Research on the Physical Properties and Internal Structure of PVP/Nb2O5 Nanocomposite Coatings
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Samples Preparation
2.2. Methods
3. Results and Discussion
3.1. XRD Analysis
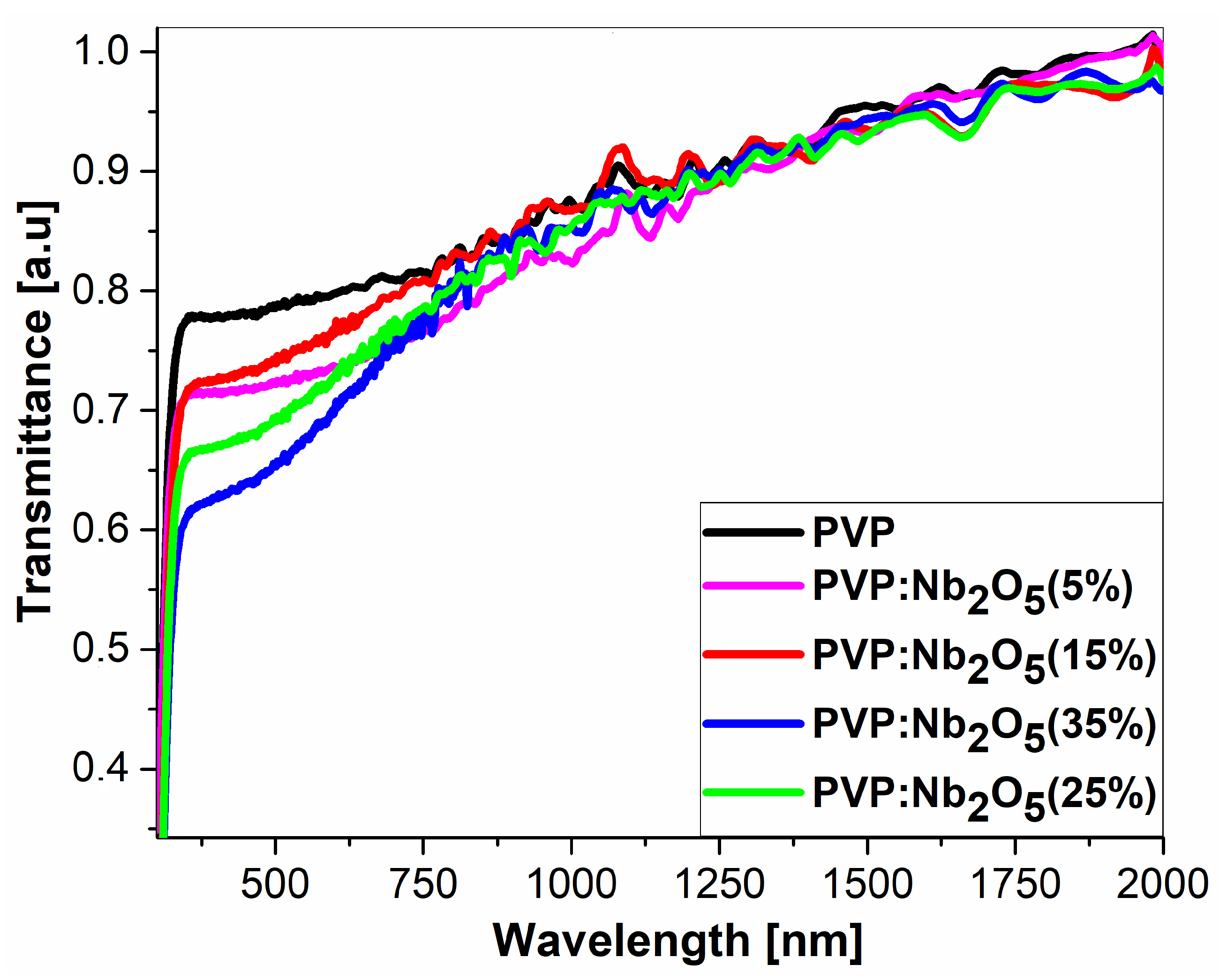
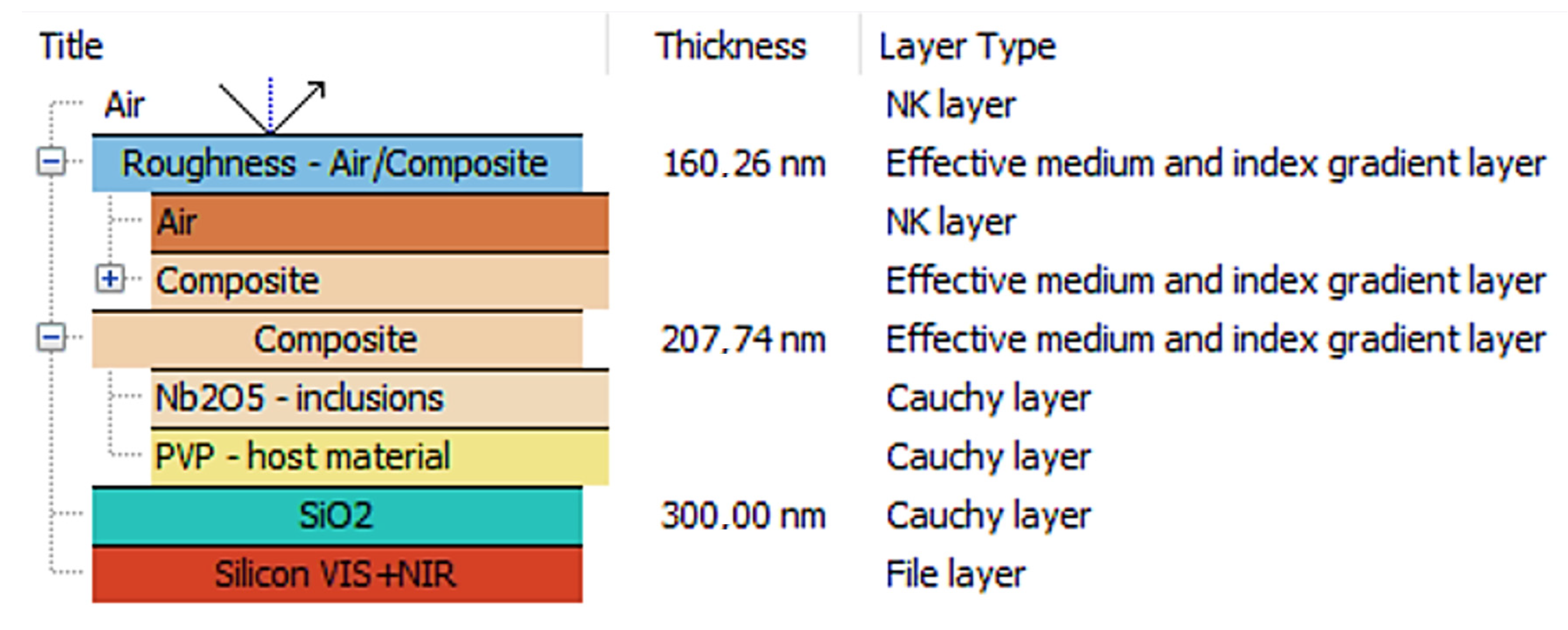
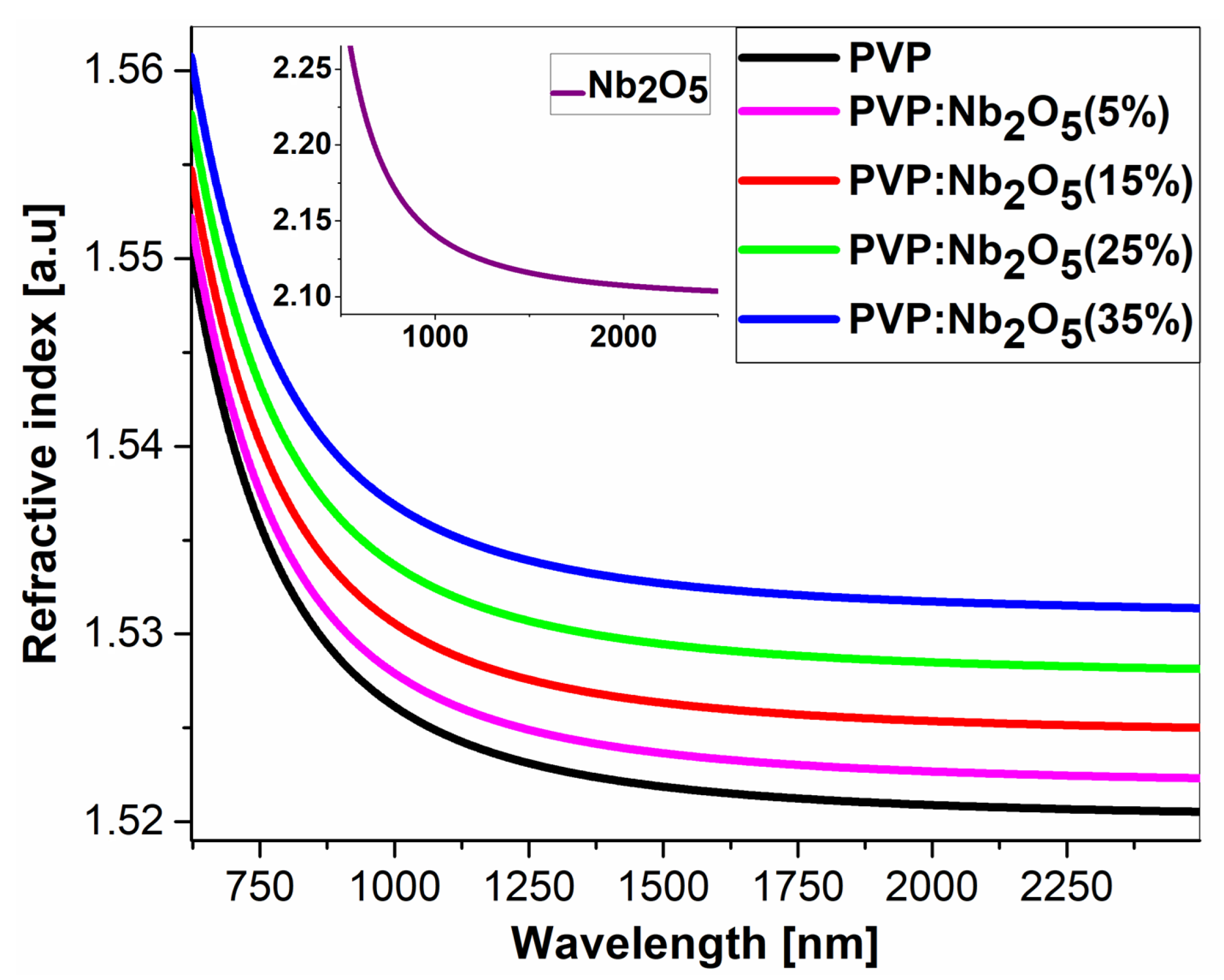
3.2. Ellipsometric Analysis
3.3. Thermal Analysis
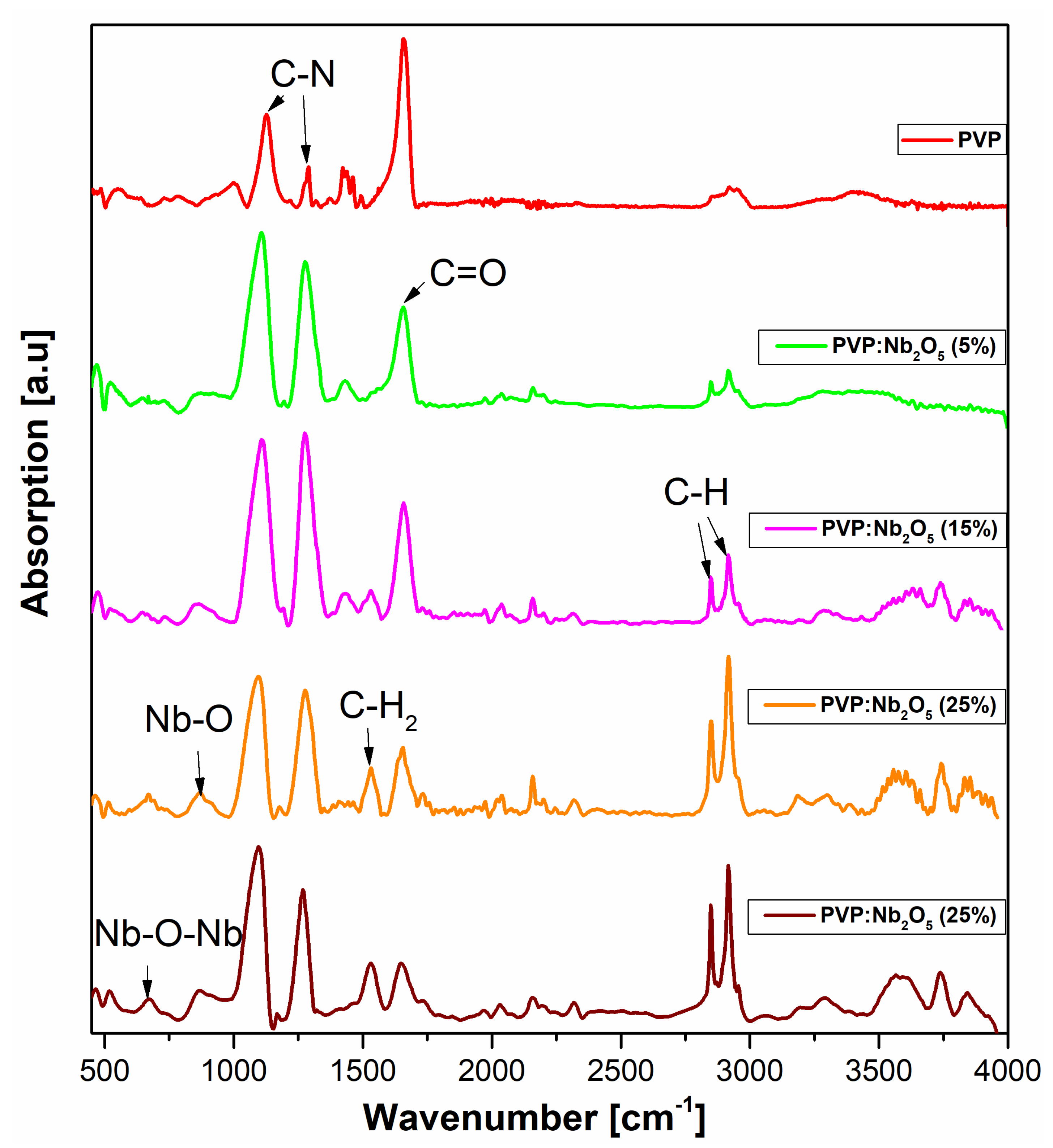
3.4. ATR-FTIR Analysis
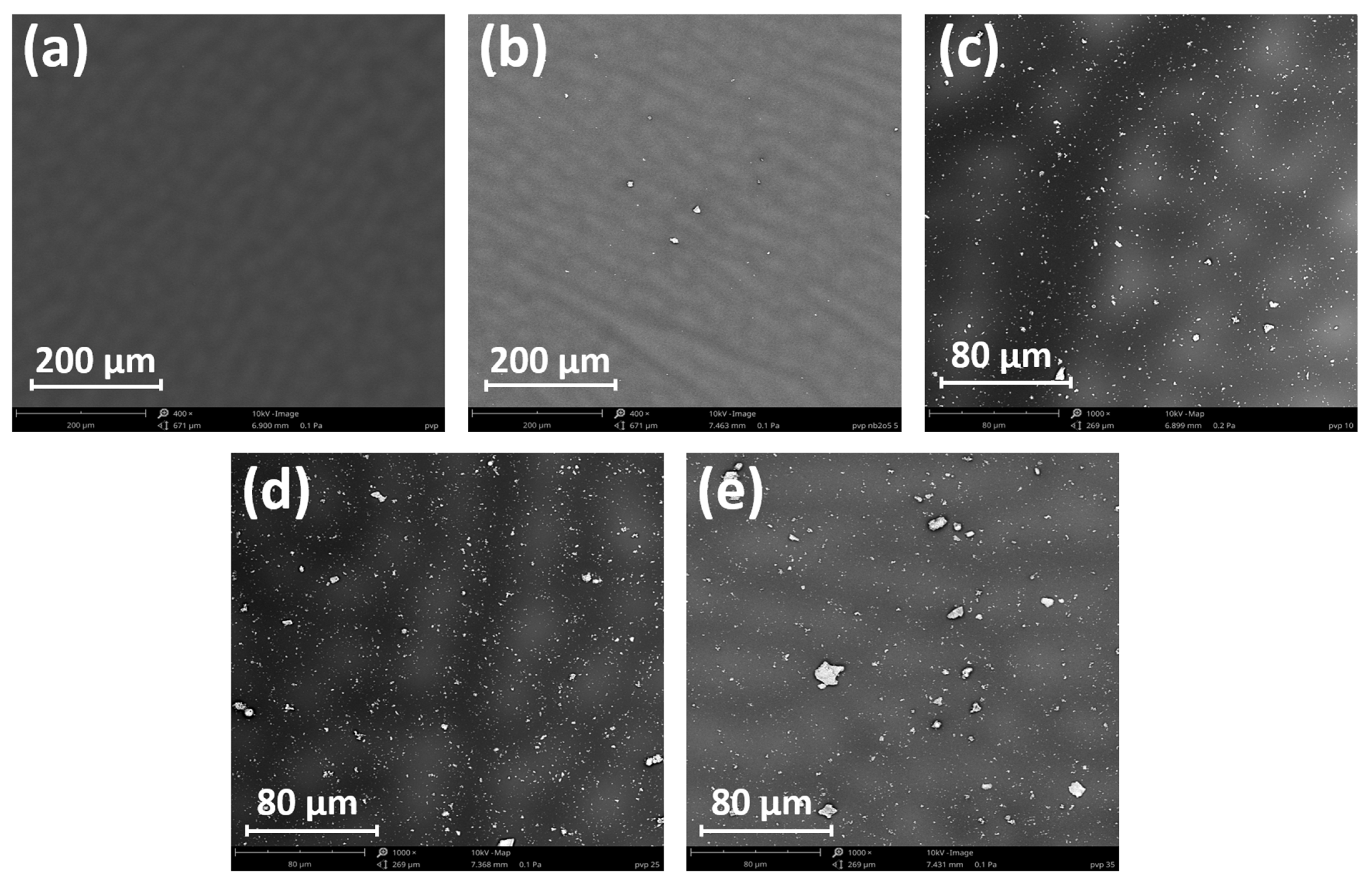
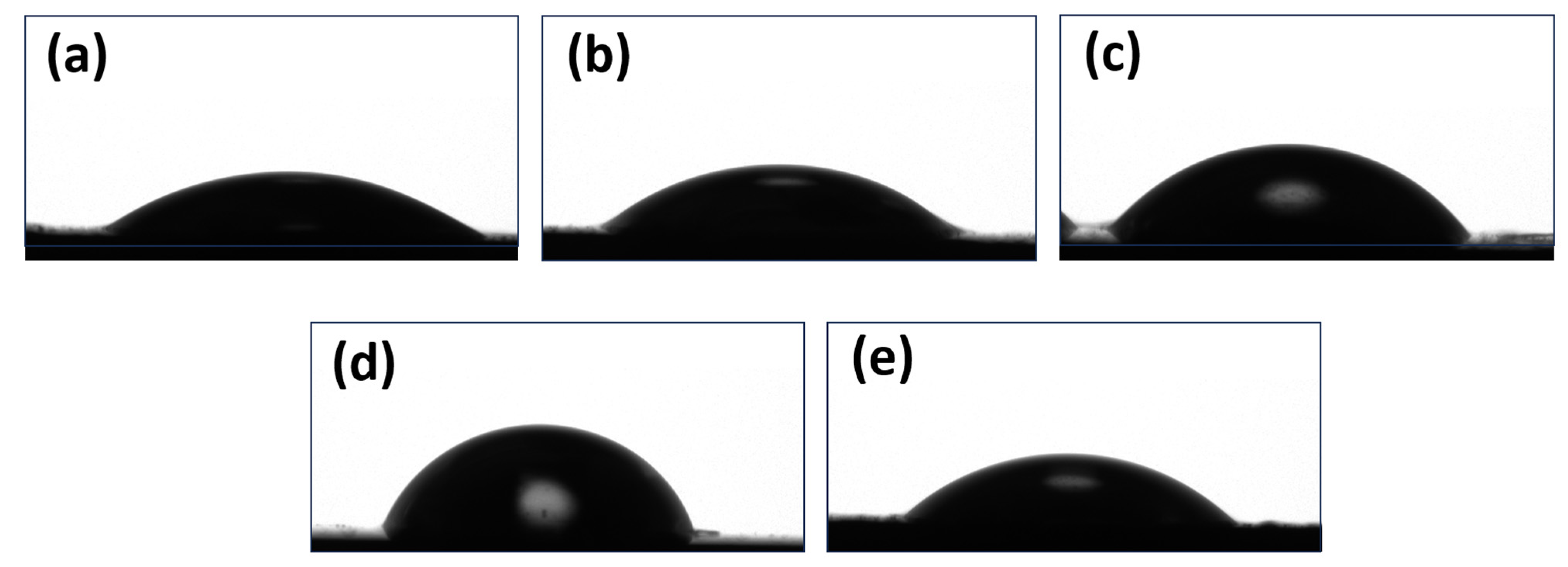
3.5. Microscopic and Contact Angle Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Manias, E. The full potential of nanoparticles in imparting new functionalities in polymer nanocomposites remains largely untapped. A widely applicable, two-solvent processing approach provides a hierarchical structure, affording unparalleled composite performance enhancement. Nanocomposites: Stiffer by design. Nat. Mater. 2007, 6, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.M.; Liou, S.J.; Lin, C.Y.; Cheng, C.Y.; Chang, Y.W.; Lee, K.R. Anticorrosively Enhanced PMMA−Clay Nanocomposite Materials with Quaternary Alkylphosphonium Salt as an Intercalating Agent. Chem. Mater. 2002, 14, 154–161. [Google Scholar] [CrossRef]
- Mathai, S.; Shaji, P.S. Polymer-Based Nanocomposite Coating Methods: A Review. J. Sci. Res. 2022, 14, 973–1002. [Google Scholar] [CrossRef]
- Bhat, A.; Budholiya, S.; Raj, S.A.; Sultan, M.; Hui, D.; Shah, A.M.; Safri, S. Review on nanocomposites based on aerospace applications. Nanotechnol. Rev. 2021, 10, 237–253. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, K.H.; Parvez, M.M.H.; Irizarry, N.; Uddin, M.N. Polymer Nanocomposites with Optimized Nanoparticle Dispersion and Enhanced Functionalities for Industrial Applications. Processes 2025, 13, 994. [Google Scholar] [CrossRef]
- Sazali, N.B.; Chan, L.W.; Wong, T.W. Nano-enabled agglomerates and compact: Design aspects of challenges. Asian J. Pharm. Sci. 2023, 18, 100794. [Google Scholar] [CrossRef]
- Silva, M.R.F.; Alves, M.F.R.P.; Cunha, J.P.G.Q.; Costa, J.L.; Silva, C.A.; Fernandes, M.H.V.; Vilarinho, P.M.; Ferreira, P. Nanostructured transparent solutions for UV-shielding: Recent developments and future challenges. Mat. Today Phys. 2023, 35, 101131. [Google Scholar] [CrossRef]
- Li, S.; Lin, M.M.; Toprak, M.S.; Kim, D.K.; Muhammed, M. Nanocomposites of polymer and inorganic nanoparticles for optical and magnetic applications. Nano Rev. 2010, 1, 5214. [Google Scholar] [CrossRef]
- Blanchard, V.; Blanchet, P. Color stability for wood products during use: Effects of inorganic nanoparticles. BioResources 2011, 6, 1219–1229. [Google Scholar] [CrossRef]
- Oladele, I.O.; Victor, O.O.; Omotosho, T.F.; Adebanjo, M.B.; Ayanleye, O.T.; Adekola, S.A. Sustainable polymer and polymer-based composite materials for extreme conditions and demanding applications—A review on pushing boundaries in materials science. Next Mater. 2025, 8, 100775. [Google Scholar] [CrossRef]
- Gore, A.H.; Prajapat, A.L. Biopolymer Nanocomposites for Sustainable UV Protective Packaging. Front. Mater. 2022, 9, 855727. [Google Scholar] [CrossRef]
- Khairy, Y.; Mohammed, M.I.; Elsaeedy, H.I.; Yahia, I.S. Optical and electrical properties of SnBr2-doped polyvinyl alcohol (PVA) polymeric solid electrolyte for electronic and optoelectronic applications. Optik 2021, 228, 166129. [Google Scholar] [CrossRef]
- Ali, H.E.; Abdel-Aziz, M.; Ibrahiem, A.M.; Sayed, M.A.; Abd-Rabboh, H.S.M.; Awwad, N.S.; Algarni, H.; Shkir, M.; Khairy, M.Y. Microstructure Study and Linear/Nonlinear Optical Performance of Bi-Embedded PVP/PVA Films for Optoelectronic and Optical Cut-Off Applications. Polymers 2022, 14, 1741. [Google Scholar] [CrossRef]
- Badawi, A. Engineering the optical properties of PVA/PVP polymeric blend in situ using tin sulfide for optoelectronics. Appl. Phys. A Mater. Sci. Process. 2020, 126, 335. [Google Scholar] [CrossRef]
- Louie, S.M.; Gorham, J.M.; Tana, J.; Hackley, V.A. Ultraviolet photo-oxidation of polyvinylpyrrolidone (PVP) coatings on gold nanoparticles. Environ. Sci. Nano 2017, 4, 1866–1875. [Google Scholar] [CrossRef]
- Hwang, J.M.; Kim, N.Y.; Shin, S.; Lee, J.H.; Ryu, J.Y.; Eom, T.; Park, B.K.; Kim, C.G.; Chung, T.-M. Synthesis of novel volatile niobium precursors containing carboxamide for Nb2O5 thin films. Polyhedron 2021, 200, 115134. [Google Scholar] [CrossRef]
- Basuvalingam, S.B.; Macco, B.; Knoops, H.C.M.; Melskens, J.; Kessels, W.M.M.; Bol, A.A. Comparison of thermal and plasma-enhanced atomic layer deposition of niobium oxide thin films. J. Vac. Sci. Technol. A 2018, 36, 041503. [Google Scholar] [CrossRef]
- Sousa Pereira, M.; Lima, A.; Almeida, R.; Martins, J.; Bagnis, D.; Barros, E.; Sombra, A.; Vasconcelos, I. Flexible, large-area organic solar cells with improved performance through incorporation of CoFe2O4 nanoparticles in the active layer. Mater. Res. 2019, 22, e20180640. [Google Scholar] [CrossRef]
- Wang, T.-C.; Su, Y.-H.; Hung, Y.-K.; Yeh, C.-S.; Huang, L.-W.; Gomulya, W.; Lai, L.-H.; Loi, M.A.; Yang, J.-S.; Wu, J.-J. Charge collection enhancement by incorporation of gold–silica core–shell nanoparticles into P3HT:PCBM/ZnO nanorod array hybrid solar cells. Phys. Chem. Chem. Phys. 2015, 17, 19854–19861. [Google Scholar] [CrossRef]
- Lu, Y.-M.; Chiang, C.-H.; Hsu, S.L.-C. The performance of polymer solar cells based on P3HT: PCBM after post-annealing and adding titanium dioxide nanoparticles. Mater. Res. Innov. 2014, 18, 102–105. [Google Scholar] [CrossRef]
- Aleshin, A.N. Organic optoelectronics based on polymer—Inorganic nanoparticle composite materials. Phys. Usp. 2013, 56, 667–674. [Google Scholar] [CrossRef]
- Asih, G.I.N.; Rafryanto, A.F.; Hartati, S.; Jiang, X.; Anggraini, A.; Yudhowijoyo, A.; Jiang, J.; Arramel. Recent advances of polymer nanocomposites in emerging applications. Compos. Funct. Mater. 2025, 1, 20250105–20250131. [Google Scholar] [CrossRef]
- Singh, N.B.; Agarwal, S. Nanocomposites: An overview. Emerg. Mater. Res. 2016, 5, 5–43. [Google Scholar] [CrossRef]
- Song, J.E.; Phenrat, T.; Marinakos, S.; Xiao, Y.; Liu, J.; Wiesner, M.R.; Tilton, R.D.; Lowry, G.V. Hydrophobic interactions increase attachment of gum arabic- and PVP-coated Ag nanoparticles to hydrophobic surfaces. Environ. Sci. Technol. 2011, 45, 5988–5995. [Google Scholar] [CrossRef]
- Jebali, S.; Vayer, M.; Belal, K.; Sinturel, C. Engineered nanocomposite coatings: From water-soluble polymer to advanced hydrophobic performances. Materials 2024, 17, 574. [Google Scholar] [CrossRef]
- Yousef, E.; Ali, M.K.M.; Allam, N.K. Tuning the optical properties and hydrophobicity of BiVO4/PVC/PVP composites as potential candidates for optoelectronics applications. Opt. Mater. 2024, 150, 115193. [Google Scholar] [CrossRef]
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-vinylpyrrolidone: Synthesis, characterization and uses. Polym. J. 1985, 17, 143–152. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Khalid, M.U.; Rudokaite, A.; da Silva, A.M.H.; Kirsnyte-Snioke, M.; Stirke, A.; Melo, W.C.M.A. A comprehensive review of niobium nanoparticles: Synthesis, characterization, applications in health sciences, and future challenges. Nanomaterials 2025, 15, 106. [Google Scholar] [CrossRef]
- Lopes, O.F.; Paris, E.C.; Ribeiro, C. Synthesis of Nb2O5 nanoparticles through the oxidant peroxide method applied to organic pollutant photodegradation: A mechanistic study. Appl. Catal. B Environ. 2014, 144, 800–808. [Google Scholar] [CrossRef]
- Hajduk, B.; Bednarski, H.; Jarząbek, B.; Janeczek, H.; Nitschke, P. P3HT:PCBM blend films phase diagram on the base of variable-temperature spectroscopic ellipsometry. Beilstein J. Nanotechnol. 2018, 9, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Hajduk, B.; Bednarski, H.; Jarząbek, B.; Nitschke, P.; Janeczek, H. Phase diagram of P3HT:PC70BM thin films based on variable-temperature spectroscopic ellipsometry. Polym. Test. 2020, 84, 106383. [Google Scholar] [CrossRef]
- Hajduk, B.; Bednarski, H.; Jarka, P.; Janeczek, H.; Godzierz, M.; Tański, T. Thermal and optical properties of PMMA films reinforced with Nb2O5 nanoparticles. Sci. Rep. 2021, 11, 22531. [Google Scholar] [CrossRef]
- Hajduk, B.; Jarka, P.; Tański, T.; Bednarski, H.; Janeczek, H.; Gnida, P.; Fijalkowski, M. An investigation of the thermal transitions and physical properties of semiconducting PDPP4T:PDBPyBT blend films. Materials 2022, 15, 8392. [Google Scholar] [CrossRef]
- Hajduk, B.; Bednarski, H.; Domański, M.; Jarząbek, B.; Trzebicka, B. Thermal transitions in P3HT: PC60BM films based on electrical resistance measurements. Polymers 2020, 12, 1458. [Google Scholar] [CrossRef]
- Richter, U. SpectraRay/3 Software Manual; Sentech Instruments GmbH: Berlin, Germany, 2011. [Google Scholar]
- Stoumbou, E.; Stavrakas, I.; Hloupis, G.; Alexandridis, A.; Triantis, D.; Moutzouris, K. A comparative study on the use of the extended-Cauchy dispersion equation for fitting refractive index data in crystals. Opt. Quantum Electron. 2013, 45, 837–859. [Google Scholar] [CrossRef]
- Johnson, D.I.; Gadd, G.E.; Town, G.E. Total differential optical properties of polymer nanocomposite materials. In Proceedings of the 2006 International Conference on Nanoscience and Nanotechnology, ICONN 2006, Brisbane, Australia, 3–7 July 2006; IEEE: Piscataway, NJ, USA, 2006; pp. 423–426. [Google Scholar] [CrossRef]
- Buera, M.D.P.; Levi, G.; Karel, M. Glass transition in poly(vinylpyrrolidone): Effect of molecular weight and diluents. Biotechnol. Prog. 1992, 8, 144–148. [Google Scholar] [CrossRef]
- Maidannyk, V.A.; Mishra, V.S.N.; Miao, S.; Djali, M.; McCarthy, N.; Nurhadi, B. The effect of polyvinylpyrrolidone addition on microstructure, surface aspects, the glass transition temperature and structural strength of honey and coconut sugar powders. J. Future Foods 2022, 2, 338–345. [Google Scholar] [CrossRef]
- Gatica, N.; Soto, L.; Moraga, C.; Vergara, L. Blends of poly(N-vinyl-2-pyrrolidone) and dihydric phenols: Thermal and infrared spectroscopic studies. Part IV. J. Chil. Chem. Soc. 2013, 58, 1978–1983. [Google Scholar] [CrossRef]
- Restrepo, I.; Velásquez, E.; Galotto, M.; Guarda, A. Influence of the molar mass and concentration of polyvinylpyrrolidone on the physical–mechanical properties of polylactic acid for food packaging. Polymers 2025, 17, 2218. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dranca, I. Probing beta relaxation in pharmaceutically relevant glasses by using DSC. Pharm. Res. 2006, 23, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.G.; Flory, P.J. The glass temperature and related properties of polystyrene. Influence of molecular weight. J. Polym. Sci. 1954, 14, 315–319. [Google Scholar] [CrossRef]
- Jarka, P.; Hajduk, B.; Kumari, P.; Janeczek, H.; Godzierz, M.; Tsekpo, Y.M.; Tański, T. Investigations on thermal transitions in PDPP4T/PCPDTBT/AuNPs composite films using variable temperature ellipsometry. Polymers 2025, 17, 704. [Google Scholar] [CrossRef] [PubMed]
- Hajduk, B.; Jarka, P.; Bednarski, H.; Godzierz, M.; Tański, T.; Staszuk, M.; Nitschke, P.; Jarząbek, B.; Fijalkowski, M.; Mazik, K. Thermal and optical properties of P3HT:PC70BM: ZnO nanoparticles composite films. Sci. Rep. 2024, 14, 66. [Google Scholar] [CrossRef]
- White, R.P.; Lipson, J.E.G.; Keddie, J.L. Spectroscopic ellipsometry as a route to thermodynamic characterization. Soft Matter 2022, 18, 6660–6673. [Google Scholar] [CrossRef]
- Wang, T.; Hu, S.; Zhang, S.; Peera, A.; Reffner, J.; Torkelson, J.M. Eliminating the Tg-Confinement Effect in Polystyrene Films: Extraordinary Impact of a 2 mol % 2-Ethylhexyl Acrylate Comonomer. Macromolecules 2022, 55, 9601–9611. [Google Scholar] [CrossRef]
- Serna, S.; Wang, T.; Torkelson, J.M. Eliminating the Tg-confinement and fragility-confinement effects in poly(4-methylstyrene) films by incorporation of 3 mol % 2-ethylhexyl acrylate comonomer. J. Chem. Phys. 2024, 160, 034903. [Google Scholar] [CrossRef]
- Ali, H.E.; Sayed, M.A.; Algarni, H.; Khairy, Y.; Abdel-Aziz, M.M. Use of niobium oxide nanoparticles as nanofillers in PVP/PVA blends to enhance UV–visible absorption, opto-linear, and nonlinear optical properties. J. Vinyl Addit. Technol. 2022, 28, 444–452. [Google Scholar] [CrossRef]




| Polymer content (%) | 100 | 90 | 85 | 75 | 65 |
| NPs content (%) | 0 | 10 | 15 | 25 | 35 |
| PVP weight (mg) | 20 | 18 | 17 | 15 | 13 |
| Nb2O5 Weight (mg) | 0 | 2 | 3 | 5 | 7 |
| Thin Film | PVP | PVP: Nb2O5 (5%) | PVP: Nb2O5 (15%) | PVP: Nb2O5 (25%) | PVP: Nb2O5 (35%) |
|---|---|---|---|---|---|
| Refractive index n [a.u] (for λ = 2500 nm) | 1.521 | 1.522 | 1.525 | 1.528 | 1.531 |
| Fraction coefficient f [a.u] | 0 | 0.004 | 0.010 | 0.017 | 0.024 |
| Thickness of films on SiO2 d [nm] | 303 | 371 | 255 | 466 | 208 |
| Roughness of films on SiO2 r [nm] | 146 | 134 | 128 | 162 | 160 |
| Thickness of films on micr. cover glass d [nm] | 257 | 302 | 270 | 285 | 340 |
| Roughness of films on micr. cover glass r [nm] | 101 | 120 | 97 | 115 | 134 |
| DSC (Powder) | Temperature Ellipsometry (Films) | ||||
|---|---|---|---|---|---|
| Sample | Tg1 (°C) | Tg2 (°C) | TgV1 (°C) | TgV2 (°C) | TgV3 (°C) |
| PVP | 88 | 188 | 92 | - | 197 |
| PVP: Nb2O5 (5%) | 88 | 181 | 104 | 142 | 204 |
| PVP: Nb2O5 (15%) | 88 | 180 | 105 | 168 | 198 |
| PVP: Nb2O5 (25%) | 89 | 183 | 95 | 151 | 207 |
| PVP: Nb2O5 (35%) | 88 | 204 | 91 | 135 | 202 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarka, P.; Kumari, P.; Łazarska, M.; Godzierz, M.; Kotowicz, S.; Marcisz, M.; Bochenek, M.; Hajduk, Ł.; Szindler, M.M.; Hajduk, B. Research on the Physical Properties and Internal Structure of PVP/Nb2O5 Nanocomposite Coatings. Polymers 2025, 17, 2939. https://doi.org/10.3390/polym17212939
Jarka P, Kumari P, Łazarska M, Godzierz M, Kotowicz S, Marcisz M, Bochenek M, Hajduk Ł, Szindler MM, Hajduk B. Research on the Physical Properties and Internal Structure of PVP/Nb2O5 Nanocomposite Coatings. Polymers. 2025; 17(21):2939. https://doi.org/10.3390/polym17212939
Chicago/Turabian StyleJarka, Paweł, Pallavi Kumari, Małgorzata Łazarska, Marcin Godzierz, Sonia Kotowicz, Marek Marcisz, Marcelina Bochenek, Łucja Hajduk, Magdalena M. Szindler, and Barbara Hajduk. 2025. "Research on the Physical Properties and Internal Structure of PVP/Nb2O5 Nanocomposite Coatings" Polymers 17, no. 21: 2939. https://doi.org/10.3390/polym17212939
APA StyleJarka, P., Kumari, P., Łazarska, M., Godzierz, M., Kotowicz, S., Marcisz, M., Bochenek, M., Hajduk, Ł., Szindler, M. M., & Hajduk, B. (2025). Research on the Physical Properties and Internal Structure of PVP/Nb2O5 Nanocomposite Coatings. Polymers, 17(21), 2939. https://doi.org/10.3390/polym17212939











