Flame-Retardant Properties of a Styrene-Vinyl Tetrazole Copolymer Additive in an LDPE/EVA Blend
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis
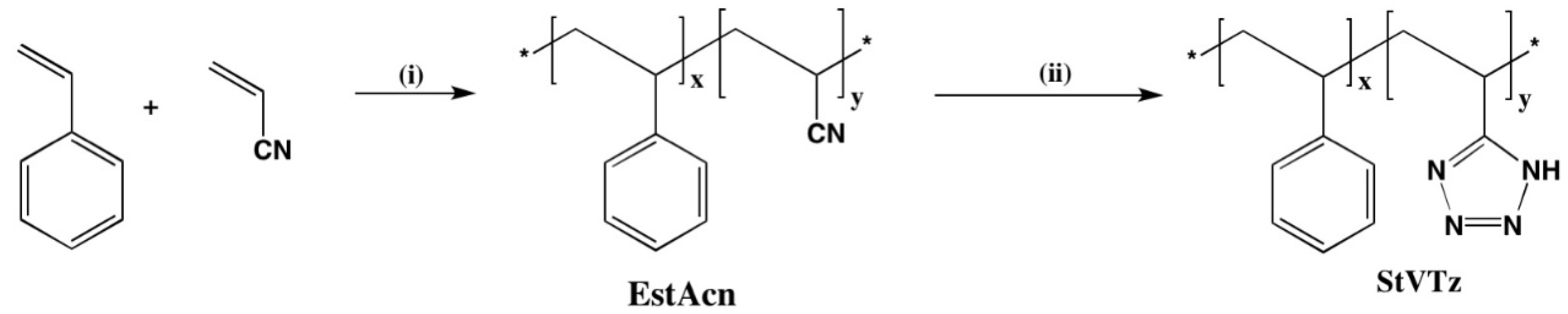
2.2.1. Synthesis of Poly(styrene-co-acrylonitrile) (EstAcn)
2.2.2. Synthesis of Copolymer Styrene-Vinyl Tetrazole (StVTz)
2.3. Preparation of LDPE/EVA Composites
2.4. Characterization
3. Results and Discussion
3.1. Chemical Structure of Copolymers
3.2. Thermal Characterization of Copolymers
3.3. LDPE/EVA Composites
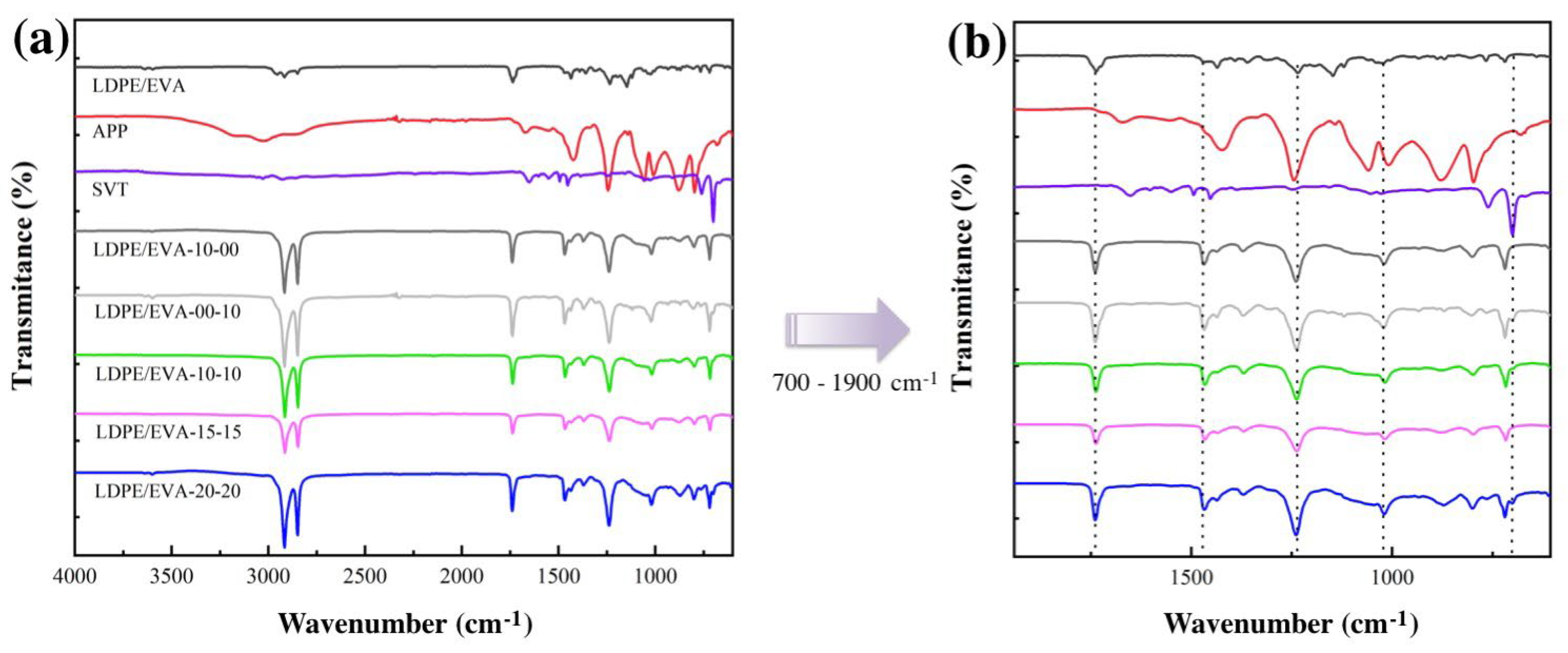
3.3.1. Chemical Characterization
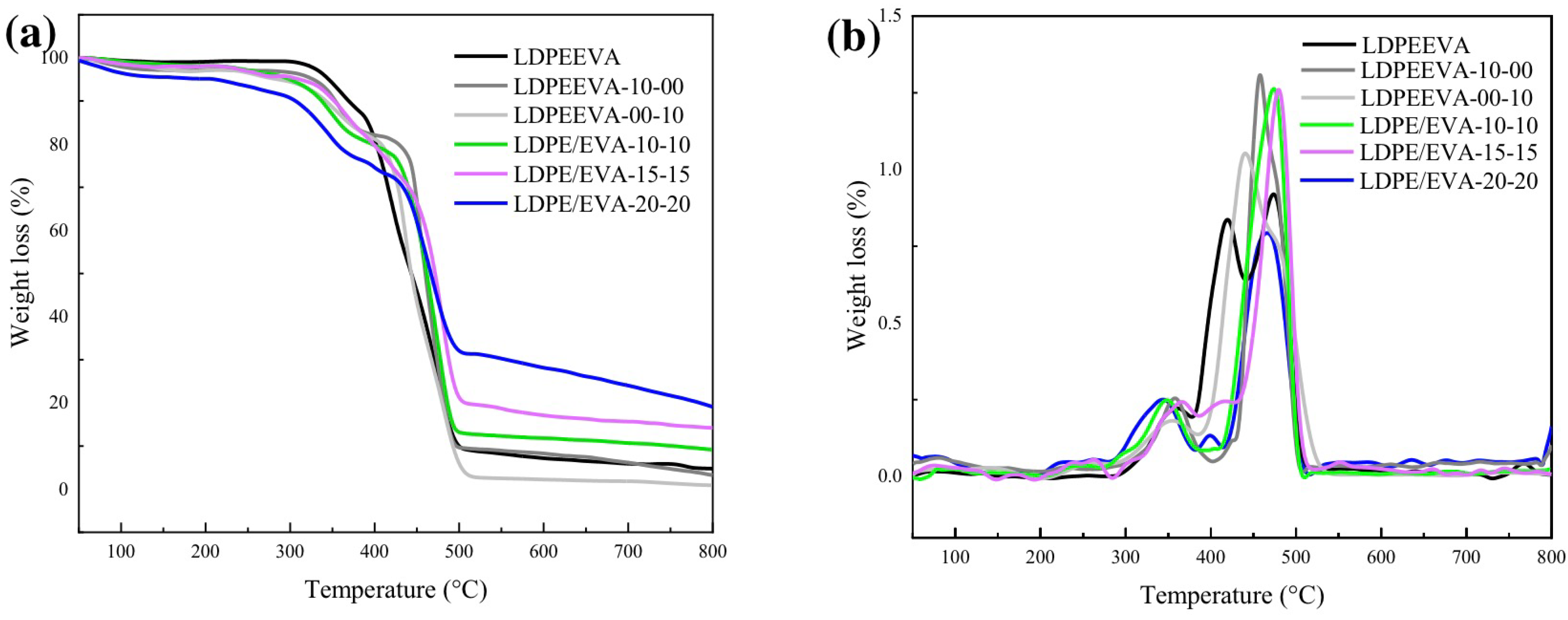
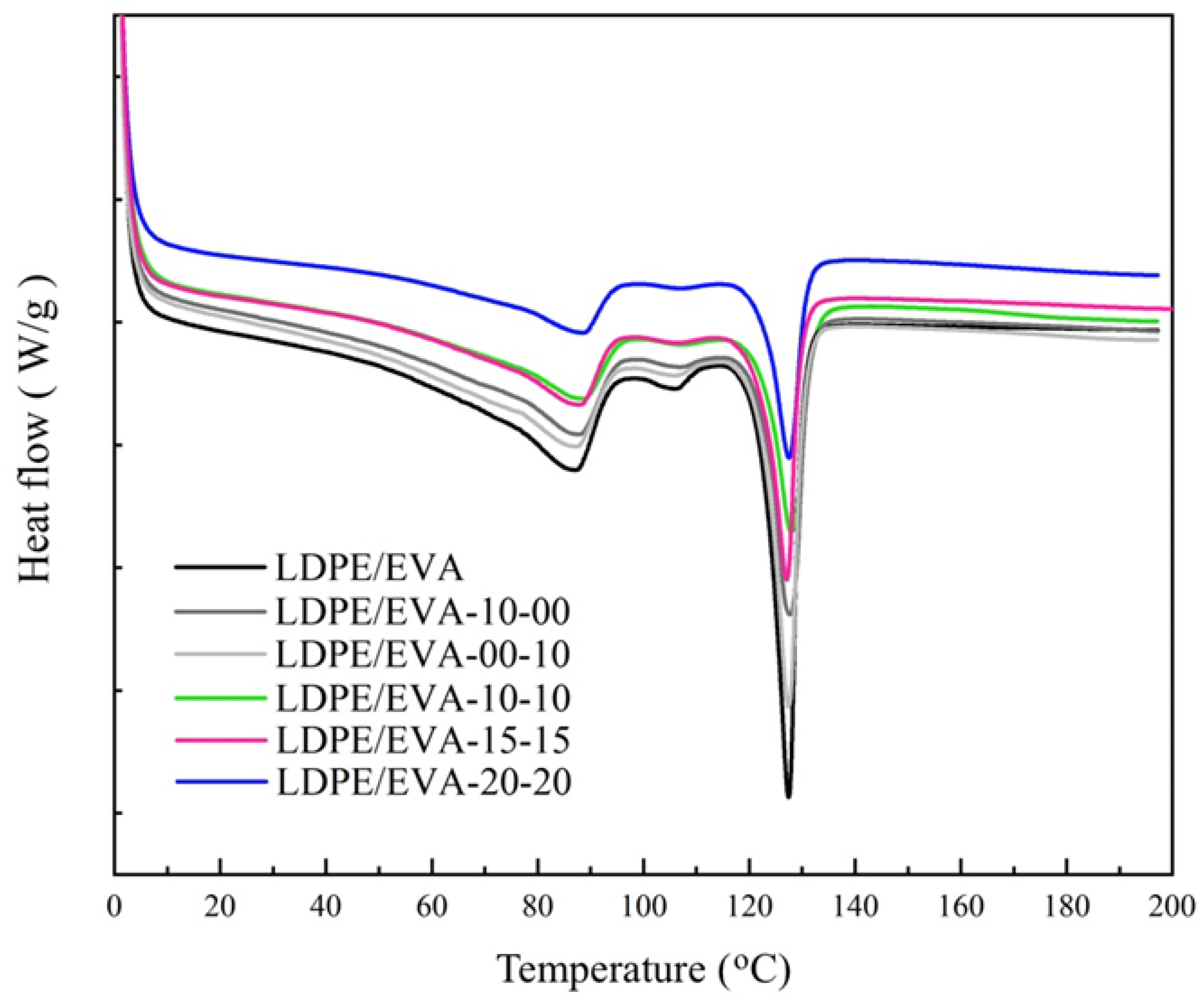
3.3.2. Thermal Characterization
3.3.3. Mechanical Properties
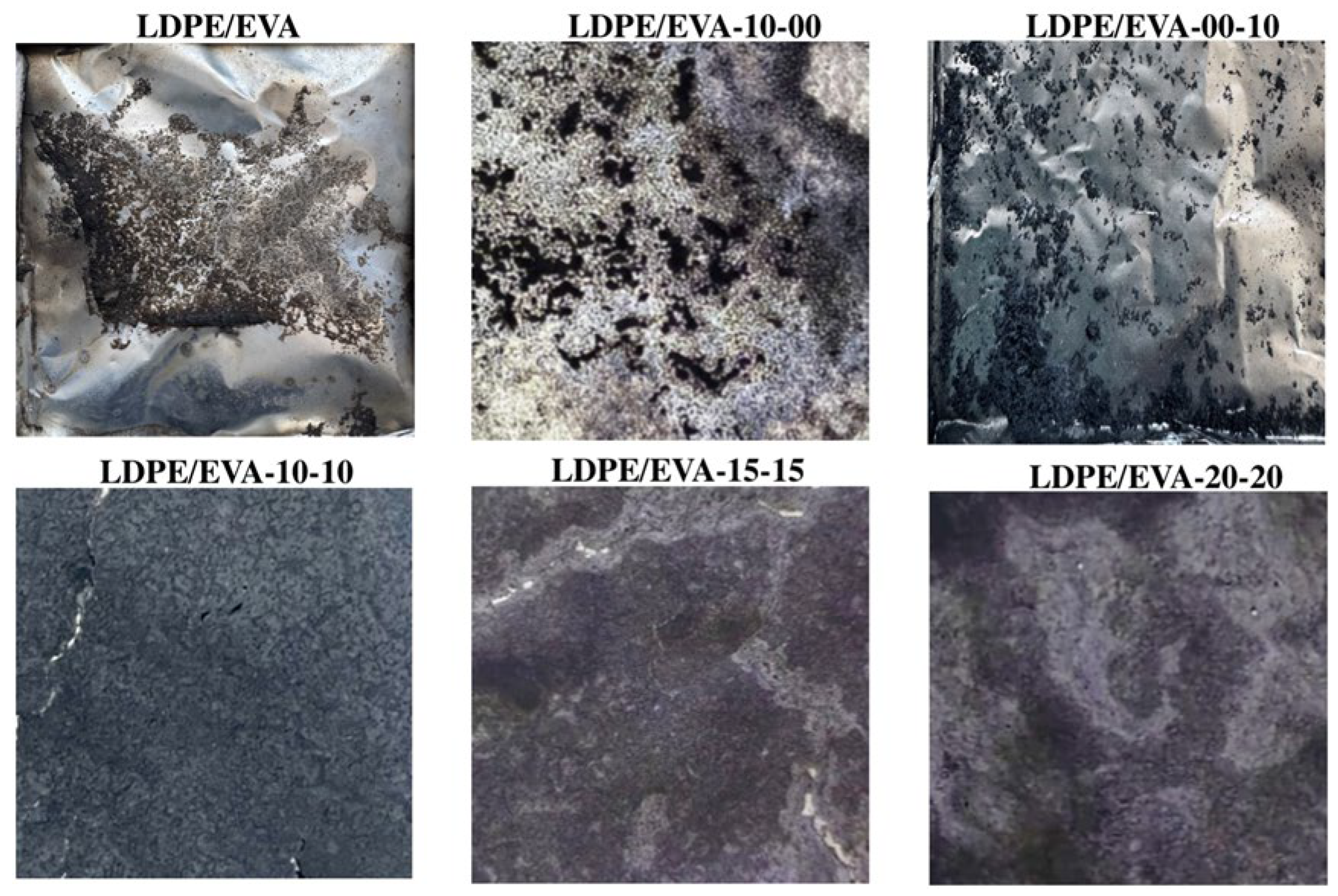
3.3.4. Flame Retardant Performance
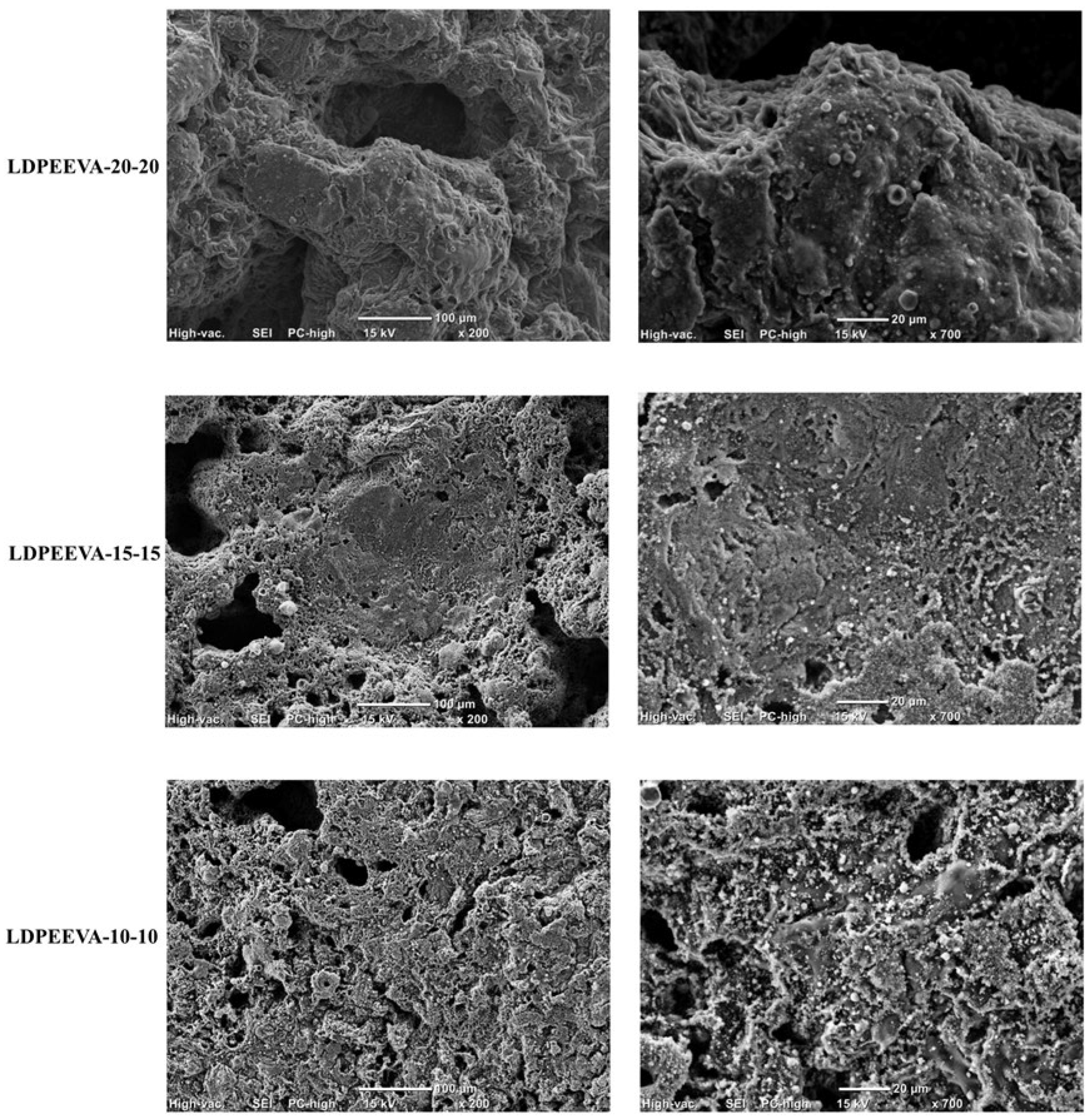
3.3.5. Flame Retardant Mechanism of LDPE/EVA Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakashima, E.; Ueno, T.; Yukumoto, M.; Takeda, K. Effect of molecular weight of polyethylene on its flammability. J. Appl. Polym. Sci. 2011, 122, 436–443. [Google Scholar] [CrossRef]
- Mensah, R.A.; Shanmugam, V.; Narayanan, S.; Renner, J.S.; Babu, K.; Neisiany, R.E.; Försth, M.; Sas, G.; Das, O. A review of sustainable and environment-friendly flame retardants used in plastics. Polym. Test. 2022, 108, 107511. [Google Scholar] [CrossRef]
- Chen, L.; Lin, Z. Polyethylene: Properties, Production and Applications. In Proceedings of the 2021 3rd International Academic Exchange Conference on Science and Technology Innovation (IAECST), Guangzhou, China, 10–12 December 2021; pp. 1191–1196. [Google Scholar] [CrossRef]
- Beach, M.W.; Rondan, N.G.; Froese, R.D.; Gerhart, B.B.; Green, J.G.; Stobby, B.G.; Shmakov, A.G.; Shvartsberg, V.M.; Korobeinichev, O.P. Studies of degradation enhancement of polystyrene by flame retardant additives. Polym. Degrad. Stab. 2008, 93, 1664–1673. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gooneie, A.; Simonetti, P.; Nazir, R.; Kaiser, J.-P.; Rippl, A.; Hirsch, C.; Lehner, S.; Rupper, P.; Hufenus, R.; et al. Comprehensive study on flame retardant polyesters from phosphorus additives. Polym. Degrad. Stab. 2018, 155, 22–34. [Google Scholar] [CrossRef]
- Morgan, A.B. Non-Halogenated Flame Retardant Handbook, 2nd ed.; Wiley-Scrivener: Austin, TX, USA, 2022. [Google Scholar]
- Ghomi, R.E.; Khosravi, F.; Mossayebi, Z.; Ardahaei, A.S.; Dehaghi, F.M.; Khorasani, M.; Neisiany, R.E.; Das, O.; Marani, A.; Mensah, R.A.; et al. The Flame Retardancy of Polyethylene Composites: From Fundamental Concepts to Nanocomposites. Molecules 2020, 25, 5157. [Google Scholar] [CrossRef]
- United Nations. UN Food Standards Body Sets New Limits for Melamine in Food. Available online: https://news.un.org/en/story/2010/07/344052 (accessed on 18 August 2024).
- European Chemicals Agency. ECHA Substance Information. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.003.288 (accessed on 4 August 2024).
- Lütjens, L.H.; Pawlowski, S.; Silvani, M.; Blumenstein, U.; Richter, I. Melamine in the environment: A critical review of available information. Environ. Sci. Eur. 2023, 35, 2. [Google Scholar] [CrossRef]
- Schartel, B.; Perret, B.; Dittrich, B.; Ciesielski, M.; Kramer, J.; Muller, P.; Altstadt, V.; Zang, L.; Doring, M. Flame Retardancy of Polymers: The Role of Specific Reactions in the Condensed Phase. Macromol. Mater. Eng. 2016, 30, 9–35. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Li, Q.; Luo, Z. A novel grafted polyethylene with diphenyl phosphoryl group: Improved flame retardancy and favorable compatibility. J. Appl. Polym. Sci. 2021, 138, e51242. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Y.Z.; Yang, B.; Liu, Y. A novel intumescent flame-retardant polyethylene system. Macromol. Mater. Eng. 2006, 291, 247–253. [Google Scholar] [CrossRef]
- Liao, S.-F.; Deng, C.; Huang, S.-C.; Cao, J.Y.; Wang, Y.-Z. An efficient halogen-free flame retardant for polyethylene: Piperazinemodified ammonium polyphosphates with different structures. Chin. J. Polym. Sci. 2016, 34, 1339–1353. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Xiao, H. Flame retardant effect and mechanism of a novel DOPO based tetrazole derivative on epoxy resin. J. Anal. Appl. Pyrolysis 2019, 139, 104–113. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.; Sanchéz, M.; Freeman, H.S. New tetrazole based dyes as efficient co-sensitizers for dsscs: Structure-properties relationship. Org. Electron. 2020, 87, 05964. [Google Scholar] [CrossRef]
- Da Silva, L.; Sánchez, M.; Ibarra-Rodriguez, M.; Freeman, H.S. Isomeric tetrazole-based organic dyes for dye-sensitized solar cells: Structure-property relationships. J. Mol. Struct. 2022, 1250, 131749. [Google Scholar] [CrossRef]
- Da Silva, L.; Freeman, H. Variation in hydrophobic chain length of co-adsorbents to improve dye-sensitized solar cell performance. Phys. Chem. Chem. Phys. 2019, 21, 16771–16778. [Google Scholar] [CrossRef]
- Wei, C.-X.; Bian, M.; Gong, G.-H. Tetrazolium Compounds: Synthesis and Applications in Medicine. Molecules 2015, 20, 5528–5553. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Ghafuri, H.; Bidgoli, N.S.S.; Pombeiro, A.J.L.; Hazra, S. Platinum and palladium complexes with tetrazole ligands: Synthesis, structure and applications. Coord. Chem. Rev. 2021, 446, 214132. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Techniques and Instrumentation in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2005; Volume 25. [Google Scholar]
- ASTM D638-Standard Test Method for Tensile Properties of Plastics. Available online: https://store.astm.org/d0638-22.html?_gl=1*1vqsd99*_gcl_au*Nzk2NDA2MjM4LjE3NjEyMDQ0NDM (accessed on 20 August 2025).
- ASTM D2863-Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index). Available online: https://store.astm.org/d2863-23e01.html?_gl=1*ei7cm0*_gcl_au*Nzk2NDA2MjM4LjE3NjEyMDQ0NDM (accessed on 20 August 2025).
- Da-Silva, L.; Salas, M.M.; Santillán, A.L.; Siller-Ceniceros, A.A.; Acosta, D.M.; Magnago, R.F.; Benavides, R. Synthesis and characterization of styrene-vinyl tetrazole copolymers for their application as a solid electrolyte. React. Func. Pol. 2021, 167, 105007. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Brebu, M.; Vasile, C. Thermal behaviour of binary and ternary copolymers containing acrylonitrile. Polym. Degrad. Stab. 2013, 98, 1889–1897. [Google Scholar] [CrossRef]
- Velázquez, D.G.; Benavides, R.; Morales-Acosta, D.; Francisco-Vieira, L.; Martínez, J.G.; Santillán, A.L.; Da-Silva, A.; Magnago, R.F.; Paula, M.M.S.; Da-Silva, L. Synthesis and characterization of tetrazole-derived solid polymeric electrolytes. J. Polym. Res. 2024, 31, 245. [Google Scholar] [CrossRef]
- Zarandi, M.B.; Bioki, H.A. Thermal and mechanical properties of blends of LDPE and EVA crosslinked by electron beam radiation. EPJ Appl. Phys. 2013, 63, 21101. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Ye, Q.; Shen, L.; Lin, H. Flame-retardant ethylene vinyl acetate composite materials by combining additions of aluminum hydroxide and melamine cyanurate: Preparation and characteristic evaluations. J. Colloid Interface Sci. 2021, 589, 525–531. [Google Scholar] [CrossRef]
- Jiang, D.; Pan, M.; Cai, X.; Zhao, Y. Flame retardancy of rice straw-polyethylene composites affected by in situ polymerization of ammonium polyphosphate/silica. Compos. Part A Appl. Sci. Manuf. 2018, 109, 1–9. [Google Scholar] [CrossRef]
- Landete-Ruiz, M.D.; Martínez-Díez, J.A.; Rodríguez-Pérez, M.Á.; De Saja, J.A.; Martín-Martínez, J.M. Improved adhesion of low-density polyethylene/EVA foams using different surface treatments. J. Adhes. Sci. Technol. 2002, 16, 1073–1101. [Google Scholar] [CrossRef]
- Berenguer, J.P.; Berman, A.; Quill, T.; Zhou, T.; Kalaitzidou, K.; Cola, B.; Bougher, T.; Smith, M. Incorporation of polyethylene fillers in all-polymer high-thermal-conductivity composites. Polym. Bull. 2021, 78, 3835–3850. [Google Scholar] [CrossRef]
- Luyt, A.S.; Malik, S.S.; Gasmi, S.A.; Porfyris, A.; Andronopoulou, A.; Korres, D.; Vouyiouka, S.; Grosshauser, M.; Pfaendner, R.; Brüll, R.; et al. Halogen-Free Flame-Retardant Compounds. Thermal Decomposition and Flammability Behavior for Alternative Polyethylene Grades. Polymers 2019, 11, 1479. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Fage, J.; Liang, S.; Gaan, S. An overview of mode of action and analytical methods for evaluation of gas phase activities of flame retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef]
- Prabhakar, M.N.; Raghavendra, G.M.; Vijaykumar, B.V.D.; Patil, K.; Seo, J.; Jung-Il, S. Synthesis of a novel compound based on chitosan and ammonium polyphosphate for flame retardancy applications. Cellulose 2019, 26, 8801–8812. [Google Scholar] [CrossRef]
- Sun, L.; Qu, Y.; Li, S. Co-microencapsulate of ammonium polyphosphate and pentaerythritol in intumescent flame-retardant coatings. J. Therm. Anal. Calorim. 2013, 111, 1099–1106. [Google Scholar] [CrossRef]
- Gupta, R.R.; Kumar, M.; Gupta, V. Heterocyclic Chemistry II; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Iji, M.; Abba, H.; Okpanachi, C. Effect of filler type on crystallinity of low-density polyethylene composites. J. Chem. Lett. 2020, 1, 155–159. [Google Scholar] [CrossRef]
- Wadkin-Snaith, D.; Mulheran, P.; Johnston, K. Filler-induced heterogeneous nucleation of polymer crystals investigated by molecular dynamics simulations. Polymer 2023, 281, 126113. [Google Scholar] [CrossRef]
- Scurti, S.; Ortolani, J.; Ghirri, A.; Maccaferri, E.; Caretti, D.; Mazzocchetti, L. Phosphorylated poly(vinyl alcohol) surface coatings as intumescent flame inhibitor for polymer matrix composites. Prog. Org. Coat. 2023, 177, 107457. [Google Scholar] [CrossRef]
- Hurley, M.J.; Gottuk, D.; Hall, J.R.; Harada, K.; Kuligowski, E.; Puchovsky, M.; Torero, J.; Watts, J.M.; Wieczorek, C. SFPE Handbook of Fire Protection Engineering, 5th ed.; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Huang, Y.W.; Song, M.L.; Ma, J.J.; Lu, Z.Y.; Yang, J.X.; Cao, K. Synthesis of a phosphorus/silicon hybrid and its synergistic effect with melamine polyphosphates on flame retardant polypropylene system. J. Appl. Polym. Sci. 2013, 129, 316–323. [Google Scholar] [CrossRef]
- Huo, S.Q.; Song, P.A.; Yu, B.; Ran, S.Y.; Chevali, V.S.; Liu, L.; Fang, Z.P.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent Advances and Future Perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Mosnácek, J.; Basfar, A.A.; Shukri, T.M.; Bahattab, M.A. Poly(Ethylene Vinyl Acetate) (EVA)/Low Density Polyethylene (LDPE)/Ammonium Polyphosphate (APP) Composites Cross-linked by Dicumyl Peroxide for Wire and Cable Applications. Polym. J. 2008, 40, 460–464. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, D.; Zhao, W.; Xie, D.; Mei, Y. A green, effective, and synergistic flame retardant for poly(ethylene-co-vinyl acetate) resin. Polym. Degrad. Stab. 2022, 206, 110201. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R-Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Wang, N.; Li, L.T.; Xu, Y.; Zhang, K.; Chen, X.L.; Wu, H. Synergistic effects of red phosphorus masterbatch with expandable graphite on the flammability and thermal stability of polypropylene/thermoplastic polyurethane blends. Polym. Polym. Compos. 2019, 28, 209–219. [Google Scholar] [CrossRef]
- Zuluaga-Parra, J.D.; Valle, L.F.R.-D.; Sánchez-Valdéz, S.; Faverzani-Magnago, R.; da Silva, A.; da Silva, L. Synergistic effect between ammonium polyphosphate-functionalized poly(lactic acid) and phosphated avocado seed on the flame-retardant properties of poly(lactic acid)/ethylene–vinyl acetate copolymer composites. Iran. Polym. J. 2025, 34, 1–15. [Google Scholar] [CrossRef]
- Albite-Ortega, J.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Medellín-Rodríguez, F.J.; Devalle, L.F.R.; Rodriguez-Fernandez, O.S.; Rivera-Salinas, J.E.; Martínez-Colunga, J.G.; Da-Silva, L.; Morales-Cepeda, A.B.; et al. Influence of ammonium polyphosphate-modified polypropylene on flammability characteristics of polypropylene keratin and chitosan sustainable composites. Fire Mater. 2023, 47, 170–181. [Google Scholar] [CrossRef]



| Sample | LDPE/EVA | APP | StVTz |
|---|---|---|---|
| LDPE/EVA | 100 | 0 | 0 |
| LDPE/EVA-10-00 | 90 | 10 | 0 |
| LDPE/EVA-00-10 | 90 | 0 | 10 |
| LDPE/EVA-10-10 | 80 | 10 | 10 |
| LDPE/EVA-15-15 | 70 | 15 | 15 |
| LDPE/EVA-20-20 | 60 | 20 | 20 |
| Sample | Td5 (°C) | Tmax (°C) | Yc (%) |
|---|---|---|---|
| LDPE/EVA | 345 | 474 | 6 |
| LDPE/EVA-10-00 | 327 | 458 | 7 |
| LDPE/EVA-00-10 | 286 | 440 | 2 |
| LDPE/EVA-10-10 | 299 | 473 | 11 |
| LDPE/EVA-15-15 | 297 | 479 | 16 |
| LDPE/EVA-20-20 | 211 | 465 | 26 |
| Sample | Cooling | 2nd Heating | ||||
|---|---|---|---|---|---|---|
| Tc (°C) | ΔHc (J·g−1) | Xc (%) | Tm (°C) | ΔHm (J g−1) | Xc (%) | |
| LDPE/EVA | 112.7 | 22.8 | 7.9 | 127.5 | 22.9 | 7.9 |
| LDPE/EVA-10-00 | 113.7 | 17.4 | 6 | 127.9 | 17.1 | 5.9 |
| LDPE/EVA-00-10 | 112.2 | 19.6 | 6.8 | 127.5 | 19.5 | 6.8 |
| LDPE/EVA-10-10 | 113.1 | 13.3 | 4.6 | 127.9 | 13.2 | 4.6 |
| LDPE/EVA-15-15 | 113.7 | 14 | 4.9 | 127.1 | 13.8 | 4.8 |
| LDPE/EVA-20-20 | 113.4 | 10.6 | 3.7 | 127.6 | 10.6 | 3.7 |
| Sample | Young’s Modulus (MPa) | Tensile Strength (Mpa) | Elongation at Break (%) |
|---|---|---|---|
| LDPE/EVA | 79.01 | 14.7 | 607 |
| LDPE/EVA-10-00 | 88.37 | 11.84 | 561 |
| LDPE/EVA-00-10 | 99.46 | 6.62 | 93 |
| LDPE/EVA-10-10 | 118.60 | 7.2 | 99 |
| LDPE/EVA-15-15 | 150.35 | 7.46 | 65 |
| LDPE/EVA-20-20 | 213.71 | 7.7 | 30 |
| Sample | CC Data | UL-94 | LOI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TTI a (s) | pHRR b (kW/m2) | TpHRR c (s) | THR d (MJ/m2) | HRR e (kW/m2) | TOF f (s) | R.M g (%) | |||
| LDPE/EVA | 53 | 2237 | 62 | 68 | 804 | 84 | 0 | HB | 17 |
| LDPE/EVA-10-00 | 37 | 1091 | 73 | 65 | 588 | 108 | 8 | HB | 19 |
| LDPE/EVA-00-10 | 34 | 1317 | 86 | 75 | 748 | 100 | 0.5 | HB | 20 |
| LDPE/EVA-10-10 | 31 | 853 | 49 | 63 | 601 | 107 | 10 | HB | 20 |
| LDPE/EVA-15-15 | 32 | 716 | 38 | 68 | 457 | 148 | 16 | HB | 23 |
| LDPE/EVA-20-20 | 29 | 664 | 61 | 68 | 459 | 150 | 22 | HB | 24 |
| Wt. (%) | |||
|---|---|---|---|
| Element | LDPE/EVA-10-10 | LDPE/EVA-15-15 | LDPE/EVA-20-20 |
| C | 10.26 | 20.71 | 7.35 |
| N | 1.17 | 1.83 | 1.38 |
| O | 21.89 | 19.25 | 33.45 |
| P | 66.69 | 58.21 | 57.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, K.F.R.; Sánchez, J.F.L.; Reyna, O.C.; Martínez, P.E.; Muñoz, J.A.E.; Parra, J.D.Z.; Magnago, R.F.; Valdés, S.S.; da Silva, L. Flame-Retardant Properties of a Styrene-Vinyl Tetrazole Copolymer Additive in an LDPE/EVA Blend. Polymers 2025, 17, 2933. https://doi.org/10.3390/polym17212933
Ramírez KFR, Sánchez JFL, Reyna OC, Martínez PE, Muñoz JAE, Parra JDZ, Magnago RF, Valdés SS, da Silva L. Flame-Retardant Properties of a Styrene-Vinyl Tetrazole Copolymer Additive in an LDPE/EVA Blend. Polymers. 2025; 17(21):2933. https://doi.org/10.3390/polym17212933
Chicago/Turabian StyleRamírez, Karla Fabiola Rodríguez, Jesús Francisco Lara Sánchez, Orlando Castro Reyna, Pedro Espinoza Martínez, Jesús Alejandro Espinosa Muñoz, José David Zuluaga Parra, Rachel Faverzani Magnago, Saul Sanchez Valdés, and Luciano da Silva. 2025. "Flame-Retardant Properties of a Styrene-Vinyl Tetrazole Copolymer Additive in an LDPE/EVA Blend" Polymers 17, no. 21: 2933. https://doi.org/10.3390/polym17212933
APA StyleRamírez, K. F. R., Sánchez, J. F. L., Reyna, O. C., Martínez, P. E., Muñoz, J. A. E., Parra, J. D. Z., Magnago, R. F., Valdés, S. S., & da Silva, L. (2025). Flame-Retardant Properties of a Styrene-Vinyl Tetrazole Copolymer Additive in an LDPE/EVA Blend. Polymers, 17(21), 2933. https://doi.org/10.3390/polym17212933





