Comparing the Printability, Biological and Physicochemical Properties of Bio-Based Photo-Crosslinkable Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photo-Crosslinkable Hydrogels
2.2.1. Methacrylation of the Biopolymers
2.2.2. Synthesis of Photo-Crosslinkable Hydrogels
2.3. Nuclear Magnetic Resonance (1H-NMR)
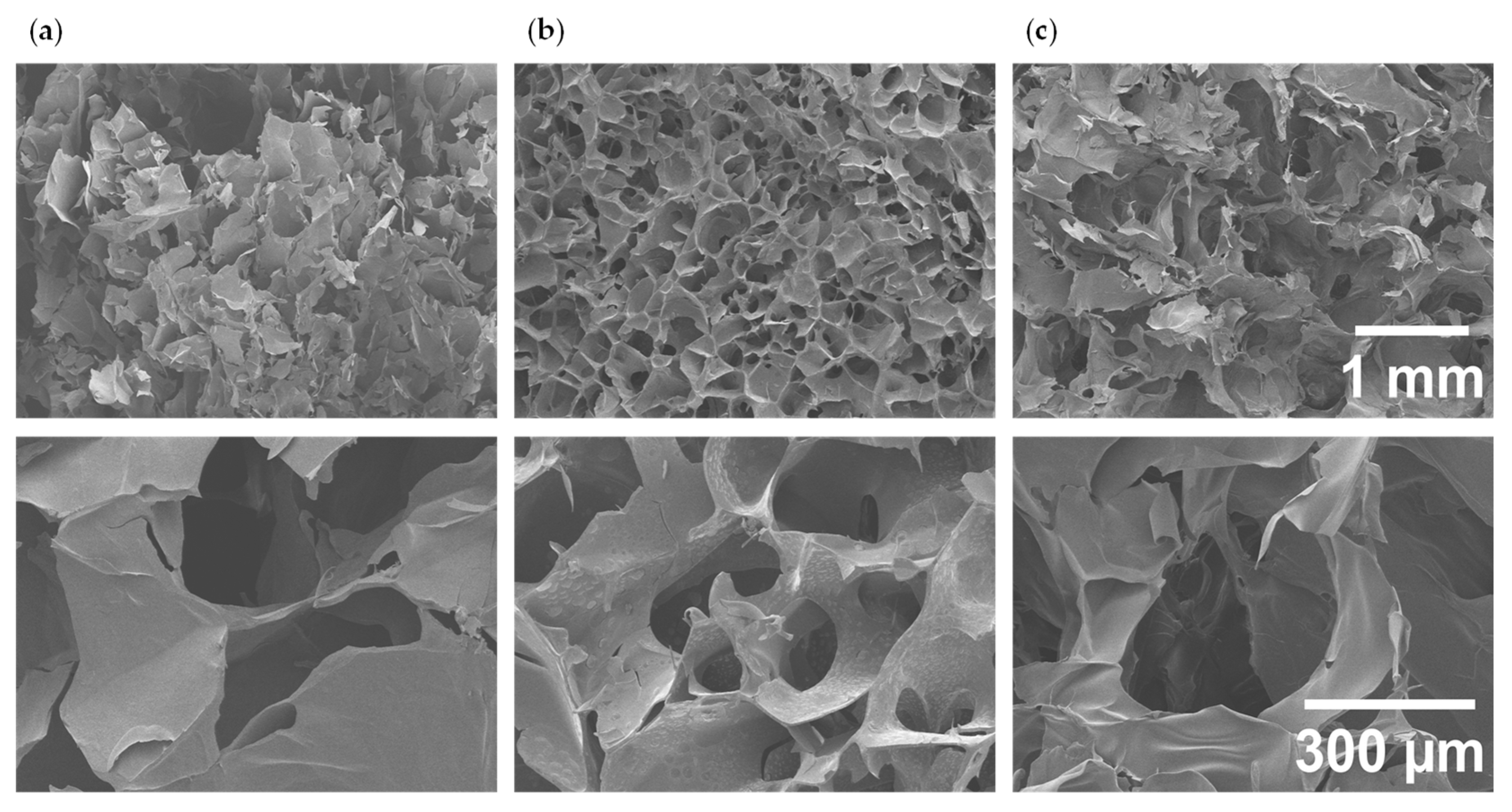
2.4. Morphological Characterization
2.5. Physico-Chemical Characterization
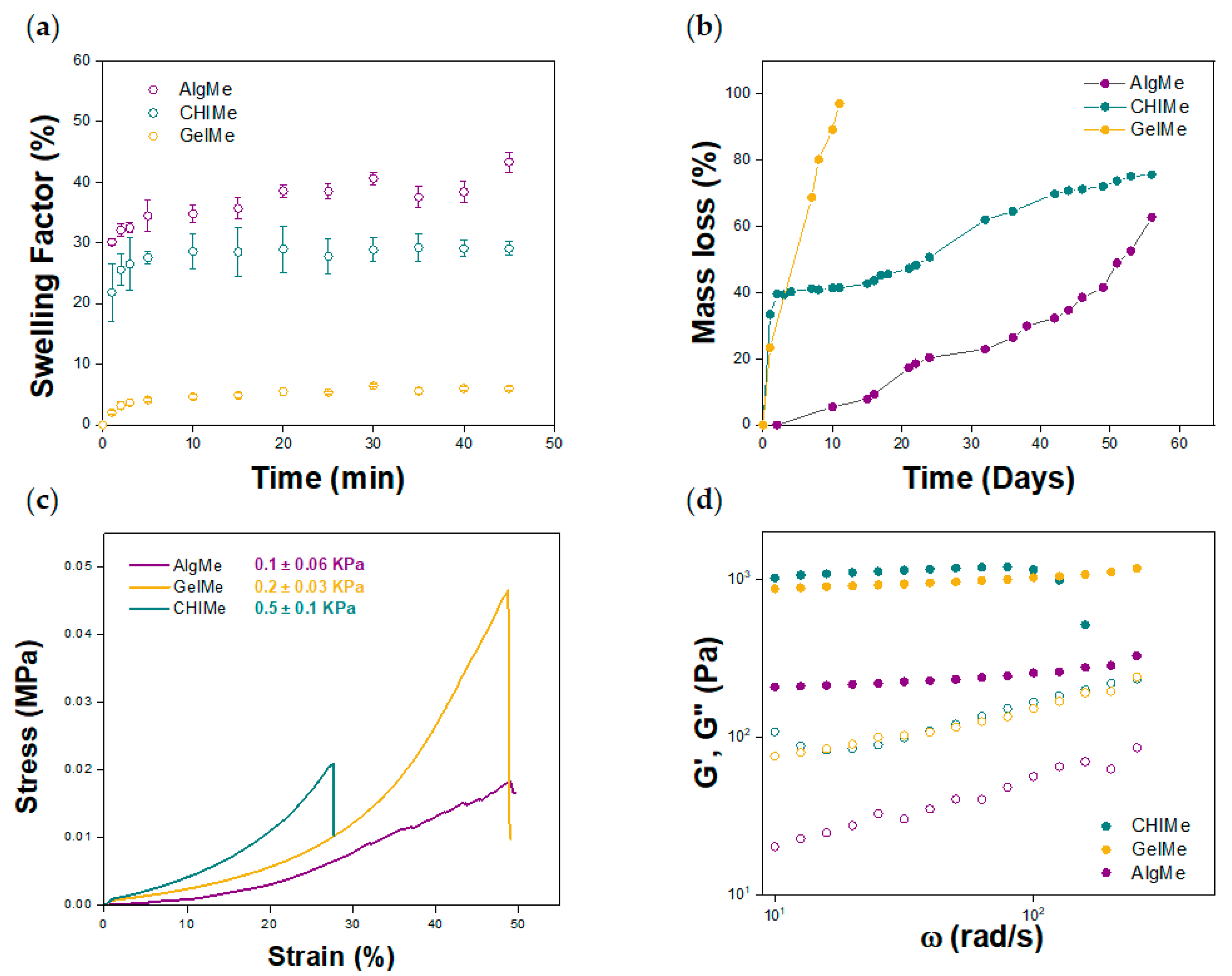
2.5.1. In Vitro Swelling
2.5.2. In Vitro Degradation
2.5.3. Compressive Stress–Strain Test
2.5.4. Rheology
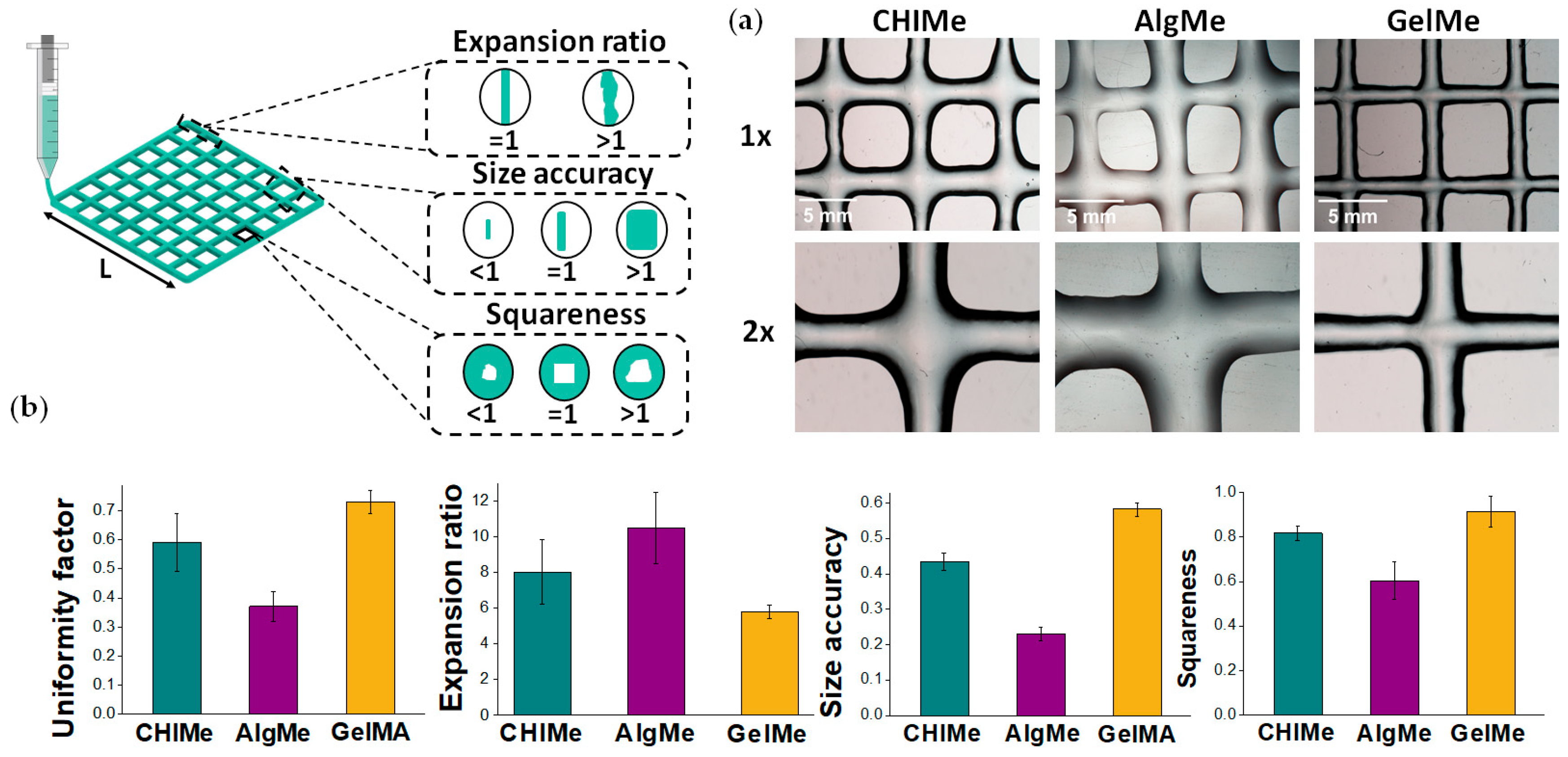
2.5.5. 3D Printing Quality
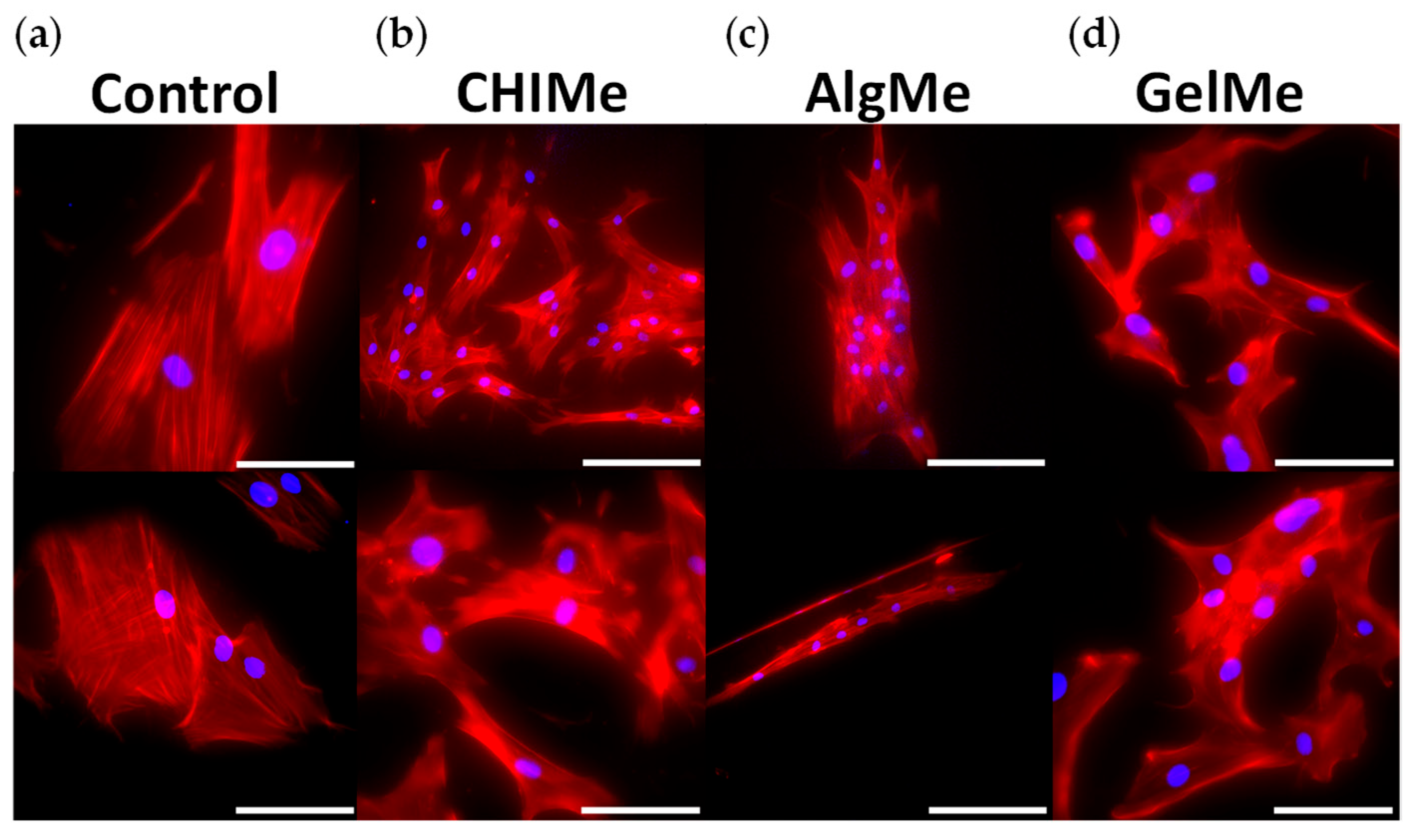
2.6. Cell Culture and Immunofluorescence
2.7. Cytotoxicity Assay of the “Medium”
3. Results
3.1. Hydrogel Preparation and Morphological Characterization
3.2. Physico-Chemical Characterization
3.3. Printability
3.4. Biocompatibility and Analysis of Cell Attachment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jose, G.; Shalumon, K.T.; Chen, J.-P. Natural Polymers Based Hydrogels for Cell Culture Applications. Curr. Med. Chem. 2019, 27, 2734–2776. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, F.; Wang, X.; Geng, X.; Zhao, S.; Liu, H.; Dou, D.; Leng, Y.; Wang, L.; Fan, Y. Biomaterial Inks for Extrusion-Based 3D Bioprinting: Property, Classification, Modification, and Selection. Int. J. Bioprint. 2022, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Pescara, T.; Gambelli, A.M.; Gaggia, F.; Asthana, A.; Perrier, Q.; Basta, G.; Moretti, M.; Senin, N.; Rossi, F.; et al. Biomaterials for Extrusion-Based Bioprinting and Biomedical Applications. Front. Bioeng. Biotechnol. 2024, 12, 1393641. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Stojanović, G.M.; Abdullah, M.F.B.; Dolatshahi-Pirouz, A.; Marei, H.E.; Ashammakhi, N.; Hasan, A. Fundamental Properties of Smart Hydrogels for Tissue Engineering Applications: A Review. Int. J. Biol. Macromol. 2024, 254, 127882. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hokmabad, V.R.; Del Bakhshayesh, A.R.; Asadi, N.S.R.; Nasrabadi, H.T. The Application of Hydrogels Based on Natural Polymers for Tissue Engineering. Curr. Med. Chem. 2020, 27, 26582680. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable Hydrogels for Tissue Engineering Applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Wu, L.; He, Y.; Mao, H.; Gu, Z. Bioactive Hydrogels Based on Polysaccharides and Peptides for Soft Tissue Wound Management. J. Mater. Chem. B 2022, 10, 7148–7160. [Google Scholar] [CrossRef]
- Rial-Hermida, M.I.; Rey-Rico, A.; Blanco-Fernandez, B.; Carballo-Pedrares, N.; Byrne, E.M.; Mano, J.F. Recent Progress on Polysaccharide-Based Hydrogels for Controlled Delivery of Therapeutic Biomolecules. ACS Biomater. Sci. Eng. 2021, 7, 4102–4127. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, A.; Muñana-González, S.; Lanceros-Mendez, S.; Ruiz-Rubio, L.; Alvarez, L.P.; Vilas-Vilela, J.L. Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation. Polymers 2024, 16, 2599. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Photocrosslinkable and Self-Healable Hydrogels of Chitosan and Hyaluronic Acid. Int. J. Biol. Macromol. 2022, 216, 291–302. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. Dynamic and Self-Healable Chitosan/Hyaluronic Acid-Based in Situ-Forming Hydrogels. Gels 2022, 8, 477. [Google Scholar] [CrossRef]
- Muñoz-Nuñez, C.; Quiroz-Pereira, Y.; Muñoz-Bonilla, A.; Fernández-García, M. Enhancing Antimicrobial and Antioxidant Properties of Chitosan-Based Films with 1-Methylimidazolium-Chitosan. Polymers 2025, 17, 2608. [Google Scholar] [CrossRef]
- Soni, B.; Mahmoud, B.; Chang, S.; El-Giar, E.M.; Hassan, E.B. Physicochemical, Antimicrobial and Antioxidant Properties of Chitosan/TEMPO Biocomposite Packaging Films. Food Packag. Shelf Life 2018, 17, 73–79. [Google Scholar] [CrossRef]
- García-García, A.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Larrea-Sebal, A.; Martin, C.; Vilas-Vilela, J.L. Extrusion-Based 3D Printing of Photocrosslinkable Chitosan Inks. Gels 2024, 10, 126. [Google Scholar] [CrossRef]
- Cosola, A.; Chiappone, A.; Martinengo, C.; Grützmacher, H.; Sangermano, M. Gelatin Type A from Porcine Skin Used as Co-Initiator in a Radical Photo-Initiating System. Polymers 2019, 11, 1901. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, B.; Zhang, X.; Huang, S.; Wa, Q. 3D Printed Concentrated Alginate/GelMA Hollow-Fibers-Packed Scaffolds with Nano Apatite Coatings for Bone Tissue Engineering. Int. J. Biol. Macromol. 2022, 202, 366–374. [Google Scholar] [CrossRef]
- Grigore, A.; Sarker, B.; Fabry, B.; Boccaccini, A.R.; Detsch, R. Behavior of Encapsulated MG-63 Cells in RGD and Gelatine-Modified Alginate Hydrogels. Tissue Eng. Part A 2014, 20, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Fatimi, A.; Liu, Y.; Musuc, A.M.; Fajardo, A.R.; Gowda, B.H.J.; Vora, L.K.; Shavandi, A.; Okoro, O.V. Recent Advances in 3D Bioprinted Polysaccharide Hydrogels for Biomedical Applications: A Comprehensive Review. Carbohydr. Polym. 2025, 348, 122845. [Google Scholar] [CrossRef] [PubMed]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-Printed Chitosan-Based Scaffolds: An in Vitro Study of Human Skin Cell Growth and an in-Vivo Wound Healing Evaluation in Experimental Diabetes in Rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef]
- Teoh, J.H.; Mozhi, A.; Sunil, V.; Tay, S.M.; Fuh, J.; Wang, C.H. 3D Printing Personalized, Photocrosslinkable Hydrogel Wound Dressings for the Treatment of Thermal Burns. Adv. Funct. Mater. 2021, 31, 2105932. [Google Scholar] [CrossRef]
- Chang, H.K.; Yang, D.H.; Ha, M.Y.; Kim, H.J.; Kim, C.H.; Kim, S.H.; Choi, J.W.; Chun, H.J. 3D Printing of Cell-Laden Visible Light Curable Glycol Chitosan Bioink for Bone Tissue Engineering. Carbohydr. Polym. 2022, 287, 119328. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Huang, X.; Hang, R.; Zhang, X.; Wang, Y.; Yao, X. DLP Printing Photocurable Chitosan to Build Bio-Constructs for Tissue Engineering. Carbohydr. Polym. 2020, 235, 115970. [Google Scholar] [CrossRef]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan:PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Rodríguez-Rego, J.M.; Macías-García, A.; Mendoza-Cerezo, L.; Díaz-Parralejo, A. Relationship between Shear-Thinning Rheological Properties of Bioinks and Bioprinting Parameters. Int. J. Bioprint. 2023, 9, 422–431. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Chen, D.; Su, T.; Chang, Q.; Huang, W.; Lu, F. 3D Bioprinting of a Gradient Stiffened Gelatin-Alginate Hydrogel with Adipose-Derived Stem Cells for Full-Thickness Skin Regeneration. J. Mater. Chem. B 2023, 11, 2989–3000. [Google Scholar] [CrossRef]
- Gharacheh, H.; Guvendiren, M. Cell-Laden Composite Hydrogel Bioinks with Human Bone Allograft Particles to Enhance Stem Cell Osteogenesis. Polymers 2022, 14, 3788. [Google Scholar] [CrossRef]
- Celikkin, N.; Mastrogiacomo, S.; Dou, W.; Heerschap, A.; Oosterwijk, E.; Walboomers, X.F.; Święszkowski, W. In Vitro and in Vivo Assessment of a 3D Printable Gelatin Methacrylate Hydrogel for Bone Regeneration Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2133–2145. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chen, C.; Xu, H.H.; Zhang, Y.S.; Zhong, L.; Hu, N.; Jia, X.L.; Wang, Y.W.; Zhong, K.H.; Liu, C.; et al. Integrated Printed BDNF/Collagen/Chitosan Scaffolds with Low Temperature Extrusion 3D Printer Accelerated Neural Regeneration after Spinal Cord Injury. Regen. Biomater. 2021, 8, rbab047. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Methacrylated Chitosan as a Polymer with Enhanced Mucoadhesive Properties for Transmucosal Drug Delivery. Int. J. Pharm. 2018, 550, 123–129. [Google Scholar] [CrossRef]
- Chou, A.I.; Nicoll, S.B. Characterization of Photocrosslinked Alginate Hydrogels for Nucleus Pulposus Cell Encapsulation. J. Biomed. Mater. Res. A 2009, 91, 187–194. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Burcu, A.; Gedikoglu, G.; Telek, H.H.; Ornek, F.; Hasirci, V. Methacrylated Gelatin Hydrogels as Corneal Stroma Substitutes: In Vivo Study. J. Biomater. Sci. Polym. Ed. 2019, 30, 1803–1821. [Google Scholar] [CrossRef]
- Jensen, H.M.; Larsen, F.H.; Engelsen, S.B. Characterization of Alginates by Nuclear Magnetic Resonance (NMR) and Vibrational Spectroscopy (IR, NIR, Raman) in Combination with Chemometrics. Methods Mol. Biol. 2015, 1308, 347–363. [Google Scholar] [CrossRef]
- Araiza-Verduzco, F.; Rodríguez-Velázquez, E.; Cruz, H.; Rivero, I.A.; Acosta-Martínez, D.R.; Pina-Luis, G.; Alatorre-Meda, M. Photocrosslinked Alginate-Methacrylate Hydrogels with Modulable Mechanical Properties: Effect of the Molecular Conformation and Electron Density of the Methacrylate Reactive Group. Materials 2020, 13, 534. [Google Scholar] [CrossRef]
- Claaßen, C.; Claaßen, M.H.; Truffault, V.; Sewald, L.; Tovar, G.E.M.; Borchers, K.; Southan, A. Quantification of Substitution of Gelatin Methacryloyl: Best Practice and Current Pitfalls. Biomacromolecules 2018, 19, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Trucco, D.; Riacci, L.; Vannozzi, L.; Manferdini, C.; Arrico, L.; Gabusi, E.; Lisignoli, G.; Ricotti, L. Primers for the Adhesion of Gellan Gum-Based Hydrogels to the Cartilage: A Comparative Study. Macromol. Biosci. 2022, 22, 2200096. [Google Scholar] [CrossRef] [PubMed]
- Giuseppe, M.D.; Law, N.; Webb, B.; Macrae, A.R.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical Behaviour of Alginate-Gelatin Hydrogels for 3D Bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of Bioink Properties on Printability and Cell Viability for 3D Bioplotting of Embryonic Stem Cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Ozdogan, C.Y.; Kenar, H.; Davun, K.E.; Yucel, D.; Doger, E.; Alagoz, S. An in Vitro 3D Diabetic Human Skin Model from Diabetic Primary Cells. Biomed. Mater. 2021, 16, 015027. [Google Scholar] [CrossRef]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked Alginate Hydrogels with Tunable Biodegradation Rates and Mechanical Properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Sanz, C.K.; Münchow, E.A.; Kalra, N.; Dubey, N.; Suárez, C.E.C.; Fenno, J.C.; Lund, R.G.; Bottino, M.C. Photocrosslinkable Methacrylated Gelatin Hydrogel as a Cell-Friendly Injectable Delivery System for Chlorhexidine in Regenerative Endodontics. Dent. Mater. 2022, 38, 1507–1517. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Barroso, N.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. 3D Printable Self-Healing Hyaluronic Acid/Chitosan Polycomplex Hydrogels with Drug Release Capability. Int. J. Biol. Macromol. 2021, 188, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Powell, C.; Solorio, L.D.; Krebs, M.D.; Alsberg, E. Affinity-Based Growth Factor Delivery Using Biodegradable, Photocrosslinked Heparin-Alginate Hydrogels. J. Control. Release 2011, 154, 258–266. [Google Scholar] [CrossRef]
- Jeon, O.; Powell, C.; Ahmed, S.M.; Alsberg, E. Biodegradable, Photocrosslinked Alginate Hydrogels with Independently Tailorable Physical Properties and Cell Adhesivity. Tissue Eng. Part A 2010, 16, 2915–2925. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Wen, Y.; Li, D.; Sun, X.; Liu, Z.; Yan, H.; Lin, Q. A Study on the Correlation between the Oxidation Degree of Oxidized Sodium Alginate on Its Degradability and Gelation. Polymers 2022, 14, 1679. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bosch, E.; Gielens, C. Gelatin Degradation at Elevated Temperature. Int. J. Biol. Macromol. 2003, 32, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Maiz-Fernández, S.; Guaresti, O.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. β-Glycerol Phosphate/Genipin Chitosan Hydrogels: A Comparative Study of Their Properties and Diclofenac Delivery. Carbohydr. Polym. 2020, 248, 116811. [Google Scholar] [CrossRef] [PubMed]
- Zoco de la Fuente, A.; García-García, A.; Pérez-Álvarez, L.; Moreno-Benítez, I.; Larrea-Sebal, A.; Martin, C.; Vilas-Vilela, J.L. Evaluation of Various Types of Alginate Inks for Light-Mediated Extrusion 3D Printing. Polymers 2024, 16, 986. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Schexnailder, P.J.; Jin, Q.; Wu, C.J.; Schmidt, G. Addition of Chitosan to Silicate Cross-Linked PEO for Tuning Osteoblast Cell Adhesion and Mineralization. ACS Appl. Mater. Interfaces 2010, 2, 3119–3127. [Google Scholar] [CrossRef]
- Aurrekoetxea, M.; Garcia-Gallastegui, P.; Irastorza, I.; Luzuriaga, J.; Uribe-Etxebarria, V.; Unda, F.; Ibarretxe, G. Dental Pulp Stem Cells as a Multifaceted Tool for Bioengineering and the Regeneration of Craniomaxillofacial Tissues. Front. Physiol. 2015, 6, 289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Jiang, D.; Ding, J.X.; Wang, S.J.; Zhang, L.; Zhang, J.Y.; Qi, Y.S.; Chen, X.S.; Yu, J.K. Role of Scaffold Mean Pore Size in Meniscus Regeneration. Acta Biomater. 2016, 43, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Datta, S. Advantage of Alginate Bioinks in Biofabrication for Various Tissue Engineering Applications. Int. J. Polym. Sci. 2023, 2023, 6661452. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, Y.; Yao, B.; Enhejirigala, B.; Li, Z.; Song, W.; Wang, Y.; Duan, X.; Yuan, X.; et al. Biophysical and Biochemical Cues of Biomaterials Guide Mesenchymal Stem Cell Behaviors. Front. Cell Dev. Biol. 2021, 9, 640388. [Google Scholar] [CrossRef] [PubMed]


| GelMe | AlgMe | CHIMe | ||
|---|---|---|---|---|
| MD (%) | 32 ± 3 | 9 ± 4 | 41 ± 4 | |
| Swelling (%) after 45 min. | 6 ± 0.2 | 38 ± 8.7 | 29 ± 1.1 | |
| Time for 50% of mass loss (days) | 5 | 52 | 24 | |
| Young Modulus (KPa) | 0.20 ± 0.03 | 0.10 ± 0.06 | 0.50 ± 0.10 | |
| Cell viability (%) | 97 ± 1 | 90 ± 2 | 89 ± 9 | |
| Printability | Uniformity Factor | ☑☑☑ | ☑ | ☑☑ |
| Squareness | ☑☑☑ | ☑ | ☑☑ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, A.; Silván, U.; Pérez-Álvarez, L.; Lanceros, S. Comparing the Printability, Biological and Physicochemical Properties of Bio-Based Photo-Crosslinkable Hydrogels. Polymers 2025, 17, 2867. https://doi.org/10.3390/polym17212867
García-García A, Silván U, Pérez-Álvarez L, Lanceros S. Comparing the Printability, Biological and Physicochemical Properties of Bio-Based Photo-Crosslinkable Hydrogels. Polymers. 2025; 17(21):2867. https://doi.org/10.3390/polym17212867
Chicago/Turabian StyleGarcía-García, Ane, Unai Silván, Leyre Pérez-Álvarez, and Senentxu Lanceros. 2025. "Comparing the Printability, Biological and Physicochemical Properties of Bio-Based Photo-Crosslinkable Hydrogels" Polymers 17, no. 21: 2867. https://doi.org/10.3390/polym17212867
APA StyleGarcía-García, A., Silván, U., Pérez-Álvarez, L., & Lanceros, S. (2025). Comparing the Printability, Biological and Physicochemical Properties of Bio-Based Photo-Crosslinkable Hydrogels. Polymers, 17(21), 2867. https://doi.org/10.3390/polym17212867








