Electrochemical Switching of Laser-Induced Graphene/Polymer Composites for Tunable Electronics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laser-Induced Composite Fabrication
2.3. Electrochemical Treatment and Characterization
2.4. Electrolyte-Gated Transistor (EGT) Demonstration
2.5. Scanning Electron Microscopy
2.6. X-Ray Photoelectron Spectroscopy
2.7. Raman Spectroscopy
2.8. Fourier-Transform Infrared Spectroscopy
3. Results
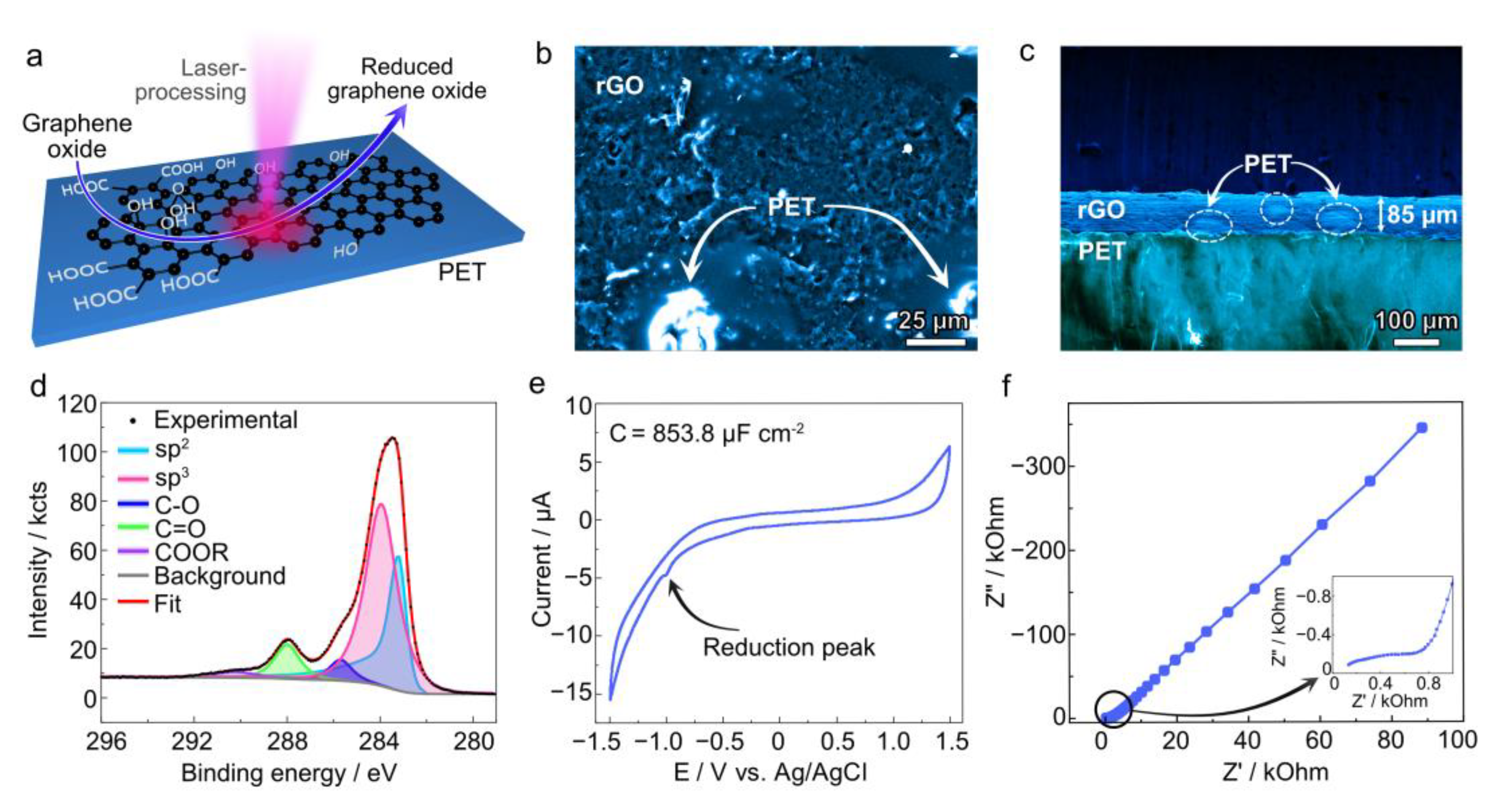
3.1. Electrode Fabrication and Characterization
3.2. Unexpected Findings on the Electrochemical Behavior of rGO/PET Electrodes
3.2.1. Activation Cycles
3.2.2. Switching Cycles
3.3. Electrochemical Treatment of rGO/PVDF Electrodes
3.4. Mechanism Behind Electrochemical Properties Switching
3.5. Application of Laser-Processed rGO/Polymer Composites in Electrolyte-Gated Transistors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasari, B.L.; Nouri, J.M.; Brabazon, D.; Naher, S. Graphene and Derivatives—Synthesis Techniques, Properties and Their Energy Applications. Energy 2017, 140, 766–778. [Google Scholar] [CrossRef]
- Seekaew, Y.; Arayawut, O.; Timsorn, K.; Wongchoosuk, C. Synthesis, Characterization, and Applications of Graphene and Derivatives. In Carbon-Based Nanofillers and Their Rubber Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–283. ISBN 9780128132487. [Google Scholar]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.-M. The Reduction of Graphene Oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Ahmed, A.; Singh, A.; Young, S.-J.; Gupta, V.; Singh, M.; Arya, S. Synthesis Techniques and Advances in Sensing Applications of Reduced Graphene Oxide (rGO) Composites: A Review. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107373. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Hooch Antink, W.; Choi, Y.; Seong, K.-D.; Kim, J.M.; Piao, Y. Recent Progress in Porous Graphene and Reduced Graphene Oxide-Based Nanomaterials for Electrochemical Energy Storage Devices. Adv. Mater. Interfaces 2018, 5, 1701212. [Google Scholar] [CrossRef]
- Radich, E.J.; Kamat, P.V. Origin of Reduced Graphene Oxide Enhancements in Electrochemical Energy Storage. ACS Catal. 2012, 2, 807–816. [Google Scholar] [CrossRef]
- Fatkullin, M.; Menzelintsev, V.; Lipovka, A.; Dogadina, E.; Plotnikov, E.; Brazovskiy, K.; Li, S.; Ma, L.; Cheng, C.; Porokhova, E.; et al. Smart Graphene Textiles for Biopotential Monitoring: Laser-Tailored Electrochemical Property Enhancement. ACS Sens. 2024, 9, 1809–1819. [Google Scholar] [CrossRef]
- Fatkullin, M.; Dogadina, E.; Bril’, I.; Ivanov, A.; Matkovic, A.; Rodriguez, R.D.; Sheremet, E. Nanomaterials/polymer-Integrated Flexible Sensors: A Full-Laser-Processing Approach for Real-Time Analyte Monitoring. IEEE Sens. J. 2024, 24, 13816–13822. [Google Scholar] [CrossRef]
- Mosa, I.M.; Pattammattel, A.; Kadimisetty, K.; Pande, P.; El-Kady, M.F.; Bishop, G.W.; Novak, M.; Kaner, R.B.; Basu, A.K.; Kumar, C.V.; et al. Ultrathin Graphene-Protein Supercapacitors for Miniaturized Bioelectronics. Adv. Energy Mater. 2017, 7, 1700358. [Google Scholar] [CrossRef]
- Silva, V.A.O.P.; Fernandes, W.S., Jr.; Rocha, D.P.; Stefano, J.S.; Munoz, R.A.A.; Bonacin, J.A.; Janegitz, B.C. 3D-Printed Reduced Graphene Oxide/polylactic Acid Electrodes: A New Prototyped Platform for Sensing and Biosensing Applications. Biosens. Bioelectron. 2020, 170, 112684. [Google Scholar] [CrossRef] [PubMed]
- Eng, A.Y.S.; Ambrosi, A.; Chua, C.K.; Saněk, F.; Sofer, Z.; Pumera, M. Unusual Inherent Electrochemistry of Graphene Oxides Prepared Using Permanganate Oxidants. Chemistry 2013, 19, 12673–12683. [Google Scholar] [CrossRef] [PubMed]
- Casero, E.; Parra-Alfambra, A.M.; Petit-Domínguez, M.D.; Pariente, F.; Lorenzo, E.; Alonso, C. Differentiation between Graphene Oxide and Reduced Graphene by Electrochemical Impedance Spectroscopy (EIS). Electrochem. Commun. 2012, 20, 63–66. [Google Scholar] [CrossRef]
- Abyzova, E.; Petrov, I.; Bril’, I.; Cheshev, D.; Ivanov, A.; Khomenko, M.; Averkiev, A.; Fatkullin, M.; Kogolev, D.; Bolbasov, E.; et al. Universal Approach to Integrating Reduced Graphene Oxide into Polymer Electronics. Polymers 2023, 15, 4622. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Cai, Q.; Ma, J.-N.; Zhu, L.; Han, D.-D.; Zhang, Y.-L. Free-Standing and Flexible Graphene Supercapacitors of High Areal Capacitance Fabricated by Laser Holography Reduction of Graphene Oxide. Appl. Phys. Lett. 2021, 118, 071601. [Google Scholar] [CrossRef]
- Zhou, A. ’an; Bai, J.; Hong, W.; Bai, H. Electrochemically Reduced Graphene Oxide: Preparation, Composites, and Applications. Carbon 2022, 191, 301–332. [Google Scholar] [CrossRef]
- Feicht, P.; Eigler, S. Defects in Graphene Oxide as Structural Motifs. ChemNanoMat 2018, 4, 244–252. [Google Scholar] [CrossRef]
- Arul, R.; Oosterbeek, R.N.; Robertson, J.; Xu, G.; Jin, J.; Simpson, M.C. The Mechanism of Direct Laser Writing of Graphene Features into Graphene Oxide Films Involves Photoreduction and Thermally Assisted Structural Rearrangement. Carbon 2016, 99, 423–431. [Google Scholar] [CrossRef]
- Ma, B.; Rodriguez, R.D.; Ruban, A.; Pavlov, S.; Sheremet, E. The Correlation between Electrical Conductivity and Second-Order Raman Modes of Laser-Reduced Graphene Oxide. Phys. Chem. Chem. Phys. 2019, 21, 10125–10134. [Google Scholar] [CrossRef]
- Lovinger, A.; Reed, D. Inhomogeneous Thermal Degradation of Poly(vinylidene Fluoride) Crystallized from the Melt. Macromolecules 1980, 13, 989–994. [Google Scholar] [CrossRef]
- Murastov, G.V.; Lipovka, A.A.; Fatkullin, M.I.; Rodriguez, R.D.; Sheremet, E.S. Laser Reduction of Graphene Oxide: Local Control of Material Properties. Phys. Usp. 2023, 66, 1105–1133. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An Improved Hummers Method for Eco-Friendly Synthesis of Graphene Oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Pud, A.A. Surface Electrochemical Reactions and the Subsequent Degradation of Solid-Phase Poly(ethylene Terephthalate) at a Cathode. Polym. Degrad. Stab. 1990, 30, 181–193. [Google Scholar] [CrossRef]
- Vasilijević, S.; Boukraa, R.; Battaglini, N.; Piro, B. Graphene-Based Materials and Their Applications in Electrolyte-Gated Transistors for Sensing. Synth. Met. 2023, 295, 117355. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, L.; Xu, D.; Duo, Y.; Gao, J.; Zhang, L.; Wang, X.; Chen, X.; Li, J.; Zhang, H. Solution-Gated Transistors of Two-Dimensional Materials for Chemical and Biological Sensors: Status and Challenges. Nanoscale 2020, 12, 11364–11394. [Google Scholar] [CrossRef]
- Furlan de Oliveira, R.; Montes-García, V.; Livio, P.A.; González-García, M.B.; Fanjul-Bolado, P.; Casalini, S.; Samorì, P. Selective Ion Sensing in Artificial Sweat Using Low-Cost Reduced Graphene Oxide Liquid-Gated Plastic Transistors. Small 2022, 18, e2201861. [Google Scholar] [CrossRef]
- Aspermair, P.; Mishyn, V.; Bintinger, J.; Happy, H.; Bagga, K.; Subramanian, P.; Knoll, W.; Boukherroub, R.; Szunerits, S. Reduced Graphene Oxide-Based Field Effect Transistors for the Detection of E7 Protein of Human Papillomavirus in Saliva. Anal. Bioanal. Chem. 2021, 413, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Reiner-Rozman, C.; Kotlowski, C.; Knoll, W. Electronic Biosensing with Functionalized rGO FETs. Biosensors 2016, 6, 17. [Google Scholar] [CrossRef]
- He, Q.; Wu, S.; Gao, S.; Cao, X.; Yin, Z.; Li, H.; Chen, P.; Zhang, H. Transparent, Flexible, All-Reduced Graphene Oxide Thin Film Transistors. ACS Nano 2011, 5, 5038–5044. [Google Scholar] [CrossRef]
- Furlan de Oliveira, R.; Livio, P.A.; Montes-García, V.; Ippolito, S.; Eredia, M.; Fanjul-Bolado, P.; González García, M.B.; Casalini, S.; Samorì, P. Liquid-gated Transistors Based on Reduced Graphene Oxide for Flexible and Wearable Electronics. Adv. Funct. Mater. 2019, 29, 1905375. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatkullin, M.; Petrov, I.; Dogadina, E.; Kogolev, D.; Vorobiev, A.; Postnikov, P.; Chen, J.-J.; de Oliveira, R.F.; Kanoun, O.; Rodriguez, R.D.; et al. Electrochemical Switching of Laser-Induced Graphene/Polymer Composites for Tunable Electronics. Polymers 2025, 17, 192. https://doi.org/10.3390/polym17020192
Fatkullin M, Petrov I, Dogadina E, Kogolev D, Vorobiev A, Postnikov P, Chen J-J, de Oliveira RF, Kanoun O, Rodriguez RD, et al. Electrochemical Switching of Laser-Induced Graphene/Polymer Composites for Tunable Electronics. Polymers. 2025; 17(2):192. https://doi.org/10.3390/polym17020192
Chicago/Turabian StyleFatkullin, Maxim, Ilia Petrov, Elizaveta Dogadina, Dmitry Kogolev, Alexandr Vorobiev, Pavel Postnikov, Jin-Ju Chen, Rafael Furlan de Oliveira, Olfa Kanoun, Raul D. Rodriguez, and et al. 2025. "Electrochemical Switching of Laser-Induced Graphene/Polymer Composites for Tunable Electronics" Polymers 17, no. 2: 192. https://doi.org/10.3390/polym17020192
APA StyleFatkullin, M., Petrov, I., Dogadina, E., Kogolev, D., Vorobiev, A., Postnikov, P., Chen, J.-J., de Oliveira, R. F., Kanoun, O., Rodriguez, R. D., & Sheremet, E. (2025). Electrochemical Switching of Laser-Induced Graphene/Polymer Composites for Tunable Electronics. Polymers, 17(2), 192. https://doi.org/10.3390/polym17020192







