Dialdehyde Cellulose Nanocrystals and Proanthocyanidins Reinforced Soy Protein Isolate Films for Blueberry Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
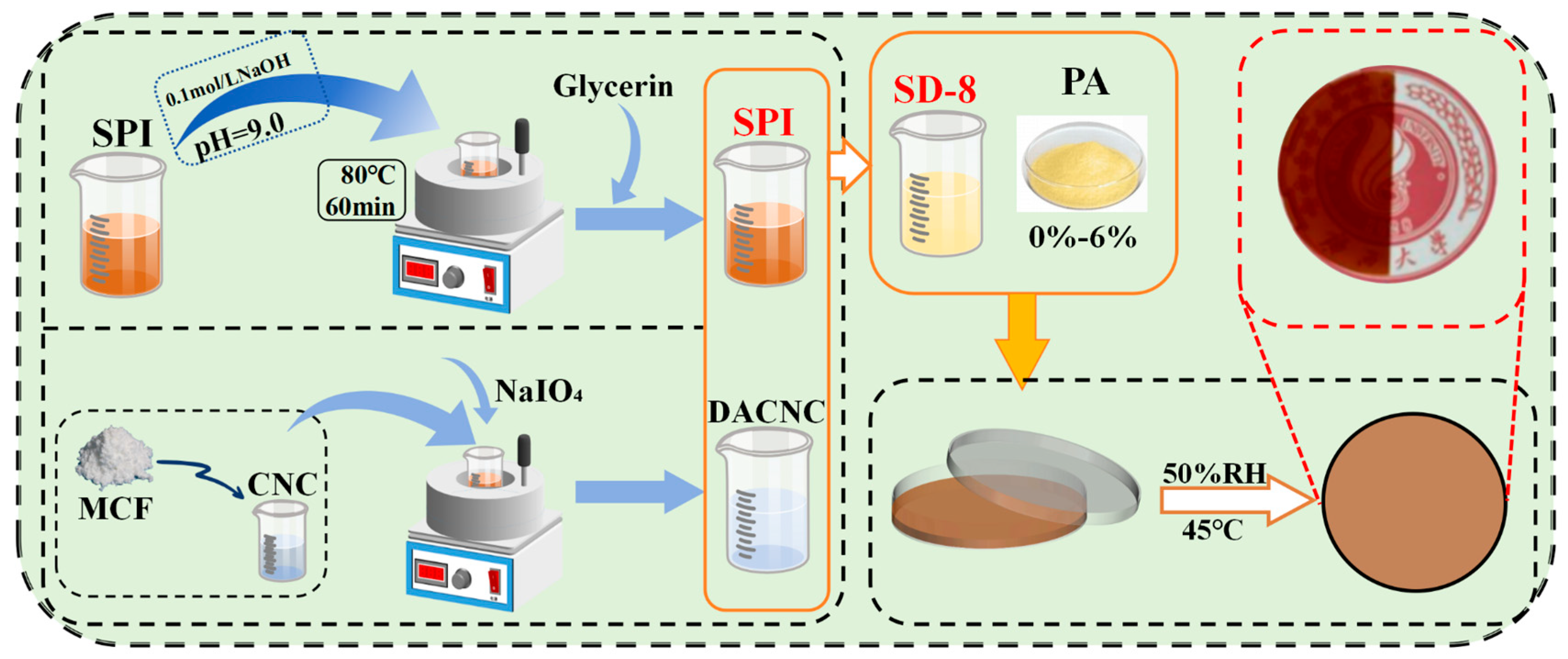
2.2. Preparation of DACNC
2.3. Fabrication of Composite Films
2.4. Characterization and Performance Evaluation
2.4.1. Structural and Morphological Analysis
2.4.2. Mechanical and Barrier Properties
2.4.3. Water Interaction and Barrier Properties
2.4.4. Antioxidant Activity
2.4.5. Antimicrobial Activity
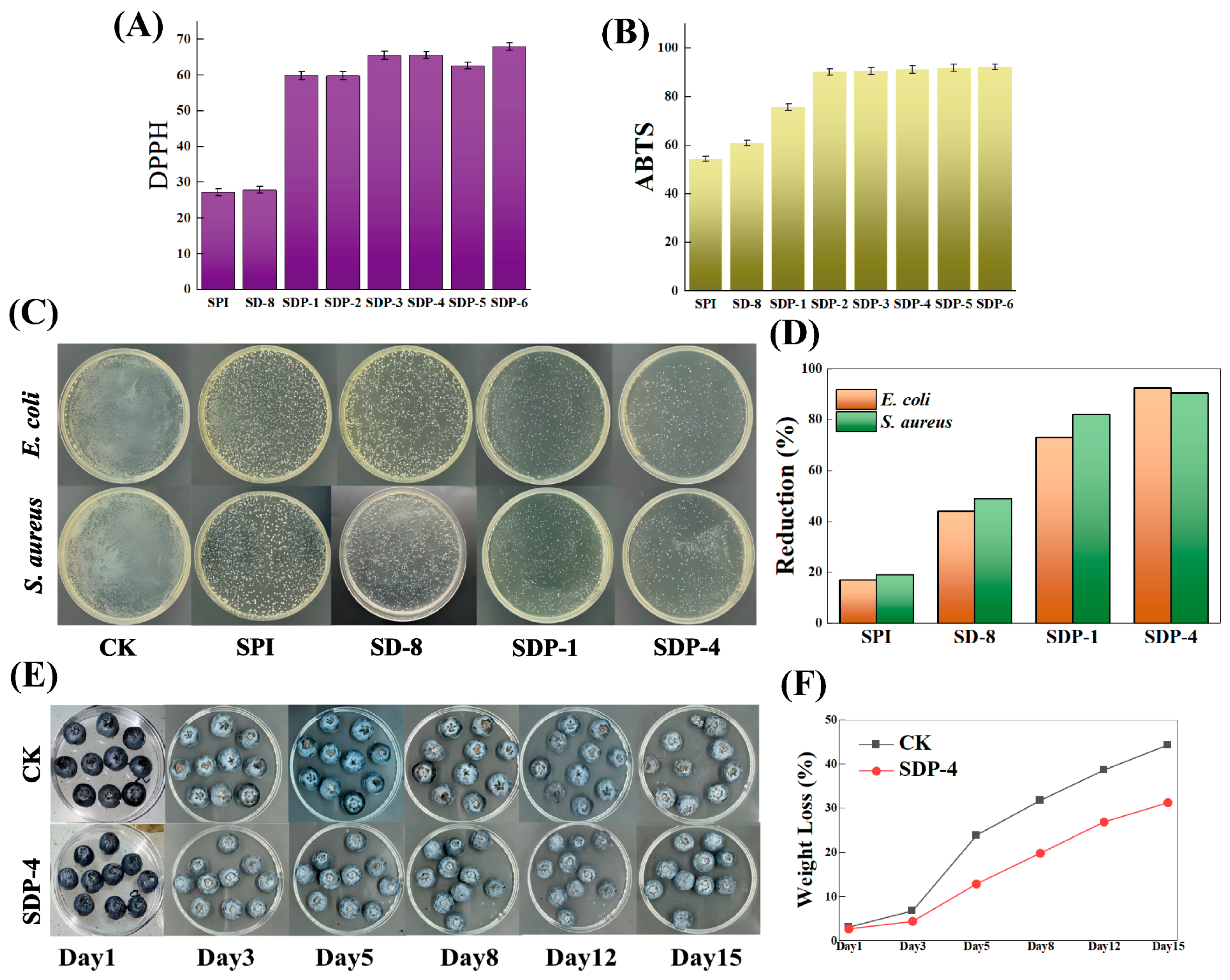
2.5. Blueberry Preservation Application
2.6. Statistical Analysis
3. Results and Discussion
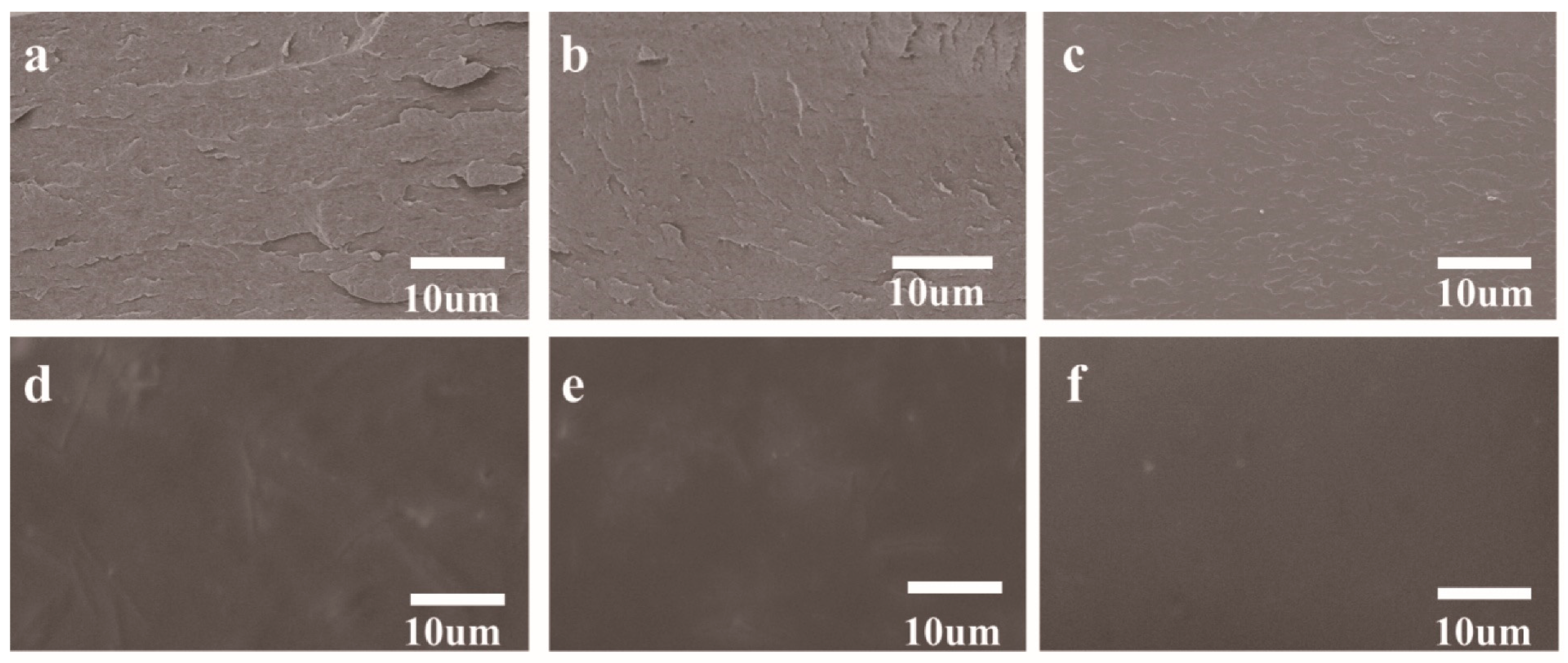
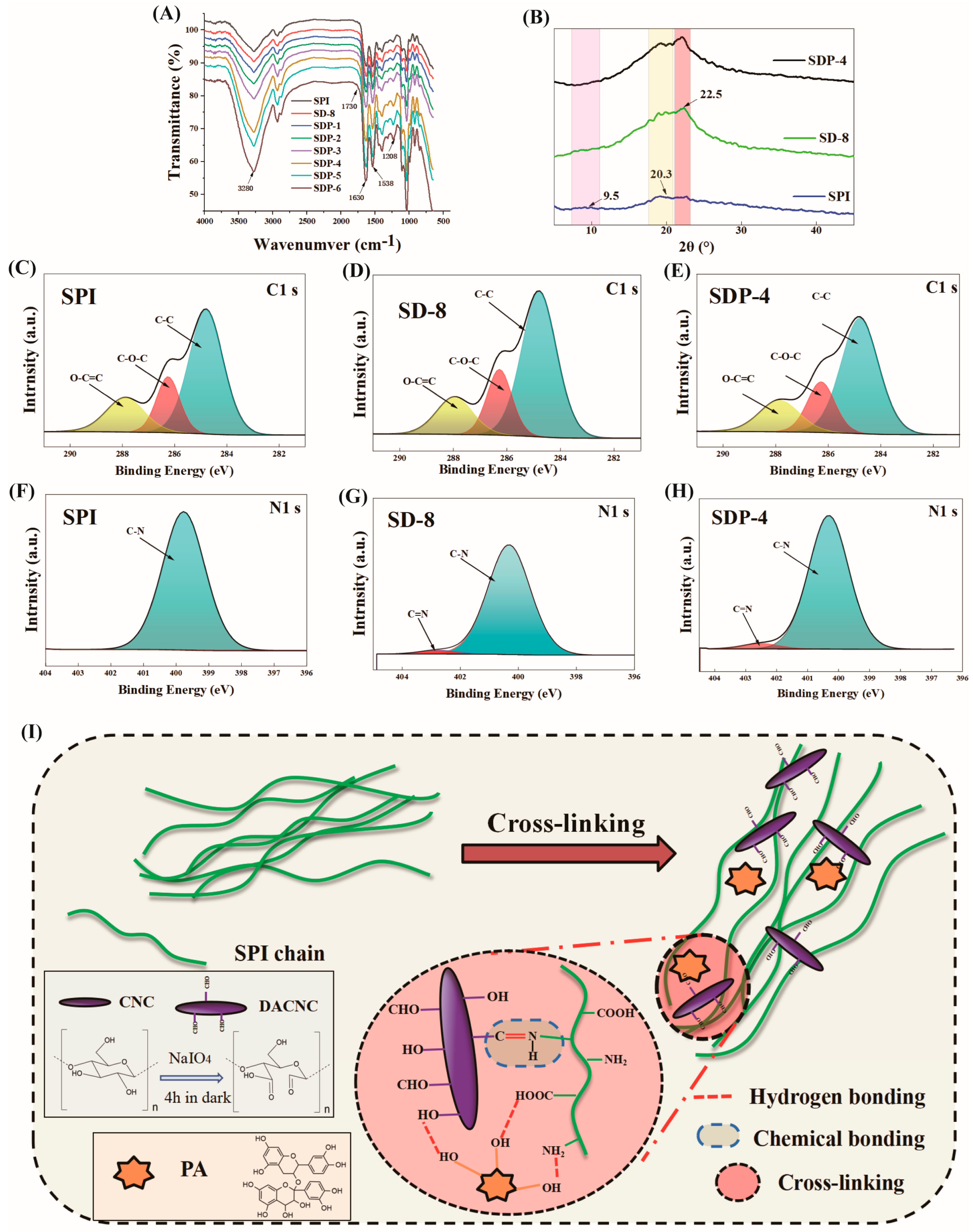
3.1. Microstructure and Intermolecular Interactions of Composite Films
3.1.1. Microstructural Morphology
3.1.2. Structural Characterization
3.2. Mechanical and Thermal Properties
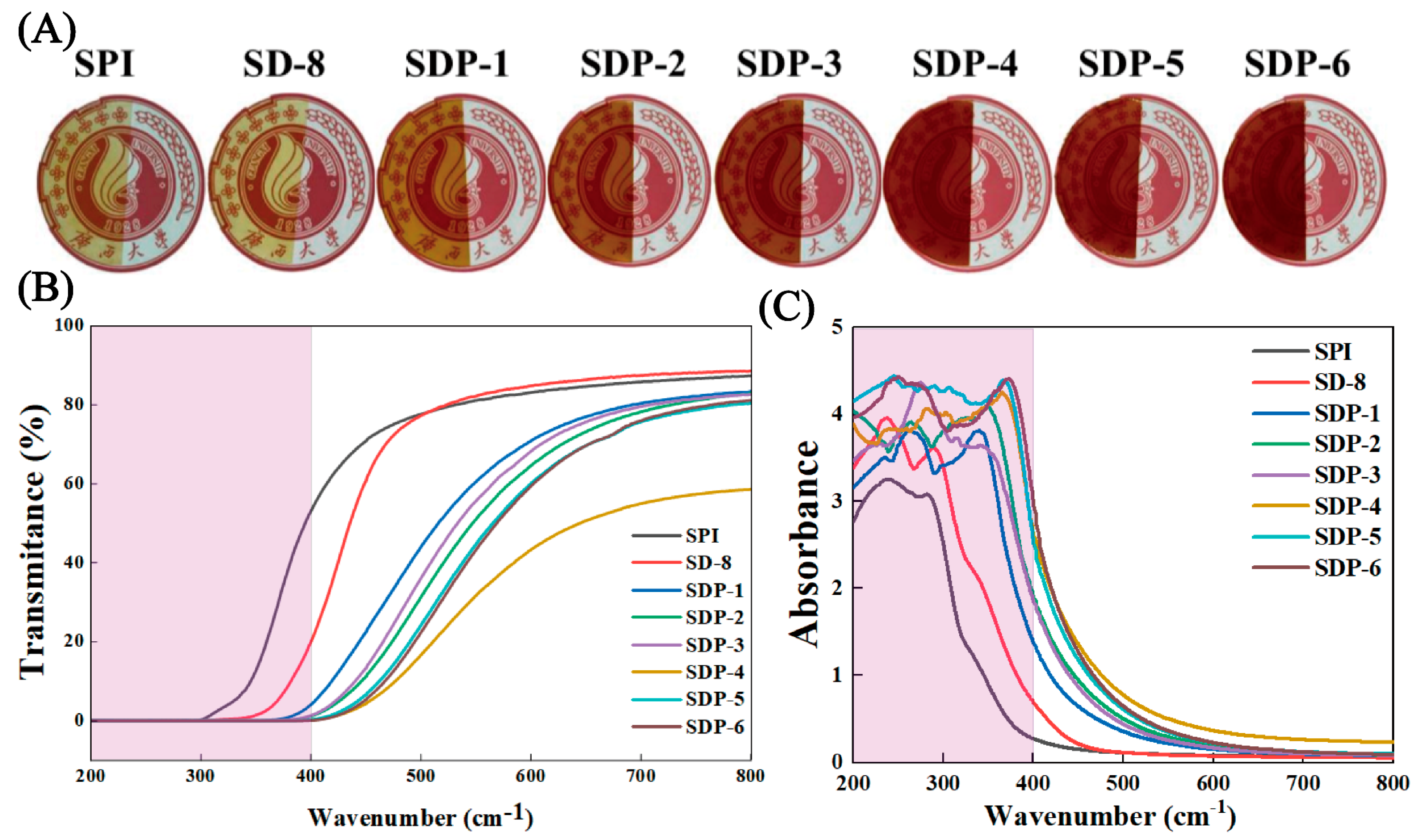
3.3. Optical Properties and UV-Shielding Capability
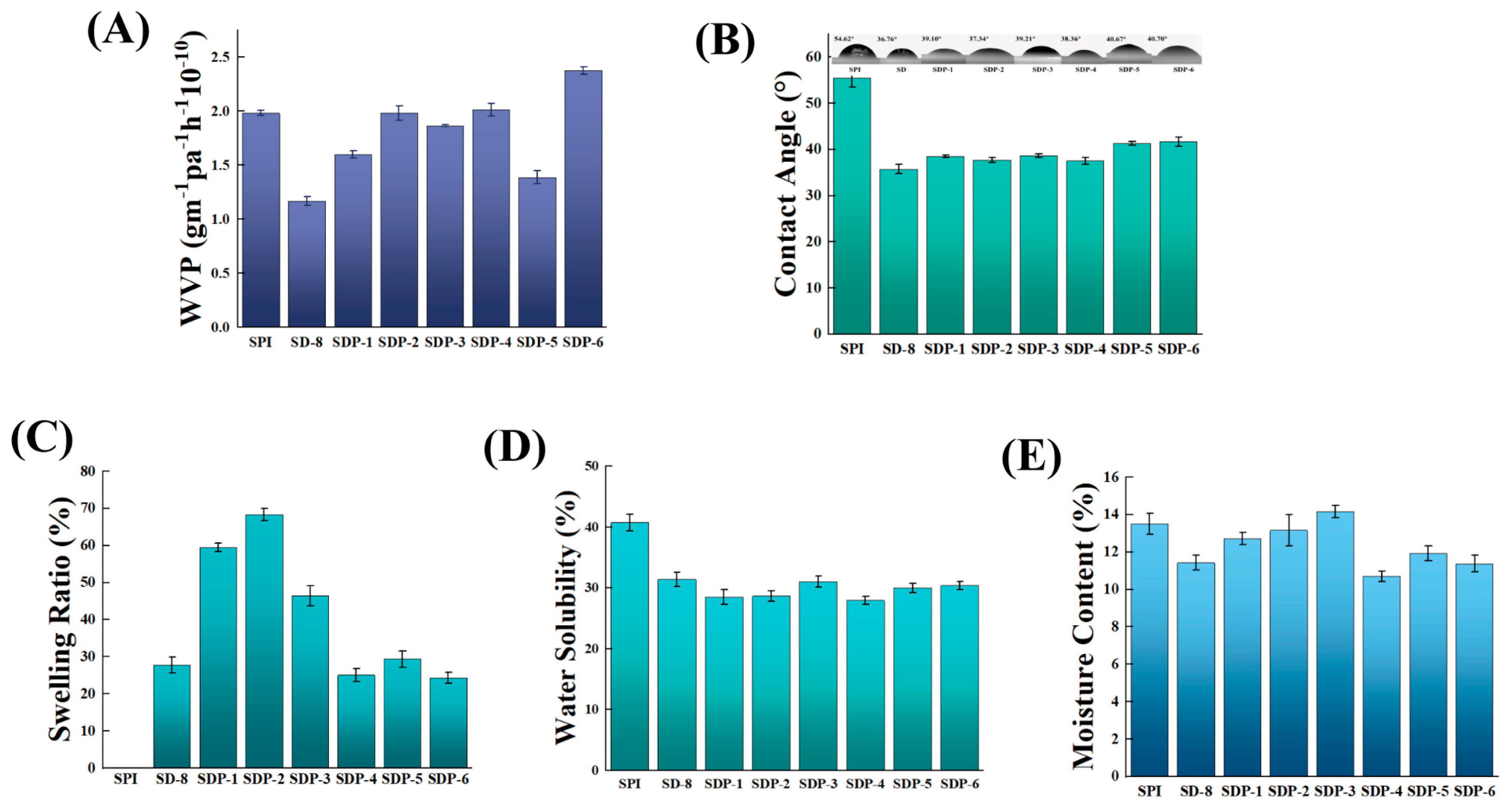
3.4. Water Responsive Properties
3.5. Antioxidant and Antimicrobial Activities
3.5.1. Antioxidant Activity
3.5.2. Antimicrobial Activity
4. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mu, Y.; Lv, S.; Liu, J.; Tong, J.; Liu, L.; Wang, J.; He, T.; Wei, D. Recent advances in research on biomass-based food packaging film materials. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70093. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, R. A review on material and antimicrobial properties of soy protein isolate film. J. Polym. Environ. 2019, 27, 1613–1628. [Google Scholar] [CrossRef]
- Yu, M.; Hou, Y.; Zheng, L.; Han, Y.; Wang, D. Soy protein isolate-based active films functionalized with Zanthoxylum bungeanum by-products: Effects on barrier, mechanical, antioxidant and cherry tomato preservation performance. Int. J. Biol. Macromol. 2023, 253, 127539. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ramezan, Y.; Pourramezan, H.; Najafi, A.; Kamkari, A.; Goksen, G.; Huang, Z.; Zhang, W. Soy Protein Isolate (SPI)-Based Films/Coatings for Food Packaging: Research Progress on Properties and Applications. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70181. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Z.; Li, W.; Li, M.; Deng, Q.; Wang, Y.; Li, C.; Chu, P.K. Ecofriendly and biodegradable soybean protein isolate films incorporated with ZnO nanoparticles for food packaging. ACS Appl. Bio Mater. 2019, 2, 2202–2207. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qi, K.; Sun, J.; Liu, C. Effects of Glycosylation of Different Oligosaccharides on Structure and Film-Forming Properties of Soy Protein Isolate. J. Agric. Food Chem. 2025, 73, 21473–21487. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Xu, H. Insight into the formation mechanism of soy protein isolate films improved by cellulose nanocrystals. Food Chem. 2021, 359, 129971. [Google Scholar] [CrossRef]
- Xue, F.; Li, C.; Adhikari, B. Physicochemical properties of active films of rose essential oil produced using soy protein isolate-polyphenol conjugates for cherry tomato preservation. Food Chem. 2024, 452, 139614. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, Z.; Jia, L.; Wang, X.; He, T.; Wang, L.; Yao, G.; Xie, F. Soybean protein isolate-sodium alginate double network emulsion gels: Mechanism of formation and improved freeze-thaw stability. Int. J. Biol. Macromol. 2024, 274, 133296. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, R.; Cui, M.; Zhang, Y.; Jiang, L.; Tian, B.; Sui, X. Insight into the formation mechanism of soy protein isolate films improved by dialdehyde starch with different degrees of aldehyde substitution. Food Hydrocoll. 2023, 145, 109071. [Google Scholar] [CrossRef]
- Ren, Z.; Ning, Y.; Jiang, A.; Xu, J.; Cheng, X.; Wang, L. Fabricating an antioxidant and bacteriostatic soy protein isolate film double-crosslinked via dialdehyde cellulose nanofibers and Tara tannins for beef tallow and cooked pork preservation. Int. J. Biol. Macromol. 2025, 301, 140366. [Google Scholar] [CrossRef]
- Liu, B.; Gao, J.; Liu, X.; Zhang, X.; Zeng, X.; Zhang, X.; Zhao, P. Preparation of soybean isolate protein/xanthan gum/agar-Lycium ruthenicum anthocyanins intelligent indicator films and its application in mutton preservation. Int. J. Biol. Macromol. 2024, 283, 137751. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Wang, Z.; Li, Y.; Chen, H. Preparation and characterization of carvacrol/soybean protein isolate composite film with efficient antimicrobial and antioxidant activities and its application in grape preservation. Food Chem. 2025, 464, 141572. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Y.; Yuan, M.; Wang, Y.; Wang, D.; Guo, Z.; Wang, Z. Film-forming mechanism of blueberry anthocyanin-added soybean isolate protein-based biodegradable packaging film and its application in edible mushroom preservation. Food Packag. Shelf Life 2025, 49, 101525. [Google Scholar] [CrossRef]
- Fan, K.; Zhao, Q.; Zou, M.; Qin, Z.; Peng, X. Gallic acid modified and green cross-linked chitosan-dialdehyde cellulose antibacterial aerogels and adsorption of anionic dyes. React. Funct. Polym. 2024, 205, 106076. [Google Scholar] [CrossRef]
- Geng, C.; Jiang, Y.; Bian, H.; Huang, G. Selective gas-permeation films with nanoMOFs as gas “Switches” for mango preservation. Chem. Eng. J. 2024, 481, 148757. [Google Scholar] [CrossRef]
- Qian, L.; Jia, R.; Zhao, Q.; Sun, N.; Yang, J.; Wen, J.; Li, H.; Yang, J.; Mo, L.; Gao, W. Tough, antibacterial, and antioxidant chitosan-based composite films enhanced with proanthocyanidin and carvacrol essential oil for fruit preservation. Food Res. Int. 2025, 208, 116269. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Huang, L.; Zou, M.; Jiang, Q.; Sun, N.; Fan, K.; Wen, J.; Mo, L.; Gao, W.; Qin, Z. Development of a high-performance natural edible film from chitosan, dialdehyde cellulose, and proanthocyanidin with enhanced antioxidant activity. Food Hydrocoll. 2025, 163, 111053. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Q.; Huang, H.; Duan, Y.; Xiao, G.; Le, T. Enhanced physico-mechanical, barrier and antifungal properties of soy protein isolate film by incorporating both plant-sourced cinnamaldehyde and facile synthesized zinc oxide nanosheets. Colloids Surf. B Biointerfaces 2019, 180, 31–38. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Lan, Y.; Wang, X. Enhancing soy protein isolate flexibility through non-covalent ferulic acid modification: Implications for interfacial characteristics and protein-based emulsion performance. Food Struct. 2024, 42, 100400. [Google Scholar] [CrossRef]
- Fu, Y.; Tian, R.; Zhao, J.; Wang, J.; Sui, X. Elucidation of the thermal aggregation processing of soy protein isolate–dialdehyde carboxymethyl cellulose cross-linked gel. Food Chem. 2025, 490, 145162. [Google Scholar] [CrossRef]
- Ao, L.; Liu, P.; Wu, A.; Zhao, J.; Hu, X. Characterization of soybean protein isolate-food polyphenol interaction via virtual screening and experimental studies. Foods 2021, 10, 2813. [Google Scholar] [CrossRef]
- Jiang, Y.; Miao, J.; Jiang, Q.; Zhang, Y.; Mo, L.; Gao, W.; Qin, Z. Dialdehyde carboxylated cellulose green crosslinked soybean protein isolate based on Schiff base reaction to improve the emulsification performance and stability of emulsion. React. Funct. Polym. 2025, 212, 106244. [Google Scholar] [CrossRef]
- Huang, S.; Tao, R.; Ismail, A.; Wang, Y. Cellulose nanocrystals derived from textile waste through acid hydrolysis and oxidation as reinforcing agent of soy protein film. Polymers 2020, 12, 958. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xia, N.; Xu, L.; Zhang, H.; Zhang, H.; Chi, Y.; Zhang, Y.; Li, L.; Li, H. Preparation, characterization and application of SPI-based blend film with antioxidant activity. Food Packag. Shelf Life 2021, 27, 100614. [Google Scholar] [CrossRef]
- Cheng, H.; McClements, D.J.; Xu, H.; Zhang, Z.; Zhang, R.; Jin, Z.; Chen, L. Effect of various natural cross-linking agents on the properties of corn starch/soy protein isolate composite films. Carbohydr. Polym. 2025, 366, 123850. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jiang, H.; Han, Z.; Gu, W.; Chitrakar, B.; Meng, X. Carbon Dots-Enhanced Soy Protein Isolate/Polyvinyl Alcohol Composite Film for Active Preservation of Oxidation-Sensitive Foods. Antioxidants 2025, 14, 669. [Google Scholar] [CrossRef]
- Rani, P.; Singh, P.; Krishna, M.M.; Tian, H.; Basak, P.; Kumar, R. Biodegradation Studies and Material Properties of Quercetin Incorporated Soy Protein Isolate Films. Biopolymers 2025, 116, e70018. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Zhang, C.; Yuan, Y.; Wang, S. Nanoparticle and dialdehyde polysaccharide synergistically improving the physicochemical properties of soy protein isolate films enriched with lingonberry extract. Food Hydrocoll. 2025, 159, 110667. [Google Scholar] [CrossRef]
- Hadavifar, S.; Abedi-Firoozjah, R.; Bahramian, B.; Jafari, N.; Sadeghi, S.M.; Majnouni, S.; Ebrahimi, B.; Ehsani, A.; Tavassoli, M. Multifunctional performance of chitosan/soy protein isolation-based films impregnated carbon dots/anthocyanin derived from purple hull pistachio for tracking and extending the shelf life of fish. Food Hydrocoll. 2025, 159, 110678. [Google Scholar] [CrossRef]
- Wei, M.; Shan, M.; Zhang, L.; Chen, N.; Tie, H.; Xiao, Y.; Li, Z. Preparation of gelatin/ĸ-carrageenan active films through procyanidins crosslinking: Physicochemical, structural, antioxidant and controlled release properties. Food Hydrocoll. 2024, 153, 110023. [Google Scholar] [CrossRef]
- Tie, S.; Zhang, Q.; Zhao, Y.; Wu, Y.; Liu, D.; Zhao, L.; Gu, S. Design and preparation of novel antioxidant and antibacterial films containing procyanidins and phycocyanin for food packaging. RSC Adv. 2024, 14, 7572–7581. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Deng, J.; Wang, D.; Yang, H.; Yang, J.; Puangsin, B.; He, X.; Shi, Z. Slow-release antimicrobial preservation composite coating based on bamboo-derived xylan–A new way to preserve blueberry freshness. Food Chem. 2025, 463, 141291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, L.; Li, J.; Wang, L.; Song, X. Extraction and characterization of cellulose from Jerusalem artichoke residue and its application in blueberry preservation. Foods 2022, 11, 1065. [Google Scholar] [CrossRef] [PubMed]

| Sample | SPI (g) | Glycerol (g) | DACNC (g) | PA (g) |
|---|---|---|---|---|
| SPI | 0.6 | 0.18 | 0 | 0 |
| SD-8 | 0.6 | 0.18 | 0.048 | 0 |
| SDP-1 | 0.6 | 0.18 | 0.048 | 0.006 (1%) |
| SDP-2 | 0.6 | 0.18 | 0.048 | 0.012 (2%) |
| SDP-3 | 0.6 | 0.18 | 0.048 | 0.018 (3%) |
| SDP-4 | 0.6 | 0.18 | 0.048 | 0.024 (4%) |
| SDP-5 | 0.6 | 0.18 | 0.048 | 0.030 (5%) |
| SDP-6 | 0.6 | 0.18 | 0.048 | 0.036 (6%) |
| Sample | Thickness (mm) | TS (MPa) | EAB (%) | E (MPa) |
|---|---|---|---|---|
| SPI | 0.116 ± 0.018 | 2.63 ± 0.40 | 83.9 ± 13.2 | 0.0314 |
| SD-8 | 0.117 ± 0.023 | 7.55 ± 0.53 | 130.8 ± 30.4 | 0.0577 |
| SDP-1 | 0.113 ± 0.016 | 10.79 ± 1.35 | 113.3 ± 20.4 | 0.0952 |
| SDP-2 | 0.119 ± 0.013 | 12.35 ± 1.06 | 116.4 ± 11.9 | 0.1061 |
| SDP-3 | 0.125 ± 0.011 | 10.68 ± 1.23 | 101.0 ± 11.2 | 0.1058 |
| SDP-4 | 0.143 ± 0.039 | 12.88 ± 0.95 | 100.6 ± 23.4 | 0.1280 |
| SDP-5 | 0.155 ± 0.026 | 15.54 ± 1.11 | 102.4 ± 21.6 | 0.1518 |
| SDP-6 | 0.149 ± 0.025 | 12.33 ± 1.18 | 99.9 ± 26.9 | 0.1235 |
| Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|
| SPI | 80.70 ± 1.19 | 6.52 ± 0.89 | 35.80 ± 2.37 | 3.96 ± 0.46 |
| SD-8 | 84.49 ± 0.37 | 2.12 ± 0.12 | 31.81 ± 1.45 | 2.4 ± 0.41 |
| SDP-1 | 63.42 ± 0.89 | 18.40 ± 0.68 | 47.67 ± 1.48 | 1.97 ± 0.20 |
| SDP-2 | 55.84 ± 1.39 | 25.07 ± 1.87 | 50.69 ± 0.80 | 2.91 ± 1.67 |
| SDP-3 | 48.36 ± 1.19 | 28.87 ± 0.43 | 54.21 ± 0.80 | 2.11 ± 0.29 |
| SDP-4 | 39.71 ± 1.11 | 33.24 ± 3.29 | 52.66 ± 2.8 | 6.72 ± 1.06 |
| SDP-5 | 40.95 ± 1.58 | 30.18 ± 0.65 | 53.15 ± 1.80 | 6.01 ± 1.69 |
| SDP-6 | 39.39 ± 2.1 | 30.62 ± 0.69 | 48.84 ± 3.3 | 3.84 ± 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Fan, K.; Meng, M.; Qin, Z.; Sun, N. Dialdehyde Cellulose Nanocrystals and Proanthocyanidins Reinforced Soy Protein Isolate Films for Blueberry Preservation. Polymers 2025, 17, 2821. https://doi.org/10.3390/polym17212821
Wei J, Fan K, Meng M, Qin Z, Sun N. Dialdehyde Cellulose Nanocrystals and Proanthocyanidins Reinforced Soy Protein Isolate Films for Blueberry Preservation. Polymers. 2025; 17(21):2821. https://doi.org/10.3390/polym17212821
Chicago/Turabian StyleWei, Jiapeng, Kehao Fan, Manting Meng, Zhiyong Qin, and Ningjing Sun. 2025. "Dialdehyde Cellulose Nanocrystals and Proanthocyanidins Reinforced Soy Protein Isolate Films for Blueberry Preservation" Polymers 17, no. 21: 2821. https://doi.org/10.3390/polym17212821
APA StyleWei, J., Fan, K., Meng, M., Qin, Z., & Sun, N. (2025). Dialdehyde Cellulose Nanocrystals and Proanthocyanidins Reinforced Soy Protein Isolate Films for Blueberry Preservation. Polymers, 17(21), 2821. https://doi.org/10.3390/polym17212821






