced via a bio-inspired strategy for heavy metal ion and dye removal. Carbohydr. Polym. 2019, 208, 404–412. [Google Scholar] [CrossRef]
- Shahzad, A.; Ullah, M.W.; Ali, J.; Aziz, K.; Javed, M.A.; Shi, Z.; Manan, S.; Ul-Islam, M.; Nazar, M.; Yang, G. The versatility of nanocellulose, modification strategies, and its current progress in wastewater treatment and environmental remediation. Sci. Total Environ. 2023, 858, 159937. [Google Scholar] [CrossRef] [PubMed]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Ong, K.K.; Noor, S.A.M.; Halim, N.A.; Shah, N.A.A.; Jamal, S.H.; Janudin, N.; Misenan, M.S.M.; et al. Emerging Developments Regarding Nanocellulose-Based Membrane Filtration Material against Microbes. Polymers 2021, 13, 3249. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, O.; Manukyan, L.; Mihranyan, A. High-Performance Virus Removal Filter Paper for Drinking Water Purification. Glob. Chall. 2018, 2, 1800031. [Google Scholar] [CrossRef]
- Manukyan, L.; Li, P.; Gustafsson, S.; Mihranyan, A. Growth media filtration using nanocellulose-based virus removal filter for upstream biopharmaceutical processing. J. Membr. Sci. 2019, 572, 464–474. [Google Scholar] [CrossRef]
- Asper, M.; Hanrieder, T.; Quellmalz, A.; Mihranyan, A. Removal of xenotropic murine leukemia virus by nanocellulose based filter paper. Biologicals 2015, 43, 452–456. [Google Scholar] [CrossRef]
- Metreveli, G.; Wågberg, L.; Emmoth, E.; Belák, S.; Strømme, M.; Mihranyan, A. A Size-Exclusion Nanocellulose Filter Paper for Virus Removal. Adv. Healthc. Mater. 2014, 3, 1546–1550. [Google Scholar] [CrossRef]
- Mautner, A.; Bismarck, A. Bacterial nanocellulose papers with high porosity for optimized permeance and rejection of nm-sized pollutants. Carbohydr. Polym. 2021, 251, 117130. [Google Scholar] [CrossRef]
- Quellmalz, A.; Mihranyan, A. Citric Acid Cross-Linked Nanocellulose-Based Paper for Size-Exclusion Nanofiltration. ACS Biomater. Sci. Eng. 2015, 1, 271–276. [Google Scholar] [CrossRef]
- Mi, X.; Albukhari, S.M.; Heldt, C.L.; Heiden, P.A. Virus and chlorine adsorption onto guanidine modified cellulose nanofibers using covalent and hydrogen bonding. Carbohydr. Res. 2020, 498, 108153. [Google Scholar] [CrossRef]
- Meingast, C.; Heldt, C.L. Arginine-enveloped virus inactivation and potential mechanisms. Biotechnol. Prog. 2020, 36, e2931. [Google Scholar] [CrossRef]
- Rosilo, H.; McKee, J.R.; Kontturi, E.; Koho, T.; Hytönen, V.P.; Ikkala, O.; Kostiainen, M.A. Cationic polymer brush-modified cellulose nanocrystals for high-affinity virus binding. Nanoscale 2014, 6, 11871–11881. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wang, H.; Li, X. Modification of cellulose microfibers by polyglutamic acid and mesoporous silica nanoparticles for Enterovirus 71 adsorption. Mater. Lett. 2020, 277, 128320. [Google Scholar] [CrossRef]
- Wang, R.; Guan, S.; Sato, A.; Wang, X.; Wang, Z.; Yang, R.; Hsiao, B.S.; Chu, B. Nanofibrous microfiltration membranes capable of removing bacteria, viruses and heavy metal ions. J. Membr. Sci. 2013, 446, 376–382. [Google Scholar] [CrossRef]
- Gouda, M.; Hebeish, A.A.; Al-Omair, M.A. Development of silver-containing nanocellulosics for effective water disinfection. Cellulose 2014, 21, 1965–1974. [Google Scholar] [CrossRef]
- Ottenhall, A.; Henschen, J.; Illergård, J.; Ek, M. Cellulose-based water purification using paper filters modified with polyelectrolyte multilayers to remove bacteria from water through electrostatic interactions. Environ. Sci. Water Res. Technol. 2018, 4, 2070–2079. [Google Scholar] [CrossRef]
- Abrishami, S.; Shirali, A.; Sharples, N.; Kartal, G.E.; Macintyre, L.; Doustdar, O. Textile Recycling and Recovery: An Eco-friendly Perspective on Textile and Garment Industries Challenges. Text. Res. J. 2024, 94, 2815–2834. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, C.; Pan, J.; Yang, G.; Sun, C.; Liu, Y.; Chen, X.; Zhao, Z. Leachate from municipal solid waste landfills in a global perspective: Characteristics, influential factors and environmental risks. J. Clean. Prod. 2022, 333, 130234. [Google Scholar] [CrossRef]
- Olhan, S.; Khatkar, V.; Behera, B.K. Review: Textile-based natural fibre-reinforced polymeric composites in automotive lightweighting. J. Mater. Sci. 2021, 56, 18867–18910. [Google Scholar] [CrossRef]
- Grover, T.; Wang, L.; Nayak, R.; Padhye, R.; Khandual, A. Sustainable strategies for the valorization of cotton and cotton blended waste: Pathways to circular economy. Cellulose 2025, 32, 4077–4100. [Google Scholar] [CrossRef]
- Stanescu, M.D. State of the art of post-consumer textile waste upcycling to reach the zero waste milestone. Environ. Sci. Pollut. Res. 2021, 28, 14253–14270. [Google Scholar] [CrossRef] [PubMed]
- Shamsuzzaman, M.; Al. Mamun, M.A.; Hasan, H.R.U.; Hassan, R.; Zulkernine, A.; Atik, M.A.R.; Islam, M. Fashion Circularity: Potential of Reusing and Recycling Remnant Fabric to Create Sustainable Products. Sustainability 2025, 17, 2010. [Google Scholar] [CrossRef]



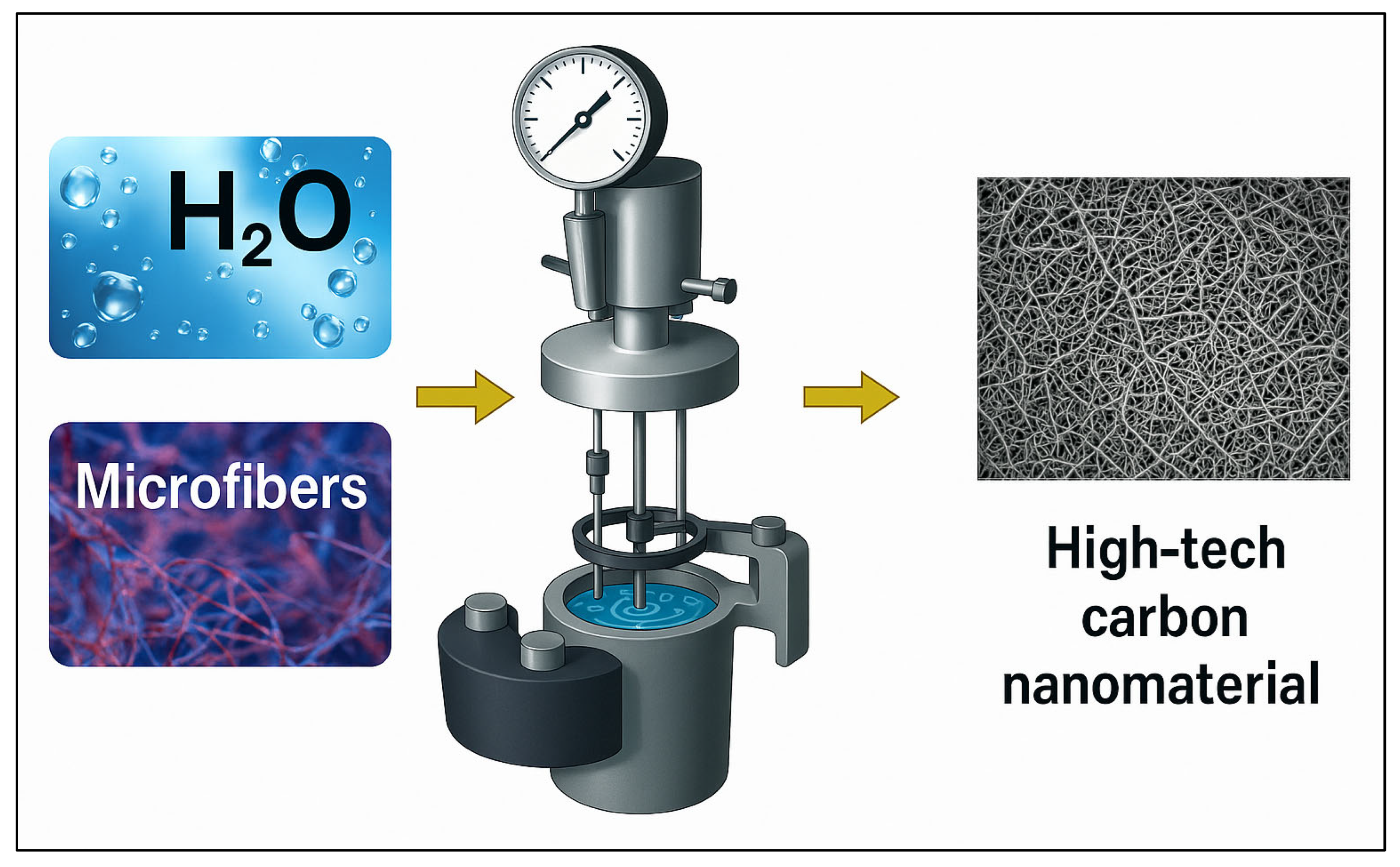
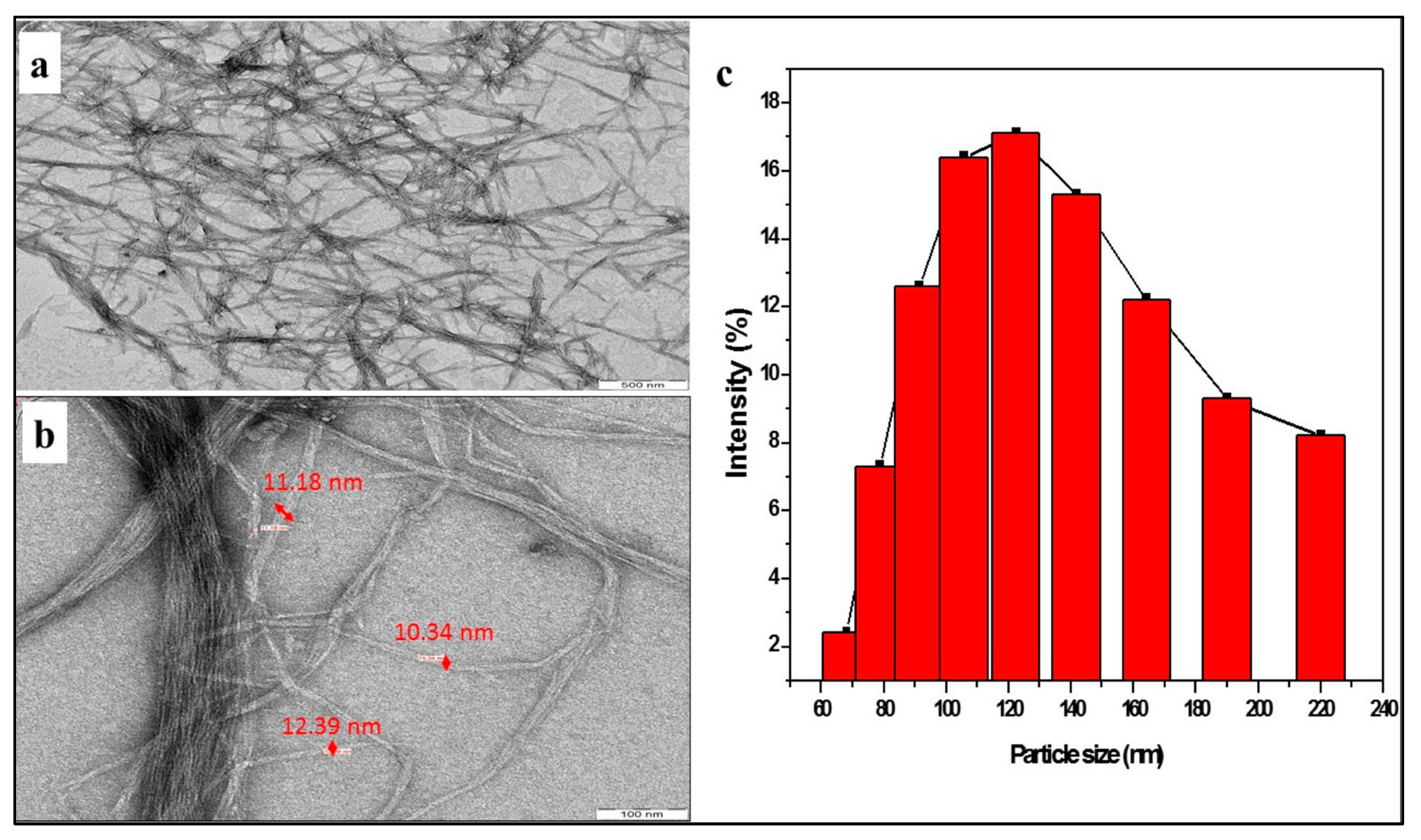

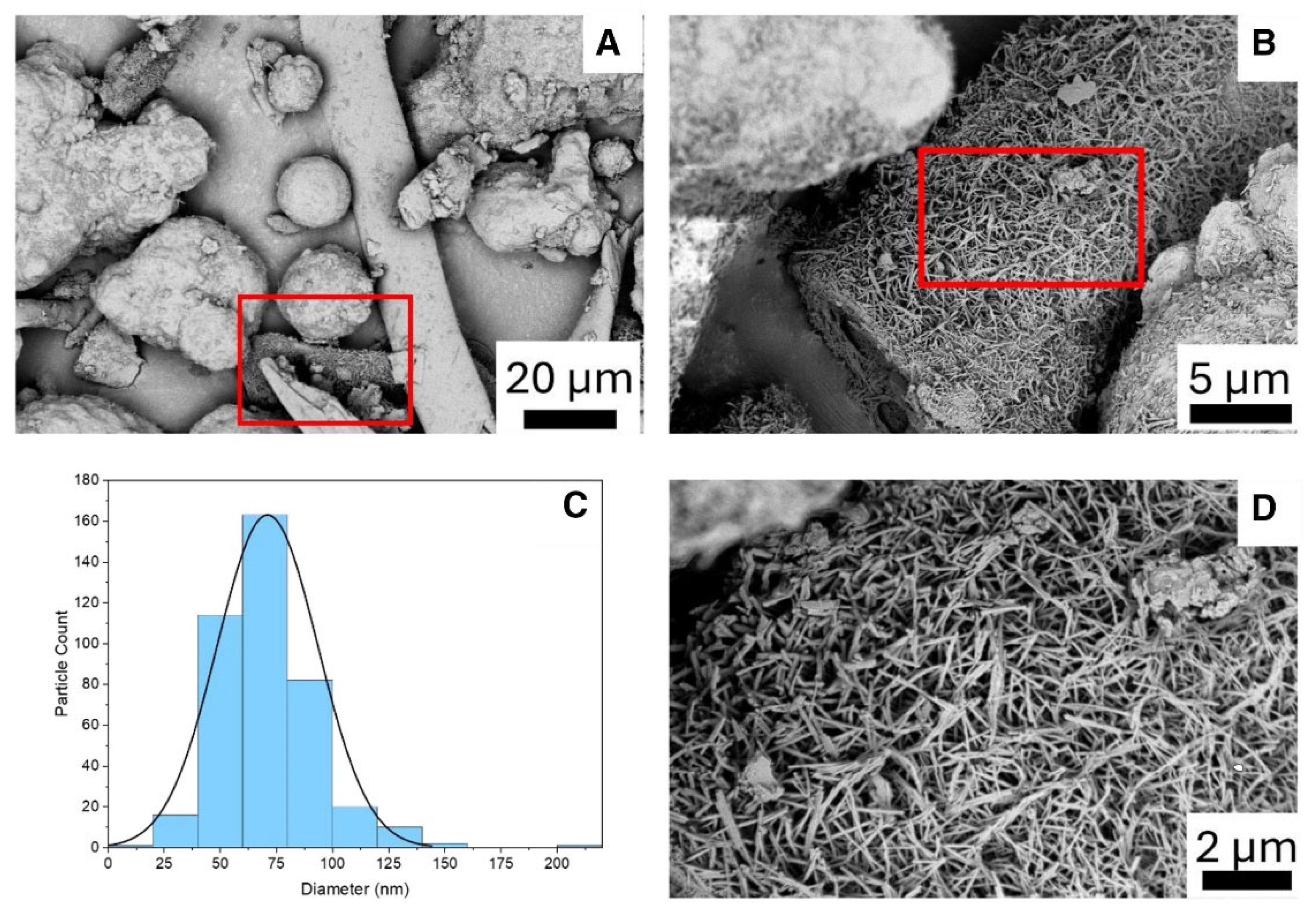
| Type of Textile Waste | Method | Shape/Size | Yield (%) | Reference |
|---|---|---|---|---|
| 100% postconsumer cotton fabric | Alkaline hydrolysis+ supercritical CO2 + high pressure homogenization | Typical rod-shaped structure 100–120 nm | Not specified | [63] |
| Waste cloth made of cotton material | H2SO4/HCl hydrolysis |
| 77 | [64] |
| Waste polyester–cotton fabric | Cellulose extraction via alkali, decolorizing, and hydrochloric acid treatment followed by H2SO4 hydrolysis. | Not specified | 56 | [4] |
| White bleached cotton fabric | TEMPO-facilitated oxidation for producing CNF |
| 73 | [65] |
| Indigo-dyed denim fabric. | TEMPO-facilitated oxidation for producing CNF |
| 79 | [65] |
| Waste cotton fabric | Combined two-step process, involving electron beam irradiation. | Low-charged cellulose nanorods Rod-like shape Length: 50–300 nm Aspect ratio 18.4 | 90 | [66] |
| Type of Textile Waste | Method | Size | Yield (%) | Reference |
|---|---|---|---|---|
| Waste cotton fabric | Alkaline bleaching and acidic hydrolysis | Length ranges from 28 to 470 nm, with a diameter between 3 and 35 nm. | 47 | [72] |
| Denim fabric | Acid hydrolysis, along with mechanical grinding | Length: 214.6–234.6; Diameter: 23.7 | 90–98 | [73] |
| White bleached cotton fabric | Chemical processes, including acid hydrolysis and TEMPO oxidation, as well as mechanical methods. |
| 40 | [65] |
| Denim fabric dyed with indigo | Chemical processes, including acid hydrolysis and TEMPO oxidation, as well as mechanical methods. |
| 38 | [65] |
| Cotton by-products | Chemical methods (oxidation bleaching, acid hydrolysis) and mechanical processes. | 50 nm | - | [74] |
| Cotton waste from industrial processes | Acid hydrolysis (chemical process) | Length: 180 ± 60 nm Diameter: 10 ± 1 nm | 45 | [71] |
| Viscose fiber textile waste | Chemical oxidation via APS | 34–49 nm | 38–40 | [75] |
| Denim Waste | Chemical (alkaline treatment, APS oxidation) | Length: 76.14 ± 8.56 nm, Diameter: 18.10 ± 3.54 nm | 21–27 | [76] |
| Recycled cotton fabric | Chemical (alkali pretreatment, acid hydrolysis) and mechanical processes | Length: 38–424 nm, Diameter: 2–17 nm | 49 | [77] |
| Cotton textile waste | Chemical (alkaline pretreatment, acid hydrolysis, chlorine-free bleaching) | Length: 203.7 ± 68.6–1819.3 ± 328.5 nm, Diameter: 16.5 ± 3.6–248.0 ± 130 nm. | 63–83 | [62] |
| Cotton linter | Alkaline pretreatment and acid hydrolysis are classified as chemical methods. | Length: 133 nm, Diameter: 10 nm | 59–72 | [78] |
| Cotton gin and waste from textile production | Chemical (acid hydrolysis) | Length: 100–300 nm Diameter: <10 nm | 50 | [79] |
| Cotton-polyester blend | Chemical (alkaline treatment, acid hydrolysis), mechanical | Length: 40–400 nm, Diameter: 40–100 nm | - | [80] |
| 100% cotton | Acid hydrolysis | Diameter: 50–150 nm Crystallinity: 80% | 73–77 | [81] |
| Cotton textile waste | Chemical (alkaline treatment, ozone bleaching, acid hydrolysis), mechanical | Length: 60–220 nm, Diameter: 10–30 nm | - | [63] |
| Viscose-rayon and nylon yarn | Chemical (acid hydrolysis), mechanical | Diameter: 65.03 ± 10.15 nm | - | [82] |
| Cotton and polyester blend (65% cotton, 35% polyester) | Chemical treatments (alkaline treatment, acid hydrolysis) | - | 56 | [4] |
| Raw and bleached cotton sliver | Chemical (acid hydrolysis) and mechanical processes | Length: 130–300 nm, Diameter: 8–34 nm | 78–88 | [83] |
| Cotton pulp fiber | Biological (enzymolysis) | Length: 250–900 Diameter: 30–45 | - | [84] |
| Cotton cloth waste scraps without dye | TEMPO-mediated oxidation | Spherical CNCs Crystallinity index: 81–85% Crystallite size: 5.1–7.8 nm | 73 | [81] |
| Waste cotton clothes | Sulfuric acid (H2SO4) hydrolysis and three-step oxidation | Rod-shaped structure Aspect ratio: 10.00 | 90 | [67] |
| Waste cotton clothes | H2SO4/HCl hydrolysis Ultrasonication |
| 49 | [77] |
| Waste cotton fabrics with a cellulose content of 94%. | Microcrystalline cellulose production through H2SO4 hydrolysis and mechanical methods. Stirring and ultrasonication | MCC particle size: 5–400 μm, MCC volume mean diameter: 49 μm SCNC dimension: 5–100 nm SCNC diameter: 35 nm | MCC: 78 SCNC: 22 | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaiya, N.G.; Al-Amin, M.; Rashed, K.; Maraveas, C. Nanomaterials from Textile Waste for Purification and Environmental Applications. Polymers 2025, 17, 3098. https://doi.org/10.3390/polym17233098
Olaiya NG, Al-Amin M, Rashed K, Maraveas C. Nanomaterials from Textile Waste for Purification and Environmental Applications. Polymers. 2025; 17(23):3098. https://doi.org/10.3390/polym17233098
Chicago/Turabian StyleOlaiya, Niyi Gideon, Md. Al-Amin, Kaifur Rashed, and Chrysanthos Maraveas. 2025. "Nanomaterials from Textile Waste for Purification and Environmental Applications" Polymers 17, no. 23: 3098. https://doi.org/10.3390/polym17233098
APA StyleOlaiya, N. G., Al-Amin, M., Rashed, K., & Maraveas, C. (2025). Nanomaterials from Textile Waste for Purification and Environmental Applications. Polymers, 17(23), 3098. https://doi.org/10.3390/polym17233098









