Additive Manufacturing in Dentistry: A Comparative Study of Polymeric Surgical Guide Fabrication
Abstract
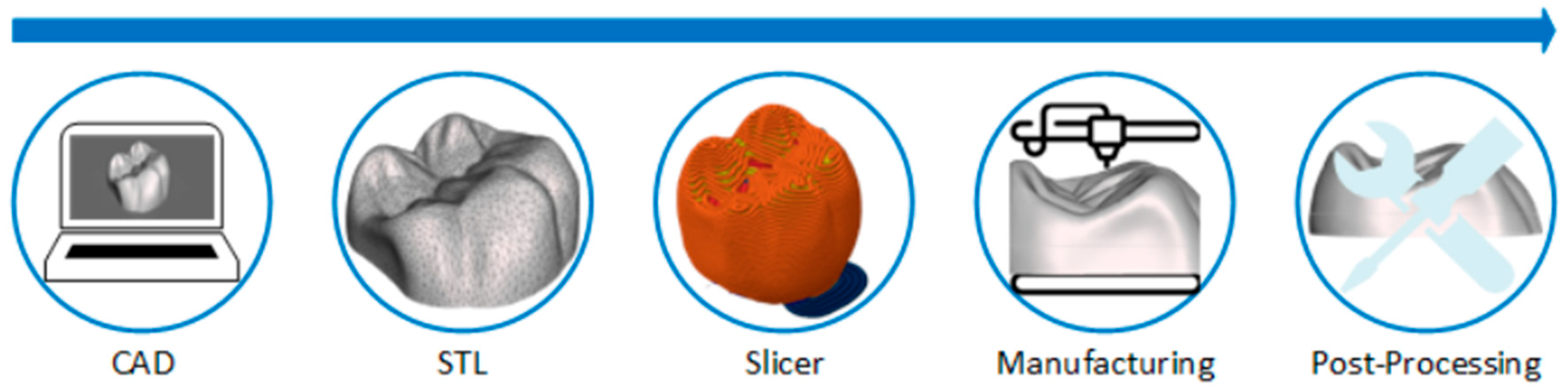
1. Introduction
2. Materials and Methods
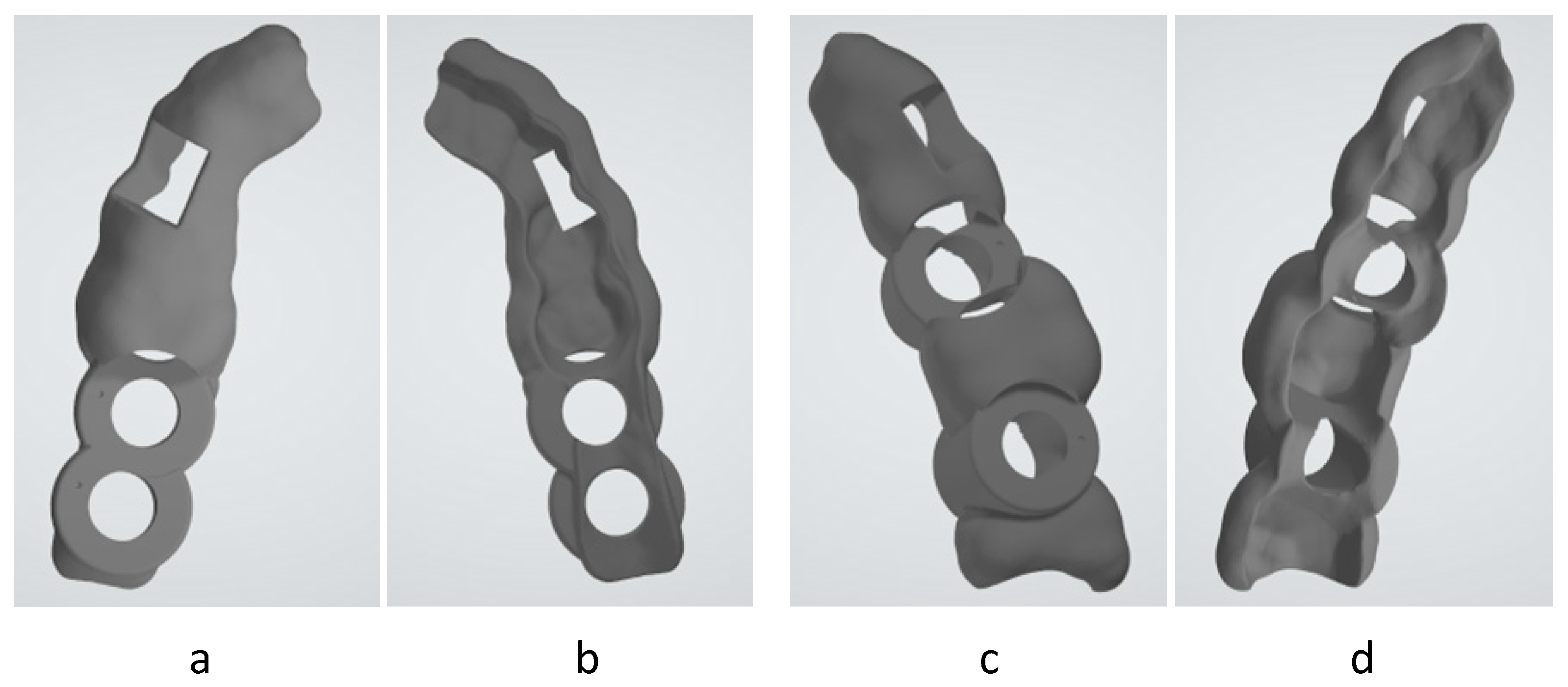
2.1. Selected Surgical Guide Model
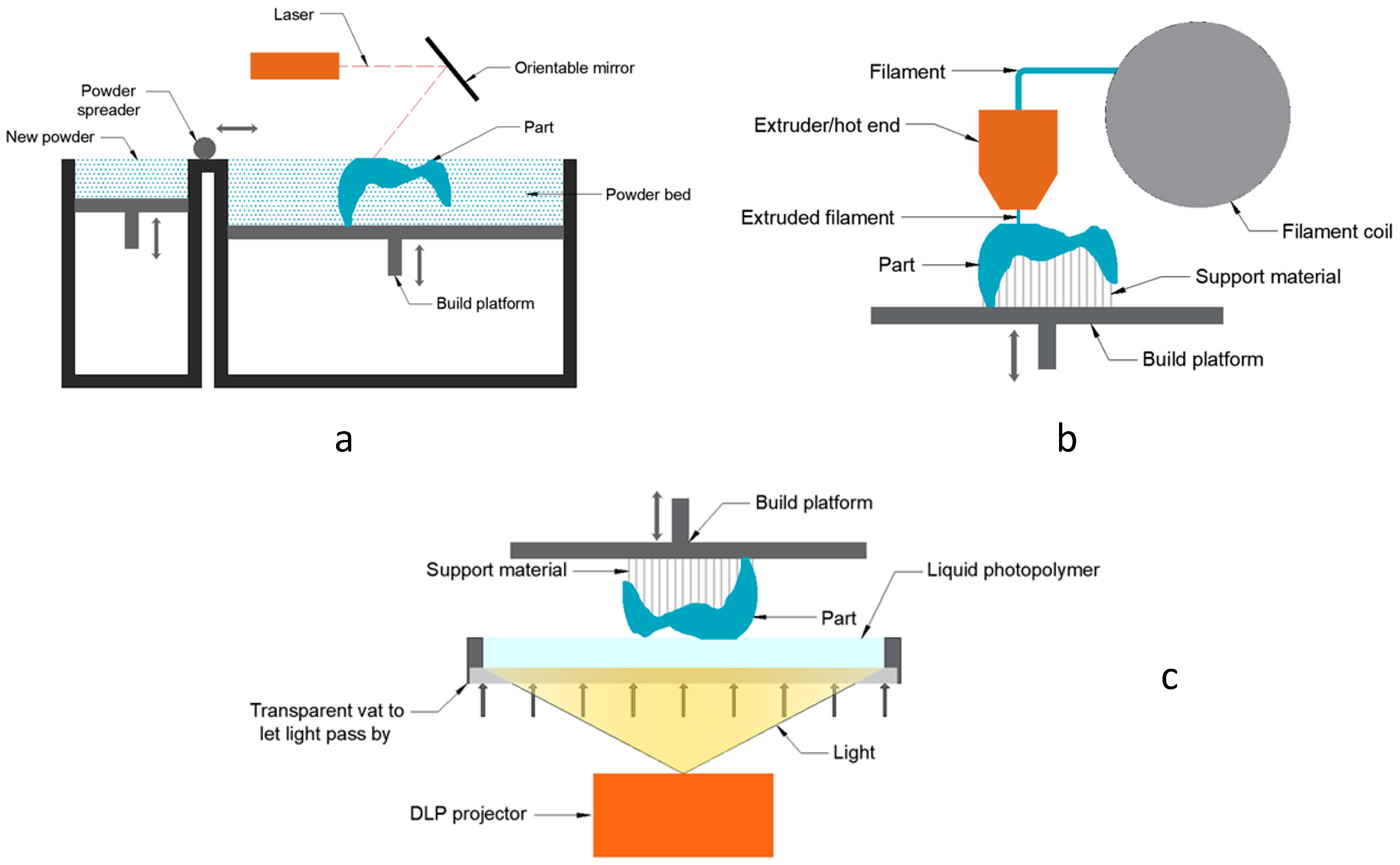
2.2. Additive Manufacturing Equipment
2.3. Selected Materials
2.4. Configuration of Manufacturing Processes
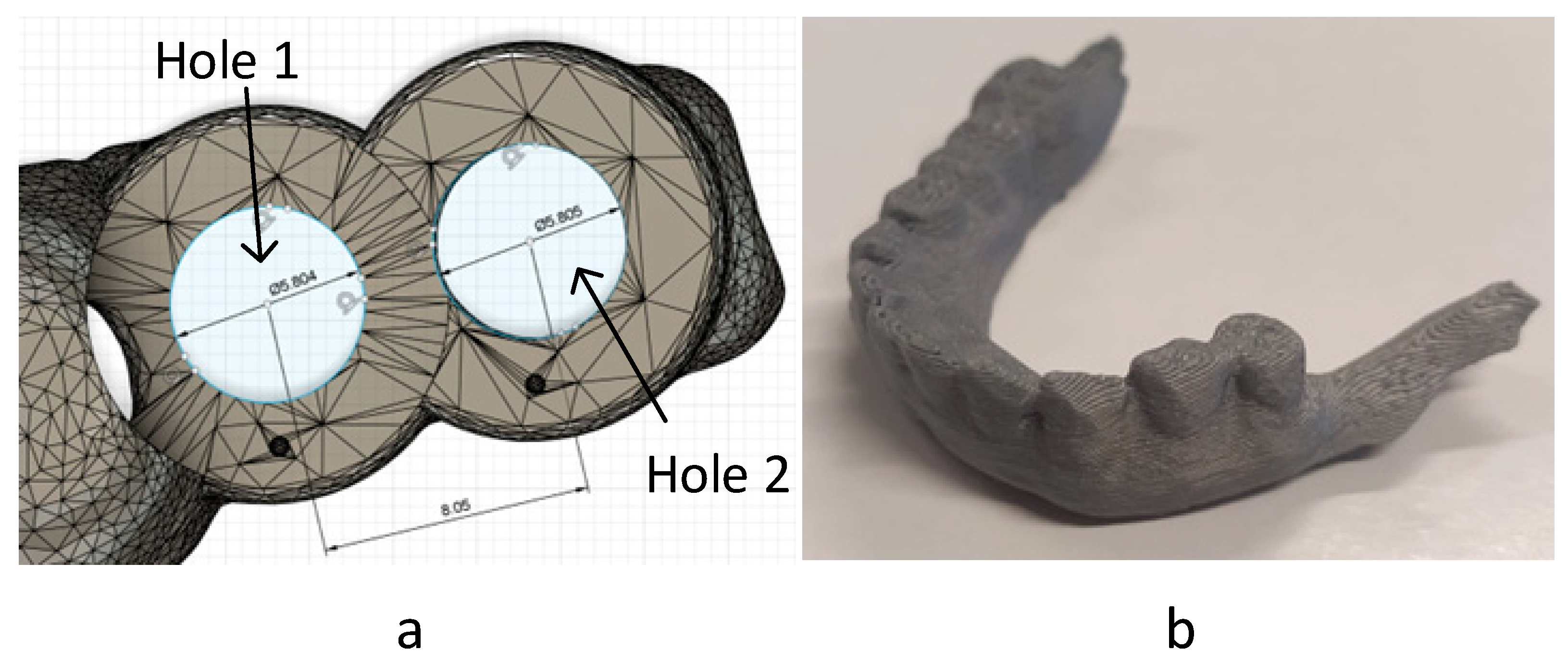
2.5. Accuracy and Dimensional Study
2.6. Methodology for Cost Analysis
- MEX: space cleaning, slicing, material feeding, machine start-up, and support removal.
- PBF: space cleaning, slicing, material feeding, machine start-up, part extraction from the powder block, and sandblasting cleaning.
- VPP: space cleaning, slicing, material feeding, machine start-up, washing in isopropyl alcohol, post-wash curing, and support removal.
- Machine use cost: The acquisition price of each technology (CA) was established, and this was used as a reference to define the maintenance and other costs during its useful life (CM). We estimated this cost at 10% of the total cost of the machine.
- As for the operator cost (CO), the salary tables of the collective bargaining agreement will be used as a reference, establishing the company cost of the operator at 12.04 EUR/h (0.2 EUR/min).
2.7. Manufacturing Processability
2.8. Criteria Weighting Method
- Manufacturing cost: lower cost, better score.
- Total manufacturing time: lower time per manufactured unit, better score.
- Dimensional accuracy: the more dimensionally similar the manufactured part and the original file, the better the score.
2.9. Moisture Content and Contact Angle
3. Results and Discussion
3.1. Results of Manufacturing Processability
3.2. Results of Accuracy and Dimensional Study
3.3. Manufacturing Cost of a Set of Parts
3.4. Manufacturing Costs of Multiple Sets of Parts
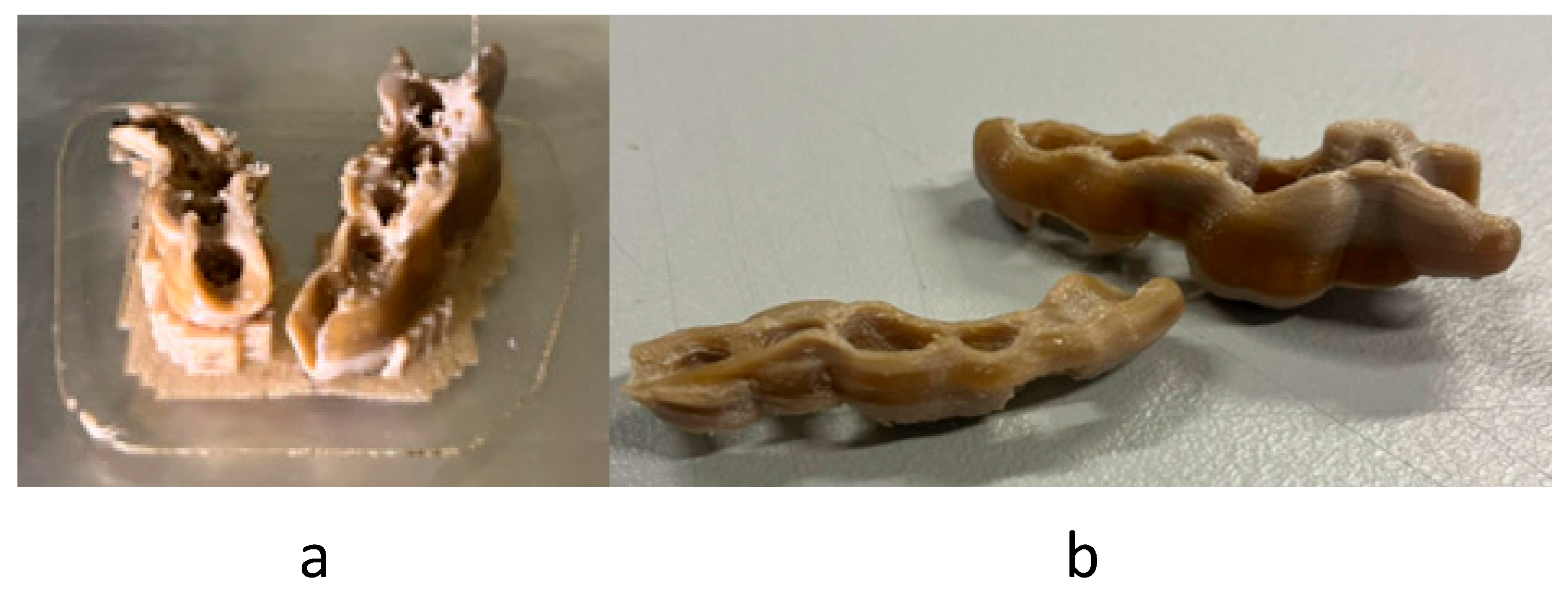
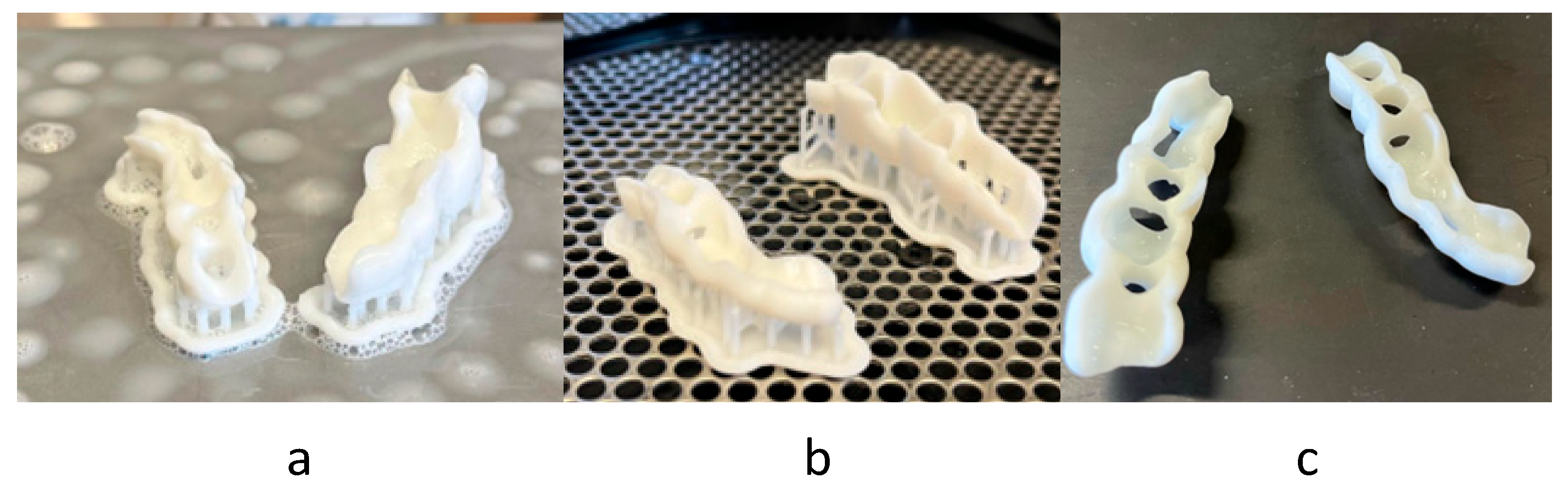
- MEX: MEX technology needs the use of supports, so only one layer of sets can be manufactured, being limited by the print area. Figure 9a shows the final arrangement. A total of 16 sets can be placed.
- VPP: This technology also needs the use of supports, so only one layer of sets was arranged, limited by the print area (Figure 9b). A total of four sets can be placed.
- PBF: This technology has the particularity of not needing supports for the manufacturing of parts, so that we can use the entire print volume of the machine. This allowed the placement of a total of 48 sets with the arrangement shown in Figure 9c.
3.5. Manufacturing Costs Depending on the Material
3.6. Scores Achieved
3.7. Clinical Practice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Al’aref, S.J.; Mosadegh, B.; Dunham, S.; Min, J.K. History of 3D Printing. In 3D Printing Applications in Cardiovascular Medicine; Academic Press: London, UK, 2018; pp. 1–10. ISBN 978-0-12-803917-5. [Google Scholar]
- Liaw, C.-Y.; Guvendiren, M. Current and Emerging Applications of 3D Printing in Medicine. Biofabrication 2017, 9, 24102. [Google Scholar] [CrossRef] [PubMed]
- Hague, R. Unlocking the Design Potential of Rapid Manufacturing. In Rapid Manufacturing; Hopkinson, N., Hague, R.J.M., Dickens, P.M., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 2005; pp. 5–18. ISBN 978-0-470-01613-8. [Google Scholar]
- Attaran, M. Additive Manufacturing: The Most Promising Technology to Alter the Supply Chain and Logistics. J. Serv. Sci. Manag. 2017, 10, 189–206. [Google Scholar] [CrossRef]
- Devine, D.M. (Ed.) Polymer-Based Additive Manufacturing: Biomedical Applications; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-24531-3. [Google Scholar]
- Kadambi, P.; Luniya, P.; Dhatrak, P. Current Advancements in Polymer/Polymer Matrix Composites for Dental Implants: A Systematic Review. Mater. Today Proc. 2021, 46, 740–745. [Google Scholar] [CrossRef]
- Hazeveld, A.; Slater, J.J.R.H.; Ren, Y. Accuracy and Reproducibility of Dental Replica Models Reconstructed by Different Rapid Prototyping Techniques. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 108–115. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, H.-W.; Seo, S.-J. Polymer-Ceramic Bionanocomposites for Dental Application. J. Nanomater. 2016, 2016, 3795976. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.N.; Hartgerink, J.D.; Schmalz, G. Scaffolds for Dental Pulp Tissue Engineering. Adv. Dent. Res. 2011, 23, 333–339. [Google Scholar] [CrossRef]
- Goodacre, B.J.; Goodacre, C.J.; Baba, N.Z.; Kattadiyil, M.T. Comparison of Denture Base Adaptation between CAD-CAM and Conventional Fabrication Techniques. J. Prosthet. Dent. 2016, 116, 249–256. [Google Scholar] [CrossRef]
- Lee, J.-S.; Hong, J.M.; Jung, J.W.; Shim, J.-H.; Oh, J.-H.; Cho, D.-W. 3D Printing of Composite Tissue with Complex Shape Applied to Ear Regeneration. Biofabrication 2014, 6, 24103. [Google Scholar] [CrossRef] [PubMed]
- Wiria, F.E.; Shyan, J.Y.M.; Lim, P.N.; Wen, F.G.C.; Yeo, J.F.; Cao, T. Printing of Titanium Implant Prototype. Mater. Des. 2010, 31, S101–S105. [Google Scholar] [CrossRef]
- Scheithauer, U.; Schwarzer, E.; Richter, H.-J.; Moritz, T. Thermoplastic 3D Printing—An Additive Manufacturing Method for Producing Dense Ceramics. Int. J. Appl. Ceram. Technol. 2015, 12, 26–31. [Google Scholar] [CrossRef]
- Maleksaeedi, S.; Eng, H.; Wiria, F.E.; Ha, T.M.H.; He, Z. Property Enhancement of 3D-Printed Alumina Ceramics Using Vacuum Infiltration. J. Mater. Process. Technol. 2014, 214, 1301–1306. [Google Scholar] [CrossRef]
- ISO/ASTM 52900:2021; Additive Manufacturing. General Principles. Fundamentals and Vocabulary. ISO International Organization for Standardization: Geneva, Switzerland, 2021.
- Alex, Y.; Shanmugam, R.; Salunkhe, S.; Čep, R. Advancement of Additive Manufacturing Technology in the Development of Personalized in Vivo and in Vitro Prosthetic Implants. Rev. Adv. Mater. Sci. 2025, 64, 20250109. [Google Scholar] [CrossRef]
- Johansson, C.; Dibes, J.; Rodriguez, L.E.L.; Papia, E. Accuracy of 3D Printed Polymers Intended for Models and Surgical Guides Printed with Two Different 3D Printers. Dent. Mater. J. 2021, 40, 339–347. [Google Scholar] [CrossRef]
- Huang, G.; Wu, L.; Hu, J.; Zhou, X.; He, F.; Wan, L.; Pan, S.-T. Main Applications and Recent Research Progresses of Additive Manufacturing in Dentistry. BioMed Res. Int. 2022, 2022, 5530188. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Donaldson, C.D.; Wertheim, D.; Naini, F.B. Accuracy and Precision of Three-Dimensionally Printed Orthognathic Surgical Splints. Appl. Sci. 2024, 14, 6089. [Google Scholar] [CrossRef]
- Pradíes, G.; Morón-Conejo, B.; Martínez-Rus, F.; Salido, M.P.; Berrendero, S. Current Applications of 3D Printing in Dental Implantology: A Scoping Review Mapping the Evidence. Clin. Oral Implant. Res. 2024, 35, 1011–1032. [Google Scholar] [CrossRef]
- Kroczek, K.; Turek, P.; Mazur, D.; Szczygielski, J.; Filip, D.; Brodowski, R.; Balawender, K.; Przeszłowski, Ł.; Lewandowski, B.; Orkisz, S.; et al. Characterisation of Selected Materials in Medical Applications. Polymers 2022, 14, 1526. [Google Scholar] [CrossRef] [PubMed]
- Lüchtenborg, J.; Burkhardt, F.; Nold, J.; Rothlauf, S.; Wesemann, C.; Pieralli, S.; Wemken, G.; Witkowski, S.; Spies, B.C. Implementation of Fused Filament Fabrication in Dentistry. Appl. Sci. 2021, 11, 6444. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Current Status and Applications of Additive Manufacturing in Dentistry: A Literature-Based Review. J. Oral Biol. Craniofacial Res. 2019, 9, 179–185. [Google Scholar] [CrossRef]
- Assari, A. Usability of Three-Dimensional Printing in Maxillofacial Surgery: A Narrative Review. Open Dent. J. 2023, 17, e187421062304190. [Google Scholar] [CrossRef]
- Hegedus, T.; Kreuter, P.; Kismarczi-Antalffy, A.A.; Demeter, T.; Banyai, D.; Vegh, A.; Geczi, Z.; Hermann, P.; Payer, M.; Zsembery, A.; et al. User Experience and Sustainability of 3D Printing in Dentistry. Int. J. Environ. Res. Public Health 2022, 19, 1921. [Google Scholar] [CrossRef]
- Anadioti, E.; Kane, B.; Soulas, E. Current and Emerging Applications of 3D Printing in Restorative Dentistry. Curr. Oral Health Rep. 2018, 5, 133–139. [Google Scholar] [CrossRef]
- Bathija, A.; Papaspyridakos, P.; Finkelman, M.; Kim, Y.; Kang, K.; Souza, A.B.D. Accuracy of Static Computer-Aided Implant Surgery (S-CAIS) Using CAD-CAM Surgical Templates Fabricated from Different Additive Manufacturing Technologies. J. Prosthet. Dent. 2025, 133, 524–529. [Google Scholar] [CrossRef]
- Chaudhary, S.; Avinashi, S.K.; Rao, J.; Gautam, C. Recent Advances in Additive Manufacturing, Applications and Challenges for Dentistry: A Review. ACS Biomater. Sci. Eng. 2023, 9, 3987–4019. [Google Scholar] [CrossRef]
- Moreira, J.P.d.A. Impressão 3D em Medicina Dentária. Master’s Thesis, Área de Medicina Dentária da Faculdade de Medicina da Universidade de Coimbra, Coimbra, Portugal, 2017. [Google Scholar]
- Huang, S.; Wei, H.; Li, D. Additive Manufacturing Technologies in the Oral Implant Clinic: A Review of Current Applications and Progress. Front. Bioeng. Biotechnol. 2023, 11, 1100155. [Google Scholar] [CrossRef]
- Demiralp, E.; Dogru, G.; Yilmaz, H. Additive Manufacturing (3D Printing) Methods and Applications in Dentistry. Clin. Exp. Health Sci. 2021, 11, 182–190. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, M.; Chohan, J.S. The Role of Additive Manufacturing for Biomedical Applications: A Critical Review. J. Manuf. Process. 2021, 64, 828–850. [Google Scholar] [CrossRef]
- Alharbi, N.; Osman, R.B. Additive Manufacturing Technologies: Where Did We Start and Where Have We Landed? A Narrative Review. Int. J. Prosthodont. 2024, 37, S243–S252. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Shin, Y.-S.; Jung, H.-D.; Hwang, C.-J.; Baik, H.-S.; Cha, J.-Y. Precision and Trueness of Dental Models Manufactured with Different 3-Dimensional Printing Techniques. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Sium-Abel, A.; Keilig, L.; Dörsam, I.; Bourauel, C. Accuracy of Template-Based Guided Dental Implant Placement—An in Vitro Comparison of Different Manufacturing Methods. Clin. Oral Implant. Res. 2024, 35, 864–875. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Chen, L.; Wang, Y.; Sun, Y. Application of FDM Three-Dimensional Printing Technology in the Digital Manufacture of Custom Edentulous Mandible Trays. Sci. Rep. 2016, 6, 19207. [Google Scholar] [CrossRef]
- Miljanovic, D.; Seyedmahmoudian, M.; Stojcevski, A.; Horan, B. Design and Fabrication of Implants for Mandibular and Craniofacial Defects Using Different Medical-Additive Manufacturing Technologies: A Review. Ann. Biomed. Eng. 2020, 48, 2285–2300. [Google Scholar] [CrossRef]
- Gao, J.; Pan, Y.; Gao, Y.; Pang, H.; Sun, H.; Cheng, L.; Liu, J. Research Progress on the Preparation Process and Material Structure of 3D-Printed Dental Implants and Their Clinical Applications. Coatings 2024, 14, 781. [Google Scholar] [CrossRef]
- Barbin, T.; Velôso, D.V.; Del Rio Silva, L.; Borges, G.A.; Presotto, A.G.C.; Barão, V.A.R.; Mesquita, M.F. 3D Metal Printing in Dentistry: An in Vitro Biomechanical Comparative Study of Two Additive Manufacturing Technologies for Full-Arch Implant-Supported Prostheses. J. Mech. Behav. Biomed. Mater. 2020, 108, 103821. [Google Scholar] [CrossRef] [PubMed]
- Henprasert, P.; Dawson, D.V.; El-Kerdani, T.; Song, X.; Couso-Queiruga, E.; Holloway, J.A. Comparison of the Accuracy of Implant Position Using Surgical Guides Fabricated by Additive and Subtractive Techniques. J. Prosthodont. 2020, 29, 534–541. [Google Scholar] [CrossRef]
- Jeong, Y.-G.; Lee, W.-S.; Lee, K.-B. Accuracy Evaluation of Dental Models Manufactured by CAD/CAM Milling Method and 3D Printing Method. J. Adv. Prosthodont. 2018, 10, 245–251. [Google Scholar] [CrossRef]
- Beefathimathul, H. Precision and Customization: The Role of 3D Printing in Modern Prosthodontics. Eur. J. Dent. 2025, 19, 580–586. [Google Scholar] [CrossRef]
- Rouzé l’Alzit, F.; Cade, R.; Naveau, A.; Babilotte, J.; Meglioli, M.; Catros, S. Accuracy of Commercial 3D Printers for the Fabrication of Surgical Guides in Dental Implantology. J. Dent. 2022, 117, 103909. [Google Scholar] [CrossRef]
- Pandey, D.; Shivarkar, A. Dental Services Market Size, Share, and Trends 2025 to 2034; Predence Research: Ottawa, ON, Canada, 2025. [Google Scholar]
- EuroStat. Healthcare Personnel Statistics—Dentists, Pharmacists and Physiotherapists; EuroStat: Luxembourg, 2024. [Google Scholar]
- La Odontología en España Según sus Profesionales. Tendencias y Evolución Empresarial. Available online: www.eldentistamoderno.com (accessed on 19 August 2025).
- Haleem, A.; Javaid, M. Polyether Ether Ketone (PEEK) and Its Manufacturing of Customised 3D Printed Dentistry Parts Using Additive Manufacturing. Clin. Epidemiol. Glob. Health 2019, 7, 654–660. [Google Scholar] [CrossRef]
- Logatharshiini, A.; Matheel, A.-R.; Nur Fatiha, G.; Rabihah, A.; Kubang, K. Revolutionizing Healthcare: Recent Advances of Polyamide 12 in Medical Applications. Glob. J. Med. Pharm. Biomed. Update 2025, 20, 19. [Google Scholar]
- Alfadhli, S.F. Evaluation of the Bond Strength of Various Denture Teeth Materials to Conventional, Cad/Cam, and 3D Printed Denture Bases. Master’s Thesis, Boston Univeristy, Boston, MA, USA, 2022. [Google Scholar]
- PLA PRO1 Ultrafuse. Available online: https://filament2print.com/es/pla/1936-9404-pla-pro1-ultrafuse.html (accessed on 23 August 2025).
- PP (Polipropileno) PPprint 721. Available online: https://filament2print.com/es/pp-polipropileno/1013-4143-ppprint-721.html (accessed on 23 August 2025).
- peek-ketaspire. Available online: https://filament2print.com/es/descatalogados/1794-8915-peek-ketaspire.html (accessed on 23 August 2025).
- Filamento PEN—Polietileno Naftalato. Available online: https://filament2print.com/es/pet-petg-cpe/2980-19036-filamento-pen-polietileno-naftalato.html (accessed on 23 August 2025).
- ABS Medical Smartfil. Available online: https://filament2print.com/es/abs/788-1494-abs-medical-smartfil.html (accessed on 23 August 2025).
- Resina KeyDenture Try-In. Available online: https://filament2print.com/es/dental-medicina/1964-9550-resina-keydenture-try-in.html (accessed on 23 August 2025).
- Resina MED413—Loctite 3D. Available online: https://filament2print.com/es/dental-medicina/2224-10653-resina-ind405hdt50-highelongation-loctite3d.html (accessed on 23 August 2025).
- Dental IBT-HARZ Labs. Available online: https://filament2print.com/es/dental-medicina/2913-18193-dental-ibt-harz-labs.html (accessed on 23 August 2025).
- PA12 Industrial—Polvo de Poliamida 12. Available online: https://filament2print.com/es/poliamida-pa12/2000-pa12-industrial-polvo-F2P-000171.html (accessed on 23 August 2025).
- PA12 Smooth—Polvo de Poliamida 12. Available online: https://filament2print.com/es/poliamida-pa12/1085-5237-pa12-smooth-polvo.html (accessed on 23 August 2025).
- PBT Optimal—Polvo para Impresión SLS. Available online: https://filament2print.com/es/polibuteno-pbt/2441-12088-pbt-optimal-polvo-para-impresion-sls.html (accessed on 23 August 2025).
- Sinterit PP—Polvo de Polipropileno. Available online: https://filament2print.com/es/polipropileno-pp/1332-6702-sinterit-pp-polvo-de-polipropileno-5904722229662.html (accessed on 23 August 2025).


| AM Category | Subcategory | Cases of Use |
|---|---|---|
| Vat Photopolymerization (VPP) | SLA | Customized implants [16] Surgical guides [21,26,27,28,29] Provisional restorations [26,30] Anatomic diagnostic models [31] Orthopedic implants [32] Occlusal splints [29] |
| DLP | Diagnostic study models [33] Dental models [25,34] Surgical guides [21,27,35] Gingival masks [21] Implants [20] | |
| Material Extrusion (MEX) | Customized implants [16] Dental models [25] Customized impression trays for edentulous jaws [31,36] Temporary crowns and bridges [28,32] Barrier membranes for bone defects [37] Flexible dental prostheses [28] | |
| Powder Bed Fusion (PBF) Polymers and metals | SLS | Customized implants [16] Dental models [23,25] Metal caps and frameworks [26,28] Crowns and dentures [28] Titanium and titanium alloy implants [38] |
| SLM | Customized implants [16] Near full density, high quality and complex geometries [16] Metal copings and frameworks [26,28] Full arch fixed dental prostheses (FAFDPs) [39] Customized titanium mesh [30] Metal primary frameworks (Ti-6-Al-4-V alloy and Co-Cr alloy) [20] | |
| EBM | Near full density, high quality and complex geometries [16] Full arch fixed dental prostheses (FAFDPs) [39] Open cell metal implants [18] Primary metal frameworks (Co-Cr alloy) [20] Dental implants [20] |
| PEEK K10 (MEX) | Sinterit PA12 (PBF) | White Resin V4 (Postcured) (VPP) | |
|---|---|---|---|
| Tensile E modulus (Mpa) (ISO 527) | 4200–4500 | 1662 | |
| Tensile E modulus (Mpa) (ASTM D 638-14) | 2800 | ||
| Tensile strength (Mpa) (ISO 527) | 70–80 | 42.3 | |
| Tensile strength (Mpa) (ASTM D 638-14) | 65 | ||
| Flexural Modulus (Mpa) (ISO 178) | 2400–2600 | 1506 | |
| Flexural Modulus (Mpa) (ASTM D 790-15) | 2200 |
| Hole 1 Diameter (mm) | Hole 1 Error (%) | Hole 2 Diameter (mm) | Hole 2 Error (%) | Distance Between Holes (mm) | Distance Between Holes Error (%) | |
|---|---|---|---|---|---|---|
| Design | 5.804 | 0 | 5.805 | 0 | 8.05 | 0 |
| VPP | 5.437 | 6.3 | 5.578 | 3.9 | 8.063 | 0.2 |
| PBF | 4.185 | 27.9 | 4.332 | 25.4 | 8.566 | 6.4 |
| MEX | 5.197 | 10.5 | 5.219 | 10.1 | 8.109 | 0.7 |
| MEX | VPP | PBF | ||||
|---|---|---|---|---|---|---|
| Printer | Printer | Curing | Printer | Sandblaster | Vacuum Cleaner | |
| Electrical consumption of the machine (kW/h) | 4.6 | 0.065 | 0.15 | 1 | - | 1.1 |
| Electrical cost per minute (EUR)—Cem | 0.01917 | 0.00027 | 0.00063 | 0.00417 | 0 | 0.00458 |
| Machine price (EUR) | 17,500 | 7750 | 1150 | 18,500 | 3530 | 4250 |
| Maintenance and others (EUR) | 1750 | 775 | 115 | 1850 | 353 | 425 |
| Depreciation of machine and maintenance per minute (EUR)—CU | 0.01082 | 0.00436 | 0.00065 | 0.01040 | 0.00198 | 0.00239 |
| MEX | VPP | PBF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Operator | Production | Operator | Production | Curing | Operator | Production | Dust Suction | Sandblaster | |
| Previous preparation (min) | 10 | 10 | 10 | ||||||
| Extraction (min) | 1 | 10 | 30 | ||||||
| Manufacturing (min) | 80 | 144 | 390 | ||||||
| Post-processing (min) | 15 | 10 | 30 | 15 | 10 | 5 | |||
| Total by process (min) | 26 | 80 | 30 | 144 | 30 | 55 | 390 | 10 | 5 |
| Total by technology (min) | 106 | 204 | 445 | ||||||
| MEX | VPP | PBF | ||||
|---|---|---|---|---|---|---|
| Printer | Printer | Curing Machine | Printer | Sandblaster | Vacuum Cleaner | |
| Machine use cost (EUR/min) | 0.011 | 0.004 | 0.0007 | 0.010 | 0.002 | 0.002 |
| Electrical cost per minute (EUR/min) | 0.019 | 0.0003 | 0.0006 | 0.004 | 0 | 0.005 |
| Manufacturing time (min) | 80 | 144 | 30 | 390 | 15 | 15 |
| Machine use and electrical costs (EUR) | 2.399 | 0.705 | 5.816 | |||
| Operator cost per minute (EUR/min) | 0.2 | 0.2 | 0.2 | |||
| Operator time (min) | 26 | 30 | 55 | |||
| Operator cost (EUR) | 5.2 | 6 | 11 | |||
| Total manufacturing cost (EUR) | 7.60 | 6.70 | 16.76 | |||
| MEX | VPP | PBF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Operator | Production | Operator | Production | Curing | Operator | Production | Dust Suction | Sandblasting | |
| Previous preparation time (min) | 10 | 10 | 10 | ||||||
| Extraction time (min) | 1 | 10 | 30 | ||||||
| Manufacturing time (min) | 2572 | 210 | 5970 | ||||||
| Post-processing time (min) | 184 | 33 | 98 | 544 | 363 | 181 | |||
| Total by process (min) | 195 | 2572 | 53 | 210 | 98 | 584 | 5970 | 363 | 181 |
| Total by technology (min) | 2767 | 361 | 6554 | ||||||
| MEX | VPP | PBF | ||||
|---|---|---|---|---|---|---|
| Printer | Printer | Cure | Printer | Sandblaster | Vacuum Cleaner | |
| Machine use cost (EUR/min) | 0.01082 | 0.00436 | 0.00065 | 0.01040 | 0.00198 | 0.00239 |
| Electrical cost per minute (EUR/min) | 0.01917 | 0.00027 | 0.00063 | 0.00417 | 0 | 0.00458 |
| Manufacturing time (min) | 2572 | 210 | 98 | 5970 | 363 | 181 |
| Machine use and electrical costs (EUR) | 77.137 | 1.097 | 88.961 | |||
| Operator cost per minute (EUR/min) | 0.2 | 0.2 | 0.2 | |||
| Operator time (min) | 195 | 53 | 584 | |||
| Operator cost(EUR) | 39 | 10.6 | 116.8 | |||
| Total manufacturing cost (EUR) | 116.14 | 11.70 | 205.76 | |||
| Manufacturing cost per set (EUR/set) | 7.26 | 2.92 | 4.29 | |||
| Technology | Material | Material Cost per Volume (EUR/cm3) | Material Cost per Set (EUR/Set) | Manufacturing and Material Costs (EUR/Set) | Average Cost (EUR/Set) |
|---|---|---|---|---|---|
| MEX | BASF PLA PRO1 Ultrafuse | 0.0592 [50] | 0.3621 | 7.96 | 9.24 EUR/set |
| MEX | PP PPprint 721 | 0.0573 [51] | 0.3507 | 7.95 | |
| MEX | MedPhen PEEK Ketaspire | 0.9126 [52] | 5.5851 | 13.18 | |
| MEX | FLXR Filament PEN–Polietileno naftalato | 0.2638 [53] | 1.6145 | 9.21 | |
| MEX | ABS Medical Smartfil | 0.0497 [54] | 0.3042 | 7.90 | |
| VPP | Resina KeyDenture Try-In | 0.3594 [55] | 3.5403 | 10.24 | 10.08 EUR/set |
| VPP | Resina MED413–Loctite 3D | 0.5160 [56] | 5.0826 | 11.79 | |
| VPP | Dental IBT–HARZ Labs | 0.1536 [57] | 1.5133 | 8.22 | |
| PBF | Sinterit PA12 Industrial | 0.0814 [58] | 0.4288 | 17.19 | 17.34 EUR/set |
| PBF | Sinterit PA12 Smooth | 0.1104 [59] | 0.5818 | 17.34 | |
| PBF | Sinterit PBT Optimal | 0.1143 [60] | 0.6022 | 17.36 | |
| PBF | Sinterit PP | 0.1350 [61] | 0.7114 | 17.47 |
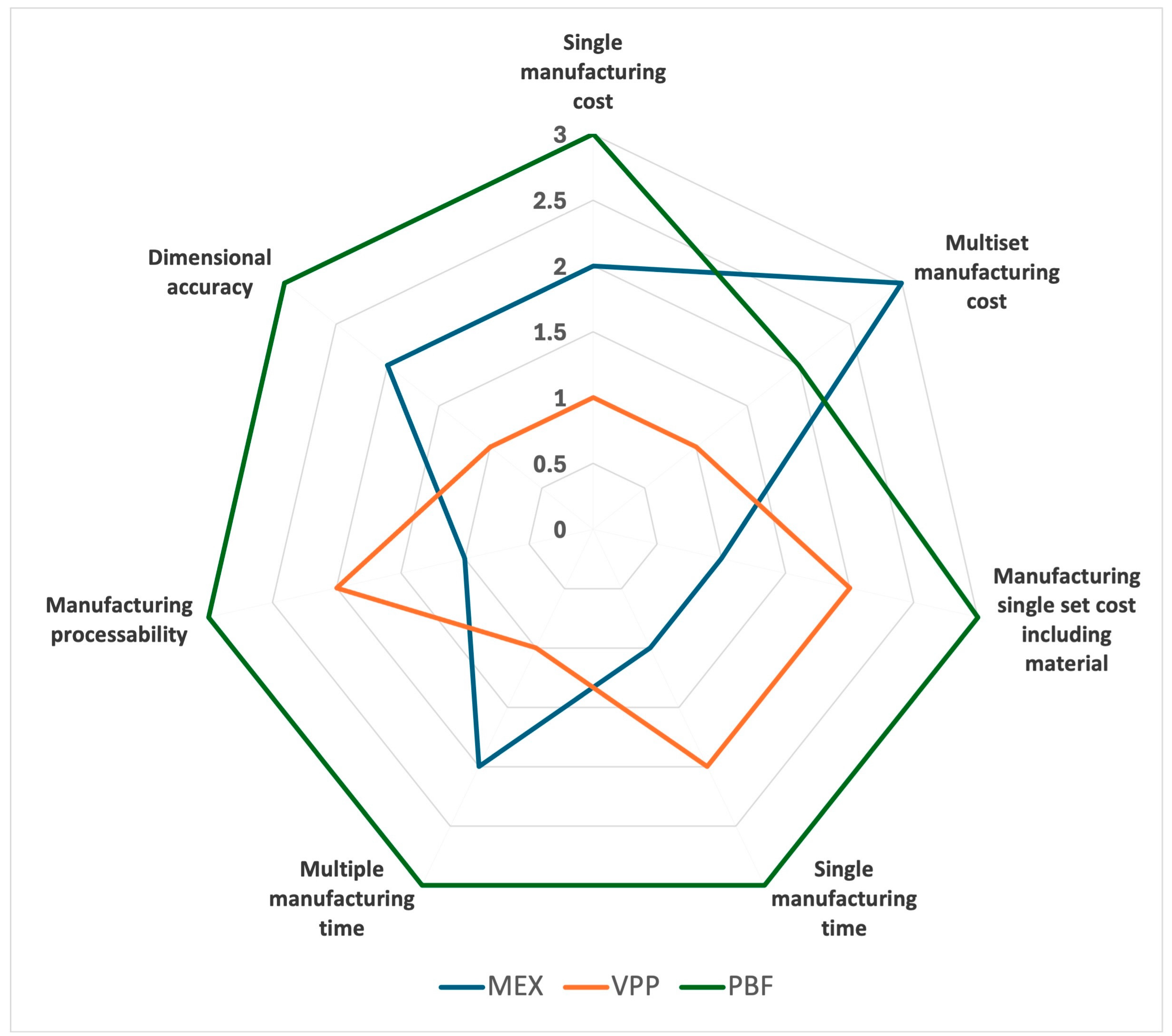
| Parameter | MEX | VPP | PBF |
|---|---|---|---|
| Single manufacturing cost | 2 | 1 | 3 |
| Multiset manufacturing cost | 3 | 1 | 2 |
| Manufacturing a single set cost including material | 1 | 2 | 3 |
| Single manufacturing time | 1 | 2 | 3 |
| Multiple manufacturing times | 2 | 1 | 3 |
| Manufacturing processability | 1 | 2 | 3 |
| Dimensional accuracy | 2 | 1 | 3 |
| Total scores | 12 | 10 | 20 |
| Graphical view | |||
 | |||
| Contact Angle (°) | Moisture Content (%) | |
|---|---|---|
| PEEK K10 (MEX) | 85.60 | 0.16 |
| Sinterit PA12 (PBF) | 138.23 | 1.23 |
| White Resin V4 (VPP) | 99.54 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Montagut, J.; González, A.; Paz, R.; Suárez, L.; Bordón, P.; Ortega, Z.; Antoniac, I.; Cacciotti, I.; Ileana, A.; Monzón, M. Additive Manufacturing in Dentistry: A Comparative Study of Polymeric Surgical Guide Fabrication. Polymers 2025, 17, 2764. https://doi.org/10.3390/polym17202764
García Montagut J, González A, Paz R, Suárez L, Bordón P, Ortega Z, Antoniac I, Cacciotti I, Ileana A, Monzón M. Additive Manufacturing in Dentistry: A Comparative Study of Polymeric Surgical Guide Fabrication. Polymers. 2025; 17(20):2764. https://doi.org/10.3390/polym17202764
Chicago/Turabian StyleGarcía Montagut, Joshua, Ana González, Rubén Paz, Luis Suárez, Pablo Bordón, Zaida Ortega, Iulian Antoniac, Ilaria Cacciotti, Adriana Ileana, and Mario Monzón. 2025. "Additive Manufacturing in Dentistry: A Comparative Study of Polymeric Surgical Guide Fabrication" Polymers 17, no. 20: 2764. https://doi.org/10.3390/polym17202764
APA StyleGarcía Montagut, J., González, A., Paz, R., Suárez, L., Bordón, P., Ortega, Z., Antoniac, I., Cacciotti, I., Ileana, A., & Monzón, M. (2025). Additive Manufacturing in Dentistry: A Comparative Study of Polymeric Surgical Guide Fabrication. Polymers, 17(20), 2764. https://doi.org/10.3390/polym17202764











