Incorporation of Butanol into Nanopores of Syndiotactic Polystyrene
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
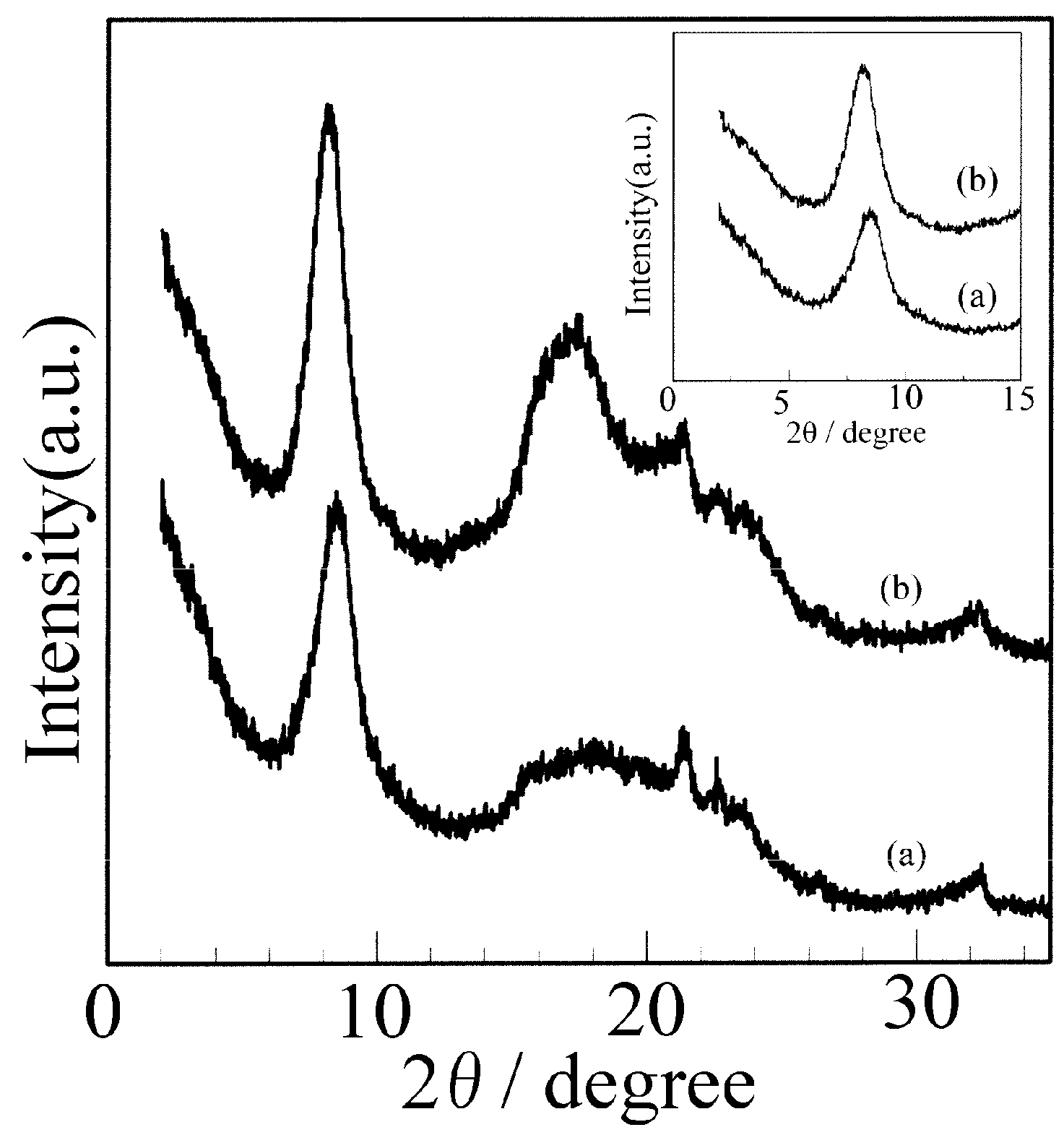
3.1. XRD Patterns of the sPS Film Before and After Immersion in Butanol
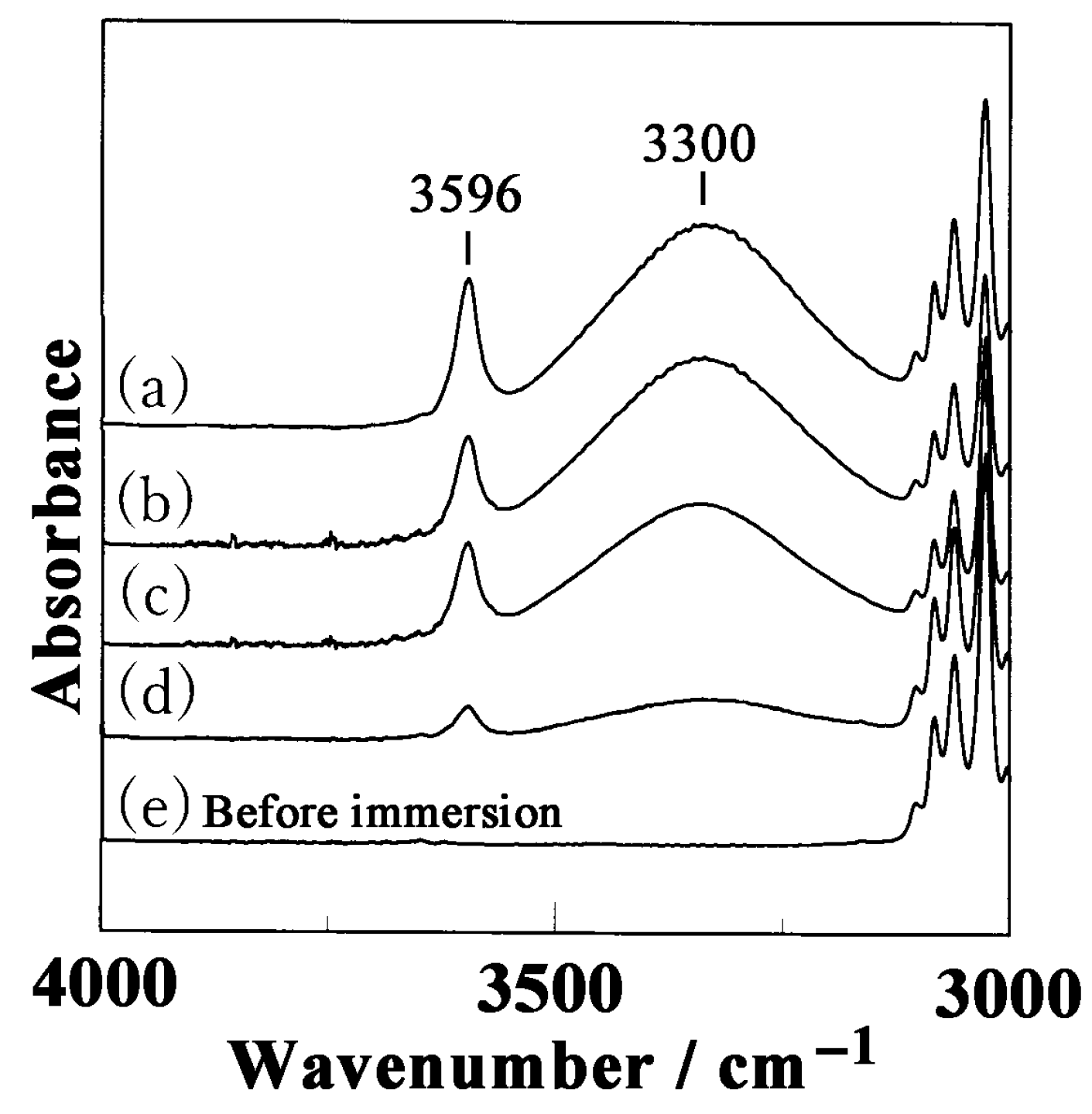
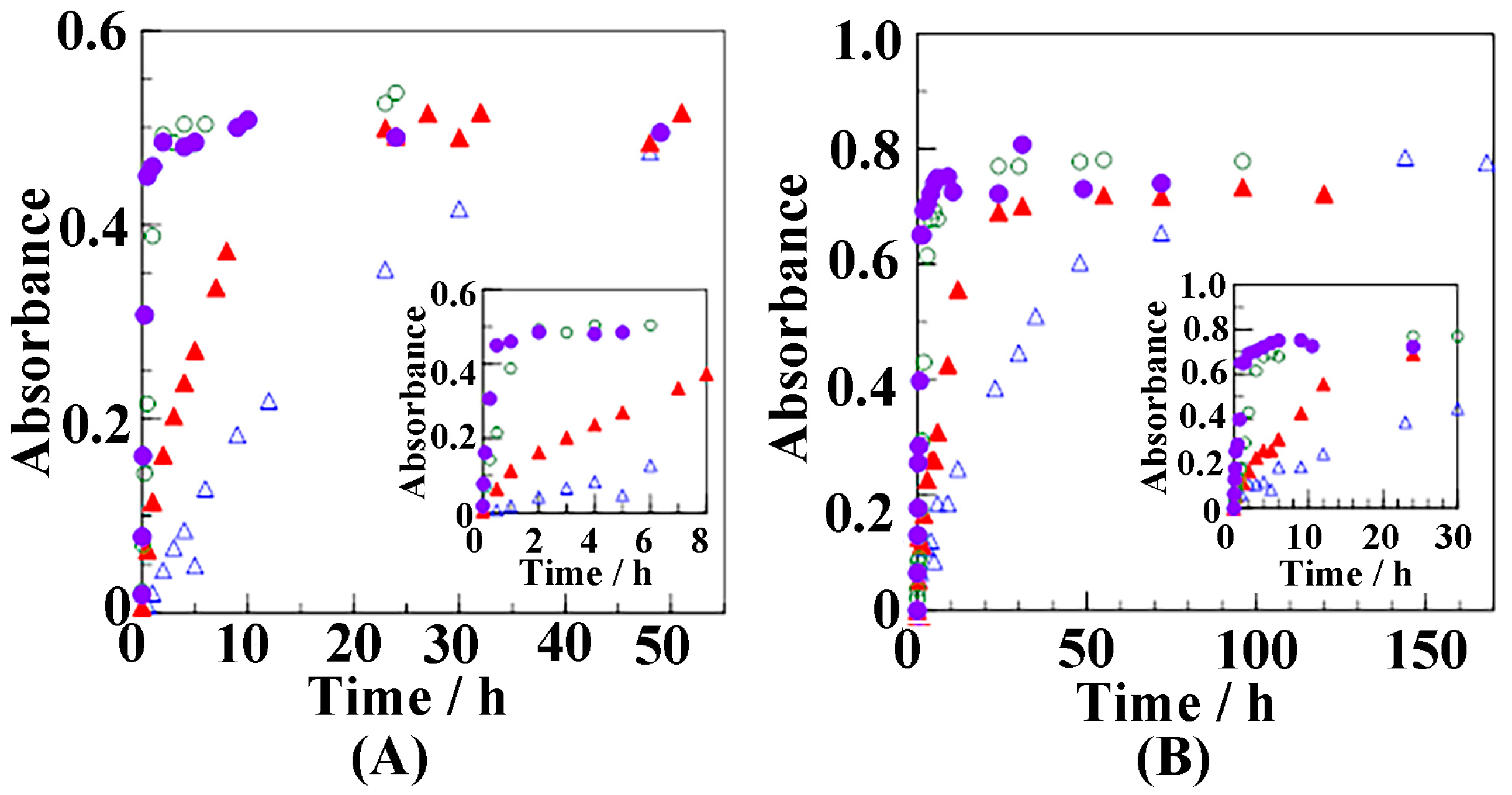
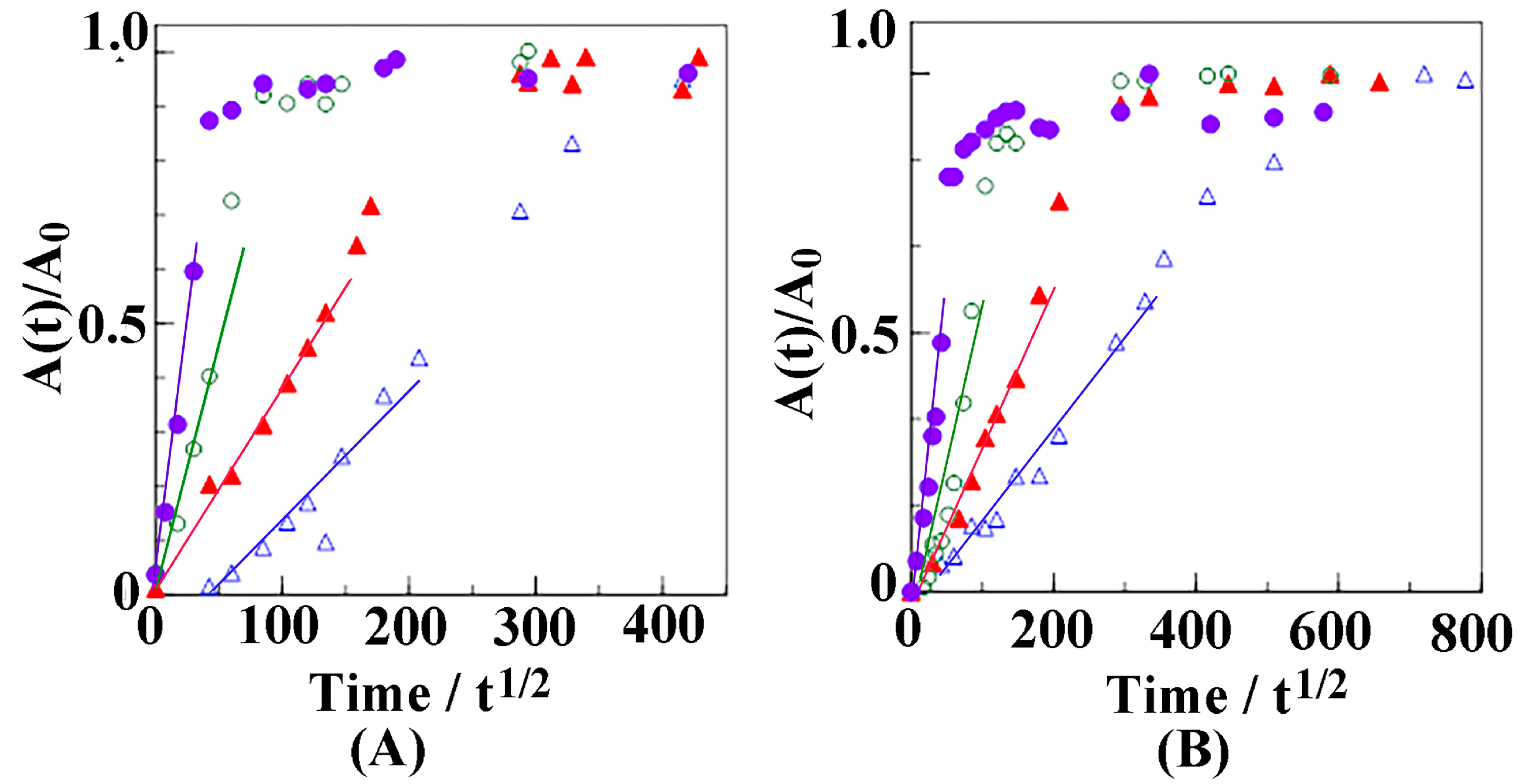
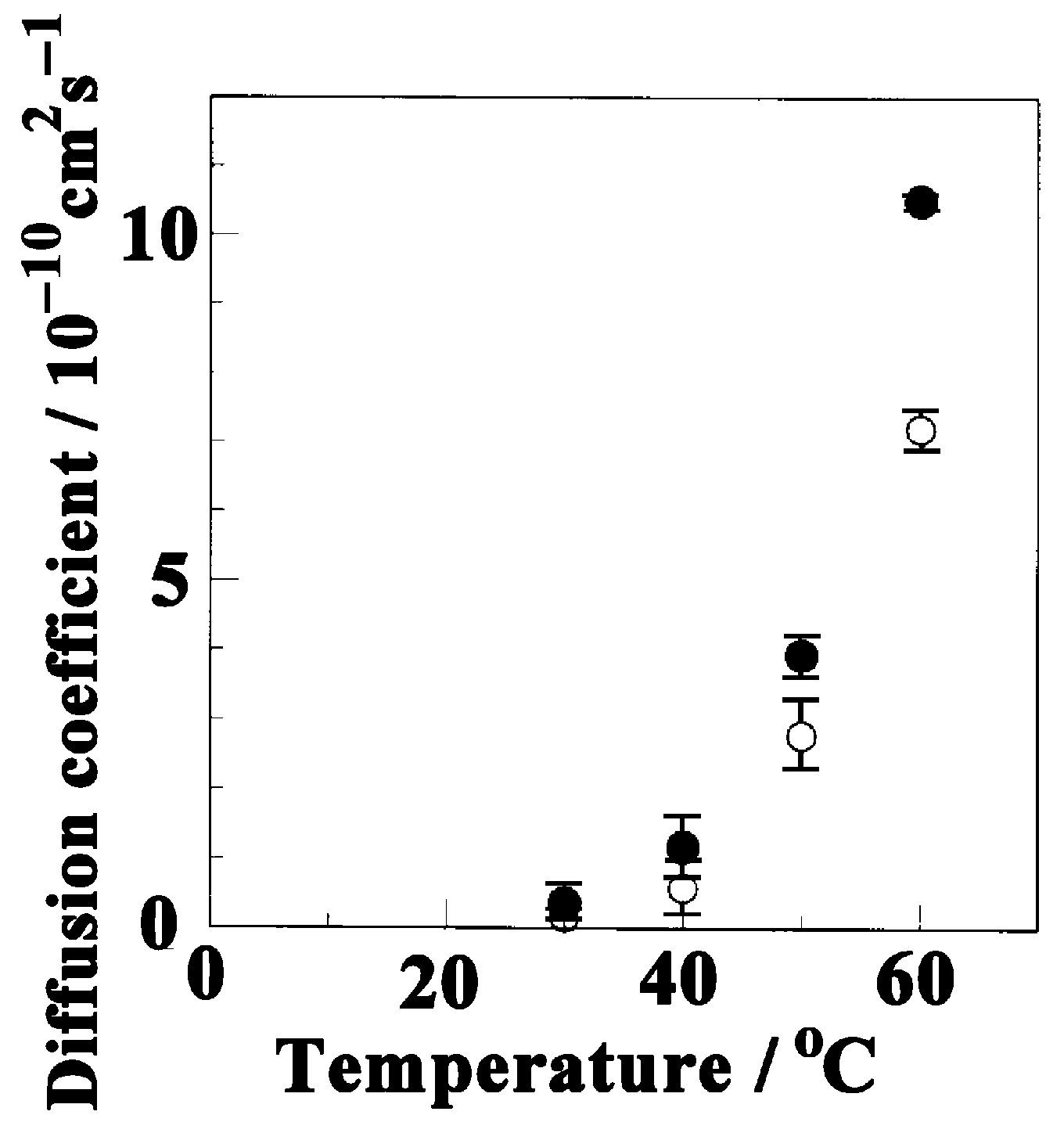
3.2. Diffusion of Butanol into the sPS Film
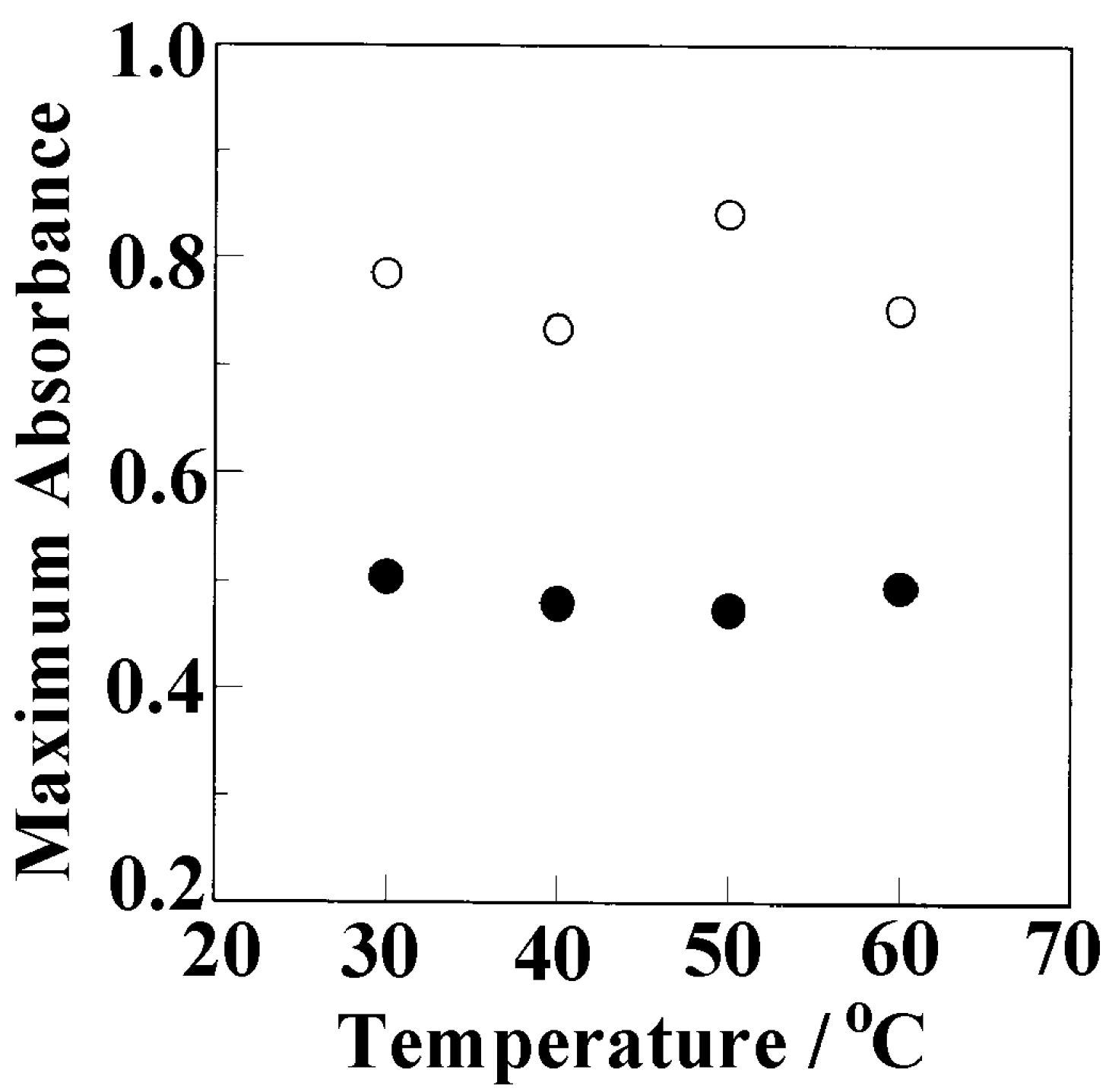
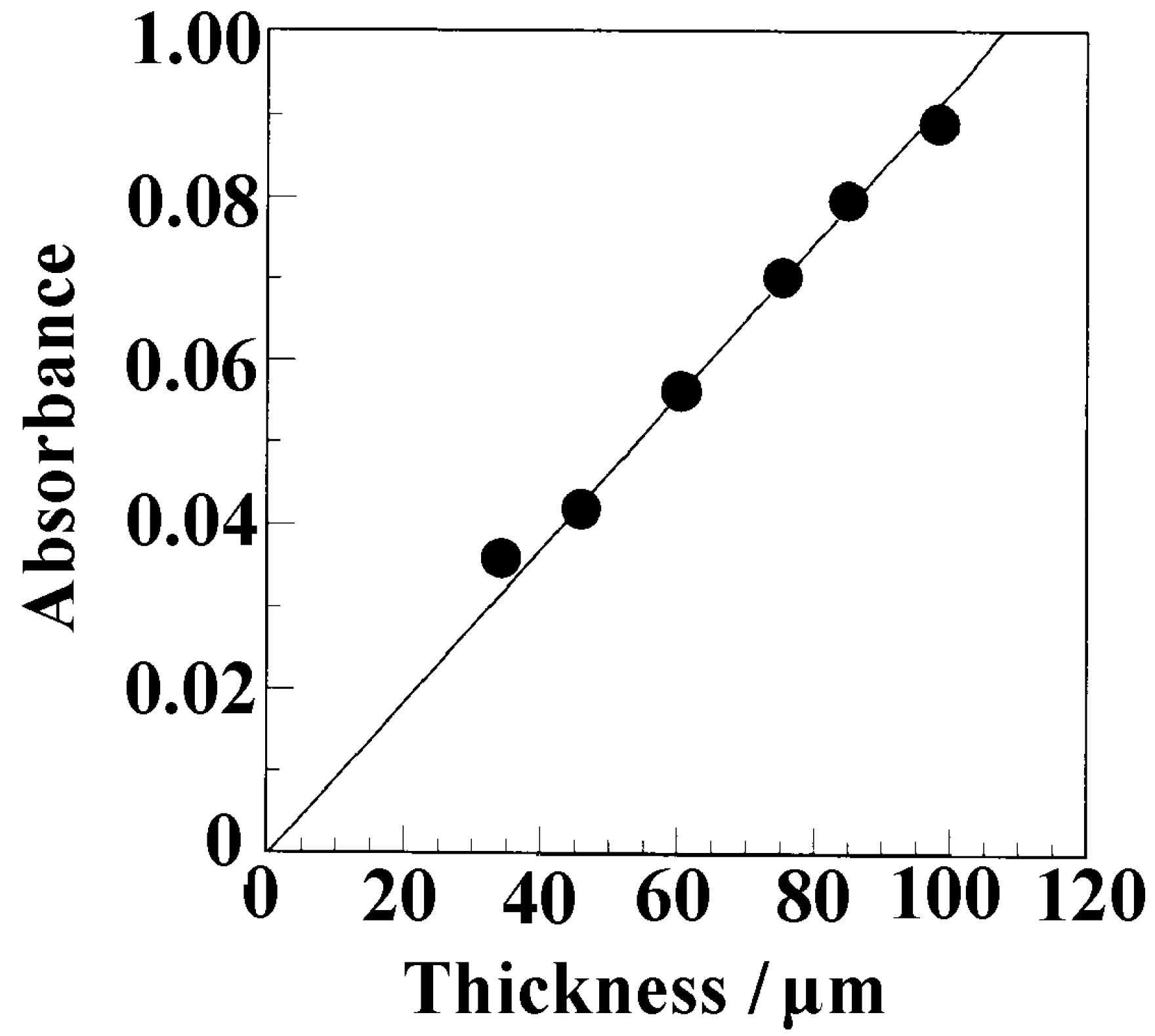
3.3. Weight Fraction of Butanol Molecules Incorporated into the Crystalline Cavity of sPS
3.4. Number of Butanol Molecules in the Crystalline Cavity of δ-sPS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- George, H.A.; Johnson, J.L.; Moore, W.E.C.; Holdeman, L.V.; Chen, J.S. Acetone, isopropanol, and butanol production by Clostridium beijerinckii (syn. Clostridium butylicum) and Clostridium aurantibutyricum. Appl. Environ. Microbiol. 1983, 45, 1160–1163. [Google Scholar] [CrossRef]
- Mitchell, W.J. Physiology of carbohydrate to solvent conversion by clostridia. Adv. Microb. Physiol. 1997, 39, 31–130. [Google Scholar]
- Lopez-Contreras, A.M.; Gabor, K.; Martens, A.A.; Renckens, B.A.M.; Claassen, P.A.M.; Oost, J.v.d.; Vos, W.M.d. Substrate-induced production and secretion of cellulases by Clostridium acetobutylicum. Appl. Environ. Microbiol. 2004, 70, 5238–5243. [Google Scholar] [CrossRef]
- Ezeji, T.; Blaschek, H.P. Fermentation of dried distillers’ grains and solubles (DDGS) hydrolysates to solvents and value-added products by solventogenic clostridia. Bioresour. Technol. 2008, 99, 5232–5242. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Goyal, Y.; Sarkar, A.; Gayen, K. Comparative economic assessment of ABE fermentation based on cellulosic and non-cellulosic feedstocks. Appl. Energy 2012, 93, 193–204. [Google Scholar] [CrossRef]
- Ndaba, B.; Chiyanzu, I.; Marx, S. n-Butanol derived from biochemical and chemical routes: A review. Biotechnol. Rep. 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Ishihara, N.; Seimiya, T.; Kuramoto, M.; Uoi, M. Crystalline syndiotactic polystyrene. Macromolecules 1986, 19, 2464–2465. [Google Scholar] [CrossRef]
- Ishihara, N.; Kuramoto, M.; Uoi, M. Stereospecific polymerization of styrene giving the syndiotactic polymer. Macromolecules 1988, 21, 3356–3360. [Google Scholar] [CrossRef]
- Immirzi, A.; de Candia, F.; Ianneli, P.; Zambelli, A.; Vittoria, V. Solvent-induced polymorphism in syndiotactic polystyrene. Makromol. Chem. Rapid Commun. 1988, 9, 761–764. [Google Scholar] [CrossRef]
- Vittoria, V.; de Candia, F.; Lanneli, P.; Immirzi, A. Solvent-induced crystallization of glassy syndiotactic polystyrene. Macromol. Chem. Rapid Commun. 1988, 9, 765–769. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nakaoki, T.; Ishihara, N. Polymorphic structures and molecular vibrations of syndiotactic polystyrene. Macromolecules 1989, 22, 4377–4382. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nakaoki, T.; Ishihara, N. Molecular conformation in glasses and gels of syndiotactic and isotactic polystyrenes. Macromolecules 1990, 23, 78–83. [Google Scholar] [CrossRef]
- De Rosa, C.; Guerra, G.; Petraccone, V.; Corradini, P. Crystal structure of the α-form of syndiotactic polystyrene. Polym. J. 1991, 23, 1435–1442. [Google Scholar] [CrossRef]
- De Rosa, C. Crystal structure of the trigonal modification (α Form) of syndiotactic polystyrene. Macromolecules 1996, 29, 8460–8465. [Google Scholar] [CrossRef]
- Cartier, L.; Okihara, T.; Lotz, B. The α Superstructure of Syndiotactic Polystyrene: A Frustrated Structure. Macromolecules 1998, 31, 3303–3310. [Google Scholar] [CrossRef]
- Wang, Y.K.; Savage, J.D.; Yang, D.; Hsu, S.L. Morphological study of phase transition behavior in syndiotactic polystyrene. Macromolecules 1992, 25, 3659–3666. [Google Scholar] [CrossRef]
- Wu, H.-D.; Tseng, C.-R.; Chang, F.-C. Chain conformation and crystallization behavior of the syndiotactic polystyrene nanocomposites studied using Fourier transform infrared analysis. Macromolecules 2001, 34, 2992–2999. [Google Scholar] [CrossRef]
- De Rosa, C.; Rapacciuolo, M.; Guerra, G.; Petraccone, V.; Corradini, P. On the crystal structure of the orthorhombic form of syndiotactic polystyrene. Polymer 1992, 33, 1423–1428. [Google Scholar] [CrossRef]
- Chatani, Y.; Shimane, Y.; Ijitsu, T.; Yukinari, T. Structural study on syndiotactic polystyrene: 3. Crystal structure of planar form I. Polymer 1993, 34, 1625–1629. [Google Scholar] [CrossRef]
- De Rosa, C.; Guerra, G.; Petraccone, V.; Pirozzi, B. Crystal Structure of the Emptied Clathrate Form (δe Form) of Syndiotactic Polystyrene. Macromolecules 1997, 30, 4147–4152. [Google Scholar] [CrossRef]
- Milano, G.; Venditto, V.; Guerra, G.; Cavallo, L.; Ciambelli, P.; Sannino, D. Shape and volume of cavities in thermoplastic molecular sieves based on syndiotactic polystyrene. Chem. Mater. 2001, 13, 1506–1511. [Google Scholar] [CrossRef]
- Gowd, E.B.; Shibayama, N.; Tashiro, K. Structural Changes during thermally induced phase transitions observed for uniaxially oriented δ form of syndiotactic polystyrene. Macromolecules 2007, 40, 6291–6295. [Google Scholar] [CrossRef]
- Sivakumar, M.; Yamamoto, Y.; Amutharani, D.; Tsujita, Y.; Yoshimizu, H.; Kinoshita, T. Study on δ-Form Complex in a Syndiotactic Polystyrene/Organic Molecules System, 1. Preferential Complexing Behavior of Xylene Isomers. Macromol. Rapid Commun. 2002, 23, 77–79. [Google Scholar] [CrossRef]
- Sivakumar, M.; Yamamoto, Y.; Amutharani, D.; Tsujita, Y.; Yoshimizu, H.; Kinoshita, T. Studies on the δ-form complex in the syndiotactic polystyrene–organic molecule system. II. Structural investigation of the δ-form with p- and m-xylene isomers. J. Appl. Polym. Sci. 2003, 89, 2882–2887. [Google Scholar] [CrossRef]
- Shaiju, P.; Gowd, E.B. Factors controlling the structure of syndiotactic polystyrene upon the guest exchange and guest extraction processes. Polymer 2015, 56, 581–589. [Google Scholar] [CrossRef]
- Jose, R.C.; Shaiju, P.; Nagendra, B.; Gowd, E.B. Influence of host preparation method on the structural phase transitions of syndiotactic polystyrene upon the guest exchange with n-alkanes. Polymer 2013, 54, 6617–6627. [Google Scholar] [CrossRef]
- Gowd, E.B.; Tashiro, K. Effect of chain-length of n-alkane on solvent-induced crystallization and solvent exchange phenomenon in syndiotactic polystyrene. Polymer 2011, 52, 822–829. [Google Scholar] [CrossRef]
- Gowd, E.B.; Shibayama, N.; Tashiro, K. Structural changes in thermally induced phase transitions of uniaxially oriented δ form of syndiotactic polystyrene investigated by temperature-dependent measurements of X-ray fiber diagrams and polarized infrared spectra. Macromolecules 2006, 39, 8412–8418. [Google Scholar] [CrossRef]
- Kaneko, F.; Radulescu, A.; Ute, K. Time-resolved SANS studies on guest exchange processes in co-crystals of syndiotactic polystyrene. Polymer 2013, 54, 3145–3149. [Google Scholar] [CrossRef]
- Kaneko, F.; Seto, N.; Sato, S.; Radulescu, A.; Schiavone, M.M.; Allgaier, J.; Ute, K. Simultaneous small-angle neutron scattering and Fourier transform infrared spectroscopic measurements on cocrystals of syndiotactic polystyrene with polyethylene glycol dimethyl ethers. J. Appl. Crystallogr. 2016, 49, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Tamai, Y.; Tsujita, Y.; Fukuda, M. Reorientational relaxation of aromatic molecules in the molecular cavity of crystalline syndiotactic polystyrene studied by molecular dynamics simulation. J. Mol. Struct. 2005, 739, 33–40. [Google Scholar] [CrossRef]
- Kobayashi, H.; Urakawa, O.; Kaneko, F.; Inoue, T. Dynamics of polar aromatic molecules confined in a nanocavity of δ-phase of syndiotactic polystyrene as studied by dielectric spectroscopy. Chem. Phys. 2016, 479, 122–128. [Google Scholar] [CrossRef]
- Kobayashi, H.; Akazawa, S.; Urakawa, O.; Kaneko, F.; Inoue, T. Anisotropic dynamics of benzonitrile confined in δ and ε clathrate phases of syndiotactic polystyrene. Macromolecules 2018, 51, 8611–8619. [Google Scholar] [CrossRef]
- Daniel, C.; Alfano, D.; Venditto, V.; Cardea, S.; Reverchon, E.; Larobina, D.; Mensitieri, G.; Guerra, G. Aerogels with a microporous crystalline host phase. Adv. Mater. 2005, 17, 1515–1518. [Google Scholar] [CrossRef]
- Daniel, C.; Sannino, D.; Guerra, G. Syndiotactic polystyrene aerogels: Adsorption in amorphous pores and absorption in crystalline nanocavities. Chem. Mater. 2008, 20, 577–582. [Google Scholar] [CrossRef]
- Itagaki, H.; Tokami, T.; Mochizuki, J. A trial to clarify a cause of forming physical gels: Morphology of syndiotactic polystyrene in n-alkylbenzene. Polymer 2012, 53, 5304–5312. [Google Scholar] [CrossRef]
- Mochizuki, J.; Sano, T.; Tokami, T.; Itagaki, H. Decisive properties of solvent able to form gels with syndiotactic polystyrene. Polymer 2015, 67, 118–127. [Google Scholar] [CrossRef]
- Wang, X.; Jana, S.C. Tailoring of morphology and surface properties of syndiotactic polystyrene aerogels. Langmuir 2013, 29, 5589–5598. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.M.; Nagendra, B.; Shaiju, P.; Surendran, K.P.; Gowd, E.B. Aerogels of hierarchically porous syndiotactic polystyrene with a dielectric constant near to air. J. Mater. Chem. C 2018, 6, 360–368. [Google Scholar] [CrossRef]
- Krishnan, V.G.; Joseph, A.M.; Peethambharan, S.K.; Gowd, E.B. Nanoporous crystalline aerogels of syndiotactic polystyrene: Polymorphism, dielectric, thermal, and acoustic properties. Macromolecules 2021, 54, 10605–10615. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, S.; Liu, H.; Yang, X.; Zhu, F. Synthesis and characterization of ultralight syndiotactic polystyrene aerogels prepared by freeze-drying. Langmuir 2025, 41, 7804–7813. [Google Scholar] [CrossRef]
- Rizzo, P.; Cozzolino, A.; Guerra, G. Chemical stabilization of hexanal molecules by inclusion as guests of nanoporous-crystalline syndiotactic polystyrene crystals. Macromolecules 2019, 52, 2255–2264. [Google Scholar] [CrossRef]
- Cozzolino, A.; Monaco, G.; Daniel, C.; Rizzo, P.; Guerra, G. Monomeric and dimeric carboxylic acid in crystalline cavities and channels of delta and epsilon forms of syndiotactic polystyrene. Polymers 2021, 13, 3330. [Google Scholar] [CrossRef]
- Cozzolino, A.; Monaco, G.; Rizzo, P.; Guerra, G. Segregation of benzoic acid in polymer crystalline cavities. Polymers 2023, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Covelli, V.; Cozzolino, A.; Rizzo, P.; Rodriquez, M.; Vestuto, V.; Bertamino, A.; Daniel, C.; Guerra, G. Salicylic acid release from syndiotactic polystyrene staple fibers. Molecules 2023, 28, 5095. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, A.; Pappalardo, S.; Rizzo, P.; Guerra, G. Linear hydrogen bonded aggregates of carboxylic acids in crystalline channels of syndiotactic polystyrene. Polymer 2022, 262, 125484. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Ianniello, G.; Rufolo, C.; Guerra, G. Syndiotactic polystyrene films with a cocrystalline phase including carvacrol guest molecules. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 657–665. [Google Scholar] [CrossRef]
- Cozzolino, A.; Botta, C.; Daniel, C.; Rizzo, P. Thymol and carvacrol: Phenolic monoterpenes extracted from the essential oil of Thymus vulgaris as natural antimicrobial guests of nanoporous crystalline syndiotactic polystyrene fibers. Macromol. Sym. 2023, 408, 2200064. [Google Scholar] [CrossRef]
- Rizzo, P.; Albunia, A.R.; Milano, G.; Venditto, V.; Guerra, G.; Mensitieri, G.; Di Maio, L. Crystalline orientation and molecular transport properties in nanoporous syndiotactic polystyrene films. Macromol. Symp. 2001, 185, 65–75. [Google Scholar] [CrossRef]
- Albunia, A.R.; D’Aniello, C.; Guerra, G.; Gatteschi, D.; Mannini, M.; Sorace, L. Ordering Magnetic Molecules within Nanoporous Crystalline Polymers. Chem. Mater. 2009, 21, 4750–4752. [Google Scholar] [CrossRef]
- Albunia, A.R.; Minucci, T.; Guerra, G. Ethylene Removal by Sorption from Polymeric Crystalline Frameworks. J. Mater. Chem. 2008, 18, 1046–1050. [Google Scholar] [CrossRef]
- Albunia, A.R.; Musto, P.; Guerra, G. FTIR Spectra of Pure Helical Crystalline Phases of Syndiotactic Polystyrene. Polymer 2006, 47, 234–242. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Bian, Z.; Xie, S.; Cai, C.; Wang, J.; Yang, H.; Li, H. Solvothermally Controllable Synthesis of Anatase TiO2 Nanocrystals with Dominant {001} Facets and Enhanced Photocatalytic Activity. CrystEngComm 2010, 12, 2219–2224. [Google Scholar] [CrossRef]
- Galdi, N.; Albunia, A.R.; Oliva, L.; Guerra, G. Selective Molecular−Complex Phase Formation of Syndiotactic Polystyrene with a Styrene Dimer. Macromolecules 2006, 39, 9171–9176. [Google Scholar] [CrossRef]
- Albunia, A.R.; Guerra, G. Spectroscopic Investigation of Guest–Guest Interactions in the Nanoporous-Crystalline δ and ε Forms of Syndiotactic Polystyrene. J. Phys. Chem. C 2014, 118, 11774–11783. [Google Scholar] [CrossRef]
- Albunia, A.R.; Graf, R.; Guerra, G.; Spiess, H.W. 2H NMR Study of Aromatic Guest Dynamics in Clathrate Phases of Syndiotactic Polystyrene. Macromol. Chem. Phys. 2005, 206, 715–724. [Google Scholar] [CrossRef]
- Albunia, A.R.; Oliva, P.; Grassi, A. Interactions of Volatile Organic Compounds with Syndiotactic Polystyrene Crystalline Nanocavities. J. Phys. Chem. A 2011, 115, 443–452. [Google Scholar] [CrossRef]
- Annunziata, L.; Albunia, A.R.; Venditto, V.; Mensitieri, G.; Guerra, G. Polymer/Gas Clathrates for Gas Storage and Controlled Release. Macromolecules 2006, 39, 9166–9170. [Google Scholar] [CrossRef]
- Nakaoki, T.; Goto, N.; Saito, K. Selective sorption and desorption of organic solvent for δ-syndiotactic polystyrene. Polym. J. 2009, 41, 214–218. [Google Scholar] [CrossRef][Green Version]
- Furukawa, K.; Nakaoki, T. Comparison of absorption kinetics of ethanol and butanol into different size nanopores present in syndiotactic polystyrene and poly(p-methylstyrene). Soft Mater. 2011, 9, 141–153. [Google Scholar] [CrossRef]
- Furukawa, K.; Nakaoki, T. Absorption kinetics of phenol into different size nanopores present in syndiotactic polystyrene and poly(p-methylstyrene). In Polymer Science; Yilmaz, F., Ed.; IntechOpen: London, UK, 2013; pp. 133–150. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujino, S.; Miyauchi, R.; Nakaoki, T.; Rizzo, P. Incorporation of Butanol into Nanopores of Syndiotactic Polystyrene. Polymers 2025, 17, 2978. https://doi.org/10.3390/polym17222978
Fujino S, Miyauchi R, Nakaoki T, Rizzo P. Incorporation of Butanol into Nanopores of Syndiotactic Polystyrene. Polymers. 2025; 17(22):2978. https://doi.org/10.3390/polym17222978
Chicago/Turabian StyleFujino, Saki, Rei Miyauchi, Takahiko Nakaoki, and Paola Rizzo. 2025. "Incorporation of Butanol into Nanopores of Syndiotactic Polystyrene" Polymers 17, no. 22: 2978. https://doi.org/10.3390/polym17222978
APA StyleFujino, S., Miyauchi, R., Nakaoki, T., & Rizzo, P. (2025). Incorporation of Butanol into Nanopores of Syndiotactic Polystyrene. Polymers, 17(22), 2978. https://doi.org/10.3390/polym17222978







