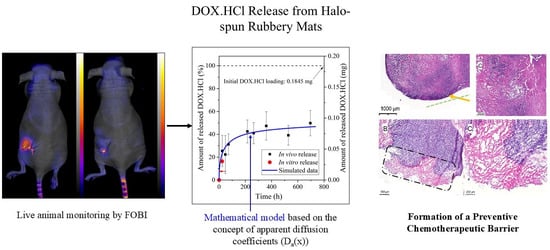
In Vivo Quantitative Monitoring of Drug Release from Halo-Spun Rubbery Mats by Fluorescent Organism Bioimaging (FOBI)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DOX.HCl-Loaded Fiber Mats
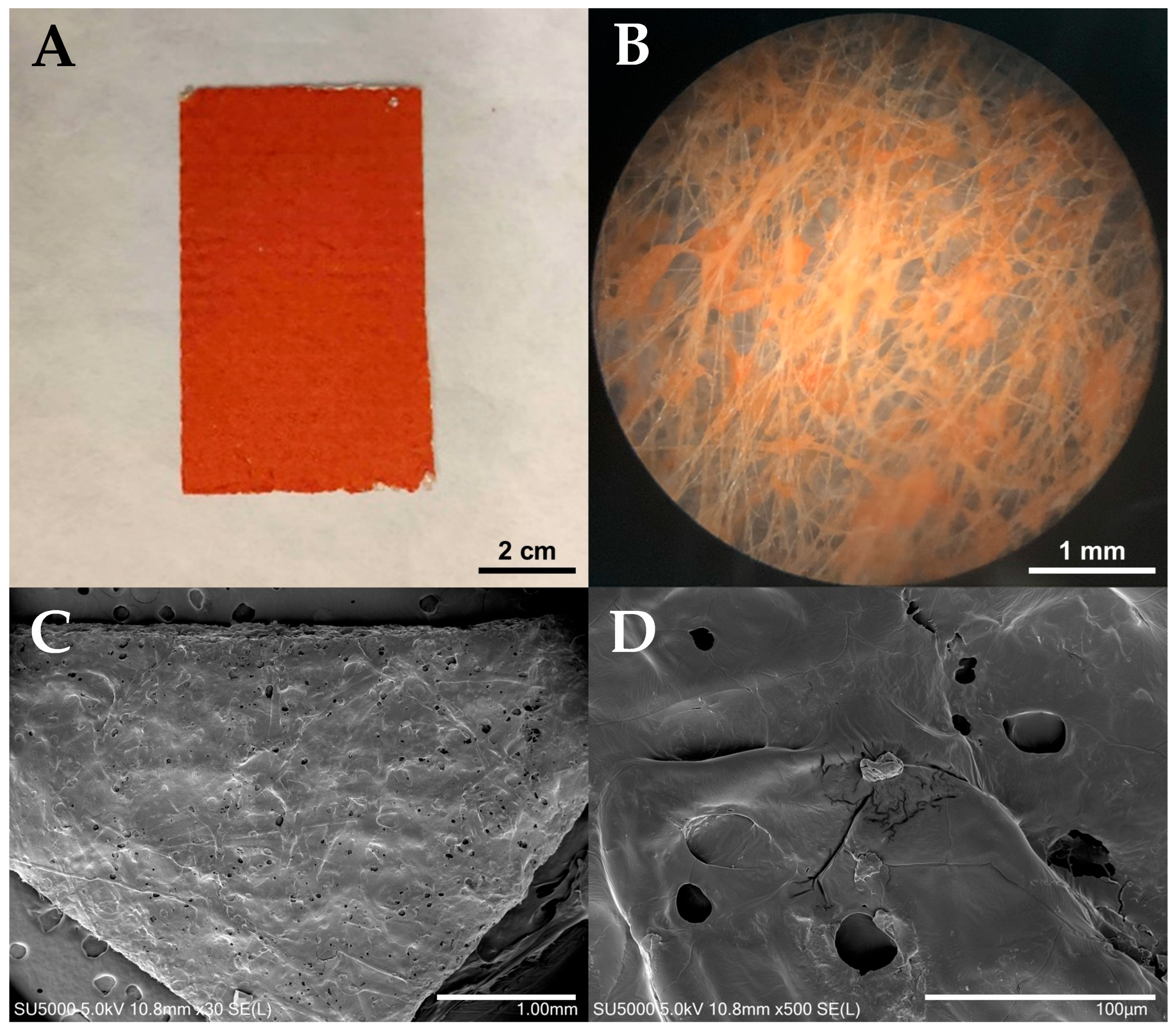
2.3. Scanning Electron Microscopy (SEM)
2.4. X-Ray Photoelectron Spectroscopy (XPS)
2.5. In Vitro Release Studies
2.6. Animal Preparation and Surgery
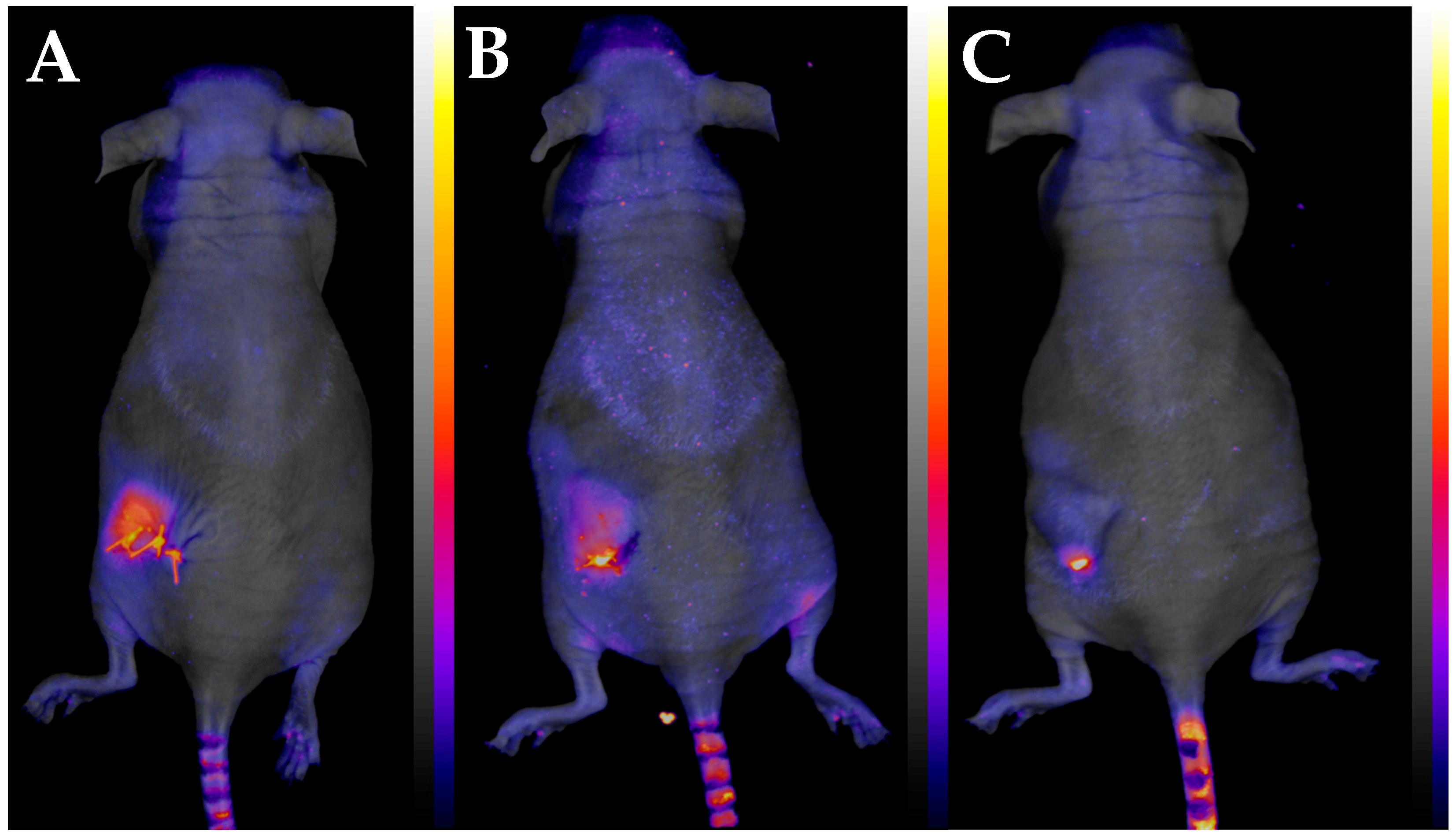
2.7. Fluorescent Organism Bioimaging (FOBI)
2.8. Histology
3. Results and Discussion
3.1. Characterization of the Halo-Spun DOX.HCl-Loaded Samples
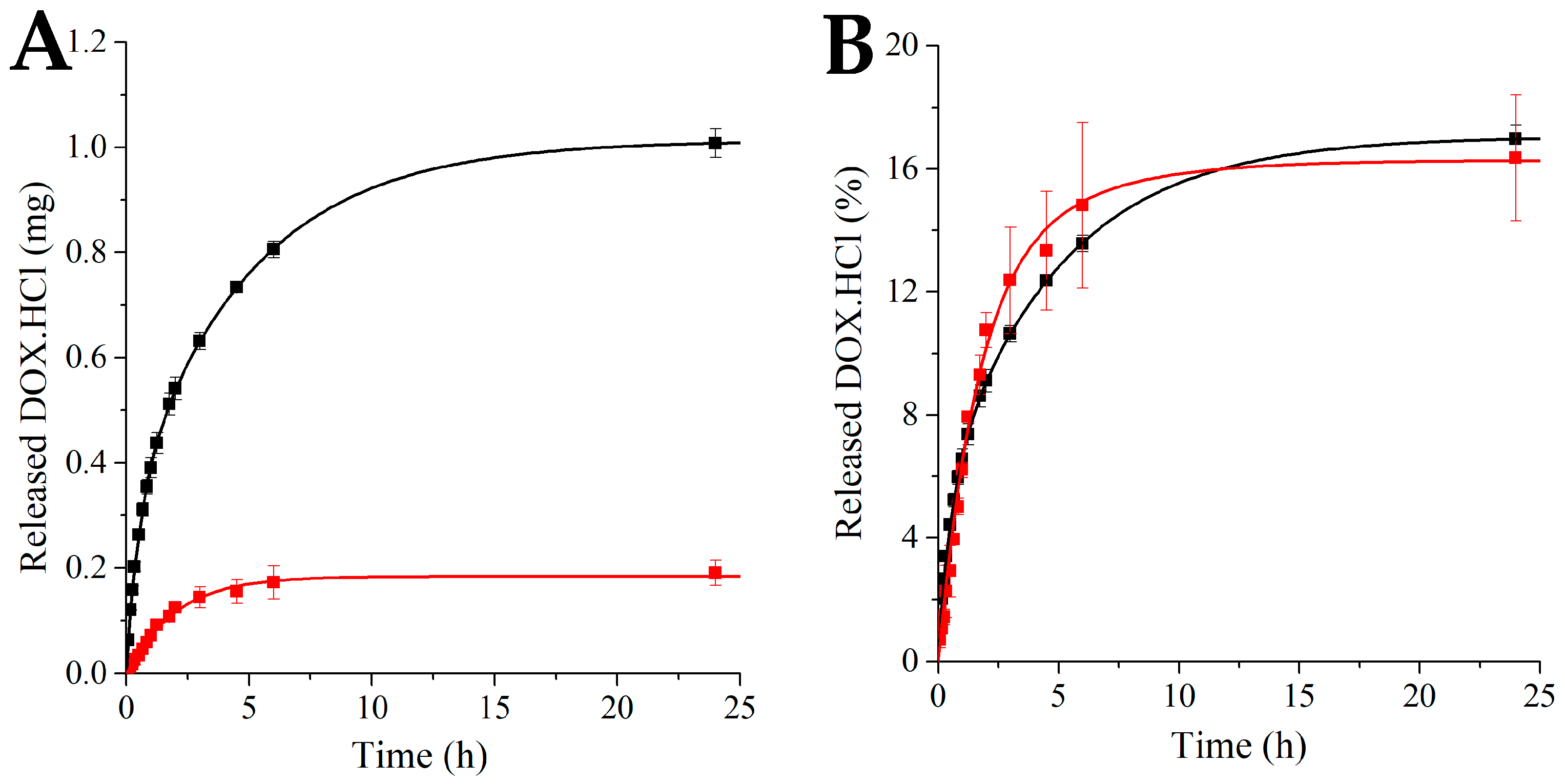
3.2. In Vitro Release Studies
3.2.1. Calibration
3.2.2. Investigation of In Vitro DOX.HCl Release
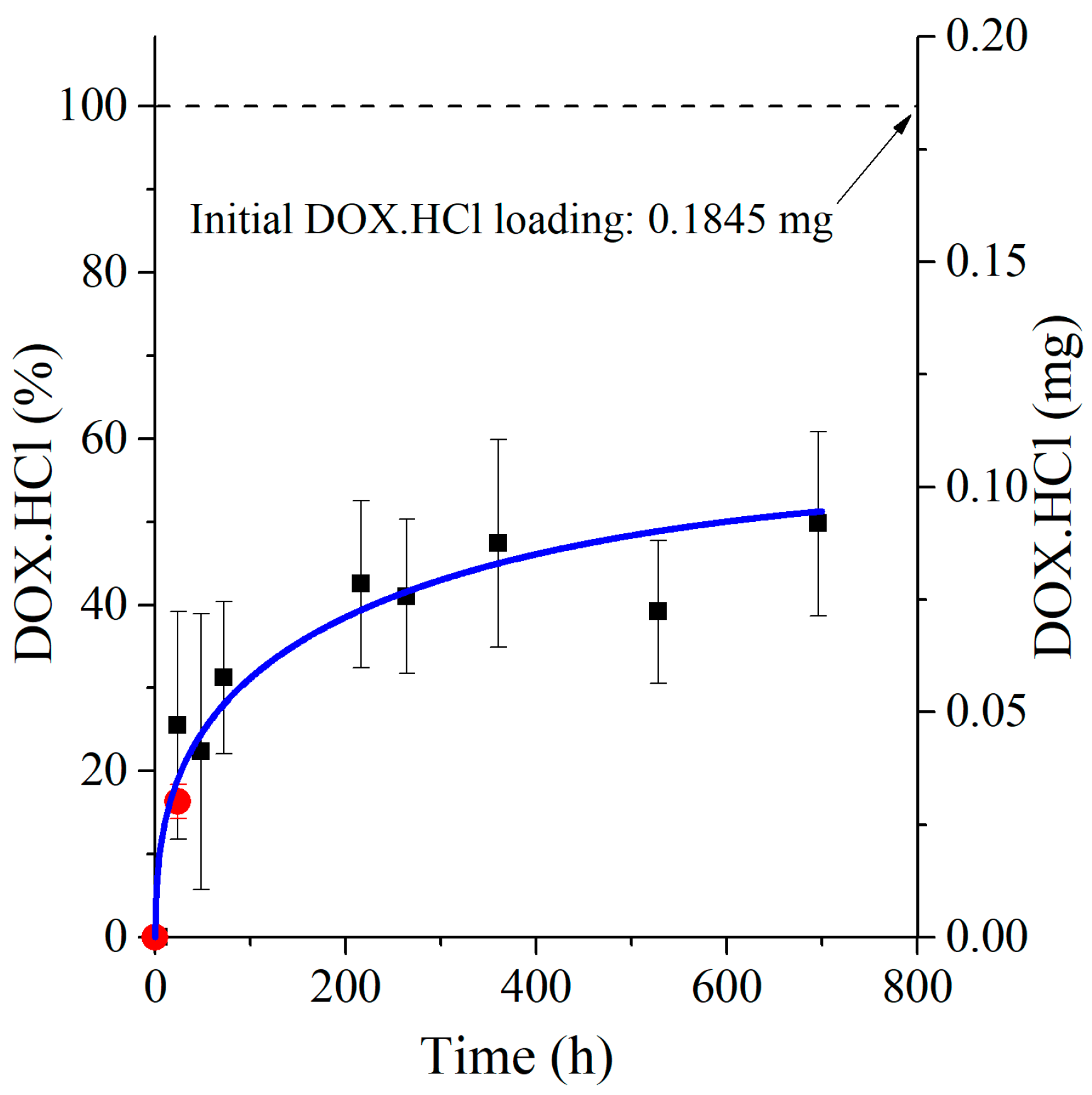
3.3. Development of the New In Vivo Monitoring Method
3.4. Mathematical Model Development and Comparison with Measured Data
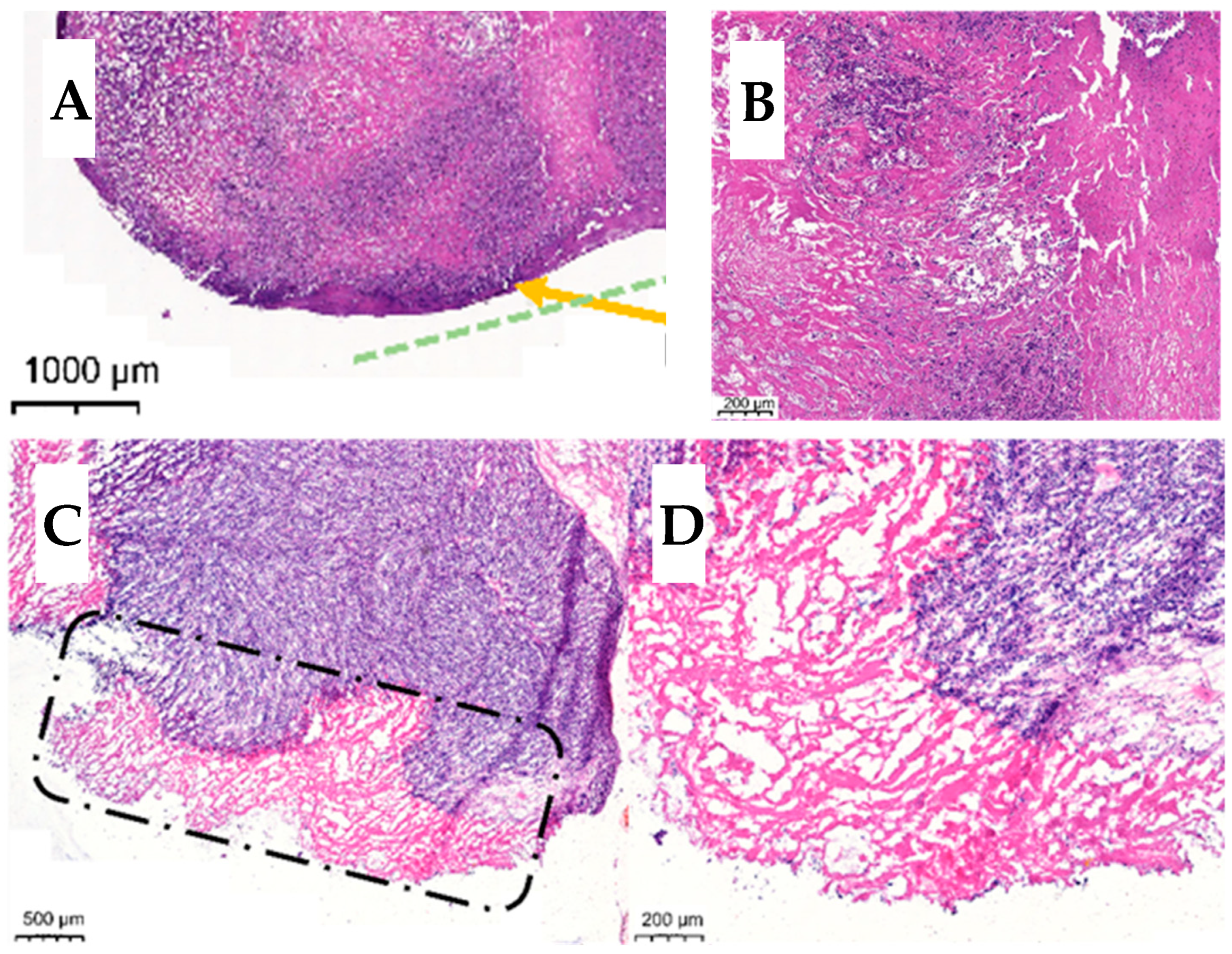
3.5. Histology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention, CDC Website. 2025. Available online: https://www.cdc.gov/cancer/ (accessed on 15 October 2025).
- Bondurant, S.; Ernster, V.; Herdman, R. Safety of Silicone Breast Implants; National Academies Press: Washington, DC, USA, 1999. [Google Scholar] [CrossRef]
- Dancey, A.; Nassimizadeh, A.; Levick, P. Capsular Contracture—What Are the Risk Factors? A 14 Year Series of 1400 Consecutive Augmentations. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.E.; Woods, J.E.; O’Fallon, W.M.; Beard, C.M.; Kurland, L.T.; Melton, L.J. Complications leading to surgery after breast implantation. N. Engl. J. Med. 1997, 336, 677–682. [Google Scholar] [CrossRef]
- American Society of Plastic Surgeons. Plastic Surgery Statistics Report. 2024. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf (accessed on 13 June 2024).
- Noskovicova, N.; Hinz, B.; Pakshir, P. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells 2021, 10, 1794. [Google Scholar] [CrossRef]
- Kim, B.H.; Park, M.; Park, H.J.; Lee, S.H.; Choi, S.Y.; Park, C.G.; Han, S.M.; Heo, C.Y.; Choy, Y.B. Prolonged, acute suppression of cysteinyl leukotriene to reduce capsular contracture around silicone implants. Acta Biomater. 2017, 51, 209–219. [Google Scholar] [CrossRef]
- McClain, A.; Jindal, A.; Durr, H.; Puskas, J.E.; Leipzig, N.D. In Vivo Release of Zafirlukast from Electrospun Polyisobutylene-Based Fiber Mats to Reduce Capsular Contracture of Silicone Breast Prostheses. ACS Appl. Bio Mater. 2024, 7, 4442–4453. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; Das, D.; Fayazzadeh, E.; Sen, S.; McClain, A.; Puskas, J.E.; Drazba, J.A.; McLennan, G. Folic acid conjugated polymeric drug delivery vehicle for targeted cancer detection in hepatocellular carcinoma. J. Biomed. Mater. Res. Part A 2019, 107, 2522–2535. [Google Scholar] [CrossRef]
- Kamath, K.R.; Barry, J.J.; Miller, K.M. The TaxusTM drug-eluting stent: A new paradigm in controlled drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 412–436. [Google Scholar] [CrossRef]
- Kantor, J.; Puskas, J.E.; Kaszas, G. The Effect of Reaction Conditions on the Synthesis of Thermoplastic Elastomers Containing Polyalloocimene, Polyisobutylene and Tapered Blocks. Chin. J. Polym. Sci. 2019, 37, 884–890. [Google Scholar] [CrossRef]
- Puskas, J.E.; Gergely, A.L.; Kaszas, G. Controlled/living carbocationic copolymerization of isobutylene with alloocimene. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 29–33. [Google Scholar] [CrossRef]
- Gergely, A.L.; Puskas, J.E. Synthesis and characterization of thermoplastic elastomers with polyisobutylene and polyalloocimene blocks. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1567–1574. [Google Scholar] [CrossRef]
- Jindal, A.; Puskas, J.E.; McClain, A.; Nedic, K.; Luebbers, M.T.; Baker, J.R.; Santos, B.P.D.; Camassola, M.; Jennings, W.; Einsporn, R.L.; et al. Encapsulation and release of Zafirlukast from electrospun polyisobutylene-based thermoplastic elastomeric fiber mat. Eur. Polym. J. 2018, 98, 254–261. [Google Scholar] [CrossRef]
- Martens, M.E.; Enn, R.; Oun, S.; Veinla, M.; Toots, K.M.; Jarvekulg, M. Device and Method for Producing Polymer Fibers and its Uses Thereof. U.S. Patent US2022049376A1, 17 February 2022. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Anand, R.; Ottani, S.; Manoli, F.; Manet, I.; Monti, S. A close-up on doxorubicin binding to γ-cyclodextrin: An elucidating spectroscopic, photophysical and conformational study. RSC Adv. 2012, 2, 2346. [Google Scholar] [CrossRef]
- Gupta, P.K.; Lam, F.C.; Hung, C.T. Investigation of the Stability of Doxorubicin Hydrochloride Using Factorial Design. Drug Dev. Ind. Pharm. 1988, 14, 1657–1671. [Google Scholar] [CrossRef]
- Janssen, M.; Crommelin, D.; Storm, G.; Hulshoff, A. Doxorubicin decomposition on storage. Effect of pH, type of buffer and liposome encapsulation. Int. J. Pharm. 1985, 23, 1–11. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Uong, T.N.T.; Yoon, M.S.; Lee, K.-H.; Hyun, H.; Nam, T.-K.; Min, J.-J.; Nguyen, H.P.Q.; Kim, S.-K. Live cell imaging of highly activated natural killer cells against human hepatocellular carcinoma in vivo. Cytotherapy 2021, 23, 799–809. [Google Scholar] [CrossRef]
- Kang, J.S. Fluorescence Detection of Cell Death in Liver of Mice Treated with Thioacetamide. Toxicol. Res. 2018, 34, 1–6. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Yun, W.S.; Choi, J.S.; Kim, W.C.; Lee, S.H.; Park, D.J.; Park, J.E.; Key, J.; Seo, Y.J. Biodistribution of poly clustered superparamagnetic iron oxide nanoparticle labeled mesenchymal stem cells in aminoglycoside induced ototoxic mouse model. Biomed. Eng. Lett. 2021, 11, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Sirianni, R.W.; Jang, E.-H.; Miller, K.M.; Saltzman, W.M. Parameter estimation methodology in a model of hydrophobic drug release from a polymer coating. J. Control. Release 2010, 142, 474–482. [Google Scholar] [CrossRef]
- Forrey, C.; Saylor, D.M.; Silverstein, J.S.; Douglas, J.F.; Davis, E.M.; Elabd, Y.A. Prediction and validation of diffusion coefficients in a model drug delivery system using microsecond atomistic molecular dynamics simulation and vapour sorption analysis. Soft Matter 2014, 10, 7480–7494. [Google Scholar] [CrossRef]
- Ranade, S.V.; Miller, K.M.; Richard, R.E.; Chan, A.K.; Allen, M.J.; Helmus, M.N. Physical characterization of controlled release of paclitaxel from the TAXUSTM Express 2 TM drug-eluting stent. J. Biomed. Mater. Res. Part A 2004, 71A, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Boden, M.; Richard, R.; Schwarz, M.C.; Kangas, S.; Huibregtse, B.; Barry, J.J. In vitro and in vivo evaluation of the safety and stability of the TAXUS® Paclitaxel-Eluting Coronary Stent. J. Mater. Sci. Mater. Med. 2009, 20, 1553–1562. [Google Scholar] [CrossRef] [PubMed]

| Nominal DOX.HCl Load (wt%) | Sample Mass (mg) | Average Mass (mg) | Nominal DOX.HCl Content (mg) |
|---|---|---|---|
| 20 wt% | 32.0 26.8 30.3 | 29.7 | 5.94 |
| 5 wt% | 24.5 23.0 22.4 | 23.3 | 1.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polyak, P.; Sasidharan Pillai, A.; Forgach, L.; Molnar, K.; Puskas, J.E.; Mathe, D. In Vivo Quantitative Monitoring of Drug Release from Halo-Spun Rubbery Mats by Fluorescent Organism Bioimaging (FOBI). Polymers 2025, 17, 2972. https://doi.org/10.3390/polym17222972
Polyak P, Sasidharan Pillai A, Forgach L, Molnar K, Puskas JE, Mathe D. In Vivo Quantitative Monitoring of Drug Release from Halo-Spun Rubbery Mats by Fluorescent Organism Bioimaging (FOBI). Polymers. 2025; 17(22):2972. https://doi.org/10.3390/polym17222972
Chicago/Turabian StylePolyak, Peter, Aswathy Sasidharan Pillai, Laszlo Forgach, Kristof Molnar, Judit E. Puskas, and Domokos Mathe. 2025. "In Vivo Quantitative Monitoring of Drug Release from Halo-Spun Rubbery Mats by Fluorescent Organism Bioimaging (FOBI)" Polymers 17, no. 22: 2972. https://doi.org/10.3390/polym17222972
APA StylePolyak, P., Sasidharan Pillai, A., Forgach, L., Molnar, K., Puskas, J. E., & Mathe, D. (2025). In Vivo Quantitative Monitoring of Drug Release from Halo-Spun Rubbery Mats by Fluorescent Organism Bioimaging (FOBI). Polymers, 17(22), 2972. https://doi.org/10.3390/polym17222972