Research on Diagnostic Methods for Gas Generation Due to Degradation of Cable PVC Materials Under Electrical and Thermal Stress
Abstract
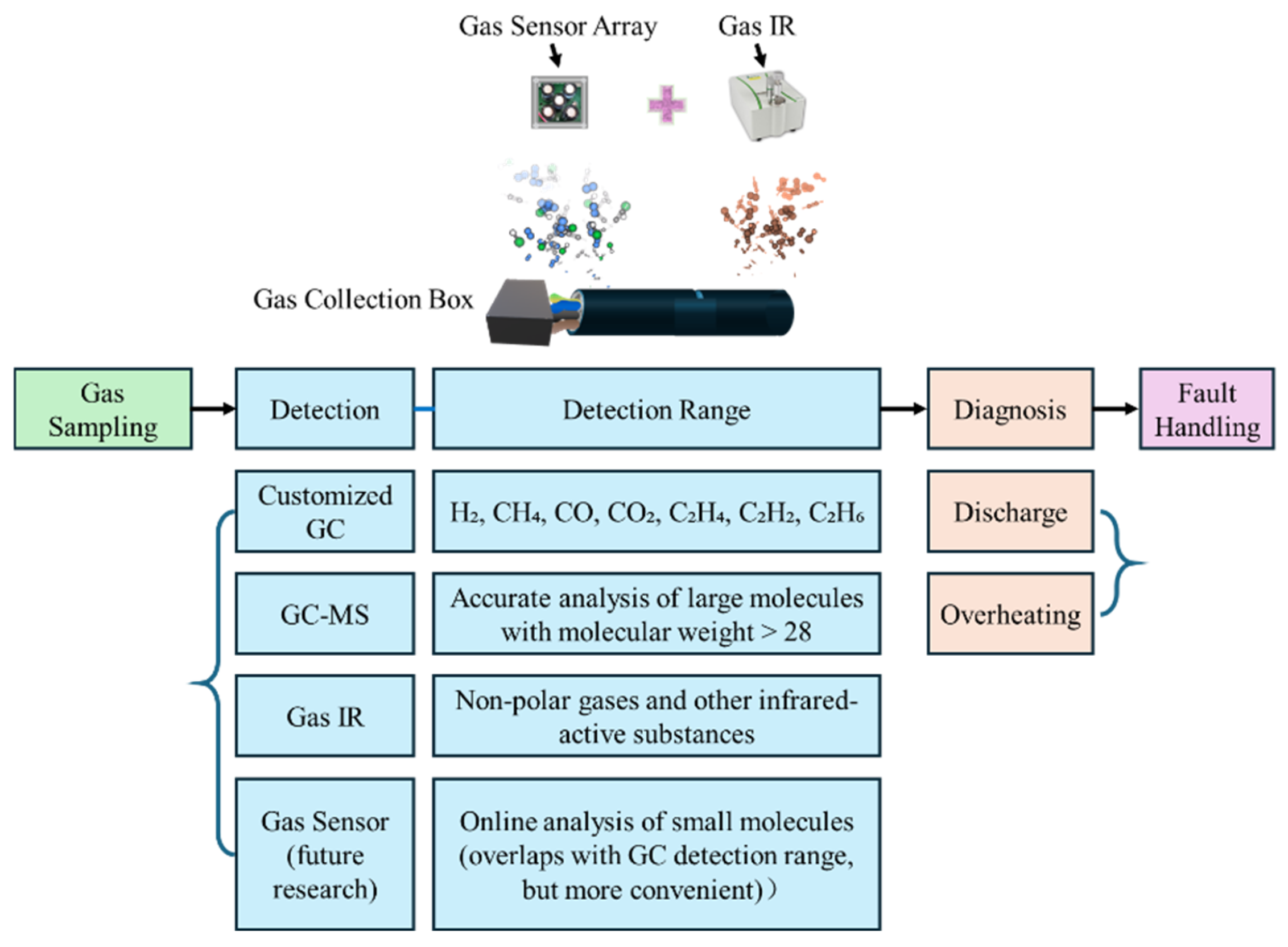
1. Introduction
2. Materials and Methods
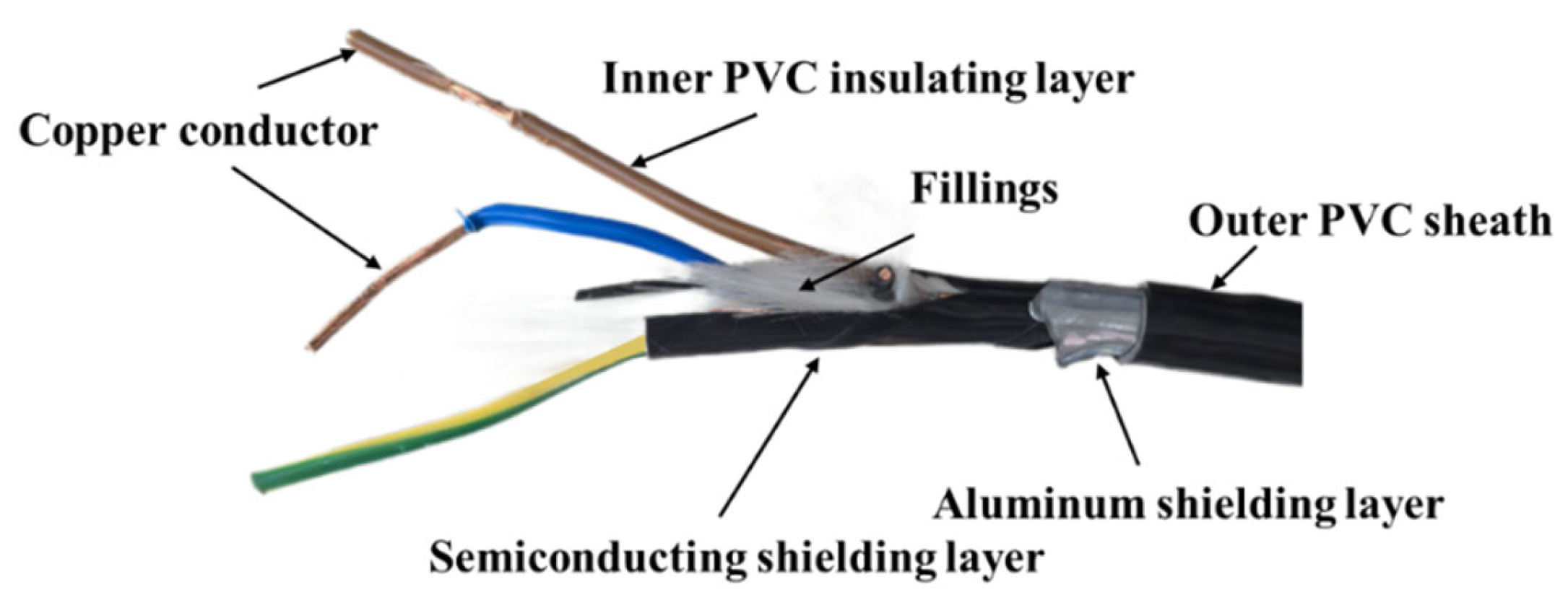
2.1. Experimental Materials
2.2. Experimental Equipment
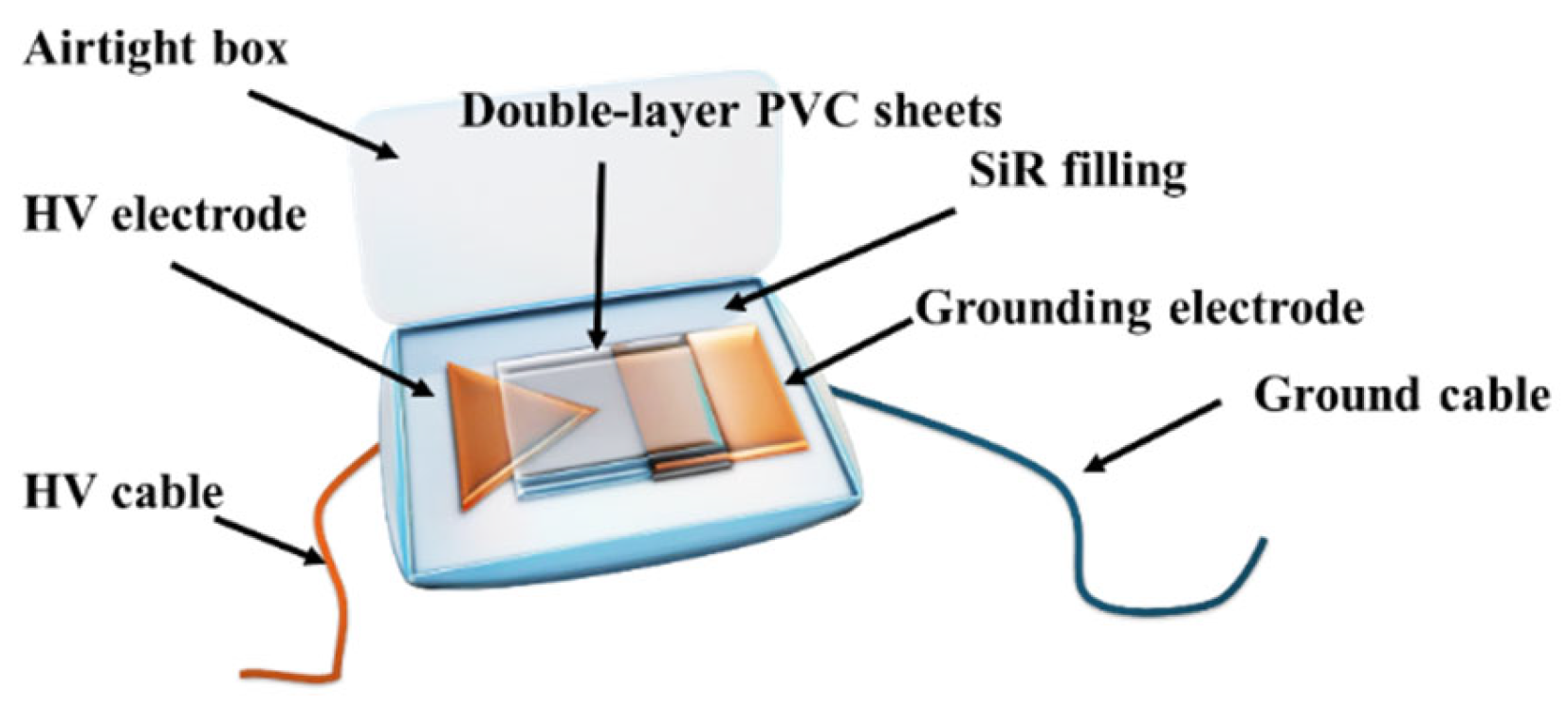
2.3. Experimental Setup for Electrically Induced Gas Generation
2.4. Experimental Setup for Thermally Induced Gas Generation
2.5. Molecular Simulation of Gas Generation from PVC
3. Results and Discussion
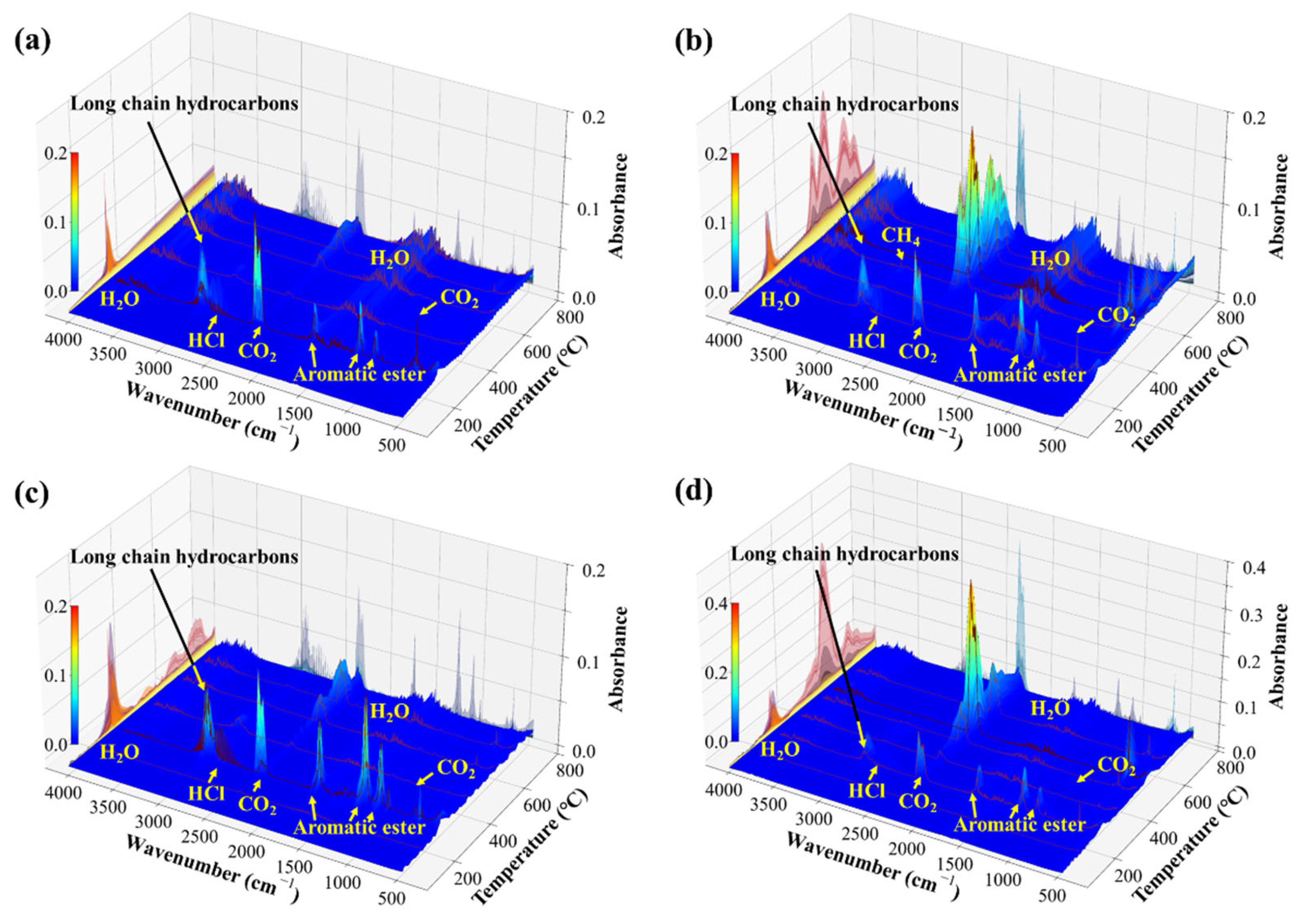
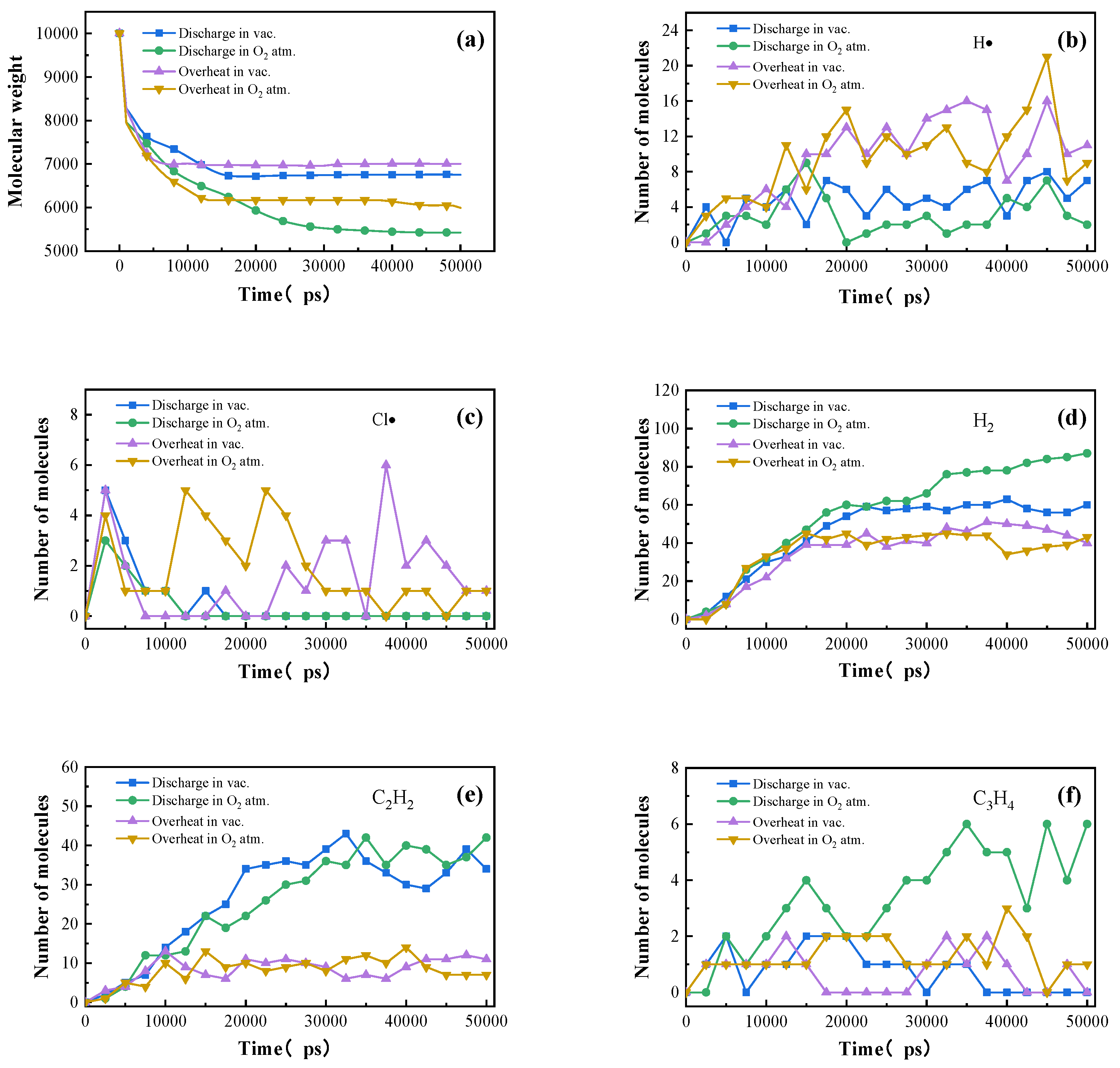
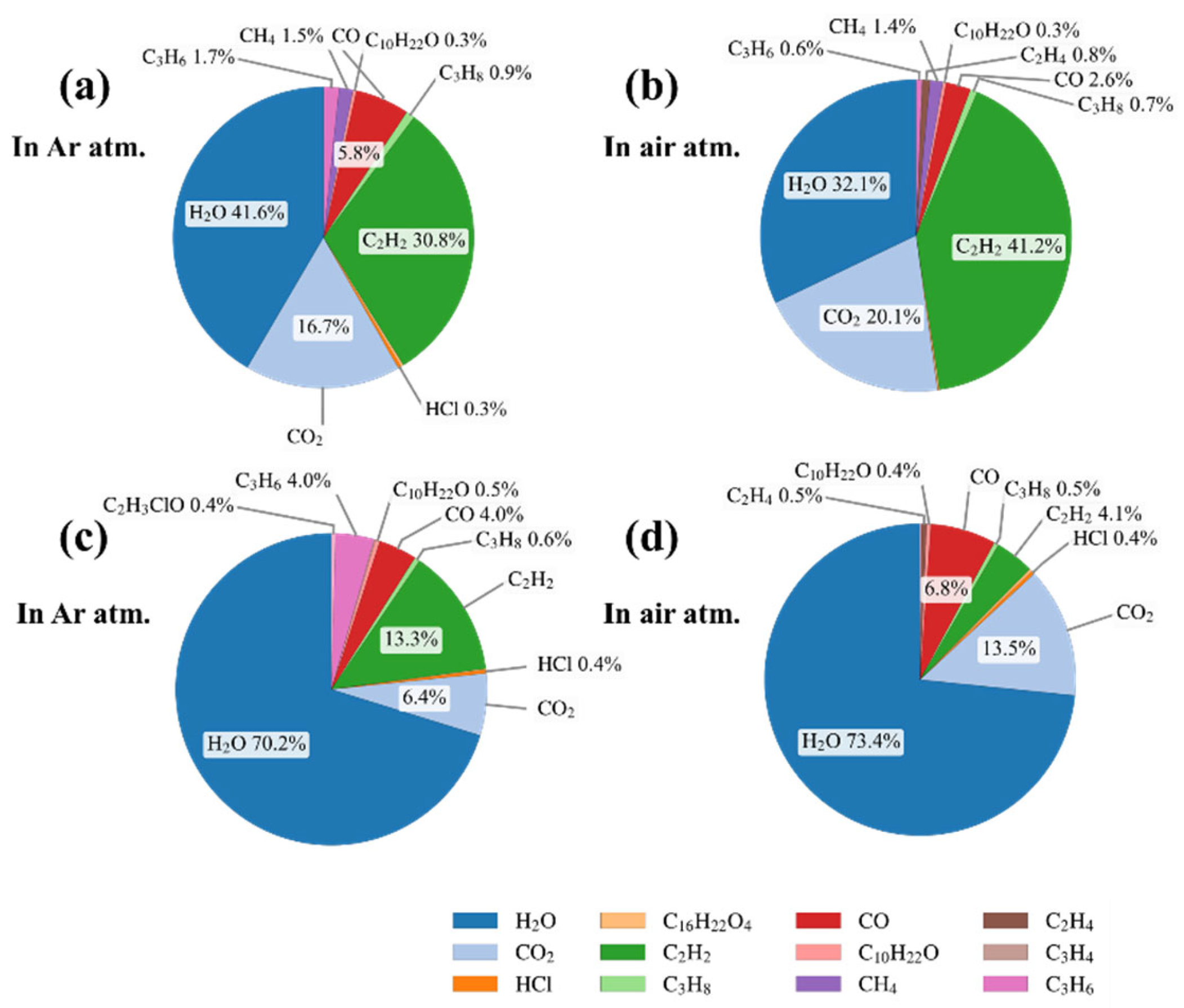
3.1. Analysis of Electrically Induced Gas Generation in PVC
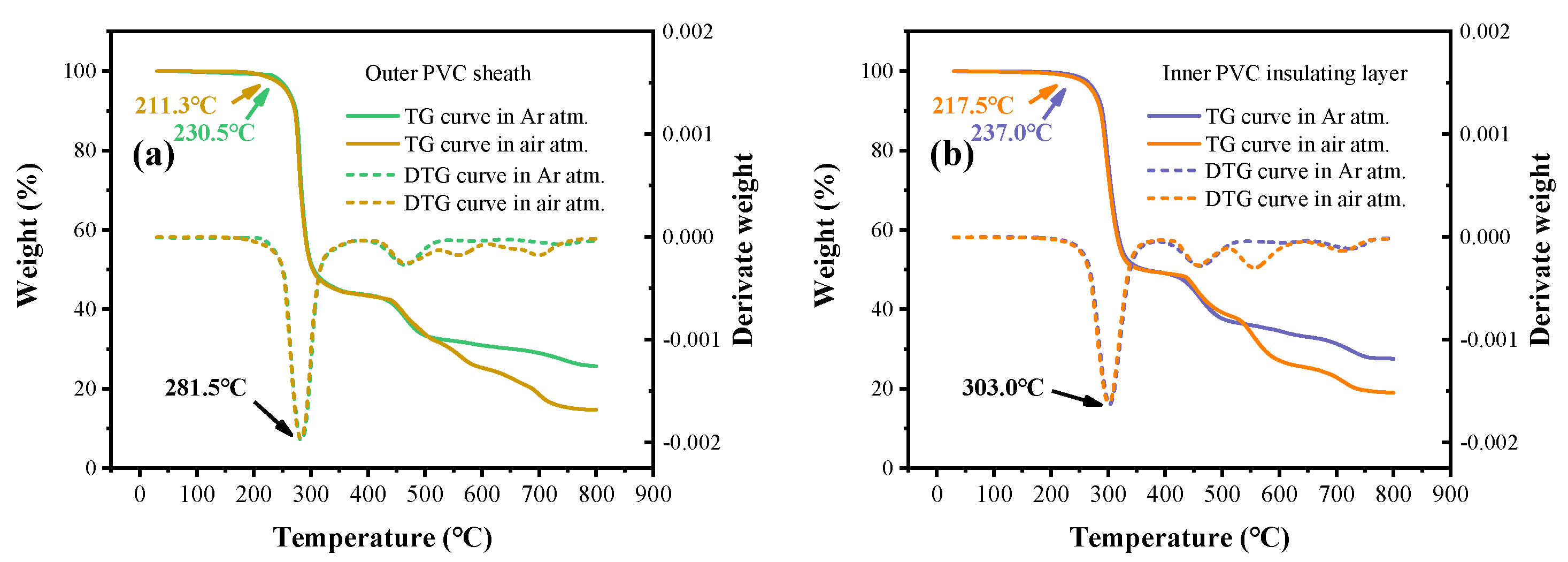
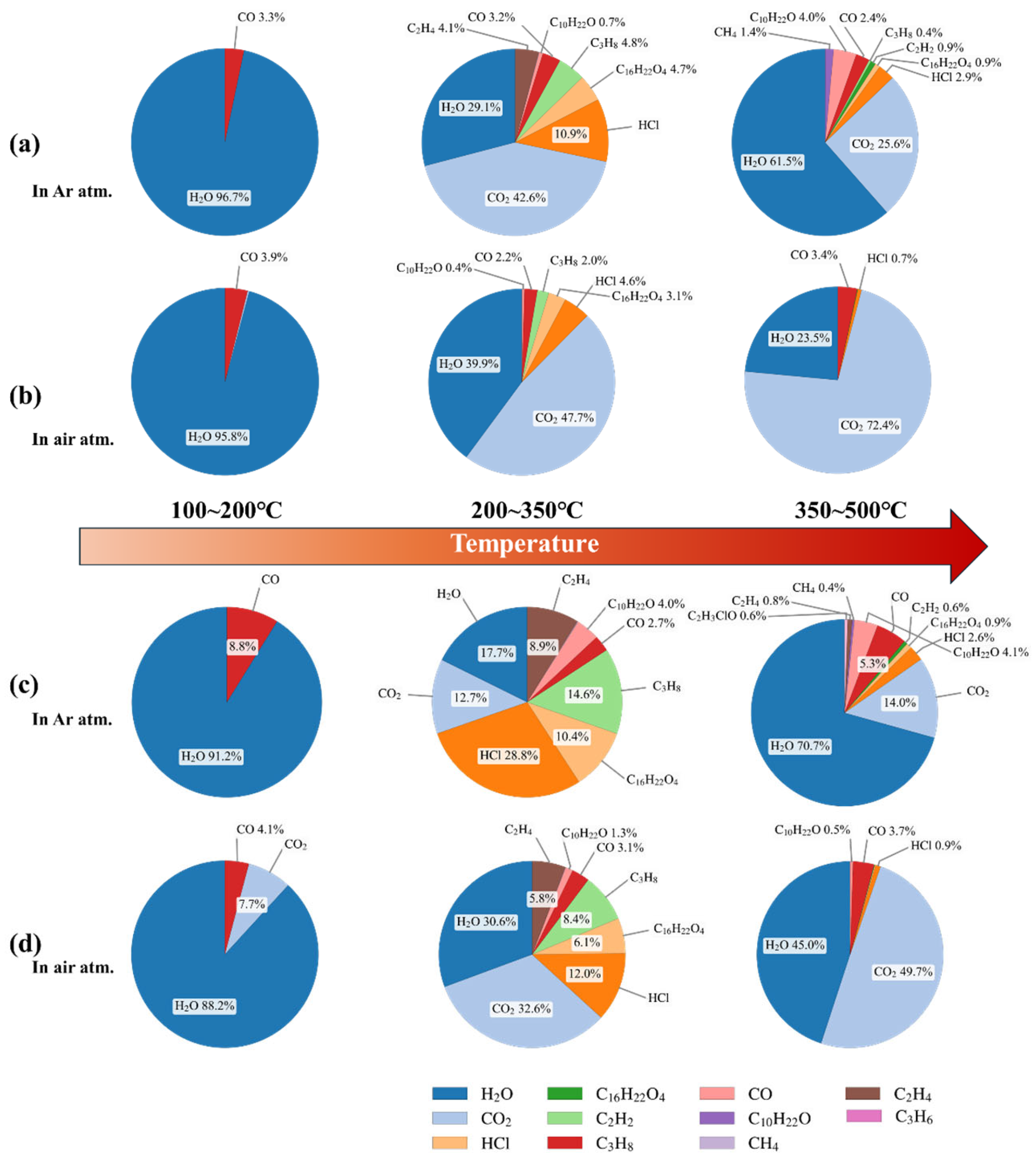
3.2. Analysis of Thermally Induced Gas Generation in PVC
3.3. Molecular Simulation of Gas Generation in PVC
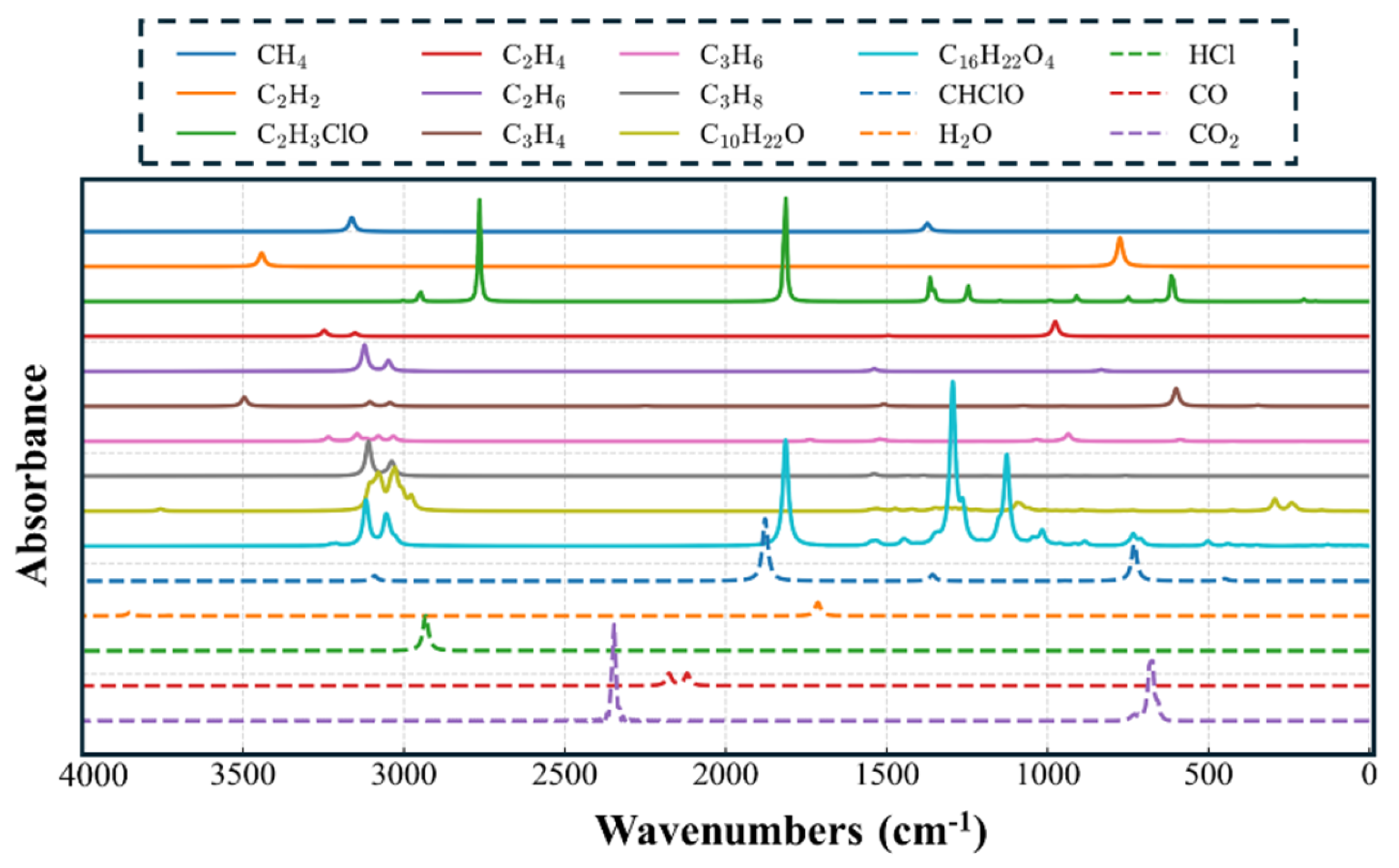
3.4. DFT Calculation Results of Infrared Spectra of PVC Characteristic Gases and Establishment of a Spectral Database
3.5. Quantitative Analysis and Fault Diagnosis of PVC
4. Conclusions
- (1)
- Under thermal and electrical stresses, the two types of PVC materials exhibited distinct multi-stage decomposition mechanisms, with significantly different gas generation characteristics strongly influenced by the surrounding atmosphere. Air promoted oxidation reactions, resulting in greater CO2 and H2O production, while discharge generated acetylene and other unsaturated hydrocarbons.
- (2)
- By integrating TG-FTIR experiments with ReaxFF and DFT molecular simulations, the radical reaction processes were elucidated, and the infrared characteristics of key products were verified. A normalized database of PVC decomposition gases was thereby established.
- (3)
- By integrating experimental results, simulations, and spectral peak-fitting using the NNLS method, this study proposed a diagnostic system based on gas concentration ratios. This system enables accurate identification of key gases such as HCl, CO, CO2, and C2H2, effectively distinguishing between different failure modes of the inner and outer PVC layers, thereby providing a reliable foundation for infrared gas diagnosis.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thai, H.-D.; Le, N.-B.; Lee, D.; Huh, J.-H. A Survey of Electrical Fire Causes Assessment Technology. IEEE Access 2024, 12, 145378–145392. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Qiu, X.; Zhu, T. A Review on Fire Research of Electric Power Grids of China: State-of-the-Art and New Insights. Fire Technol. 2024, 60, 1027–1076. [Google Scholar] [CrossRef]
- Kong, J.; Zhou, K.; Ren, X.; Chen, Y.; Li, Y.; Meng, P. Insight into gaseous product distribution of cross-linked polyethylene pyrolysis using ReaxFF MD simulation and TG-MS. J. Anal. Appl. Pyrolysis 2023, 169, 105847. [Google Scholar] [CrossRef]
- Miskolczi, N.; Bartha, L.; Angyal, A. Pyrolysis of Polyvinyl Chloride (PVC)-Containing Mixed Plastic Wastes for Recovery of Hydrocarbons. Energy Fuels 2009, 23, 2743–2749. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Y.; Zhu, J.; Yang, H.; Li, Y.; Jin, L.; Hu, H. Insight into the pyrolysis behavior of polyvinyl chloride using in situ pyrolysis time-of-flight mass spectrometry: Aromatization mechanism and Cl evolution. Fuel 2023, 331, 125994. [Google Scholar] [CrossRef]
- An, W.; Tang, Y.; Liang, K.; Wang, T.; Zhou, Y.; Wen, Z. Experimental Study on Flammability and Flame Spread Characteristics of Polyvinyl Chloride (PVC) Cable. Polymers 2020, 12, 2789. [Google Scholar] [CrossRef]
- Honus, S.; Kumagai, S.; Fedorko, G.; Molnár, V.; Yoshioka, T. Pyrolysis gases produced from individual and mixed PE, PP, PS, PVC, and PET—Part I: Production and physical properties. Fuel 2018, 221, 346–360. [Google Scholar] [CrossRef]
- Hong, D.; Gao, P.; Wang, C. A comprehensive understanding of the synergistic effect during co-pyrolysis of polyvinyl chloride (PVC) and coal. Energy 2022, 239, 122258. [Google Scholar] [CrossRef]
- Yao, Z.; Ma, X. A new approach to transforming PVC waste into energy via combined hydrothermal carbonization and fast pyrolysis. Energy 2017, 141, 1156–1165. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, Y.; Cai, N.; Wang, X.; Chen, Y.; Yang, H.; Chen, H. Preparation of carbon nanotubes by catalytic pyrolysis of dechlorinated PVC. Waste Manag. 2023, 169, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Li, W.; Bai, Z.; Zhang, T.; Jia, Y.; Hou, Y.; Chen, J.; Guo, Z.; Kong, L.; Bai, J.; Li, W. Comparative study on pyrolysis behaviors and chlorine release of pure PVC polymer and commercial PVC plastics. Fuel 2023, 340, 127555. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Wang, S.; Zhang, H.; Xu, F. TG-FTIR and Py-GC/MS study of the pyrolysis mechanism and composition of volatiles from flash pyrolysis of PVC. J. Energy Inst. 2020, 93, 2362–2370. [Google Scholar] [CrossRef]
- Ji, M.; Chen, L.; Que, J.; Zheng, L.; Chen, Z.; Wu, Z. Effects of transition metal oxides on pyrolysis properties of PVC. Process. Saf. Environ. Prot. 2020, 140, 211–220. [Google Scholar] [CrossRef]
- Ma, S.; Lu, J.; Gao, J. Study of the Low Temperature Pyrolysis of PVC. Energy Fuels 2002, 16, 338–342. [Google Scholar] [CrossRef]
- Miranda, R.; Yang, J.; Roy, C.; Vasile, C. Vacuum pyrolysis of commingled plastics containing PVC I. Kinetic study. Polym. Degrad. Stab. 2001, 72, 469–491. [Google Scholar] [CrossRef]
- Miranda, R.; Pakdel, H.; Roy, C.; Vasile, C. Vacuum pyrolysis of commingled plastics containing PVC II. Product analysis. Polym. Degrad. Stab. 2001, 73, 47–67. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, L. Molecular Mechanism Study of the Kinetics and Product Yields during Copyrolysis of Biomass and Solid Wastes: ReaxFF-MD Method Approach. ACS Omega 2023, 8, 36126–36135. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Cao, Q.; Yuan, G.; Yin, L.; Chen, D.; He, P.; Wang, H. Morphological characteristics of polyvinyl chloride (PVC) dechlorination during pyrolysis process: Influence of PVC content and heating rate. Waste Manag. 2016, 58, 241–249. [Google Scholar] [CrossRef]
- Gui, B.; Qiao, Y.; Wan, D.; Liu, S.; Han, Z.; Yao, H.; Xu, M. Nascent tar formation during polyvinylchloride (PVC) pyrolysis. Proc. Combust. Inst. 2013, 34, 2321–2329. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.; Laresgoiti, M.; Adrados, A. Dechlorination of fuels in pyrolysis of PVC containing plastic wastes. Fuel Process. Technol. 2011, 92, 253–260. [Google Scholar] [CrossRef]
- Ma, C.; Kumagai, S.; Saito, Y.; Yoshioka, T.; Huang, X.; Shao, Y.; Ran, J.; Sun, L. Recent Advancements in Pyrolysis of Halogen-Containing Plastics for Resource Recovery and Halogen Upcycling: A State-of-the-Art Review. Environ. Sci. Technol. 2024, 58, 1423–1440. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, B.; Zhu, M. An overview on the recycling of waste poly(vinyl chloride). Green Chem. 2023, 25, 6971–7025. [Google Scholar] [CrossRef]
- Xu, S.; Hu, Y.; Tahir, M.H.; Hu, W.; Zhang, P.; Tang, Y. Copyrolysis characteristics of polyvinyl chloride, polyethylene and polypropylene based on ReaxFF molecular simulation. Comput. Theor. Chem. 2023, 1229, 114350. [Google Scholar] [CrossRef]
- Hwang, G.-J.; Lim, S.-G.; Bong, S.-Y.; Ryu, C.-H.; Choi, H.-S. Preparation of anion exchange membrane using polyvinyl chloride (PVC) for alkaline water electrolysis. Korean J. Chem. Eng. 2015, 32, 1896–1901. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Liu, Z.; Yu, J.; Zheng, G.; Ma, Y.; Xie, Z.; Tao, C.; Qu, R.; Li, S.; et al. Elucidating synergistic effects and environmental value enhancement in infrared-Assisted Co-Pyrolysis of coal and polyvinyl chloride. Sep. Purif. Technol. 2025, 357, 130071. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, D.; Yin, L.; Wang, Z.; Zhao, L.; Wang, J. High efficiency chlorine removal from polyvinyl chloride (PVC) pyrolysis with a gas–liquid fluidized bed reactor. Waste Manag. 2014, 34, 1045–1050. [Google Scholar] [CrossRef]
- Wu, J.; Chen, T.; Luo, X.; Han, D.; Wang, Z.; Wu, J. TG/FTIR analysis on co-pyrolysis behavior of PE, PVC and PS. Waste Manag. 2014, 34, 676–682. [Google Scholar] [CrossRef]
- Yang, Z.; Niu, S.; Han, K.; Wang, Y.; Yu, H. Mechanism research on the co-pyrolysis of PVC and cellulose through ReaxFF MD combined with DFT simulation. Process Saf. Environ. Prot. 2025, 194, 469–485. [Google Scholar] [CrossRef]
- Liu, L.; Xu, G.; Wang, J.; Li, G.; He, C.; Jiao, Y. Co-pyrolysis of biomass and plastic waste based on ReaxFF MD: Insights into hydrogen migration, radicals interactions and synergistic mechanism. Energy 2025, 325, 136103. [Google Scholar] [CrossRef]
- Wolf, M.; Vallverdu, G.S. Reactive molecular dynamics simulations of plastics pyrolysis with additives: Comparison of ReaxFF branches and experimental results. J. Anal. Appl. Pyrolysis 2024, 177, 106266. [Google Scholar] [CrossRef]
- Wu, J.; Papanikolaou, K.G.; Cheng, F.; Addison, B.; Cuthbertson, A.A.; Mavrikakis, M.; Huber, G.W. Kinetic Study of Polyvinyl Chloride Pyrolysis with Characterization of Dehydrochlorinated PVC. ACS Sustain. Chem. Eng. 2024, 12, 7402–7413. [Google Scholar] [CrossRef]
- Chidara, A.; Cheng, K.; Gallear, D. Ontology-Based Modelling and Analysis of Sustainable Polymer Systems: PVC Comparative Polymer and Implementation Perspectives. Polymers 2025, 17, 2612. [Google Scholar] [CrossRef] [PubMed]
- Vikhareva, I.N.; Abramian, A.; Manojlović, D.; Bol’shakov, O. Features of Thermal Stabilization of PVC Modified with Microstructured Titanium Phosphate. Polymers 2025, 17, 2140. [Google Scholar] [CrossRef]
- Charitopoulou, M.-A.; Vouvoudi, E.C.; Achilias, D.S. Isoconversional Analysis of the Catalytic Pyrolysis of ABS, HIPS, PC and Their Blends with PP and PVC. Polymers 2024, 16, 2299. [Google Scholar] [CrossRef]
- Dobrotă, D.; Dimulescu, C.S.; Stăncioiu, A. Reusability of Scrap Rubber, Tire Shredding, Recycled PVC and Fly Ash for Development of Composites with Vibration Damping Ability. Polymers 2024, 16, 2167. [Google Scholar] [CrossRef] [PubMed]
- Bajetto, G.; Scutera, S.; Menotti, F.; Banche, G.; Chiaradia, G.; Turesso, C.; De Andrea, M.; Vallino, M.; Van Es, D.S.; Biolatti, M.; et al. Antimicrobial Efficacy of a Vegetable Oil Plasticizer in PVC Matrices. Polymers 2024, 16, 1046. [Google Scholar] [CrossRef]
- Gardi, S.; Giannone, L.; Sarti, G.; Sarti, G. Surface Chalking upon Weathering of Dark-Colored PVC Articles and Relevant Stabilizers. Polymers 2024, 16, 1047. [Google Scholar] [CrossRef]
- Ait-Touchente, Z.; Khellaf, M.; Raffin, G.; Lebaz, N.; Elaissari, A. Recent advances in polyvinyl chloride (PVC) recycling. Polym. Adv. Technol. 2024, 35, e6228. [Google Scholar] [CrossRef]
- Lu, L.; Li, W.; Cheng, Y.; Liu, M. Chemical recycling technologies for PVC waste and PVC-containing plastic waste: A review. Waste Manag. 2023, 166, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Edo, G.I.; Ndudi, W.; Ali, A.B.M.; Yousif, E.; Zainulabdeen, K.; Onyibe, P.N.; Ekokotu, H.A.; Isoje, E.F.; Igbuku, U.A.; Essaghah, A.E.A.; et al. Poly(vinyl chloride) (PVC): An updated review of its properties, polymerization, modification, recycling, and applications. J. Mater. Sci. 2024, 59, 21605–21648. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C. Progress in the modification of polyvinyl chloride (PVC) membranes: A performance review for wastewater treatment. J. Water Process Eng. 2022, 45, 102466. [Google Scholar] [CrossRef]


| PVC Layer | Condition | Atmosphere | Diagnostic Features (Gas Types and Concentrations) | Infrared Peak Position (cm−1) |
|---|---|---|---|---|
| Outer layer | Overheating | – | HCl is the dominant gas, accompanied by alkanes, plasticizer fragments, and a small amount of CO at low temperature. | HCl ≈ 2900; CO ≈ 2100; CH stretch (alkanes) ≈ 2850–2960; C=O (plasticizer) ≈ 1730 |
| Outer layer | Electrical discharge | – | C2H2 is the main gas, with CH4 and C2H4 also present; CO2 and H2O are nearly absent. | C2H2 ≈ 730 & 610; CH4 ≈ 3010; C2H4 ≈ 950–1000 |
| Inner layer | Overheating | – | HCl is the primary product, with alkanes and a small amount of CO formed at low temperature. | HCl ≈ 2900; CO ≈ 2100; CH stretch (alkanes) ≈ 2850–2960 |
| Inner layer | Electrical discharge | – | C2H2, CH4, and HCl are the main gases, while CO2 and H2O remain at very low levels. | C2H2 ≈ 730 & 610; CH4 ≈ 3010; HCl ≈ 2900 |
| Overall criterion | Oxidizing atmosphere | Air | CO2 and H2O are the major products, accompanied by small amounts of CH4, C2H2, and CO. Oxidation promotes CO2 formation and reduces unsaturated hydrocarbons. | CO2 ≈ 2350; H2O ≈ 3700 & 1630; CH4 ≈ 3010; C2H2 ≈ 730; CO ≈ 2100 |
| Overall criterion | Inert atmosphere | Argon | HCl and C2H2 are the main diagnostic gases. HCl indicates thermal decomposition, while C2H2 is typical of discharge-induced degradation under non-oxidizing conditions. | HCl ≈ 2900; C2H2 ≈ 730 & 610 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Huang, X.; Su, J.; Liu, Z.; Pang, X.; Wang, Z.; Chen, Y. Research on Diagnostic Methods for Gas Generation Due to Degradation of Cable PVC Materials Under Electrical and Thermal Stress. Polymers 2025, 17, 3021. https://doi.org/10.3390/polym17223021
Zhang P, Huang X, Su J, Liu Z, Pang X, Wang Z, Chen Y. Research on Diagnostic Methods for Gas Generation Due to Degradation of Cable PVC Materials Under Electrical and Thermal Stress. Polymers. 2025; 17(22):3021. https://doi.org/10.3390/polym17223021
Chicago/Turabian StyleZhang, Peng, Xingwang Huang, Jingang Su, Zhen Liu, Xianhai Pang, Zihao Wang, and Yidong Chen. 2025. "Research on Diagnostic Methods for Gas Generation Due to Degradation of Cable PVC Materials Under Electrical and Thermal Stress" Polymers 17, no. 22: 3021. https://doi.org/10.3390/polym17223021
APA StyleZhang, P., Huang, X., Su, J., Liu, Z., Pang, X., Wang, Z., & Chen, Y. (2025). Research on Diagnostic Methods for Gas Generation Due to Degradation of Cable PVC Materials Under Electrical and Thermal Stress. Polymers, 17(22), 3021. https://doi.org/10.3390/polym17223021






