Glyoxal as Single Crosslinker for Mechanically Blown, Condensed and Hydrolyzable Tannin Foams
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Foam Synthesis
2.3. Density and Porosity
2.4. Cell Measurements
2.5. Scanning Electron Microscopy (SEM)
2.6. Solid-State 13C NMR
2.7. Compression Test
2.8. Thermal Conductivity
2.9. Leaching Resistance and Acid Recovery
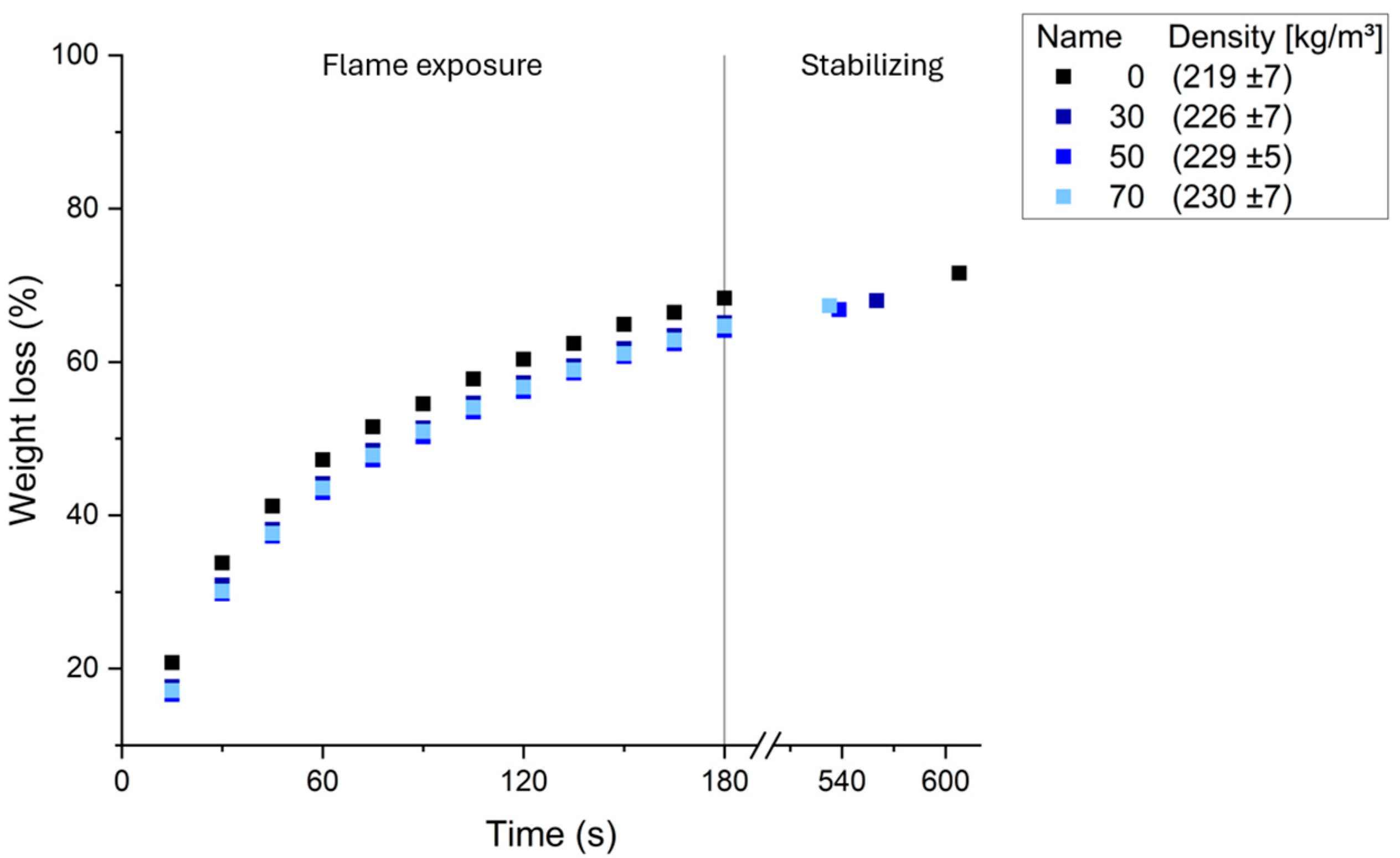
2.10. Fire Resistance
2.11. Statistical Analysis
3. Results and Discussion
3.1. Foam Structure and Morphology
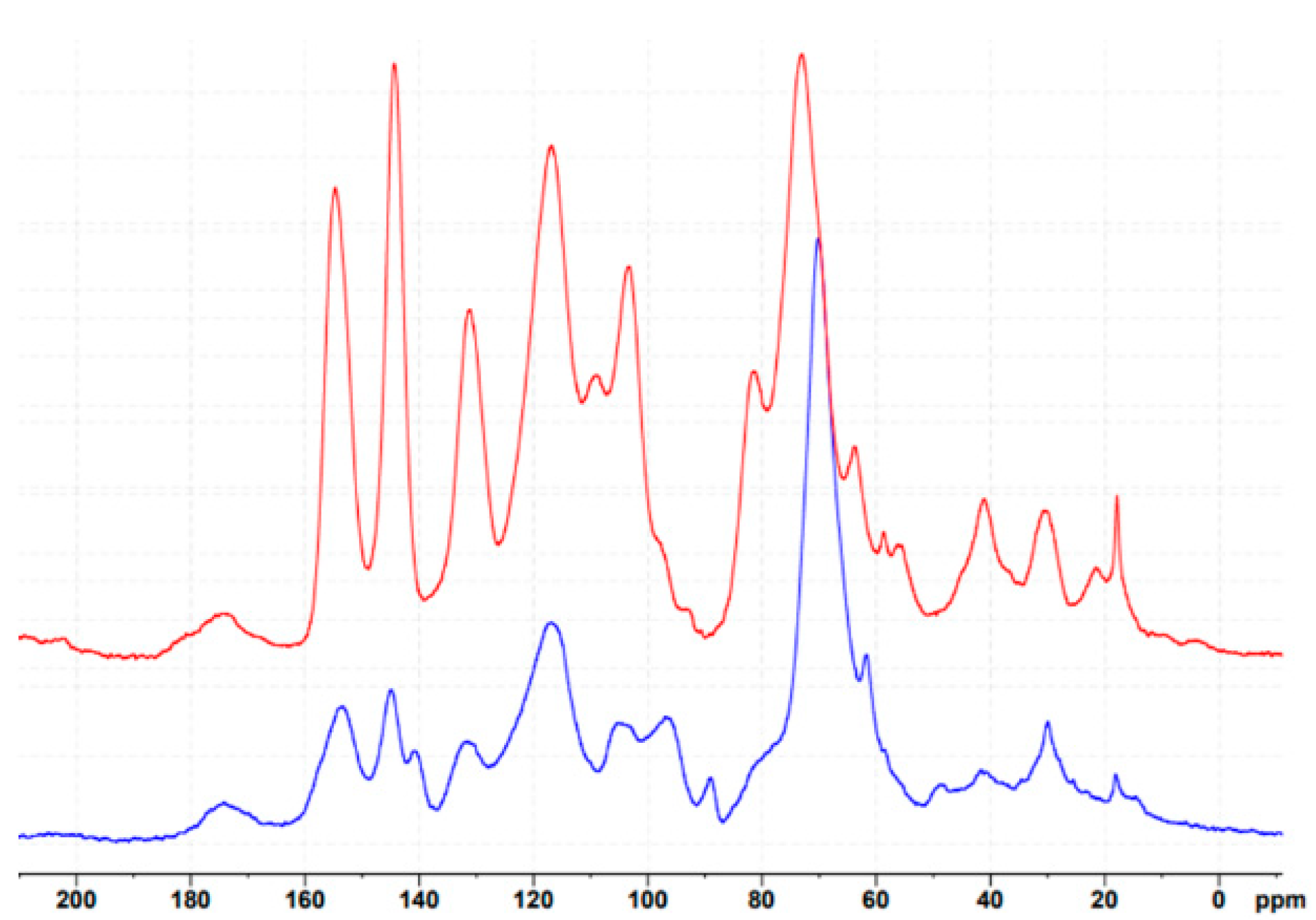
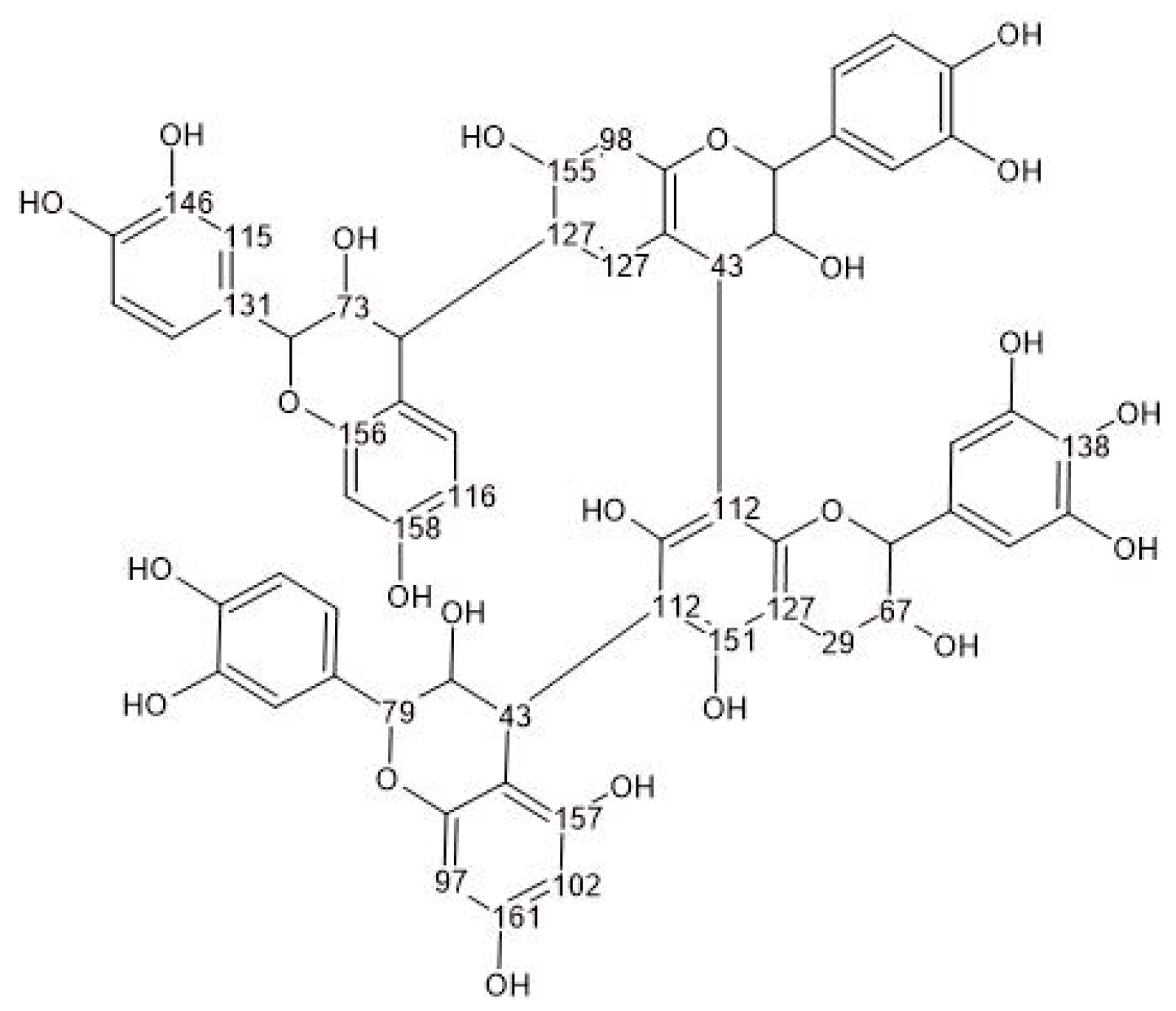
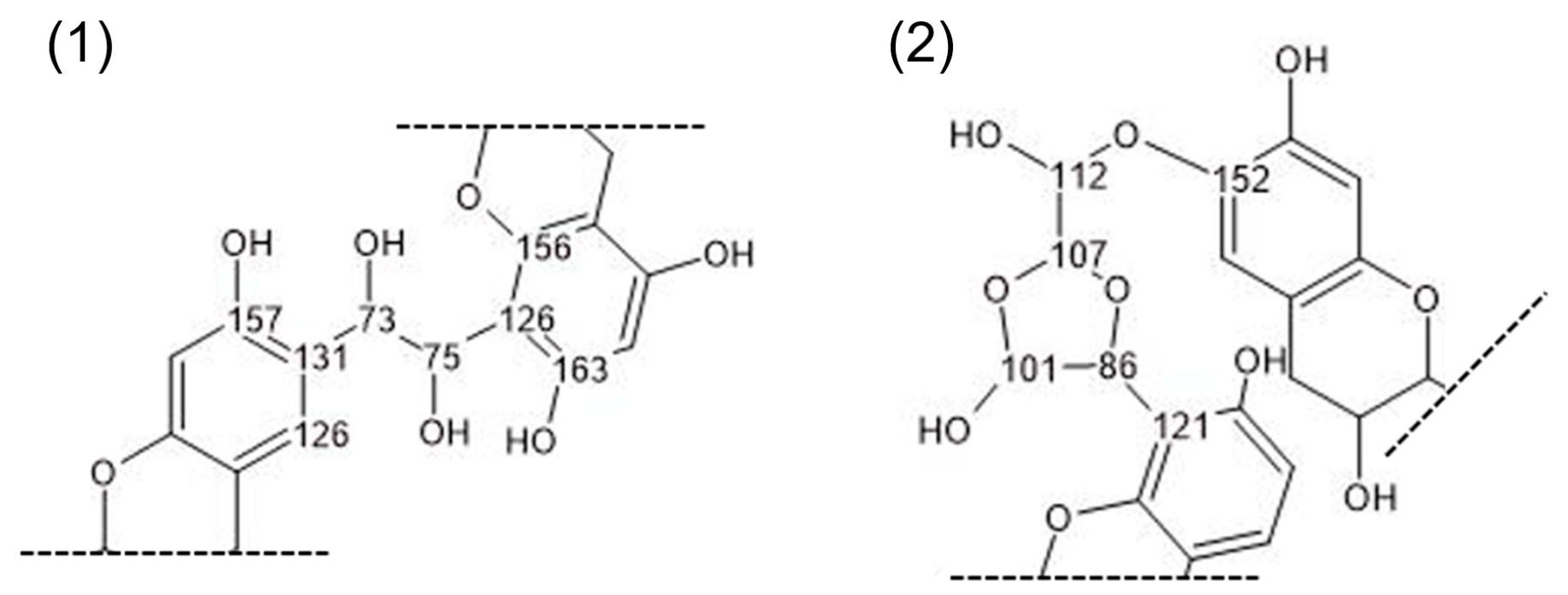
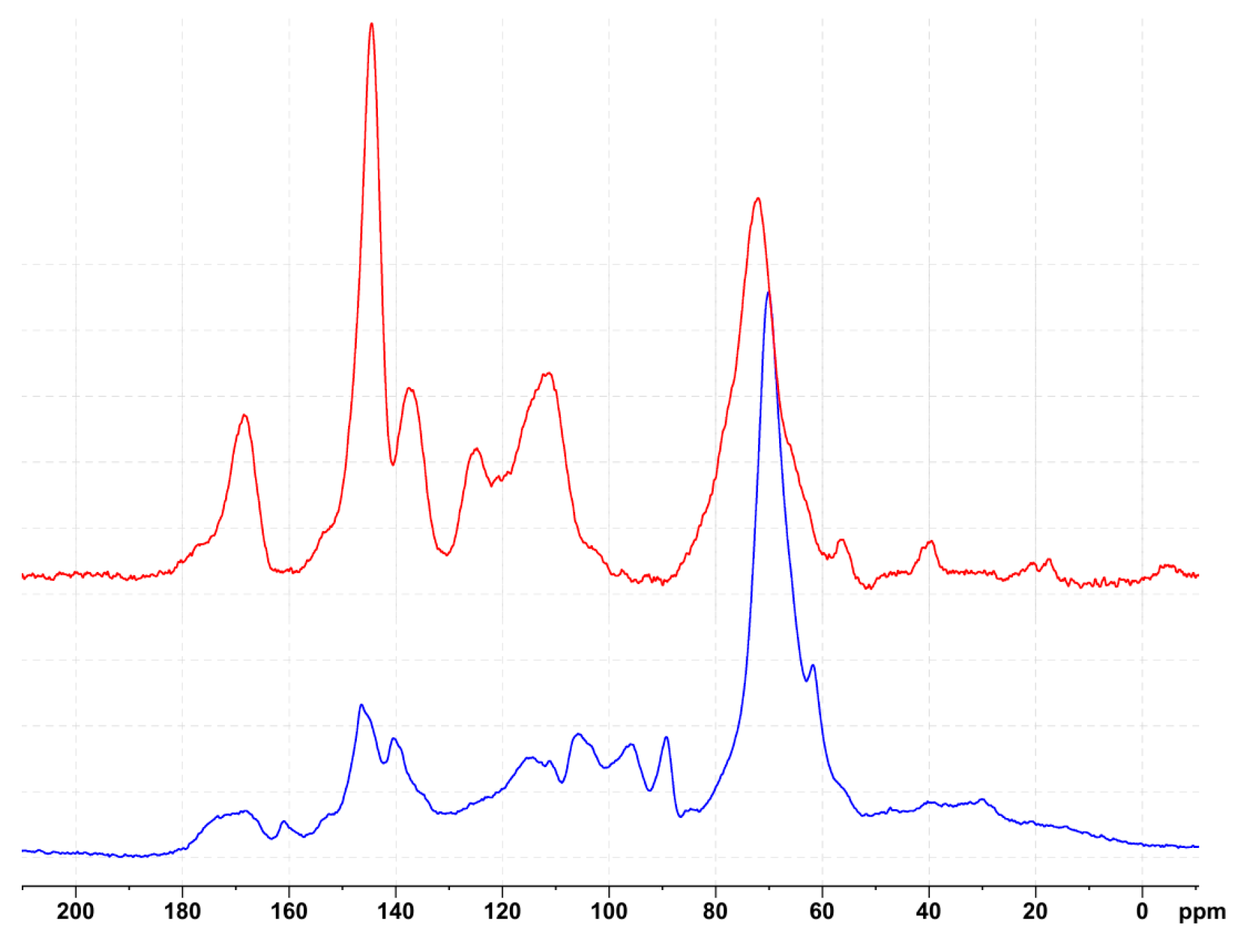
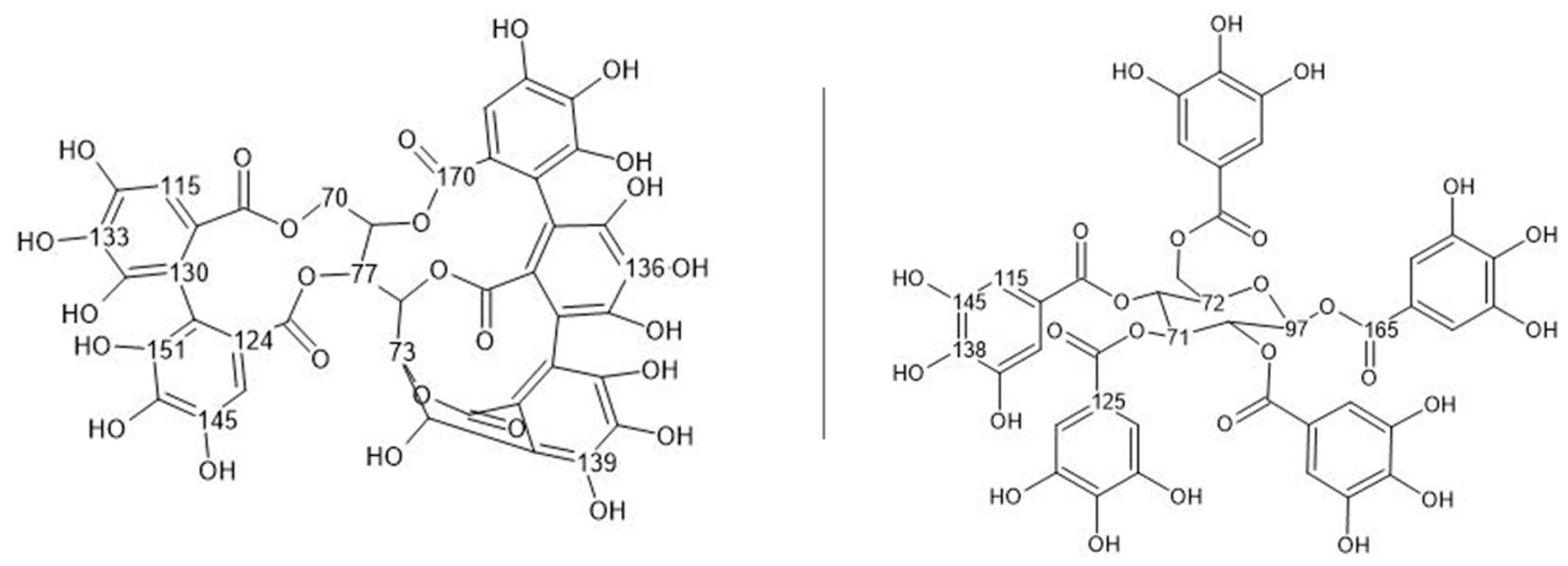
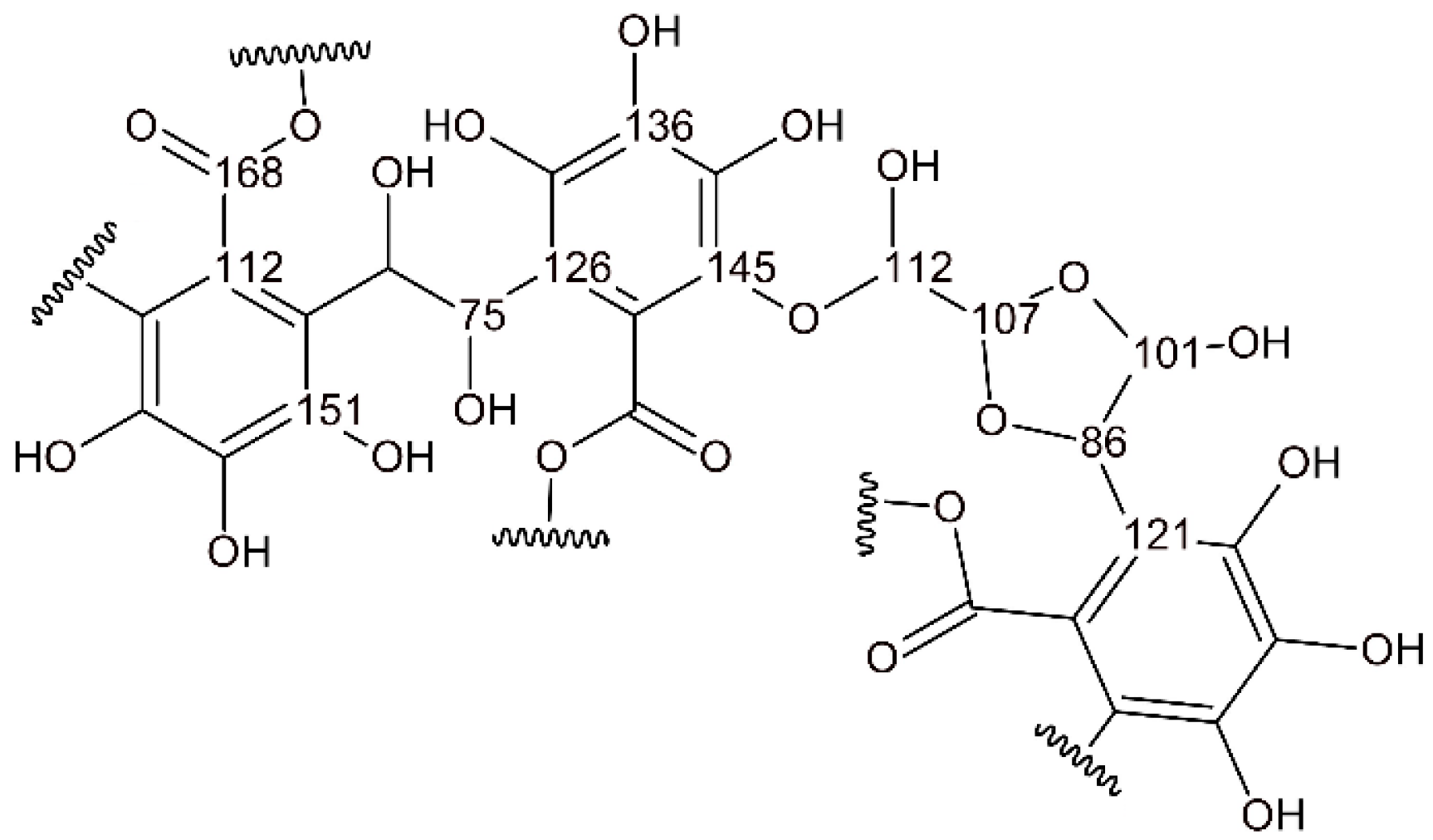
3.2. Chemical Characterization
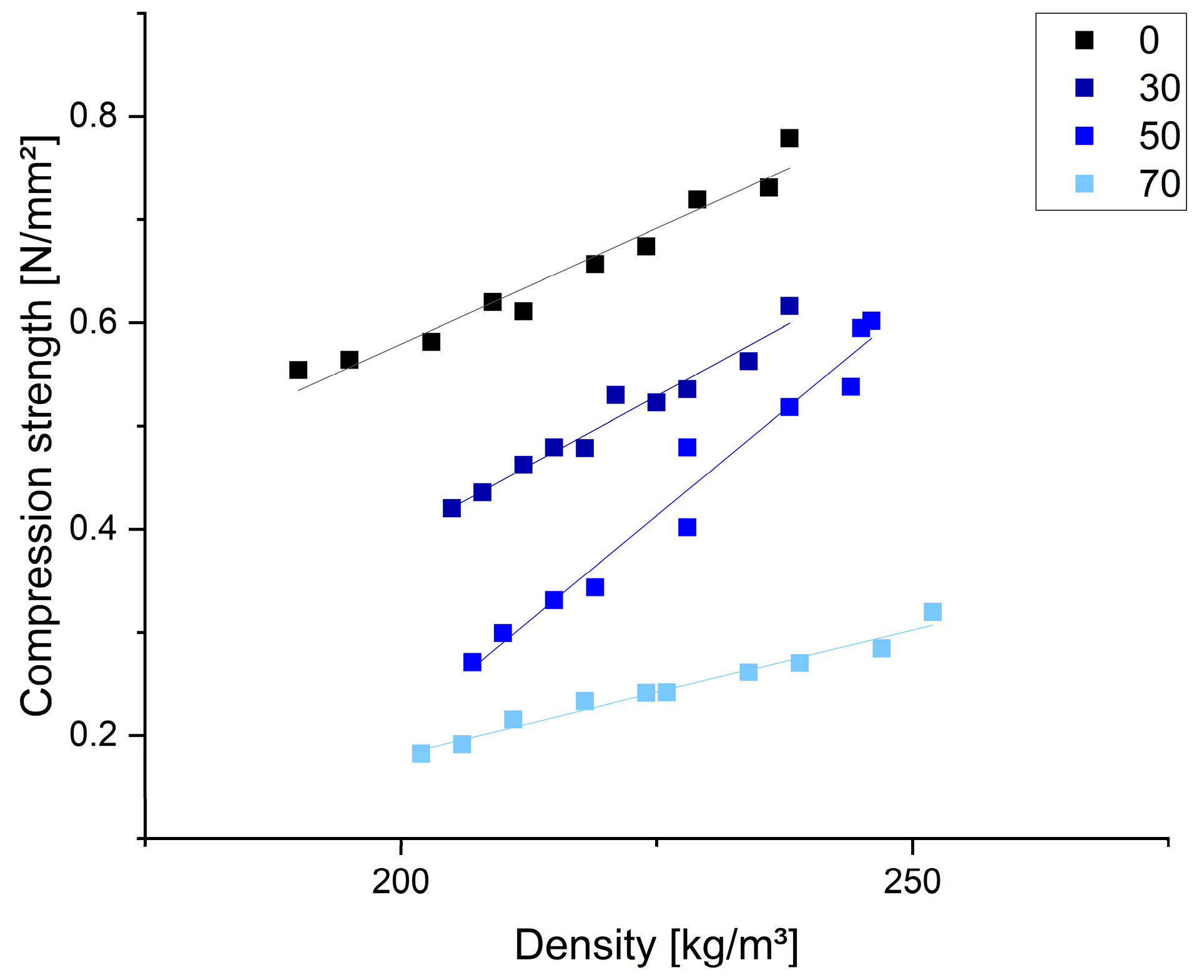
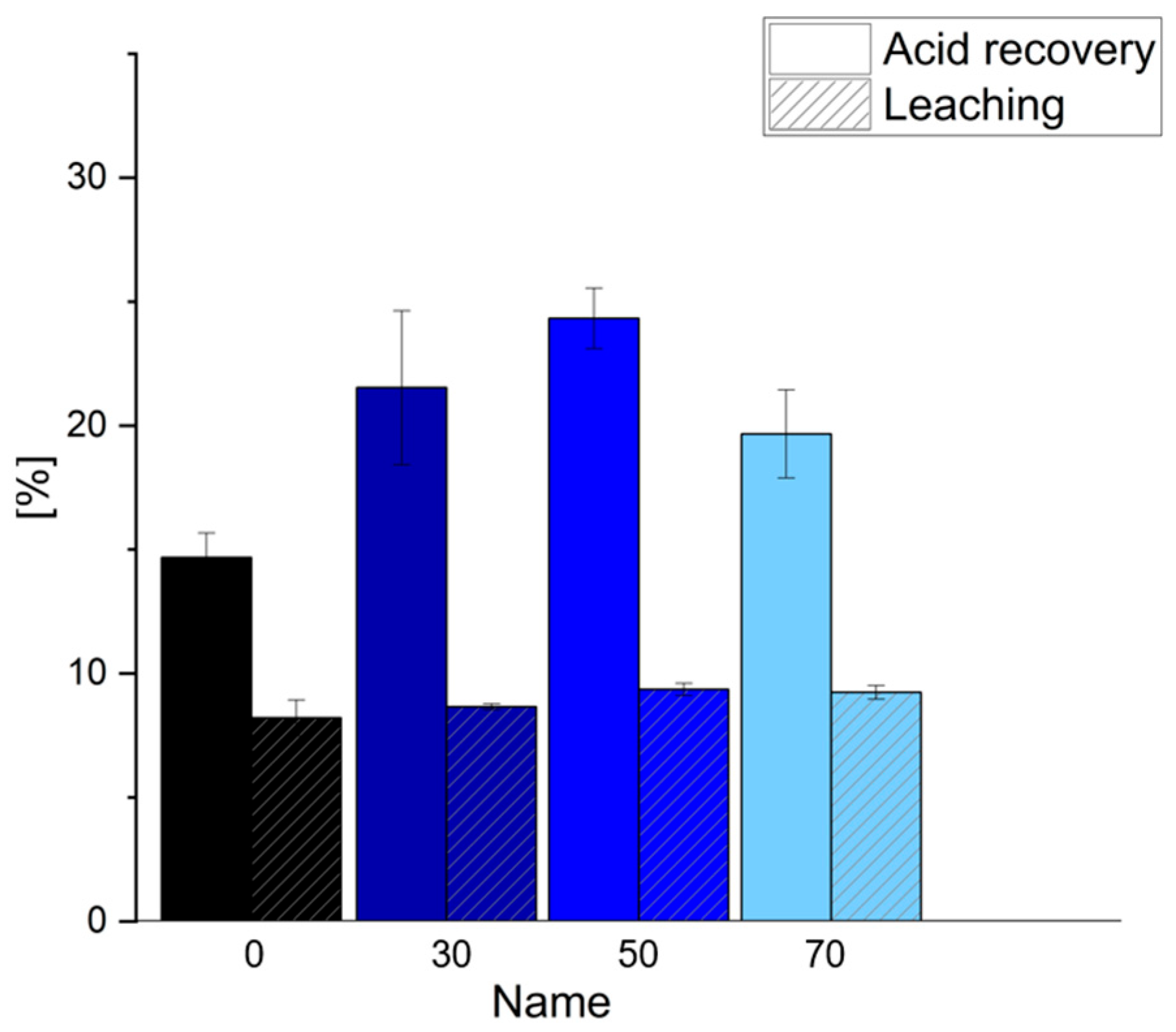
3.3. Functional Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pizzi, A. Tannin-Based Biofoams—A Review. J. Renew. Mater. 2019, 7, 477–492. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Nicolas, V.; Marie, Z.; Celzard, A.; Fierro, V. A Review of Rigid Polymeric Cellular Foams and Their Greener Tannin-Based Alternatives. Polymers 2022, 14, 3974. [Google Scholar] [CrossRef]
- Tondi, G.; Petutschnigg, A. Tannin—Based Foams: The Innovative Material for Insulation Purposes. In Handbook of Composites from Renewable Materials; Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 93–105. ISBN 978-1-119-22436-5. [Google Scholar]
- Tondi, G.; Zhao, W.; Pizzi, A.; Du, G.; Fierro, V.; Celzard, A. Tannin-Based Rigid Foams: A Survey of Chemical and Physical Properties. Bioresour. Technol. 2009, 100, 5162–5169. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.B.B.; Côrrea, R.; De Cademartori, P.H.G.; Ribeiro, A.C.R.; Coldebella, R.; Delucis, R.A.; Lunkes, N.; Missio, A.L. Bio-Based Tannin Foams: Comparing Their Physical and Thermal Response to Polyurethane Foams in Lightweight Sandwich Panels. Compounds 2023, 4, 1–16. [Google Scholar] [CrossRef]
- Basso, M.C.; Li, X.; Fierro, V.; Pizzi, A.; Giovando, S.; Celzard, A. Green, Formaldehyde-Free, Foams for Thermal Insulation. Adv. Mater. Lett. 2011, 2, 378–382. [Google Scholar] [CrossRef]
- Delgado-Sánchez, C.; Santiago-Medina, F.; Fierro, V.; Pizzi, A.; Celzard, A. Optimisation of “Green” Tannin-Furanic Foams for Thermal Insulation by Experimental Design. Mater. Des. 2018, 139, 7–15. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A.; Olives, R. Natural Tannin-Based Rigid Foams as Insulation for Doors and Wall Panels. Maderas Cienc. Tecnol. 2008, 10, 219–227. [Google Scholar] [CrossRef]
- Tondi, G.; Oo, C.W.; Pizzi, A.; Trosa, A.; Thevenon, M.F. Metal Adsorption of Tannin Based Rigid Foams. Ind. Crops Prod. 2009, 29, 336–340. [Google Scholar] [CrossRef]
- Sepperer, T.; Neubauer, J.; Eckardt, J.; Schnabel, T.; Petutschnigg, A.; Tondi, G. Pollutant Absorption as a Possible End-Of-Life Solution for Polyphenolic Polymers. Polymers 2019, 11, 911. [Google Scholar] [CrossRef]
- Sepperer, T.; Tondi, G.; Petutschnigg, A.; Young, T.M.; Steiner, K. Mitigation of Ammonia Emissions from Cattle Manure Slurry by Tannins and Tannin-Based Polymers. Biomolecules 2020, 10, 581. [Google Scholar] [CrossRef]
- Sepperer, T.; Petutschnigg, A.; Steiner, K. Long-Term Study on the Nitrogen Retention Potential of Bark Extracts and a Polymer Based Thereof in Cattle Manure Slurry. Bioresour. Technol. Rep. 2022, 18, 101085. [Google Scholar] [CrossRef]
- Basso, M.C.; Pizzi, A.; Al-Marzouki, F.; Abdalla, S. Horticultural/Hydroponics and Floral Natural Foams from Tannins. Ind. Crops Prod. 2016, 87, 177–181. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.-C.; Pizzi, A.; Celzard, A.; Ella Ebang, E.; Gallon, N.; Charrier, B. Pine (P. pinaster) and Quebracho (S. lorentzii) Tannin-Based Foams as Green Acoustic Absorbers. Ind. Crops Prod. 2015, 67, 70–73. [Google Scholar] [CrossRef]
- Zhou, X.; Pizzi, A.; Sauget, A.; Nicollin, A.; Li, X.; Celzard, A.; Rode, K.; Pasch, H. Lightweight Tannin Foam/Composites Sandwich Panels and the Coldset Tannin Adhesive to Assemble Them. Ind. Crops Prod. 2013, 43, 255–260. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Khanbabaee, K. Tannins: Classification and Definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [CrossRef]
- Molino, S.; Casanova, N.A.; Rufián Henares, J.Á.; Fernandez Miyakawa, M.E. Natural Tannin Wood Extracts as a Potential Food Ingredient in the Food Industry. J. Agric. Food Chem. 2020, 68, 2836–2848. [Google Scholar] [CrossRef]
- Arbenz, A.; Avérous, L. Chemical Modification of Tannins to Elaborate Aromatic Biobased Macromolecular Architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Li, X.; Luo, J.; Gao, Q.; Li, J. “Green” Bio-Thermoset Resins Derived from Soy Protein Isolate and Condensed Tannins. Ind. Crops Prod. 2017, 108, 363–370. [Google Scholar] [CrossRef]
- Azadeh, E.; Chen, X.; Pizzi, A.; Gérardin, C.; Gérardin, P.; Essawy, H. Self-Blowing Non-Isocyanate Polyurethane Foams Based on Hydrolysable Tannins. J. Renew. Mater. 2022, 10, 3217–3227. [Google Scholar] [CrossRef]
- Eckardt, J.; Sepperer, T.; Cesprini, E.; Šket, P.; Tondi, G. Comparing Condensed and Hydrolysable Tannins for Mechanical Foaming of Furanic Foams: Synthesis and Characterization. Molecules 2023, 28, 2799. [Google Scholar] [CrossRef] [PubMed]
- Azadeh, E.; Pizzi, A.; Gerardin-Charbonnier, C.; Gerardin, P. Hydrolysable Chestnut Tannin Extract Chemical Complexity in Its Reactions for Non-Isocyanate Polyurethanes (NIPU) Foams. J. Renew. Mater. 2023, 11, 2823–2848. [Google Scholar] [CrossRef]
- Dorini Falavinha, J.V.; Cademartori, P.H.G.D.; Gérardin, P.; Pizzi, A.; Gérardin-Charbonnier, C. New Rigid Furan Biofoams Based on Hydrolysable Chesnut (Castanea sativa) Tannin by Chemical Expansion. J. Renew. Mater 2025, 13, 687–697. [Google Scholar] [CrossRef]
- Meikleham, N.E.; Pizzi, A. Acid- and Alkali-Catalyzed Tannin-Based Rigid Foams. J. Appl. Polym. Sci. 1994, 53, 1547–1556. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Pizzi, A.; Fredon, E.; Gerardin, C.; Zhou, X.; Du, G. Tannin-Furanic Foams Modified by Soybean Protein Isolate (SPI) and Industrial Lignin Substituting Formaldehyde Addition. Ind. Crops Prod. 2021, 168, 113607. [Google Scholar] [CrossRef]
- Yuan, W.; Xi, X.; Zhang, J.; Pizzi, A.; Essawy, H.; Du, G.; Zhou, X.; Chen, X. A Novel Strategy Inspired by Steaming Chinese Steamed Bread for Preparation of Tannin-Furanic Rigid Bio-Foam. Constr. Build. Mater. 2023, 376, 131035. [Google Scholar] [CrossRef]
- Link, M.; Kolbitsch, C.; Tondi, G.; Ebner, M.; Wieland, S.; Petutschnigg, A. Formaldehyde-Free Tannin-Based Foams and Their Use as Lightweight Panels. BioResources 2011, 6, 4218–4228. [Google Scholar] [CrossRef]
- Szczurek, A.; Fierro, V.; Pizzi, A.; Stauber, M.; Celzard, A. A New Method for Preparing Tannin-Based Foams. Ind. Crops Prod. 2014, 54, 40–53. [Google Scholar] [CrossRef]
- Merle, J.; Birot, M.; Deleuze, H.; Mitterer, C.; Carré, H.; Bouhtoury, F.C.-E. New Biobased Foams from Wood Byproducts. Mater. Des. 2016, 91, 186–192. [Google Scholar] [CrossRef]
- Goes Lopes, P.J.; Berger, C.; Dalla Costa, H.W.; Coldebella, R.; Ibeiro De Oliveira, A.; Lunkes, N.; Gatto, D.A.; De Avila Delucis, R.; Missio, A.L. Effect of the pH Value of Tannin Extracts on Properties of Classic Tannin Foams. J. Cell. Plast. 2022, 58, 861–875. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Laborie, M.-P.; Garcia, D.; Celzard, A. Bioresourced Pine Tannin/Furanic Foams with Glyoxal and Glutaraldehyde. Ind. Crops Prod. 2013, 45, 401–405. [Google Scholar] [CrossRef]
- Basso, M.C.; Giovando, S.; Pizzi, A.; Lagel, M.C.; Celzard, A. Alkaline Tannin Rigid Foams. J. Renew. Mater. 2014, 2, 182–185. [Google Scholar] [CrossRef]
- Li, X.; Pizzi, A.; Zhou, X.; Fierro, V.; Celzard, A. Formaldehyde-Free Prorobitenidin/Profi Setinidin Tannin/Furanic Foams Based on Alternative Aldehydes: Glyoxal and Glutaraldehyde. J. Renew. Mater. 2015, 3, 142–150. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, B.; Essawy, H.; Huang, Z.; Tang, J.; Miao, Z.; Chen, F.; Zhang, J. Preparation and Characterization of Biomass Tannin-Based Flexible Foam Insoles for Athletes. Polymers 2023, 15, 3480. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guigo, N.; Pizzi, A.; Sbirrazzuoli, N.; Li, B.; Fredon, E.; Gerardin, C. Ambient Temperature Self-Blowing Tannin-Humins Biofoams. Polymers 2020, 12, 2732. [Google Scholar] [CrossRef]
- Li, X.; Basso, M.C.; Fierro, V.; Pizzi, A.; Celzard, A. Chemical Modification of Tannin/Furanic Rigid Foams by Isocyanates and Polyurethanes. Maderas Cienc. Tecnol. 2012, 14, 257–266. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Celzard, A.; Laborie, M.-P. Natural Albumin/Tannin Cellular Foams. Ind. Crops Prod. 2015, 73, 41–48. [Google Scholar] [CrossRef]
- Eckardt, J.; Moro, L.; Colusso, E.; Šket, P.; Giovando, S.; Tondi, G. Comparing Hydrolysable and Condensed Tannins for Tannin Protein-Based Foams. Polymers 2025, 17, 153. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Essawy, H.; Pizzi, A.; Fredon, E.; Gerardin, C.; Du, G.; Zhou, X. Flame-Retardant and Thermally-Insulating Tannin and Soybean Protein Isolate (SPI) Based Foams for Potential Applications in Building Materials. Constr. Build. Mater. 2022, 315, 125711. [Google Scholar] [CrossRef]
- Basso, M.C.; Dssouli-Lagel, M.-C.; Pizzi, A.P.; Celzard, A.; Abdalla, S. First Tools for Tannin-Furanic Foams Design. BioResources 2015, 10, 5233–5241. [Google Scholar] [CrossRef]
- Chen, X.; Xi, X.; Pizzi, A.; Fredon, E.; Zhou, X.; Li, J.; Gerardin, C.; Du, G. Preparation and Characterization of Condensed Tannin Non-Isocyanate Polyurethane (NIPU) Rigid Foams by Ambient Temperature Blowing. Polymers 2020, 12, 750. [Google Scholar] [CrossRef]
- Sepperer, T.; Šket, P.; Petutschnigg, A.; Hüsing, N. Tannin-Furanic Foams Formed by Mechanical Agitation: Influence of Surfactant and Ingredient Ratios. Polymers 2021, 13, 3058. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Medina, F.J.; Delgado-Sánchez, C.; Basso, M.C.; Pizzi, A.; Fierro, V.; Celzard, A. Mechanically Blown Wall-Projected Tannin-Based Foams. Ind. Crops Prod. 2018, 113, 316–323. [Google Scholar] [CrossRef]
- Li, X.; Pizzi, A.; Lacoste, C.; Fierro, V.; Celzard, A. Physical Properties of Tannin/Furanic Resin Foamed With Different Blowing Agents. BioResources 2012, 8, 743–752. [Google Scholar] [CrossRef]
- Zhao, W.; Pizzi, A.; Fierro, V.; Du, G.; Celzard, A. Effect of Composition and Processing Parameters on the Characteristics of Tannin-Based Rigid Foams. Part I: Cell Structure. Mater. Chem. Phys. 2010, 122, 175–182. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Xi, X.; Pizzi, A.; Zhou, X.; Fredon, E.; Du, G.; Gerardin, C. Condensed Tannin-Glucose-Based NIPU Bio-Foams of Improved Fire Retardancy. Polym. Degrad. Stab. 2020, 175, 109121. [Google Scholar] [CrossRef]
- Merle, J.; Trinsoutrot, P.; Charrier-El Bouhtoury, F. Optimization of the Formulation for the Synthesis of Bio-Based Foams. Eur. Polym. J. 2016, 84, 577–588. [Google Scholar] [CrossRef]
- Eckardt, J.; Neubauer, J.; Sepperer, T.; Donato, S.; Zanetti, M.; Cefarin, N.; Vaccari, L.; Lippert, M.; Wind, M.; Schnabel, T.; et al. Synthesis and Characterization of High-Performing Sulfur-Free Tannin Foams. Polymers 2020, 12, 564. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A. Tannin-Based Rigid Foams: Characterization and Modification. Ind. Crops Prod. 2009, 29, 356–363. [Google Scholar] [CrossRef]
- ISO 844:2021; Rigid Cellular Plastics—Determination of Compression Properties. International Organization for Standardization: Geneva, Switzerland, 2021.
- Gustafsson, S.E. Transient Plane Source Techniques for Thermal Conductivity and Thermal Diffusivity Measurements of Solid Materials. Rev. Sci. Instrum. 1991, 62, 797–804. [Google Scholar] [CrossRef]
- Gustafsson, S.E.; Mihiretie, B.M.; Gustavsson, M.K. Measurement of Thermal Transport in Solids with the Hot Disc Method. Int. J. Thermophys. 2024, 45, 1. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, Y.; Essawy, H.; Feng, S.; Li, X.; Liao, J.; Zhou, X.; Zhang, J.; Xie, S. Formaldehyde Free Renewable Thermosetting Foam Based on Biomass Tannin with a Lignin Additive. J. Renew. Mater. 2022, 10, 3009–3024. [Google Scholar] [CrossRef]
- Sain, S.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Öman, T.; Skrifvars, M. Spruce Bark-Extracted Lignin and Tannin-Based Bioresin-Adhesives: Effect of Curing Temperatures on the Thermal Properties of the Resins. Molecules 2021, 26, 3523. [Google Scholar] [CrossRef]
- Van Nieuwenhove, I.; Renders, T.; Lauwaert, J.; De Roo, T.; De Clercq, J.; Verberckmoes, A. Biobased Resins Using Lignin and Glyoxal. ACS Sustain. Chem. Eng. 2020, 8, 18789–18809. [Google Scholar] [CrossRef]
- Khiari, R.; Baaka, N.; Ammar, M.; Saad, M.K. Properties of Tannin-Glyoxal Resins Prepared from Lyophilized and Condensed Tannin. J. Text. Eng. Fash. Technol. 2017, 3, 705–711. [Google Scholar] [CrossRef]
- Tondi, G. Tannin-Based Copolymer Resins: Synthesis and Characterization by Solid State 13C NMR and FT-IR Spectroscopy. Polymers 2017, 9, 223. [Google Scholar] [CrossRef]
- Pizzi, A.; Stephanou, A. A Comparative C13 NMR Study of Polyflavonoid Tannin Extracts for Phenolic Polycondensates. J. Appl. Polym. Sci. 1993, 50, 2105–2113. [Google Scholar] [CrossRef]
- Prigione, V.; Trocini, B.; Spina, F.; Poli, A.; Romanisio, D.; Giovando, S.; Varese, G.C. Fungi from Industrial Tannins: Potential Application in Biotransformation and Bioremediation of Tannery Wastewaters. Appl. Microbiol. Biotechnol. 2018, 102, 4203–4216. [Google Scholar] [CrossRef]
- Venter, P.B.; Sisa, M.; Van Der Merwe, M.J.; Bonnet, S.L.; Van Der Westhuizen, J.H. Analysis of Commercial Proanthocyanidins. Part 1: The Chemical Composition of Quebracho (Schinopsis lorentzii and Schinopsis balansae) Heartwood Extract. Phytochemistry 2012, 73, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; Mayo, B.; et al. Safety and Efficacy of a Feed Additive Consisting of an Extract of Condensed Tannins from Schinopsis balansae Engl. and Schinopsis lorentzii (Griseb.) Engl. (Red Quebracho Extract) for Use in All Animal Species (FEFANA Asbl). EFSA J. 2022, 20, e07699. [Google Scholar] [CrossRef] [PubMed]
- Banfi, D.; Patiny, L. www.nmrdb.org: Resurrecting and Processing NMR Spectra On-Line. Chimia 2008, 62, 280. [Google Scholar] [CrossRef]
- Avzianova, E.; Brooks, S.D. Raman Spectroscopy of Glyoxal Oligomers in Aqueous Solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 101, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Khatib, M.; Campo, M.; Bellumori, M.; Cecchi, L.; Vignolini, P.; Innocenti, M.; Mulinacci, N. Tannins from Different Parts of the Chestnut Trunk (Castanea sativa Mill.): A Green and Effective Extraction Method and Their Profiling by High-Performance Liquid Chromatography-Diode Array Detector-Mass Spectrometry. ACS Food Sci. Technol. 2023, 3, 1903–1912. [Google Scholar] [CrossRef]
- Campo, M.; Pinelli, P.; Romani, A. Hydrolyzable Tannins from Sweet Chestnut Fractions Obtained by a Sustainable and Eco-Friendly Industrial Process. Nat. Prod. Commun. 2016, 11, 409–415. [Google Scholar] [CrossRef]
- Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, Á.M.; Fernández De Simón, B.; Hernández, T.; Estrella, I. Phenolic Compounds in Chestnut (Castanea Sativa Mill.) Heartwood. Effect of Toasting at Cooperage. J. Agric. Food Chem. 2010, 58, 9631–9640. [Google Scholar] [CrossRef]
- Mujić, I.; Rudić, D.; Zivković, J.; Jukić, H.; Jug, T.; Nikolić, G.; Trutić, N. Antioxidant and antibacterial properties of Castanea sativa mill. catkins extracts. Acta Hortic. 2014, 1043, 167–173. [Google Scholar] [CrossRef]
- Matsuo, Y.; Wakamatsu, H.; Omar, M.; Tanaka, T. Reinvestigation of the Stereochemistry of the C -Glycosidic Ellagitannins, Vescalagin and Castalagin. Org. Lett. 2015, 17, 46–49. [Google Scholar] [CrossRef]
- Tondi, G.; Link, M.; Kolbitsch, C.; Lesacher, R.; Petutschnigg, A. Pilot Plant Up-Scaling of Tannin Foams. Ind. Crops Prod. 2016, 79, 211–218. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Laborie, M.-P.; Celzard, A.; Fierro, V. Pine Tannin-Based Rigid Foams: Mechanical and Thermal Properties. Ind. Crops Prod. 2013, 43, 245–250. [Google Scholar] [CrossRef]
- Tondi, G.; Link, M.; Kolbitsch, C.; Gavino, J.; Luckeneder, P.; Petutschnigg, A.; Herchl, R.; Van Doorslaer, C. Lignin-Based Foams: Production Process and Characterization. BioResources 2016, 11, 2972–2986. [Google Scholar] [CrossRef]

| Tannin [%] | Water [%] | Glyoxal [%] | Ethylene Glycol [%] | Glycerol [%] | Sulfuric Acid [%] | Tween80 [%] |
|---|---|---|---|---|---|---|
| 39.6 | 30.7 | 9.5 | 5 | 3.6 | 3 | 8.6 |
| Name | Bulk Density [kg/m3] | Skeletal Density [kg/m3] | Total Porosity [%] | Open Porosity [%] | Calc. Cell Diameter [µm] | Orthotropicity |
|---|---|---|---|---|---|---|
| 0 | 208 (23) | 1431 (2) | 85.5 (1.6) | 83.3 (2) | 189 (65) | 1.17 (0.30) |
| 30 | 216 (3) | 1442 (2) | 85 (0.2) | 82.7 (0.2) | 247 (74) | 1.21 (0.23) |
| 50 | 220 (2) | 1444 (2) | 84.8 (0.1) | 83 (0.9) | 261 (94) | 1.21 (0.30) |
| 70 | 241 (30) | 1454 (3) | 83.4 (2.1) | 82.9 (2.5) | 365 (126) | 1.25 (0.30) |
| Name | Density [kg/m3] | Thermal Conductivity [mW/(m·K)] |
|---|---|---|
| 0 | 190 (1.61) | 56.4 (0.2) |
| 30 | 225 (2.15) | 68.4 (0.4) |
| 50 | 210 (3.49) | 67.4 (0.3) |
| 70 | 300 (5.12) | 71.2 (0.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckardt, J.; De Nato, M.; Colusso, E.; Moro, L.; Šket, P.; Giovando, S.; Tondi, G. Glyoxal as Single Crosslinker for Mechanically Blown, Condensed and Hydrolyzable Tannin Foams. Polymers 2025, 17, 3008. https://doi.org/10.3390/polym17223008
Eckardt J, De Nato M, Colusso E, Moro L, Šket P, Giovando S, Tondi G. Glyoxal as Single Crosslinker for Mechanically Blown, Condensed and Hydrolyzable Tannin Foams. Polymers. 2025; 17(22):3008. https://doi.org/10.3390/polym17223008
Chicago/Turabian StyleEckardt, Jonas, Michele De Nato, Elena Colusso, Lorenzo Moro, Primož Šket, Samuele Giovando, and Gianluca Tondi. 2025. "Glyoxal as Single Crosslinker for Mechanically Blown, Condensed and Hydrolyzable Tannin Foams" Polymers 17, no. 22: 3008. https://doi.org/10.3390/polym17223008
APA StyleEckardt, J., De Nato, M., Colusso, E., Moro, L., Šket, P., Giovando, S., & Tondi, G. (2025). Glyoxal as Single Crosslinker for Mechanically Blown, Condensed and Hydrolyzable Tannin Foams. Polymers, 17(22), 3008. https://doi.org/10.3390/polym17223008






