Thermosetting Resin for Plug and Abandonment of Oil Wells with Reduced Environmental Impact
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication
2.2.1. Preparation of the Liquid Resin
2.2.2. Preparation of the Solid Resin
2.3. Characterization Techniques
2.3.1. Density
2.3.2. Miscibility
2.3.3. Hardness
2.3.4. Environmental Hazard Identification of Chemicals
2.3.5. Imaging Methods
2.3.6. Water Pressure Resistance
2.3.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.8. Thermogravimetric Analysis
2.3.9. Rheology
2.3.10. Compressive Strength
2.3.11. Lap Shear Strength
3. Results and Discussion
3.1. Environmental Hazard Identification of Chemicals
3.2. Optimal Initiator Concentration
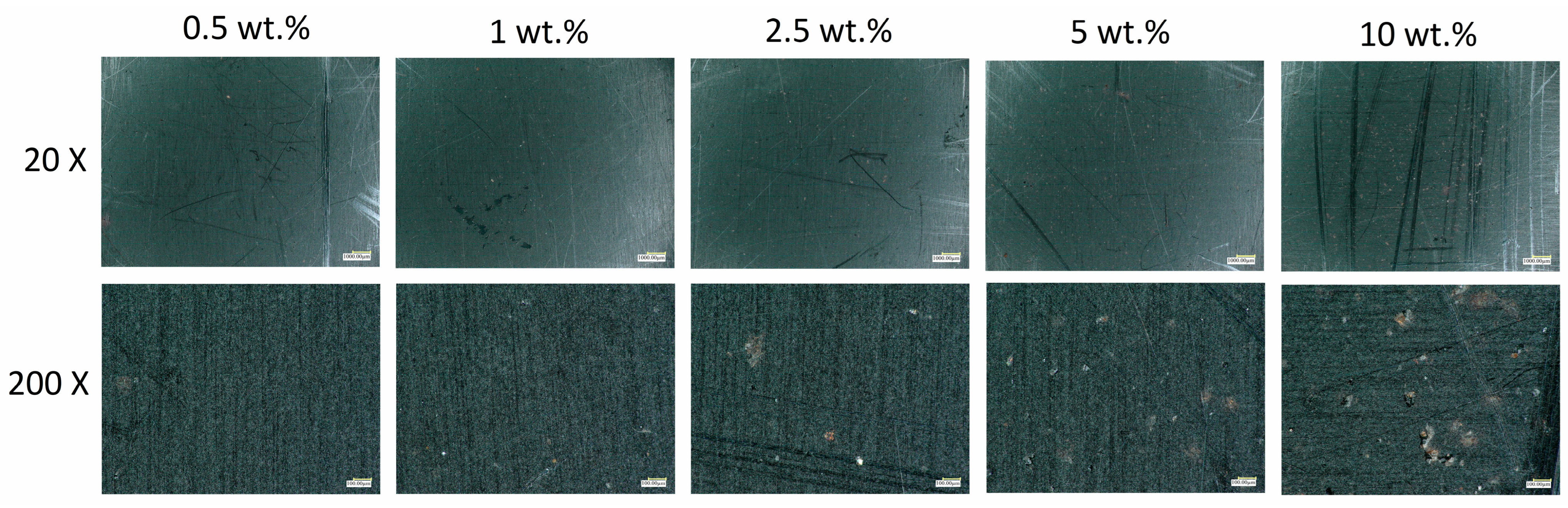
3.2.1. Microscopy
3.2.2. Computed Tomography and Water Pressure Resistance
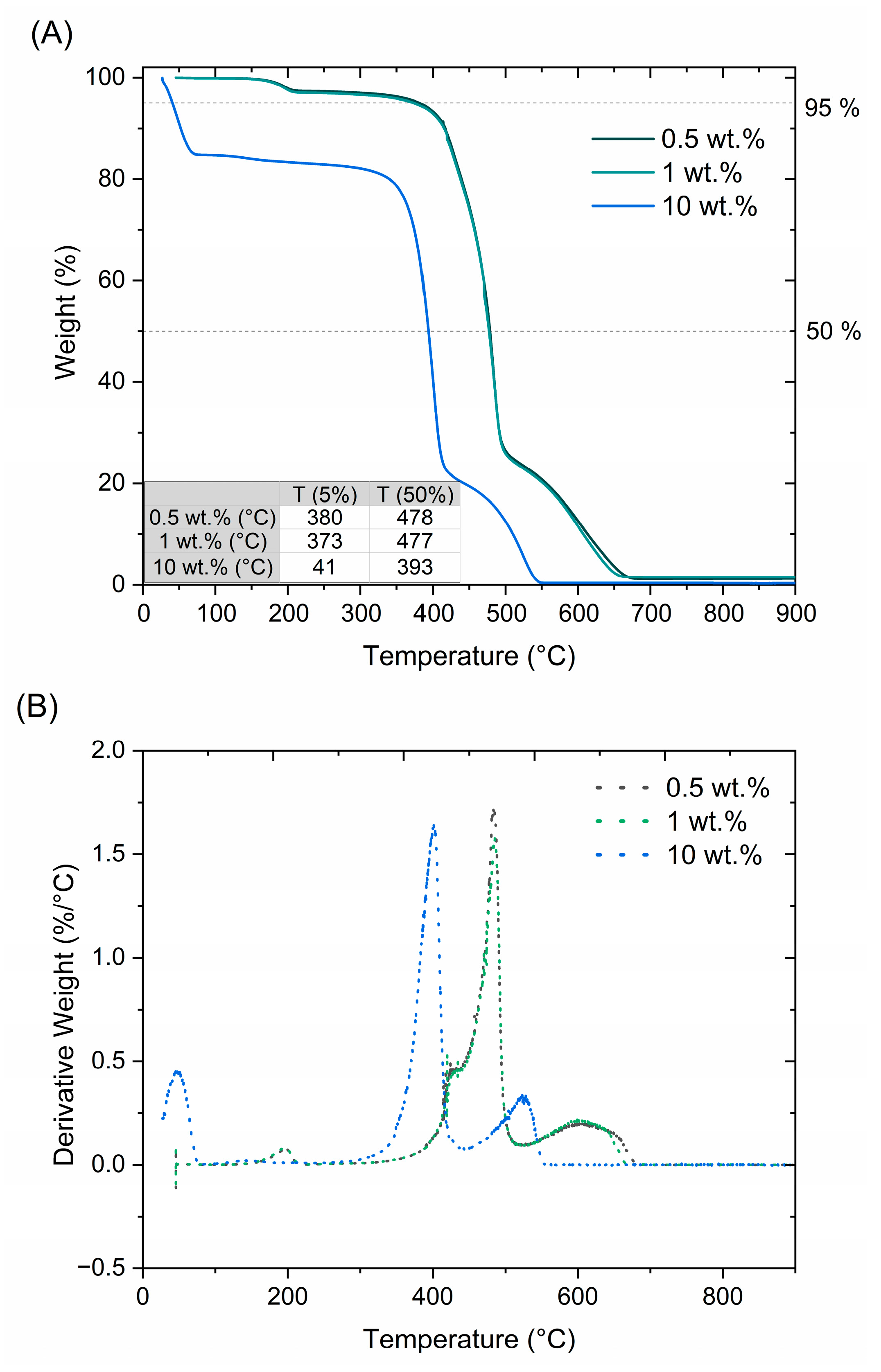
3.2.3. Thermal Stability
3.3. Rheological Characterization
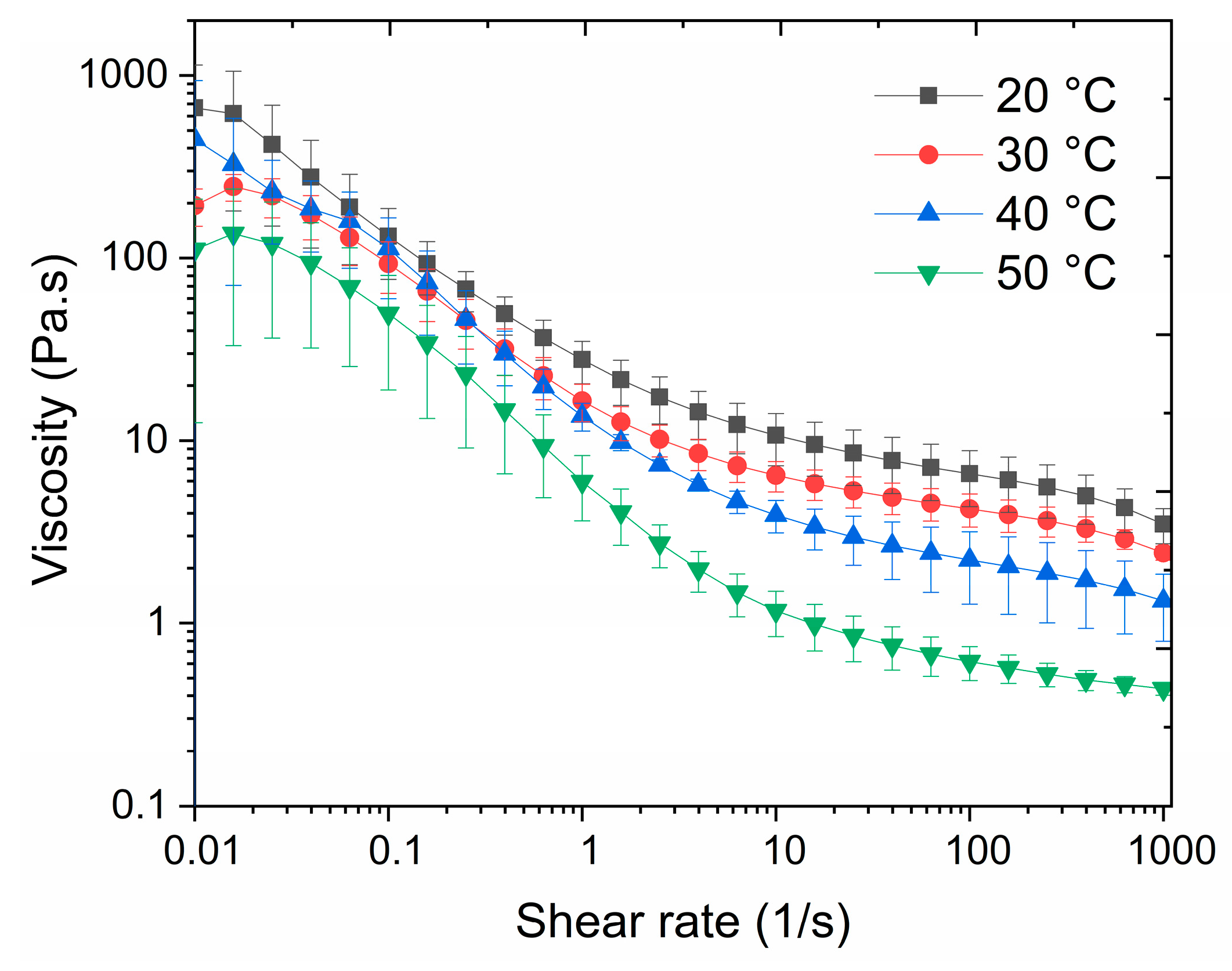
3.3.1. Viscosity of the Resin
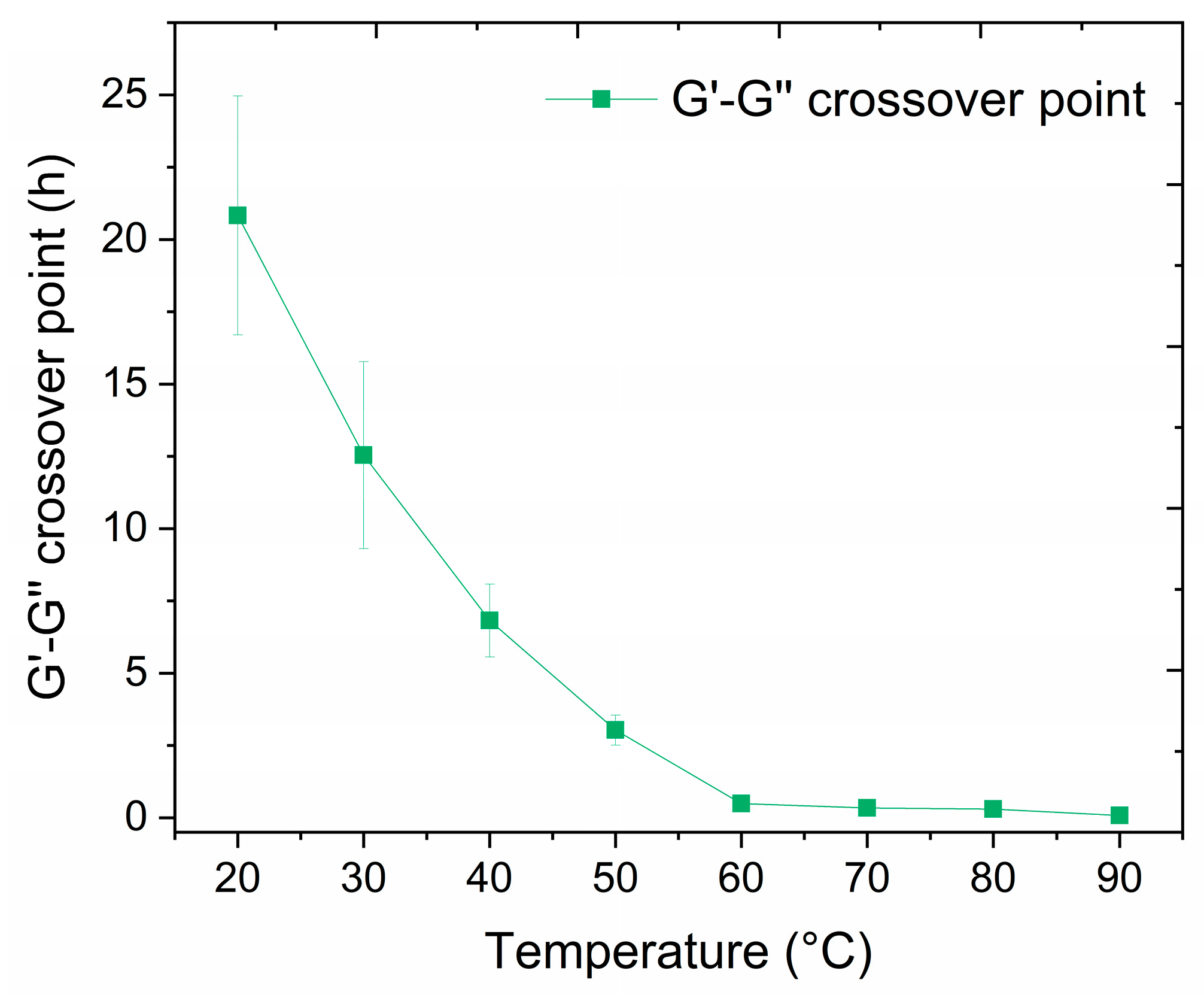
3.3.2. Cross-Linking Time Dependence on Temperature
3.4. Solid Polymer Plug Characterization
3.4.1. Compressive Strength
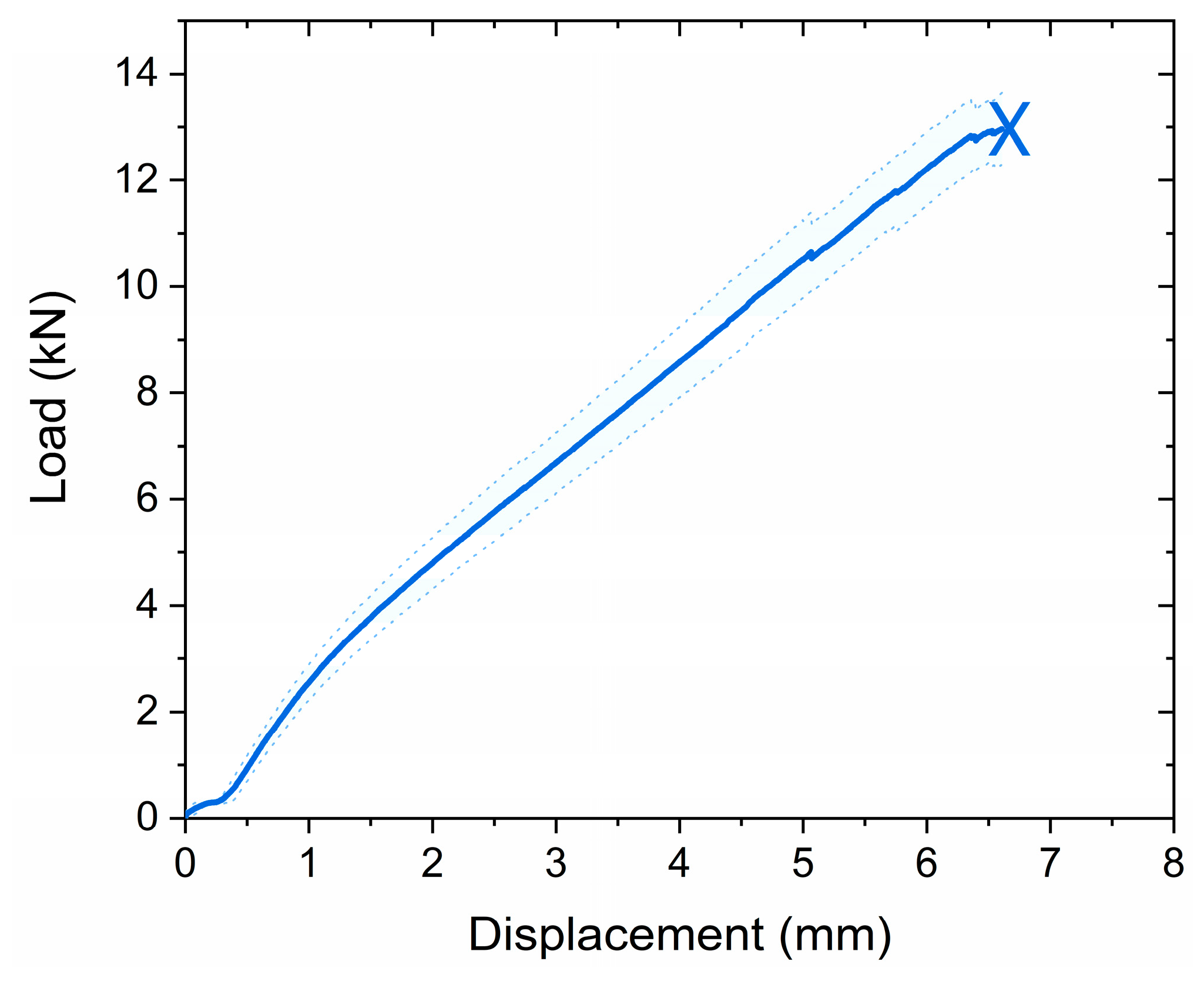
3.4.2. Lap Shear Strength
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalifeh, M.; Saasen, A. Introduction to Permanent Plug and Abandonment of Wells; Springer Nature: Cham, Switzerland, 2020; Volume 12. [Google Scholar] [CrossRef]
- Davison, J.M.; Salehabadi, M.; De Gennaro, S.; Wilkinson, D.; Hogg, H.; Hunter, C.; Schutjens, P. Plugging and abandonment of oil and gas wells: A geomechanics perspective. In Proceedings of the US Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 25–28 June 2017. [Google Scholar]
- Liversidge, D.; Taoutaou, S.; Agarwal, S. Permanent plug and abandonment solution for the North Sea. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Adelaide, Australia, 11–13 September 2006. [Google Scholar] [CrossRef]
- Vrålstad, T.; Saasen, A.; Fjær, E.; Øia, T.; Ytrehus, J.D.; Khalifeh, M. Plug & abandonment of offshore wells: Ensuring long-term well integrity and cost-efficiency. J. Pet. Sci. Eng. 2019, 173, 478–491. [Google Scholar] [CrossRef]
- Kiran, R.; Teodoriu, C.; Dadmohammadi, Y.; Nygaard, R.; Wood, D.; Mokhtari, M.; Salehi, S. Identification and evaluation of well integrity and causes of failure of well integrity barriers (A review). J. Nat. Gas Sci. Eng. 2017, 45, 511–526. [Google Scholar] [CrossRef]
- Yousuf, N.; Olayiwola, O.; Guo, B.; Liu, N. A comprehensive review on the loss of wellbore integrity due to cement failure and available remedial methods. J. Pet. Sci. Eng. 2021, 207, 109123. [Google Scholar] [CrossRef]
- Shryock, S.H.; Slagle, K.A. Problems Related to Squeeze Cementing. J. Pet. Technol. 1968, 20, 801–807. [Google Scholar] [CrossRef]
- Alkhamis, M.; Imqam, A.; Milad, M. Evaluation of an ultra-high performance epoxy resin sealant for wellbore integrity applications. In Proceedings of the SPE Symposium Decommissioning and Abandonment, Kuala Lumpur, Malaysia, 3–4 December 2021. [Google Scholar] [CrossRef]
- Ab Rahman, S.; Pang, A.; Arsad, A.; Sidek, A.; Saidu, A.; Awang, N.; Mohsin, R.; Abdurrahman, M. The chemistry insight: Epoxy sealant as an alternative remedial operation for well integrity. Rev. Chem. Eng. 2023, 39, 857–873. [Google Scholar] [CrossRef]
- Alsaihati, Z.A.; Al-Yami, A.S.; Wagle, V.; BinAli, A.; Mukherjee, T.S.; Al-Kubaisi, A.; Alsafran, A. Wagle. An Overview of Polymer Resin Systems Deployed for Remedial Operations in Saudi Arabia. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 24–27 April 2017. [Google Scholar] [CrossRef]
- Genedy, M.; Kandil, U.F.; Matteo, E.N.; Stormont, J.; Taha, M.M.R. A new polymer nanocomposite repair material for restoring wellbore seal integrity. Int. J. Greenh. Gas Control 2017, 58, 290–298. [Google Scholar] [CrossRef]
- Khan, D.A.; Acuna, M.C.; Hasan, S.I.; Merino, L.; Chica, P.; Hussein, A.M.; Normann, S. Restoring Well Integrity: The Innovative Design of Thermally Activated Resin in a Sour Environment. In Proceedings of the Middle East Oil, Gas and Geosciences Show, Manama, Bahrain, 19–21 February 2023. [Google Scholar] [CrossRef]
- Sanabria, A.E.; Knudsen, K.; Leon, G.A. Thermal Activated Resin to Repair Casing Leaks in the Middle East. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 7–10 November 2016. [Google Scholar] [CrossRef]
- Todorovic, J.; Raphaug, M.; Lindeberg, E.; Vrålstad, T.; Buddensiek, M.L. Remediation of leakage through annular cement using a polymer resin: A laboratory study. Energy Procedia 2016, 86, 442–449. [Google Scholar] [CrossRef]
- Bhaisora, D.; Aly, A.; Morsy, A.; Shamma, H.; El Nashar, R.; Abdel Karim, S.E.E. Innovative Application of Ultra-Lightweight Epoxy Resin-Cement Slurry Mixture Achieved Zonal Isolation Objectives and Secured Well Integrity: Case History From Offshore Gulf of Suez. In Proceedings of the International Petroleum Technology Conference, Doha, Qatar, 6–9 December 2015. [Google Scholar] [CrossRef]
- Moradi, S.S.T.; Nikolaev, N.I.; Leusheva, E.L. Improvement of cement properties using a single multi-functional polymer. Int. J. Eng. Trans. 2018, 31, 181–187. [Google Scholar] [CrossRef]
- Yami, A.; Buwaidi, H.; Al-Herz, A.; Mukherjee, T.S.; Bedford, D.; Viso, R.; Hugentobler, K. Application of Heavy Weight Cement-Resin Blend System to Prevent CCA Pressure in Saudi Arabia Deep Gas Fields. In Proceedings of the SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 4–6 April 2017. [Google Scholar] [CrossRef]
- Shanbhag, R.; Melrose, Z.; Nutt, S.; Cleveland, M.; Keese, R. Thermosetting polymer based flexible synthetic cement for successful zonal isolation in thermal wells—A new approach. In Proceedings of the SPE Thermal Well Integrity and Design Symposium, Banff, AB, Canada, 23–25 November 2015. [Google Scholar] [CrossRef]
- Kamali, M.; Khalifeh, M.; Saasen, A.; Godøy, R.; Delabroy, L. Alternative setting materials for primary cementing and zonal isolation–Laboratory evaluation of rheological and mechanical properties. J. Pet. Sci. Eng. 2021, 201, 108455. [Google Scholar] [CrossRef]
- de Morais, S.C.; do Nascimento Marques, N.; da Câmara, P.C.F.; de Souza, E.A.; de Carvalho Balaban, R. Versatile hydrolyzed polyacrylamide-polyethyleneimine gels reinforced with glycerol for plugging and abandonment of oil wells. J. Mol. Liq. 2023, 388, 122748. [Google Scholar] [CrossRef]
- Al-Yami, A.; Al-Jubran, M.; Aswad, H.; Looni, Q. Innovative Epoxy Resin Formulation for Downhole Casing Repair Applications. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 11–15 November 2018. [Google Scholar] [CrossRef]
- Al-Ansari, A.A.; Al-Refai, I.M.; Al-Beshri, M.H.; Pino, R.M.; Leon, G.A.; Knudsen, K.; Sanabria, A.E. Thermal activated resin to avoid pressure build-up in Casing-Casing Annulus (CCA). In Proceedings of the SPE Offshore Europe Conference and Exhibition, Aberdeen, Scotland, UK, 8–11 September 2015. [Google Scholar] [CrossRef]
- Beharie, C.; Francis, S.; Øvestad, K.H. Resin: An alternative barrier solution material. In Proceedings of the SPE Bergen One Day Seminar, Bergen, Norway, 22 April 2015. [Google Scholar] [CrossRef]
- Morris, K.A.; Deville, J.P.; Jones, P. Resin-Based Cement Alternatives for Deepwater Well Construction. In Proceedings of the SPE Deepwater Drilling and Completions Conference, Galveston, TX, USA, 20–21 June 2012. [Google Scholar] [CrossRef]
- Young, S.; Chew, A.; Mettath, S.; De Stefano, G. Thermo-settable cross-linked polymer system for optimization of wellbore stability and zonal isolation. In Proceedings of the Offshore Mediterranean Conference and Exhibition, Ravenna, Italy, 20–22 March 2013. [Google Scholar]
- Ying, X.; Yuan, X.; Yadong, Z.; Ziyi, F. Study of Gel Plug for Temporary Blocking and Well-Killing Technology in Low-Pressure, Leakage-Prone Gas Well. SPE Prod. Oper. 2021, 36, 234–244. [Google Scholar] [CrossRef]
- Brewis, D.M.; Mathieson, I.; Sutherland, I.; Cayless, R.A.; Dahm, R.H. Pretreatment of poly(vinyl fluoride) and poly(vinylidene fluoride) with potassium hydroxide. Int. J. Adhes. Adhes. 1996, 16, 87–95. [Google Scholar] [CrossRef]
- Li, D.; Liao, M. Dehydrofluorination mechanism, structure and thermal stability of pure fluoroelastomer (poly(VDF-ter-HFP-ter-TFE) terpolymer) in alkaline environment. J. Fluor. Chem. 2017, 201, 55–67. [Google Scholar] [CrossRef]
- ASTM D2250-15; Standard Test Method for Rubber Property Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2021.
- OSPAR Commission. Guidelines for Completing the Harmonised Offshore Chemical Notification Format (HOCNF) (OSPAR agreement: 2012/05. Update 2023). 2023, pp. 1–42. Available online: https://ospar.org/documents?v=51408 (accessed on 18 November 2024).
- Danish (Q)SAR Database, Research Group for Chemical Risk Assessment and GMO, National Food Institute, Technical University of Denmark. Available online: https://qsar.food.dtu.dk/ (accessed on 15 October 2024).
- ASTM D695-23; Standard Test Method for Compressive Properties of Rigid Plastics. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D1002-10; Standard Test Method for Apparent Shear Strength of Single-Lap-Joint Adhesively Bonded Metal Specimens by Tension Loading (Metal-to-Metal). ASTM International: West Conshohocken, PA, USA, 2019.
- De Vries, K.J.; Gellings, P.J. The thermal decomposition of potassium and sodium-pyrosulfate. J. Inorg. Nucl. Chem. 1969, 31, 1307–1313. [Google Scholar] [CrossRef]
- Maafa, I.M. Inhibition of Free Radical Polymerization: A Review. Polymer 2023, 15, 488. [Google Scholar] [CrossRef]
- Khalifeh, M.; Todorovic, J.; Vrålstad, T.; Saasen, A.; Hodne, H. Long-term durability of rock-based geopolymers aged at downhole conditions for oil well cementing operations. J. Sustain. Cem.-Based Mater. 2017, 6, 217–230. [Google Scholar] [CrossRef]
- Nascimento, J.D.; Rocha, C.A.A.; Simão, C.A.; Toledo Filho, R.D. Behavior of oil well cement slurry systems aged in CO2-saturated water under high pressure and temperature. J. Therm. Anal. Calorim. 2022, 147, 95–107. [Google Scholar] [CrossRef]
- Alberdi-Pagola, P.; Fischer, G. Quantification of Casing Expansion-Induced Cracking in Oilwell Cement Sheaths. SPE J. 2024, 29, 714–724. [Google Scholar] [CrossRef]
- Kamali, M.; Khalifeh, M.; Eid, E.; Saasen, A. Experimental Study of Hydraulic Sealability and Shear Bond Strength of Cementitious Barrier Materials. J. Energy Resour. Technol. Trans. 2022, 144, 23007. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Wang, X.; Cao, Y.; He, P.; Li, H.; Wang, L. Versatile Underwater Pressure Sensitive Adhesive: UV Curing Synthesis and Substrate-Independent Adhesion. ACS Appl. Mater. Interfaces. 2024, 16, 41461–41474. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]




| Compound | Aquatic Toxicity (mg/L) | Potential Bioaccumulation from Kow (QSAR) | Biodegradability (Level 0) | ||
|---|---|---|---|---|---|
| Microtox EC30min, 50 (A. fischeri) | Freshwater Algae EC72h, 50 (R. subcapitata) | Freshwater Crustacean EC48h, 50 (D. magna) | |||
| fPVDF | >100 | >100 | >100 | - | Not pass |
| Cross-linking agent (PETA) | >100 | 103 [66–140] | >100 | No | Not pass |
| Initiator (PP) | >100 | 29 [20–38] | >100 | No | - |
| Additive (Silica) | >100 * | >100 * | >100 * | No | Not pass |
| Temperature | ||||||||
|---|---|---|---|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | 50 °C | 60 °C | 70 °C | 80 °C | 90 °C | |
| G′-G″ crossover point (h) | 20.8 ± 4.1 | 12.5 ± 3.2 | 6.8 ± 1.3 | 3.0 ± 0.5 | 0.49 ± 0.03 | 0.34 ± 0.07 | 0.30 ± 0.03 | 0.08 ± 0.03 |
| G′ after 72 h (kPa) | - | 53.9 | - | - | 61.9 | - | - | 17,600 |
| Property | Value |
|---|---|
| Density at 25 °C | 1.33 g/cm3 |
| Viscosity (0.1–1000 1/s, 20–50 °C) | 800–0.15 Pa·s |
| Setting time (20–90 °C) | 20.8–0.08 h |
| Miscibility with water and oil | No |
| Pumpability | Yes |
| Target oil-well temperature | 90 °C |
| Thermal stability up to | 180 °C |
| Hardness—Shore A | 93 ± 5 A |
| Compressive strength | 54 ± 4 MPa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echarri-Giacchi, M.; Frederiksen, C.H.; Skjolding, L.M.; Skov, A.L.; Skowyra, M. Thermosetting Resin for Plug and Abandonment of Oil Wells with Reduced Environmental Impact. Polymers 2025, 17, 212. https://doi.org/10.3390/polym17020212
Echarri-Giacchi M, Frederiksen CH, Skjolding LM, Skov AL, Skowyra M. Thermosetting Resin for Plug and Abandonment of Oil Wells with Reduced Environmental Impact. Polymers. 2025; 17(2):212. https://doi.org/10.3390/polym17020212
Chicago/Turabian StyleEcharri-Giacchi, Maria, Christian Husum Frederiksen, Lars Michael Skjolding, Anne Ladegaard Skov, and Magdalena Skowyra. 2025. "Thermosetting Resin for Plug and Abandonment of Oil Wells with Reduced Environmental Impact" Polymers 17, no. 2: 212. https://doi.org/10.3390/polym17020212
APA StyleEcharri-Giacchi, M., Frederiksen, C. H., Skjolding, L. M., Skov, A. L., & Skowyra, M. (2025). Thermosetting Resin for Plug and Abandonment of Oil Wells with Reduced Environmental Impact. Polymers, 17(2), 212. https://doi.org/10.3390/polym17020212






