Impact of Different Parameters to Enhanced Corrosion Resistance of Zinc-PVDF-G Coating for the Protection of Mild Steel Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation of Zn-PVDF-G Coating and Methods
3. Results and Discussion
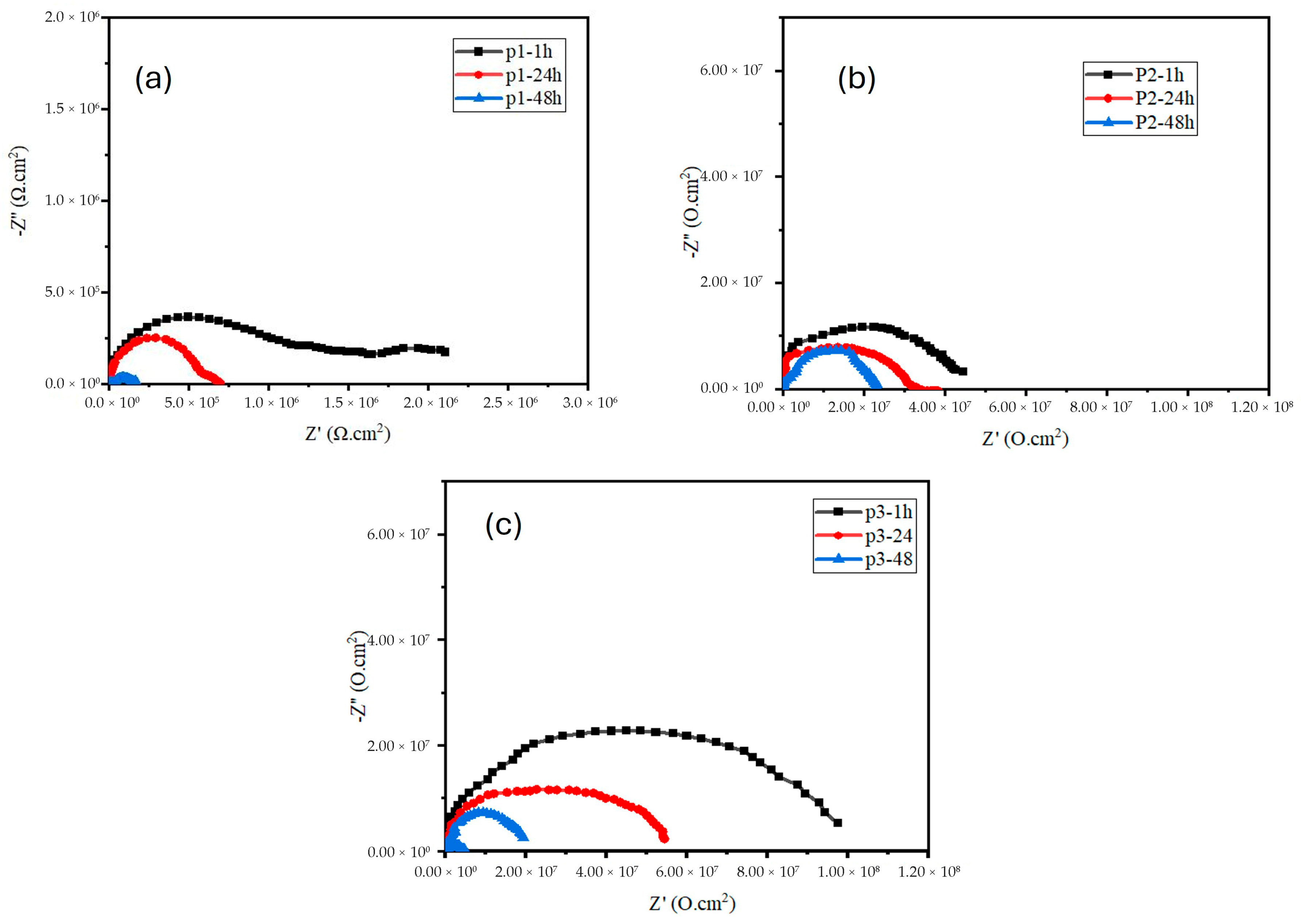
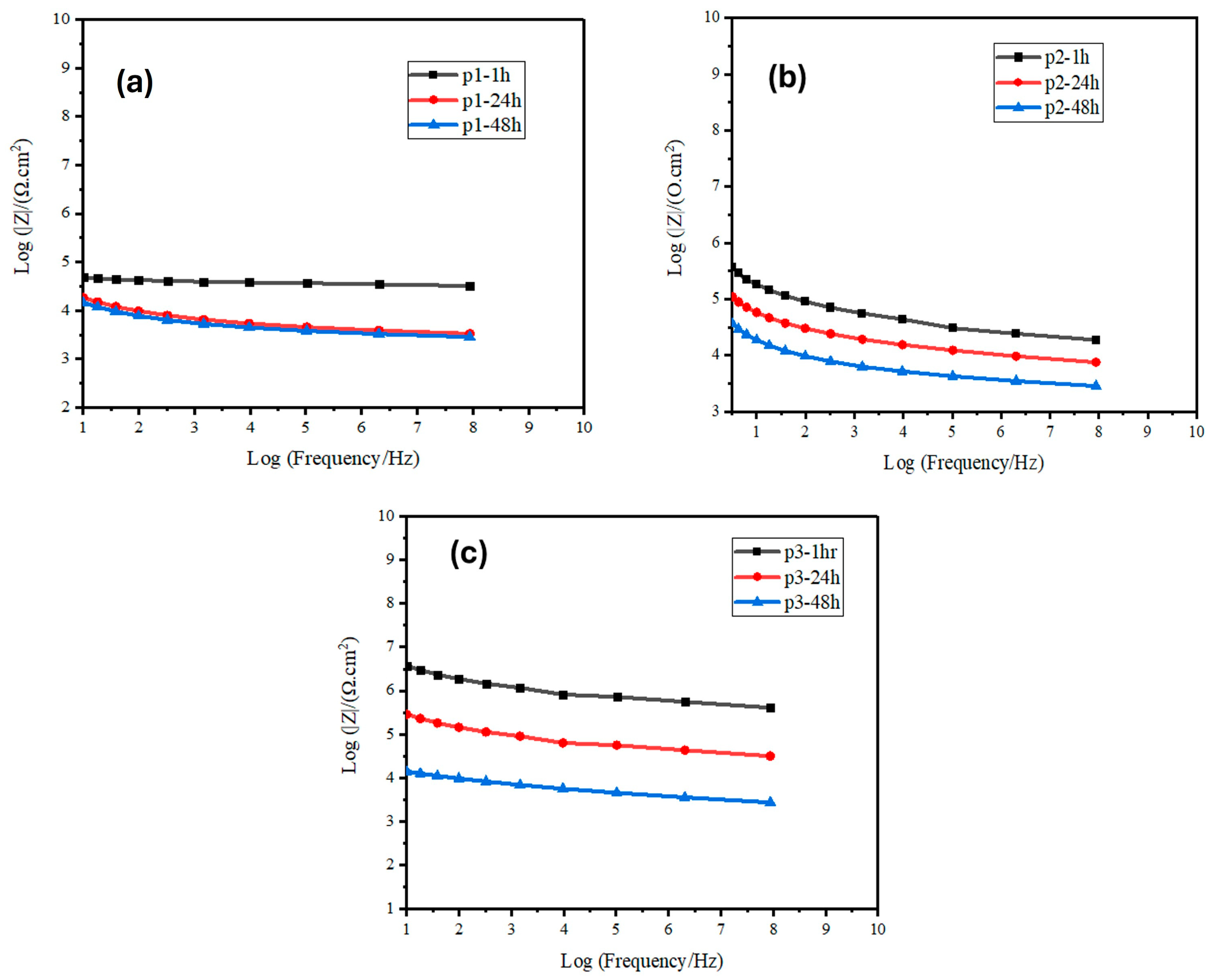
3.1. EIS Measurement of the Zinc-PVDF-G Coated Samples
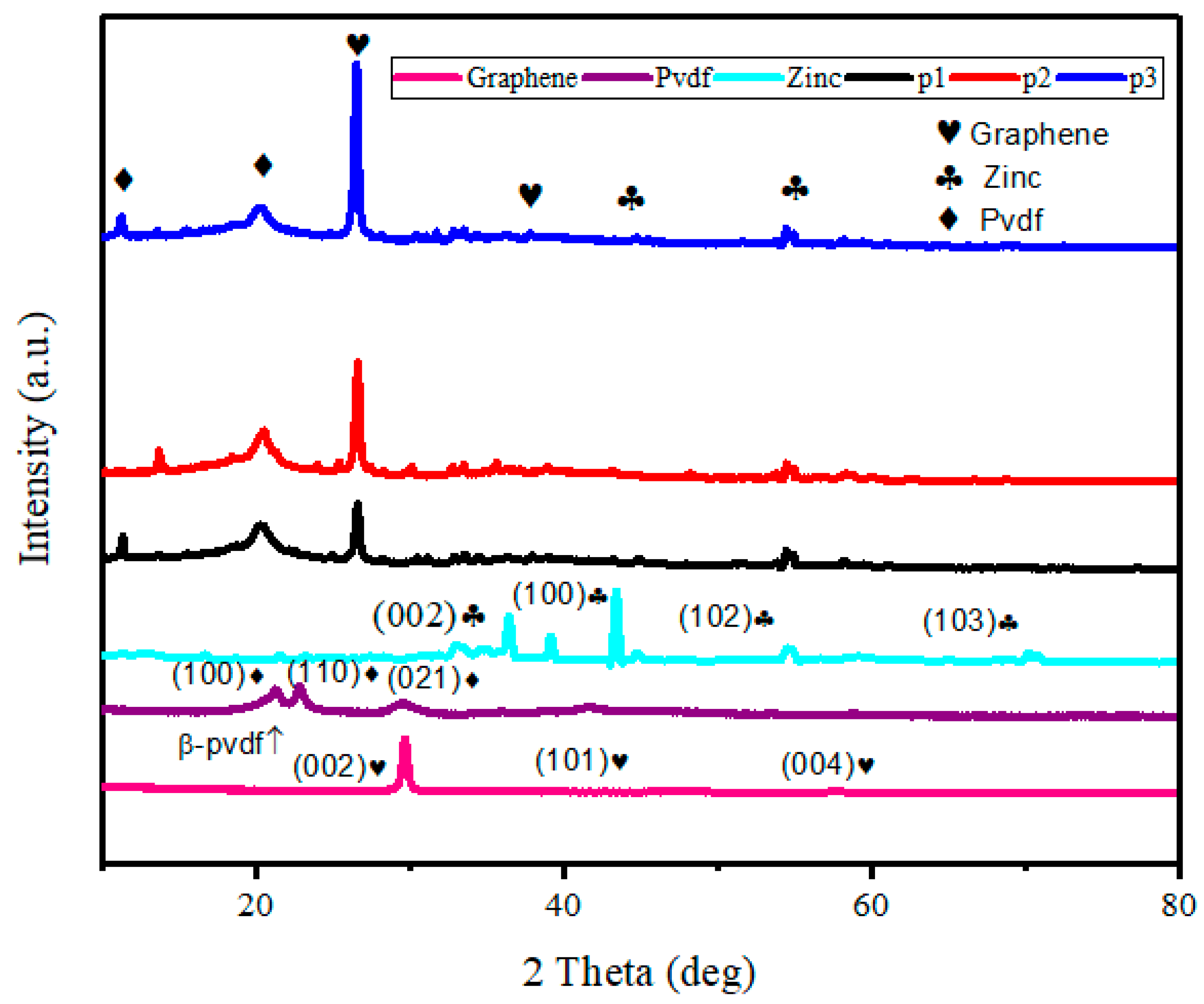
3.2. XRD Analysis
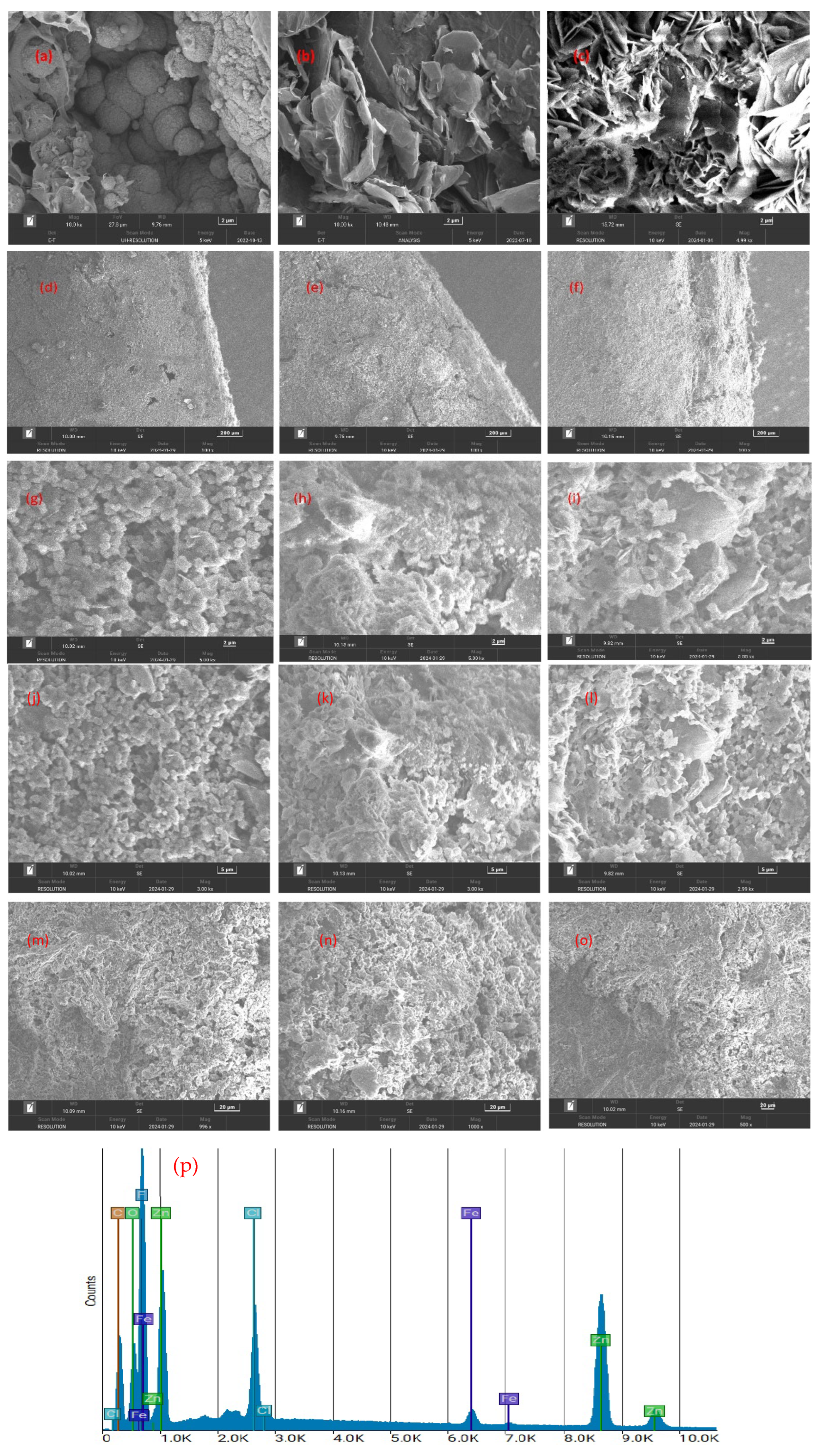
3.3. SEM Analysis
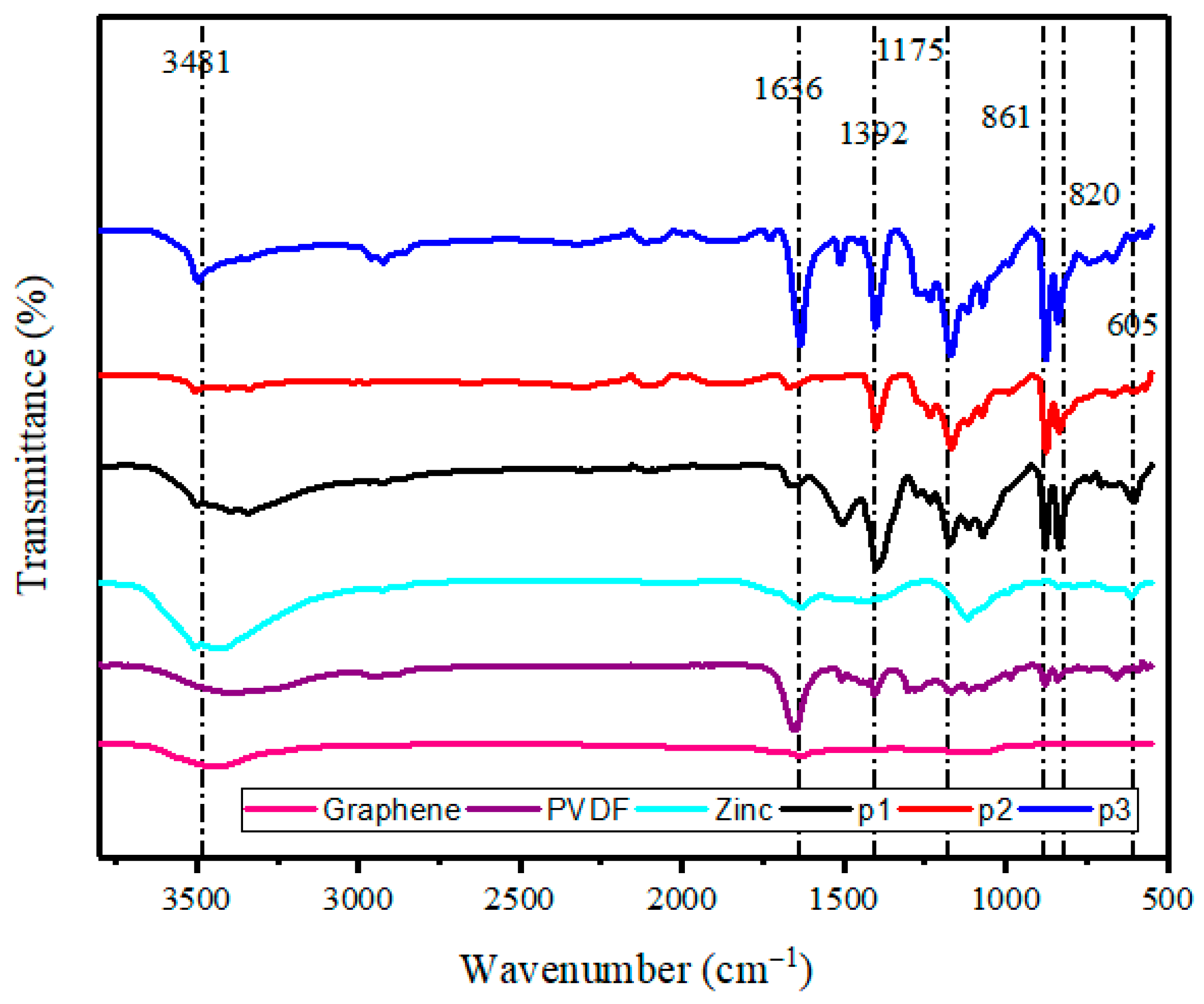
3.4. FTIR Analysis
3.5. Response Surface Methodology Modelling and Optimisation
3.5.1. Analysis of Variance (ANOVA)
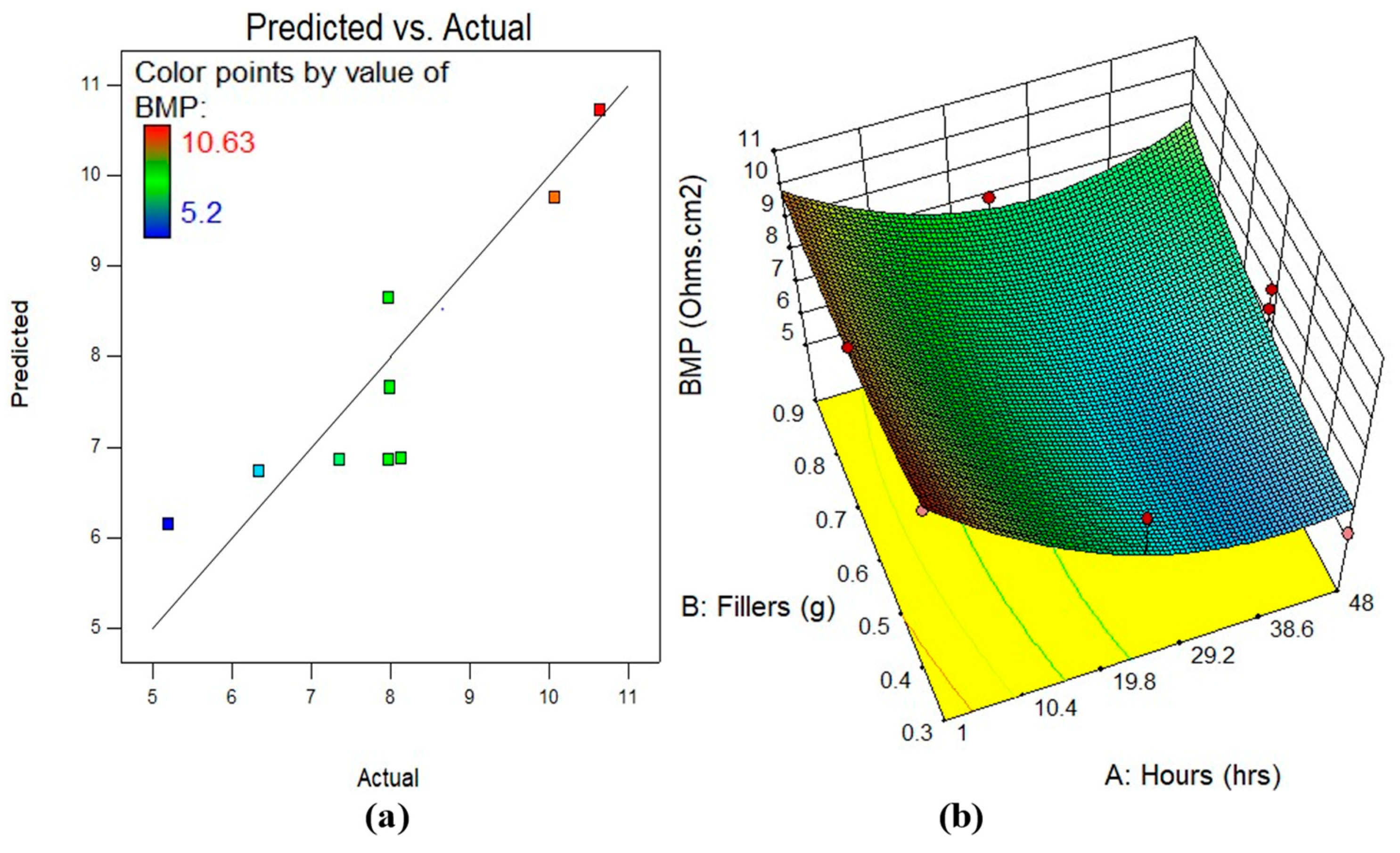
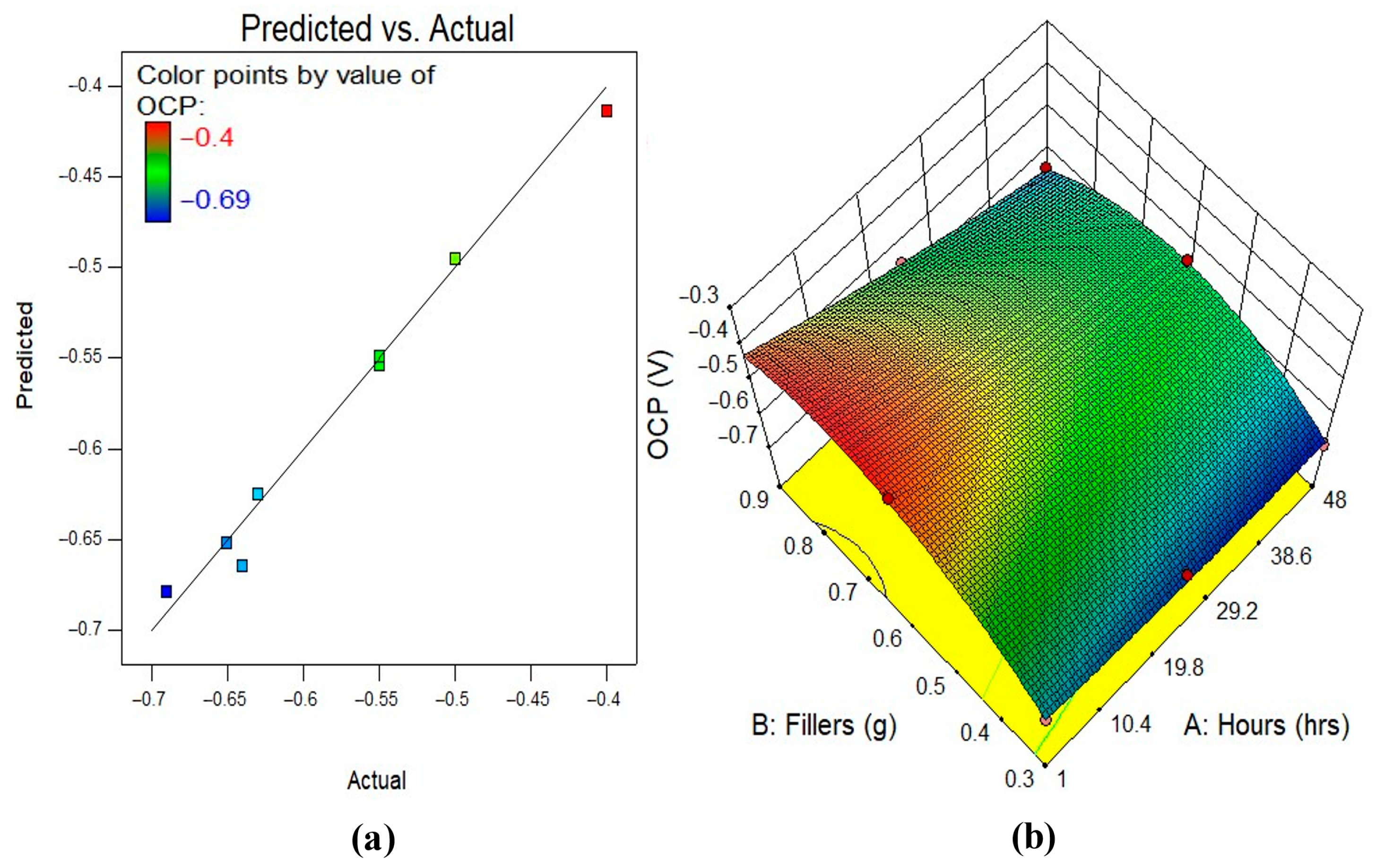
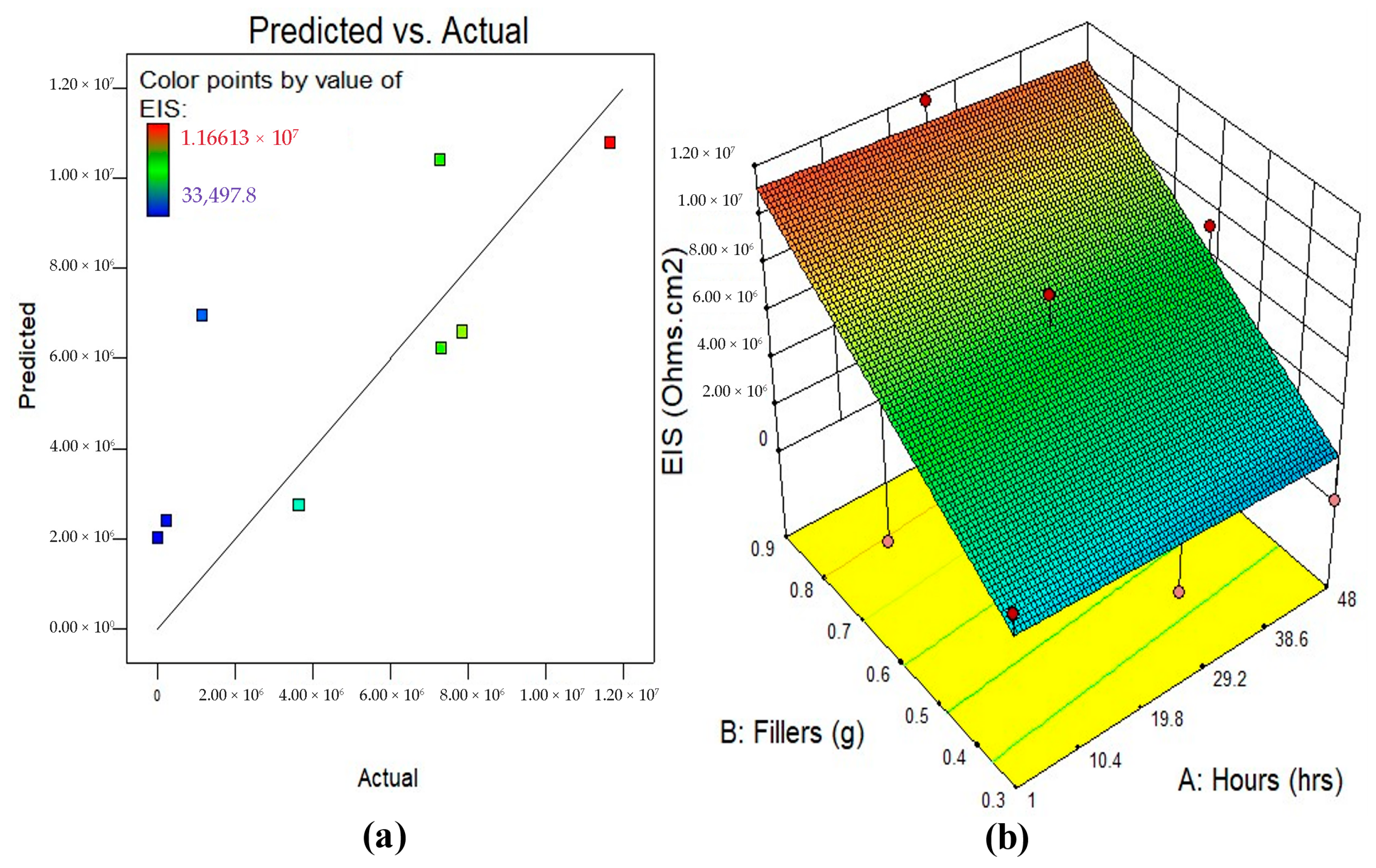
3.5.2. Model Diagnostics and Response Surface Plots
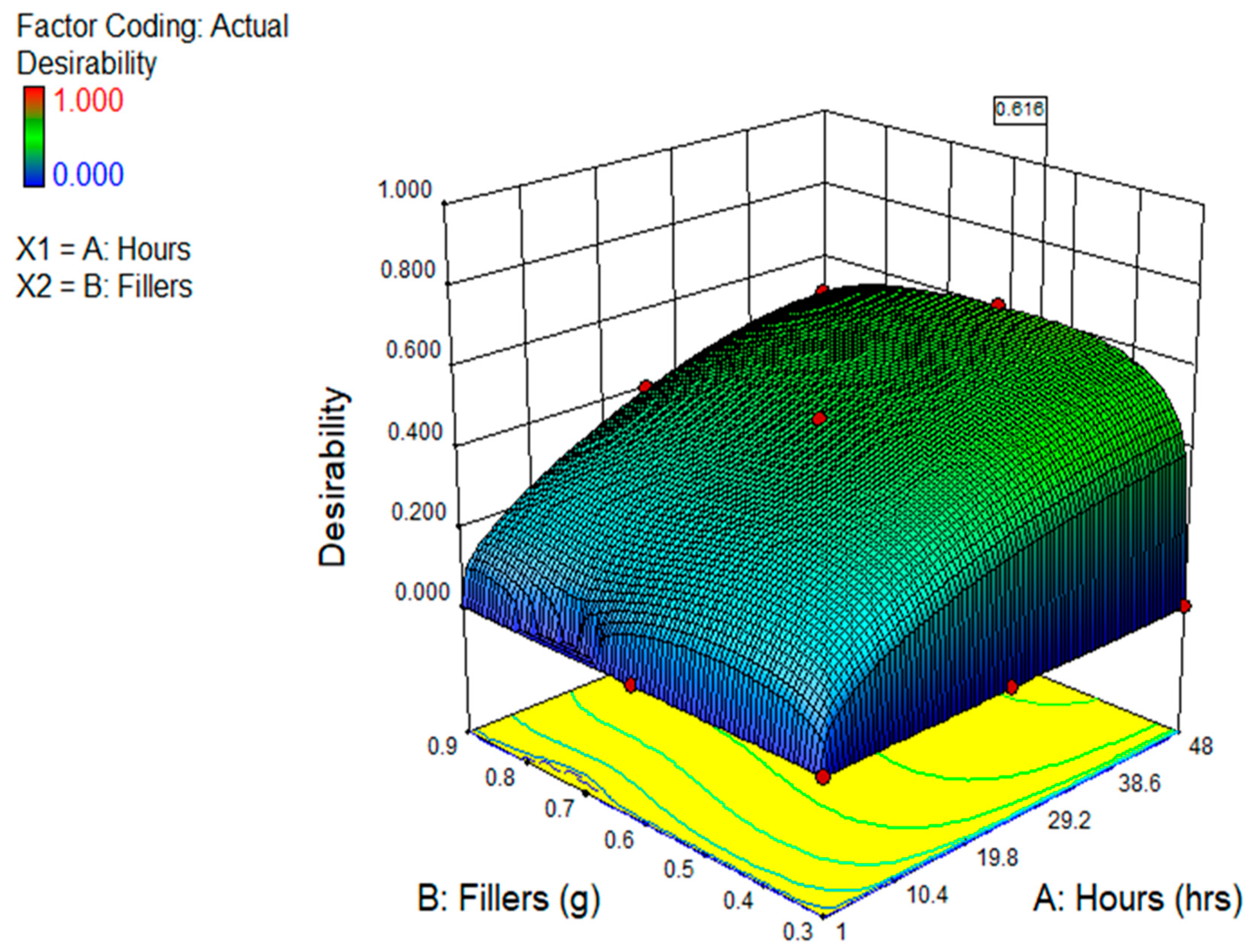
3.5.3. Multi-Objective Optimisation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fayomi, O.; Popoola, A. An investigation of the properties of Zn coated mild steel. Int. J. Electrochem. Sci. 2012, 7, 6555–6570. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.; Hussain, C.M. Recent developments in sustainable corrosion inhibitors: Design, performance and industrial scale applications. Mater. Adv. 2021, 2, 3806–3850. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef]
- Jena, G.; Philip, J. A review on recent advances in graphene oxide-based composite coatings for anticorrosion applications. Prog. Org. Coat. 2022, 173, 107208. [Google Scholar] [CrossRef]
- Choudhary, S.; Garg, A.; Mondal, K. Relation between open circuit potential and polarization resistance with rust and corrosion monitoring of mild steel. J. Mater. Eng. Perform. 2016, 25, 2969–2976. [Google Scholar] [CrossRef]
- Izadi, M.; Shahrabi, T.; Ramezanzadeh, B. Active corrosion protection performance of an epoxy coating applied on the mild steel modified with an eco-friendly sol-gel film impregnated with green corrosion inhibitor loaded nanocontainers. Appl. Surf. Sci. 2018, 440, 491–505. [Google Scholar] [CrossRef]
- Stern, K.H. Metallurgical and Ceramic Protective Coatings; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Handbook, A. Surface Engineering; ASM International, Handbook Committee: Materials Park, OH, USA, 1994; Volume 5. [Google Scholar]
- Popoola, A.; Fayomi, O. Effect of some process variables on zinc coated low carbon steel substrates. Sci. Res. Essays 2011, 6, 4264–4272. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Kappl, H. Corrosion protection with hard coatings on steel: Past approaches and current research efforts. Surf. Coat. Technol. 2014, 257, 182–205. [Google Scholar] [CrossRef]
- Farh, H.M.H.; Seghier, M.E.A.B.; Zayed, T. A comprehensive review of corrosion protection and control techniques for metallic pipelines. Eng. Fail. Anal. 2023, 143, 106885. [Google Scholar] [CrossRef]
- Ayub, S.; Guan, B.H.; Ahmad, F.; Oluwatobi, Y.A.; Nisa, Z.U.; Javed, M.F.; Mosavi, A. Graphene and iron reinforced polymer composite electromagnetic shielding applications: A review. Polymers 2021, 13, 2580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, Y.; Zhu, H.; Li, X.; Zou, Z.; Luo, C.; Wei, J.; Lu, B.; Zhang, D.; Zhou, M. Graphene oxide and its derivatives films for sustained-release trace element zinc based on cation-π interaction. Sci. Rep. 2025, 15, 4255. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-e.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, A.K. Material selection and performance in oil and gas industry. In Applied Metallurgy and Corrosion Control: A Handbook for the Petrochemical Industry; Springer: Berlin/Heidelberg, Germany, 2017; pp. 269–347. [Google Scholar]
- Cheng, Y.; Zhang, Z.; Cao, F.; Li, J.; Zhang, J.; Wang, J.; Cao, C. A study of the corrosion of aluminum alloy 2024-T3 under thin electrolyte layers. Corros. Sci. 2004, 46, 1649–1667. [Google Scholar] [CrossRef]
- Brindha, R.; Selvii, B.K.; Selvam, M. Corrosion Behavior of Zn/Graphene Composite in Aqueous Electrolyte System. J. Nanosci. Nanotechnol. 2018, 2, 303. [Google Scholar]
- Fernández-Solis, C.D.; Vimalanandan, A.; Altin, A.; Mondragón-Ochoa, J.S.; Kreth, K.; Keil, P.; Erbe, A. Fundamentals of electrochemistry, corrosion and corrosion protection. In Soft Matter at Aqueous Interfaces; Springer: Cham, Switzerland, 2016; pp. 29–70. [Google Scholar]
- Zhang, R.; Yu, X.; Yang, Q.; Cui, G.; Li, Z. The role of graphene in anti-corrosion coatings: A review. Constr. Build. Mater. 2021, 294, 123613. [Google Scholar] [CrossRef]
- Tanjil, M.R.-E.; Jeong, Y.; Yin, Z.; Panaccione, W.; Wang, M.C. Ångström-scale, atomically thin 2D materials for corrosion mitigation and passivation. Coatings 2019, 9, 133. [Google Scholar] [CrossRef]
- Liu, T.; Lyu, W.; Li, Z.; Wang, S.; Wang, X.; Jiang, J.; Jiang, X. Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites. Nanotechnol. Rev. 2023, 12, 20220566. [Google Scholar] [CrossRef]
- Bairagi, H.; Vashishth, P.; Sehrawat, R.; Mangla, B. Advanced applications of chitosan and derivative based bioinspired, sustainable corrosion inhibitor for metal surface protection. Can. Metall. Q. 2025, 64, 1999–2038. [Google Scholar] [CrossRef]
- Liang, S.; Kang, Y.; Tiraferri, A.; Giannelis, E.P.; Huang, X.; Elimelech, M. Highly hydrophilic polyvinylidene fluoride (PVDF) ultrafiltration membranes via postfabrication grafting of surface-tailored silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 6694–6703. [Google Scholar] [CrossRef]
- Ayub, S.; Guan, B.H.; Ahmad, F.; Javed, M.F.; Mosavi, A.; Felde, I. Preparation methods for graphene metal and polymer based composites for emi shielding materials: State of the art review of the conventional and machine learning methods. Metals 2021, 11, 1164. [Google Scholar] [CrossRef]
- Das, T.K.; Prusty, S. Graphene-based polymer composites and their applications. Polym. Plast. Technol. Eng. 2013, 52, 319–331. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Madkour, M. Potential use of smart coatings for corrosion protection of metals and alloys: A review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Boruah, P.K.; Borthakur, P.; Das, M.R. Magnetic metal/metal oxide nanoparticles and nanocomposite materials for water purification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 473–503. [Google Scholar]
- Xie, Y.; Chen, M.; Xie, D.; Zhong, L.; Zhang, X. A fast, low temperature zinc phosphate coating on steel accelerated by graphene oxide. Corros. Sci. 2017, 128, 1–8. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Wang, C.; Zhang, W.; Lv, C.; Zhu, Y. A novel antiscaling and anti-corrosive polymer-based functional coating. J. Taiwan Inst. Chem. Eng. 2019, 97, 397–405. [Google Scholar] [CrossRef]
- Kumar, M.P.; Singh, M.P.; Srivastava, C. Electrochemical behavior of Zn–graphene composite coatings. RSC Adv. 2015, 5, 25603–25608. [Google Scholar] [CrossRef]
- Priyadharshini, A.; Xavier, J.R. Recent Innovations in Graphene-Based Nanocomposite Coatings for Enhanced Flame Retardancy in Industrial Applications. Polym. Degrad. Stab. 2025, 240, 111479. [Google Scholar] [CrossRef]
- Ayub, S.; Mohammed, B.S.; Bheel, N. Influence of various factors on the corrosion rate of zinc-coated mild steel. AIP Conf. Proc. 2025, 3317, 060013. [Google Scholar]
- Ali, A.A.; Madkour, M.; Al Sagheer, F.; Nazeer, A.A. Alumina lath-like structure-rGO–PVDF hybrid film formation with high-performance corrosion protection for 316L stainless-steel alloy. J. Mater. Res. Technol. 2021, 15, 3694–3707. [Google Scholar] [CrossRef]
- Geenen, F.M. Characterisation of Organic Coatings with Impedance Measurements: A Study of Coating Structure, Adhesion and Underfilm Corrosion. Ph.D. Thesis, Technische Universiteit Delft, Delft, The Netherlands, 1991. [Google Scholar]
- Kavirajwar, J.S.; Suvitha, A.; Trilaksana, H.; Alzahrani, H.; Alharbi, N.; Siddiq, H.; Florence, S.S. Enhancing the corrosion resistance of mild steel coated zinc studies. Meas. Sens. 2024, 34, 101264. [Google Scholar] [CrossRef]
- Zhan, Y.; Chen, Y.; Dong, H.; Li, Y.; Sun, A.; Chen, X.; Yang, X.; Zhu, F.; Jia, H. In situ growth of flower-like ZnO onto graphene oxide for the synergistically enhanced anti-corrosion ability of epoxy coating. Ceram. Int. 2024, 50, 5914–5926. [Google Scholar] [CrossRef]
- Yen, P.-J.; Ting, C.-C.; Chiu, Y.-C.; Tseng, T.-Y.; Hsu, Y.-J.; Wu, W.-W.; Wei, K.-H. Facile production of graphene nanosheets comprising nitrogen-doping through in situ cathodic plasma formation during electrochemical exfoliation. J. Mater. Chem. C 2017, 5, 2597–2602. [Google Scholar] [CrossRef]
- Ayub, S.; Guan, B.H.; Ahmad, F.; Soleimani, H.; You, K.Y.; Nisa, Z.U.; Yusuf, J.Y.; Hamid, M.A.B. Optimization of magnetite with modified graphene for microwave absorption properties. J. Alloys Compd. 2023, 936, 168182. [Google Scholar] [CrossRef]
- Ban, F.; Majid, S.R.; Huang, N.M.; Lim, H. Graphene oxide and its electrochemical performance. Int. J. Electrochem. Sci 2012, 7, 4345–4351. [Google Scholar] [CrossRef]
- Fu, Y.; Cheng, Y.; Chen, C.; Li, D.; Zhang, W. Study on preparation process and enhanced piezoelectric performance of pine-needle-like ZnO@ PVDF composite nanofibers. Polym. Test. 2022, 108, 107513. [Google Scholar] [CrossRef]
- Jaffari, G.H.; Shawana, H.; Mumtaz, F.; Can, M.M. Electrical response of PVDF/BaTiO3 nanocomposite flexible free-standing films. Bull. Mater. Sci. 2023, 46, 227. [Google Scholar] [CrossRef]
- Azhagan, S.A.C.; Ganesan, S. Effect of zinc acetate addition on crystal growth, structural, optical, thermal properties of glycine single crystals. Arab. J. Chem. 2017, 10, S2615–S2624. [Google Scholar] [CrossRef]
- Wang, C.; Qin, M.; Yi, Z.; Deng, H.; Wang, J.; Sun, Y.; Luo, G.; Shen, Q. Oxidation Mechanism of Core-Shell Structured Al@ PVDF Powders Synthesized by Solvent/Non-Solvent Method. Materials 2022, 15, 3036. [Google Scholar] [CrossRef]
- El Achaby, M.; Arrakhiz, F.; Vaudreuil, S.; Essassi, E.; Qaiss, A. Piezoelectric β-polymorph formation and properties enhancement in graphene oxide–PVDF nanocomposite films. Appl. Surf. Sci. 2012, 258, 7668–7677. [Google Scholar] [CrossRef]
- Hasegawa, R.; Takahashi, Y.; Chatani, Y.; Tadokoro, H. Crystal structures of three crystalline forms of poly(vinylidene fluoride). Polym. J. 1972, 3, 600–610. [Google Scholar] [CrossRef]
- Sun, X.; Shiraz, H.; Wong, R.; Zhang, J.; Liu, J.; Lu, J.; Meng, N. Enhancing the Performance of PVDF/GO Ultrafiltration Membrane via Improving the Dispersion of GO with Homogeniser. Membranes 2022, 12, 1268. [Google Scholar] [CrossRef]
- Tan, K.; Gan, W.; Velayutham, T.; Abd Majid, W. Pyroelectricity enhancement of PVDF nanocomposite thin films doped with ZnO nanoparticles. Smart Mater. Struct. 2014, 23, 125006. [Google Scholar] [CrossRef]
- Bheel, N.; Mohammed, B.S.; Mohamad, H.; Sutanto, M.H.; Tafsirojjaman, T. Synergetic effect of multiwalled carbon nanotubes on mechanical and deformation properties of engineered cementitious composites: RSM modelling and optimization. Diam. Relat. Mater. 2024, 147, 111299. [Google Scholar] [CrossRef]


| Material | Composition |
|---|---|
| Na2SO4 | 3.75 g |
| NaCl | 1.75 g |
| ZnSO4 | 22.5 g |
| Deionized water | 250 ml |
| Sample | Voltage (V) | Time (min) | Average Measured Thickness (µm) |
|---|---|---|---|
| CZ1 | 3 | 15 | 64.9 |
| CZ2 | 3 | 30 | 109 |
| CZ3 | 3 | 45 | 260 |
| CZ4 | 5 | 15 | 411 |
| CZ5 | 5 | 30 | 489 |
| CZ6 | 5 | 45 | 501 |
| CZ7 | 10 | 15 | 612 |
| CZ8 | 10 | 30 | 627 |
| CZ9 | 10 | 45 | 640 |
| S.No | Input Factors | Ranges |
|---|---|---|
| 1. | Hours | 1 to 48 |
| 2. | Graphene | 0.30 to 0.90 |
| Response | Source | Sum of Squares | Df | Mean Square | F-Value | p-Value > F | Significance |
|---|---|---|---|---|---|---|---|
| BMP | Model | 41.22 | 5 | 8.24 | 15.04 | 0.0002 | significant |
| A-Hours | 9.03 | 1 | 9.03 | 16.47 | 0.0023 | significant | |
| B-Fillers | 0.84 | 1 | 0.84 | 1.53 | 0.2449 | Not significant | |
| AB | 1.65 | 1 | 1.65 | 3.01 | 0.1134 | not significant | |
| A2 | 6.55 | 1 | 6.55 | 11.95 | 0.0062 | significant | |
| B2 | 0.97 | 1 | 0.97 | 1.78 | 0.2123 | Not significant | |
| Residual | 5.48 | 10 | 0.55 | - | - | - | |
| Lack of Fit | 5.29 | 2 | 2.64 | 110.06 | <0.0001 | significant | |
| Pure Error | 0.19 | 8 | 0.024 | - | - | ||
| Cor Total | 46.70 | 15 | - | ||||
| OCP | Model | 0.089 | 5 | 0.018 | 158.35 | <0.0001 | significant |
| A-Hours | 0.021 | 1 | 0.021 | 189.18 | <0.0001 | significant | |
| B-Fillers | 0.016 | 1 | 0.016 | 145.85 | <0.0001 | significant | |
| AB | 4.429 × 10−3 | 1 | 4.429 × 10−3 | 39.42 | <0.0001 | significant | |
| A2 | 4.252 × 10−4 | 1 | 4.252 × 10−4 | 3.78 | 0.0804 | Not significant | |
| B2 | 0.042 | 1 | 0.042 | 373.37 | <0.0001 | significant | |
| Residual | 1.124 × 10−3 | 10 | 1.124 × 10−4 | - | - | - | |
| Lack of Fit | 1.124 × 10−3 | 2 | 5.619 × 10−4 | - | - | - | |
| Pure Error | 0.000 | 8 | 0.000 | - | - | - | |
| Cor Total | 0.090 | 15 | - | - | - | - | |
| EIS | Model | 1.286 × 1014 | 2 | 6.431 × 1013 | 12.64 | 0.0009 | significant |
| A-Hours | 9.179 × 1011 | 1 | 9.179 × 1011 | 0.18 | 0.6779 | Not significant | |
| B-Fillers | 1.174 × 1014 | 1 | 1.174 × 1014 | 23.08 | 0.0003 | significant | |
| Residual | 6.612 × 1013 | 13 | 5.086 × 1012 | - | - | - | |
| Lack of Fit | 6.612 × 1013 | 5 | 1.322 × 1013 | - | - | - | |
| Pure Error | 0.000 | 8 | 0.000 | - | - | - | |
| Cor Total | 1.947 × 1014 | 15 | - | - | - |
| Model Validation Constraints | BMP | OCP | EIS |
|---|---|---|---|
| Std. Dev. | 0.74 | 0.011 | 2.255 × 106 |
| Mean | 7.90 | −0.56 | 6.054 × 106 |
| C.V. % | 9.38 | 1.89 | 37.25 |
| PRESS | 47.52 | 9.380 × 10−3 | 1.120 × 1014 |
| −2 Log Likelihood | 28.26 | −107.61 | 510.20 |
| R-Squared | 0.8826 | 0.9875 | 0.6605 |
| Adj R-Squared | 0.8240 | 0.9813 | 0.6082 |
| Pred R-Squared | −0.0176 | 0.8959 | 0.4247 |
| Adeq Precision | 10.099 | 40.877 | 8.981 |
| Factors | Input Factors | Responses (Output Factors) | ||||
|---|---|---|---|---|---|---|
| Hours (h) | GO (g) | BMP (Ohms·cm2) | OCP (V) | EIS (Ohms·cm2) | ||
| Value | Min. | 1 | 0.30 | 5.20 | −0.69 | 33,497.80 |
| Max | 48 | 0.90 | 10.63 | −0.40 | 1.16613 × 107 | |
| Goal | Maximum | maximum | Minimum | Minimum | Minimum | |
| Results | 48 | 0.531 | 6.604 | −0.563 | 5.2443 × 106 | |
| Desirability | 0.616 (61.60%) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayub, S.; Mohammed, B.S.; Al-Yacouby, A.M.; Bheel, N. Impact of Different Parameters to Enhanced Corrosion Resistance of Zinc-PVDF-G Coating for the Protection of Mild Steel Substrates. Polymers 2025, 17, 2914. https://doi.org/10.3390/polym17212914
Ayub S, Mohammed BS, Al-Yacouby AM, Bheel N. Impact of Different Parameters to Enhanced Corrosion Resistance of Zinc-PVDF-G Coating for the Protection of Mild Steel Substrates. Polymers. 2025; 17(21):2914. https://doi.org/10.3390/polym17212914
Chicago/Turabian StyleAyub, Saba, Bashar S. Mohammed, Ahmad Mahamad Al-Yacouby, and Naraindas Bheel. 2025. "Impact of Different Parameters to Enhanced Corrosion Resistance of Zinc-PVDF-G Coating for the Protection of Mild Steel Substrates" Polymers 17, no. 21: 2914. https://doi.org/10.3390/polym17212914
APA StyleAyub, S., Mohammed, B. S., Al-Yacouby, A. M., & Bheel, N. (2025). Impact of Different Parameters to Enhanced Corrosion Resistance of Zinc-PVDF-G Coating for the Protection of Mild Steel Substrates. Polymers, 17(21), 2914. https://doi.org/10.3390/polym17212914






