Isothermal Crystallization Kinetics and Their Effect on the Molding Process and Mechanical Properties of PAEK and PEEK
Abstract
1. Introduction
2. Materials and Methods
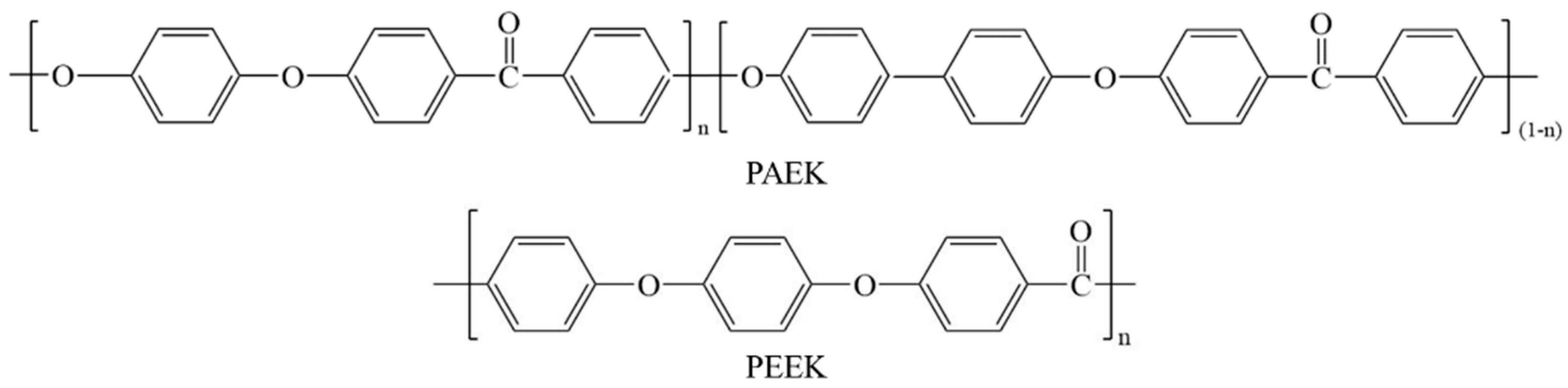
2.1. Raw Materials
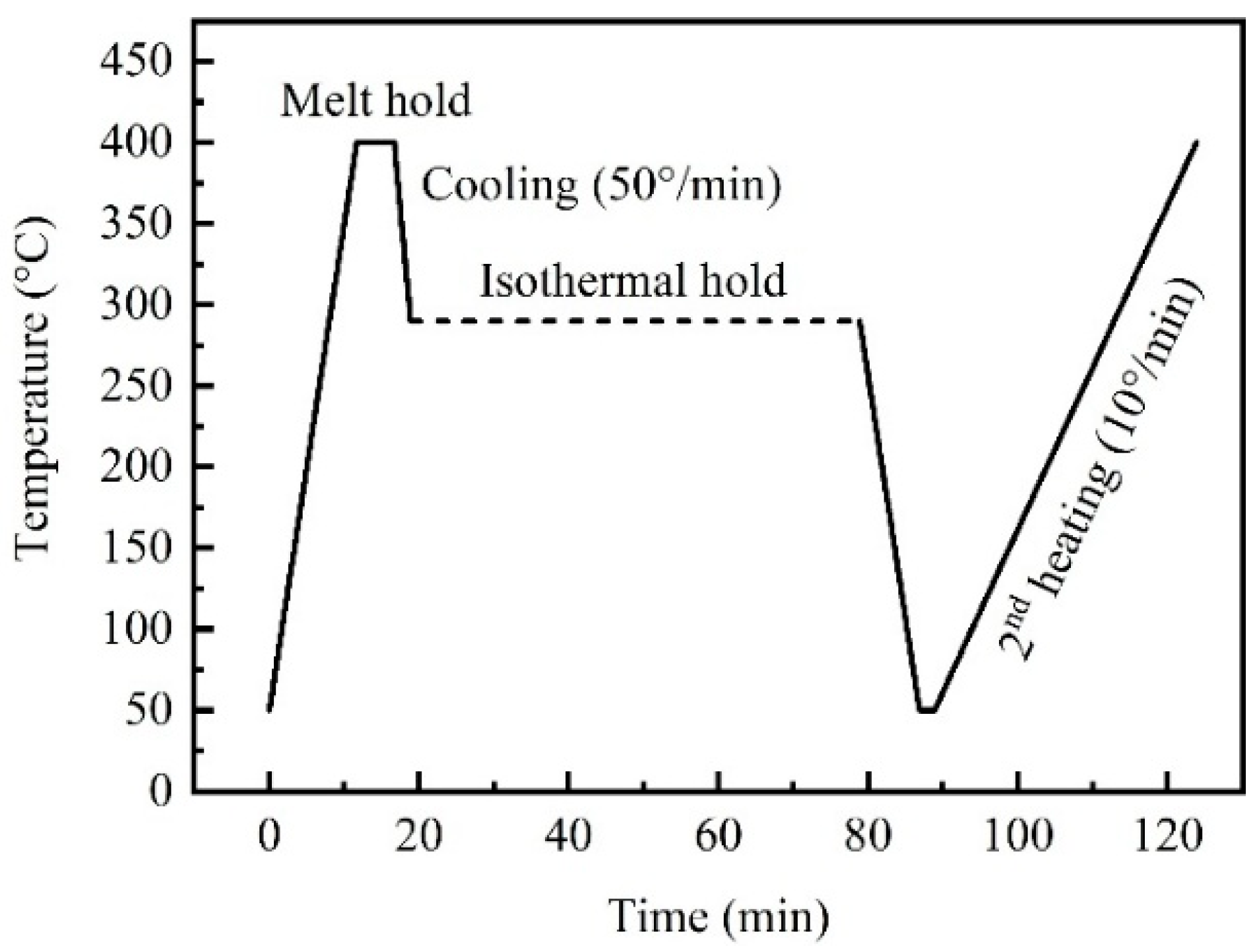
2.2. Differential Scanning Calorimetry (DSC) Testing
2.3. Isothermal Crystallization Kinetics Model
2.4. Microscope AnalysisTensile Testing
2.5. Specimens Preparation
2.6. Tensile Testing
3. Results and Discussion
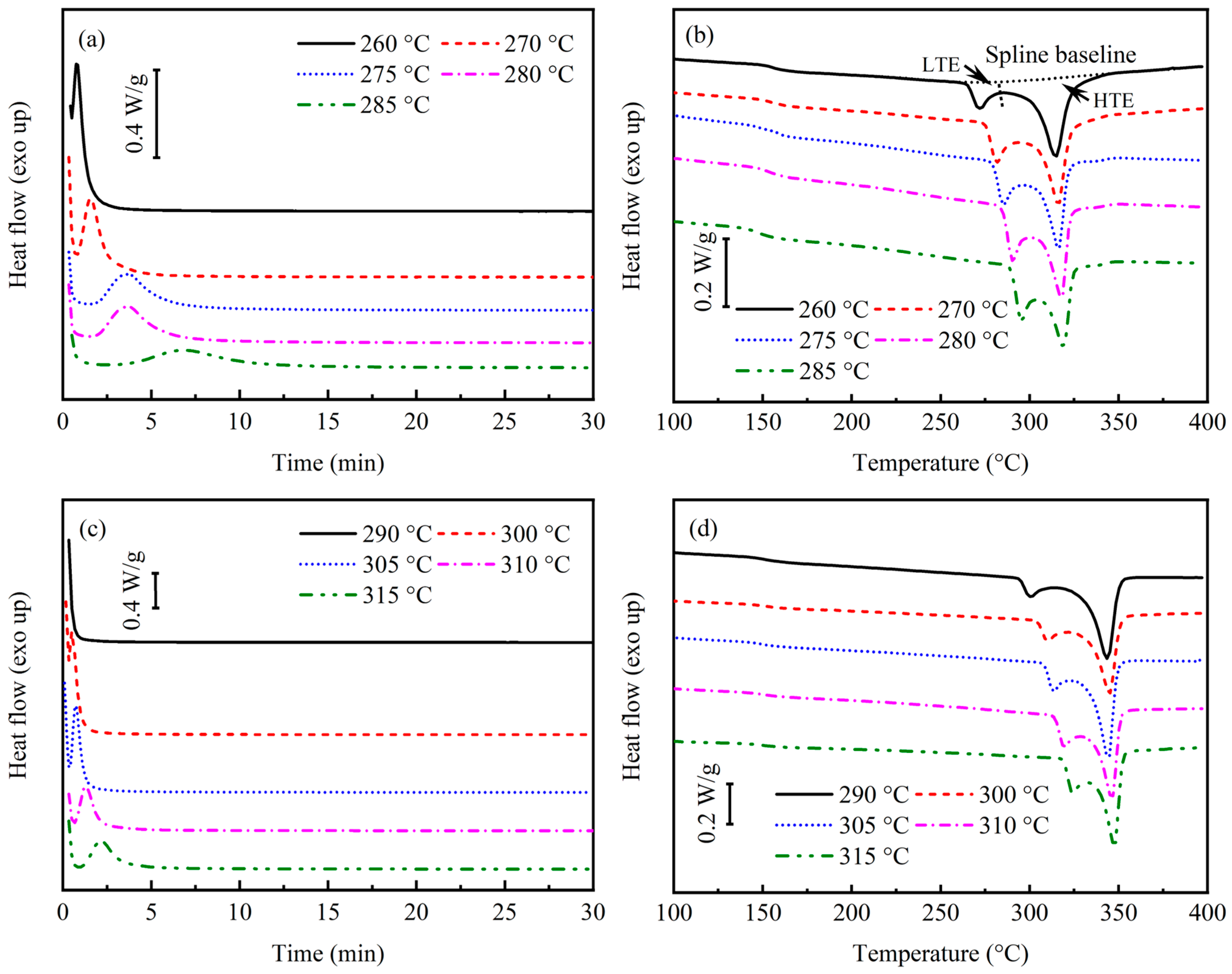
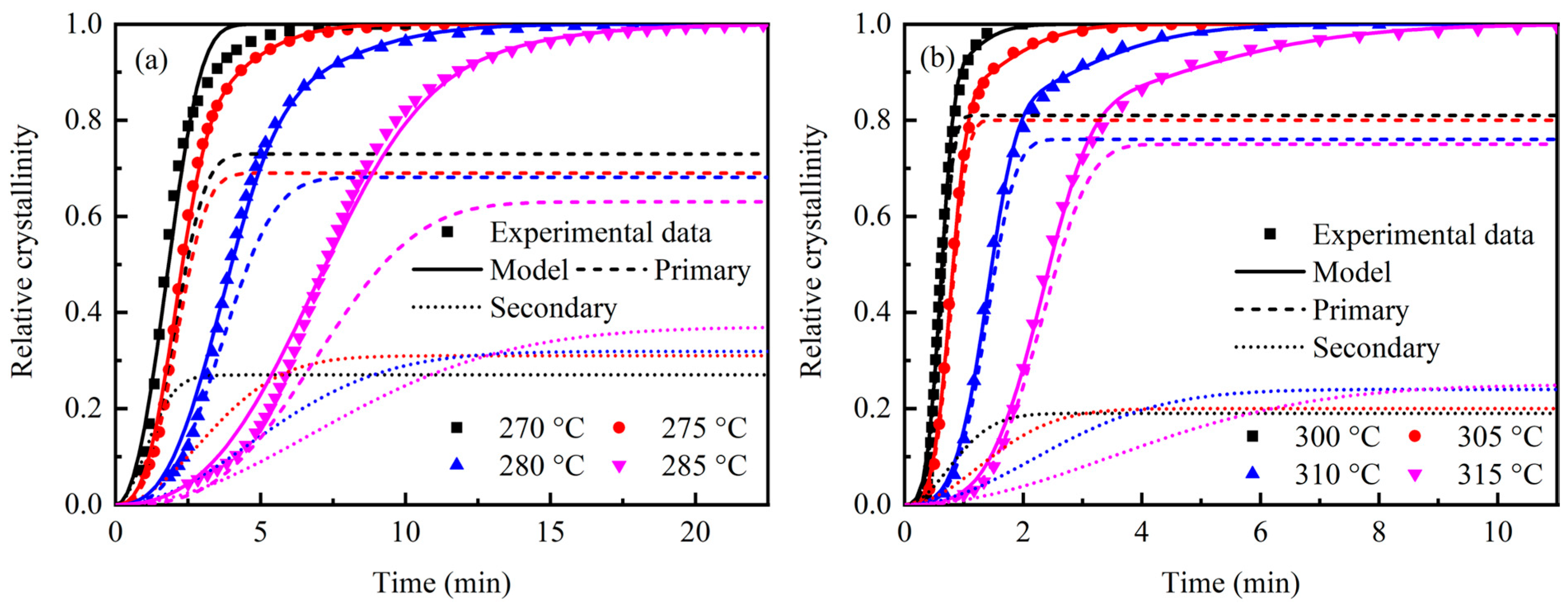
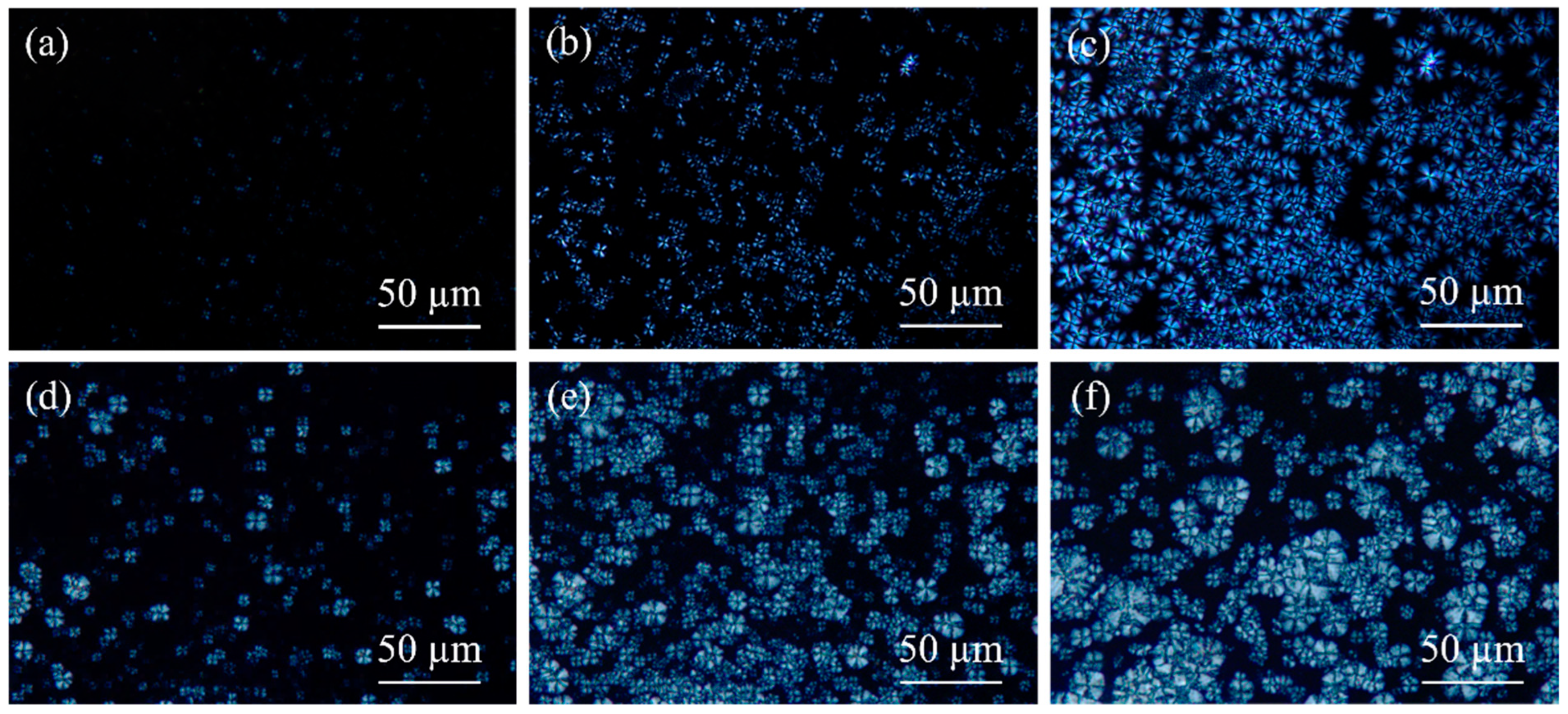
3.1. Isothermal Crystallization Kinetics
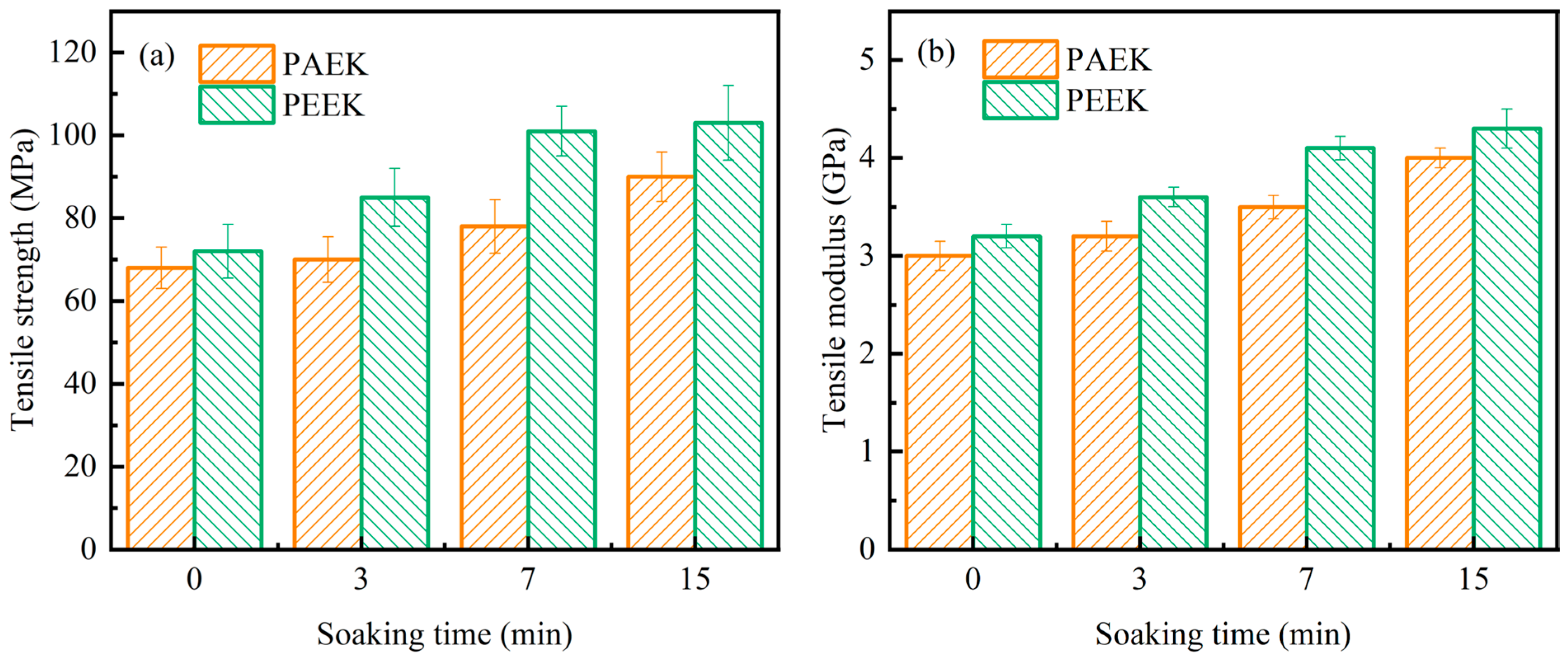
3.2. Crystallization Behavior and Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Miranda, R.; Scalici, T.; Di Franco, F.; Santamaria, M.; Valenza, A. Enhancing the PEEK composites-titanium interface performances through electrochemical treatment in fibre-metal laminates for aerospace applications. Int. J. Adhes. Adhes. 2024, 134, 103767. [Google Scholar]
- Cao, Y.; Yang, H.; Wan, K.; Li, D.; He, Q.; Wu, H. High-performance PEEK composite materials research on 3D printing for neutron and photon radiation shielding. Compos. Part A—Appl. S. 2024, 185, 108352. [Google Scholar] [CrossRef]
- Eko, A.J.; Epaarachchi, J.; Jewewantha, J.; Zeng, X. A review of type IV composite overwrapped pressure vessels. Int. J. Hydrogen Energ. 2025, 109, 551–573. [Google Scholar]
- Zhang, H.; Zhang, Z.; Long, Y.; Ran, X.; Guo, Y.; Li, Y. Enhanced mechanical properties of continuous carbon fiber-reinforced PEEK composites via process parameters optimization and assisted infrared irradiation heating. Compos. Commun. 2025, 58, 102531. [Google Scholar] [CrossRef]
- Nikonovich, M.; Ramalho, A.; Emami, N. Effect of cryogenic aging and test-environment on the tribological and mechanical properties of PEEK composites. Tribol. Int. 2024, 194, 109554. [Google Scholar] [CrossRef]
- Dandy, L.O.; Oliveux, G.; Wood, J.; Jenkins, M.J.; Leeke, G.A. Accelerated degradation of polyetheretherketone (PEEK) composite materials for recycling applications. Polym. Degrad. Stabil. 2015, 112, 52–62. [Google Scholar]
- Dai, J.N.; Kou, S.Q.; Yang, H.Y.; Xu, Z.B.; Shu, S.L.; Qiu, F.; Jiang, Q.C.; Zhang, L.C. High-content continuous carbon fibers reinforced PEEK matrix composite with ultra-high mechanical and wear performance at elevated temperature. Compos. Struct. 2022, 295, 115837. [Google Scholar]
- Quan, D.; Deegan, B.; Binsfeld, L.; Li, X.; Atkinson, J.; Ivanković, A.; Murphy, N. Effect of interlaying UV-irradiated PEEK fibres on the mechanical, impact and fracture response of aerospace-grade carbon fibre/epoxy composites. Compos. Part B—Eng. 2020, 191, 107923. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, B.; Li, Z.; Dang, Z.; Zhang, C. Evaluating the loading rate dependency of mode I delamination for composite laminates at different temperatures. Compos. Sci. Technol. 2024, 249, 110505. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Wang, J.; Ding, A. A focused review on the thermo-stamping process and simulation progresses of continuous fibre reinforced thermoplastic composites. Compos. Part B—Eng. 2021, 224, 109196. [Google Scholar]
- Driezen, J.; Herrmann, A.S. Characterization and modelling of the crystallization behavior of PEEK for polymer processing techniques. Polymer. 2023, 284, 126298. [Google Scholar] [CrossRef]
- Arquier, R.; Iliopoulos, I.; Régnier, G.; Miquelard-Garnier, G. Consolidation of continuous-carbon-fiber-reinforced PAEK composites: A review. Mater. Today Commun. 2022, 32, 104036. [Google Scholar] [CrossRef]
- Heathman, N.; Koirala, P.; Yap, T.; Emami, A.; Tehrani, M. In situ consolidation of carbon fiber PAEK via laser-assisted automated fiber placement. Compos. Part B-Eng. 2023, 249, 110405. [Google Scholar] [CrossRef]
- Yi, N.; Davies, R.; Chaplin, A.; McCutchion, P.; Ghita, O. Slow and fast crystallising poly aryl ether ketones (PAEKs) in 3D printing: Crystallisation kinetics, morphology, and mechanical properties. Addit. Manuf. 2021, 39, 101843. [Google Scholar] [CrossRef]
- Wang, W.; Yao, J.; Zhang, J.; Wang, Q.; Liu, G.; Wang, M. High-performance CF/LM-PAEK L-shaped corners by the hot stamping technique with low stamping temperature. Polym. Compos. 2025, 46, S429–S443. [Google Scholar] [CrossRef]
- Pérez-Martín, H.; Mackenzie, P.; Baidak, A.; Brádaigh, C.M.Ó.; Ray, D. Crystallisation behaviour and morphological studies of PEKK and carbon fibre/PEKK composites. Compos. Part A—Appl. S. 2022, 159, 106992. [Google Scholar] [CrossRef]
- Choupin, T.; Fayolle, B.; Régnier, G.; Paris, C.; Cinquin, J.; Brulé, B. A more reliable DSC-based methodology to study crystallization kinetics: Application to poly(ether ketone ketone) (PEKK) copolymers. Polymer 2018, 155, 109–115. [Google Scholar] [CrossRef]
- Gao, S.L.; Kim, J.K. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Compos. Part A—Appl. S. 2000, 31, 517–530. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wang, K. Interfacial crystallization behavior of poly(ether-etherketone) on polyimide-modified CCF300 carbon fibers. Polym. Compos. 2020, 41, 2433–2445. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Y.; Wei, K.; Fang, W.; Sun, H. Non-isothermal crystallization kinetics of short glass fiber reinforced poly (ether ether ketone) composites. Materials 2018, 11, 2094. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Yao, J.; Liu, G.; Wang, M. The effect of tool temperature and dwell time on the quality and CBS properties of L-shaped CF/LM-PAEK corners manufactured by the thermo-stamping process. J. Compos. Mater. 2025. [Google Scholar] [CrossRef]
- Velisaris, C.N.; Seferis, J.C. Crystallization kinetics of polyetheretherketone (peek) matrices. Polym. Eng. Sci. 1986, 26, 1574–1581. [Google Scholar] [CrossRef]
- Hsiao, B.S.; Chang, I.Y.; Sauer, B.B. Isothermal crystallization kinetics of poly(ether ketone ketone) and its carbon-fibre-reinforced composites. Polymer 1991, 32, 2799–2805. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–220. [Google Scholar] [CrossRef]
- Panda, J.N.; Bijwe, J.; Pandey, R.K. Optimization of the amount of short glass fibers for superior wear performance of PAEK composites. Compos. Part A—Appl. S. 2019, 116, 158–168. [Google Scholar] [CrossRef]
- Audoit, J.; Rivière, L.; Dandurand, J. Thermal, mechanical and dielectric behaviour of poly(aryl ether ketone) with low melting temperature. J. Therm Anal. Calorim. 2019, 135, 2147–2157. [Google Scholar] [CrossRef]
- Cortés, L.Q.; Caussé, N.; Dantras, E. Morphology and dynamical mechanical properties of poly ether ketone ketone (PEKK) with meta phenyl links. J. Appl. Polym. Sci. 2016, 133, 43396. [Google Scholar] [CrossRef]
- GB/T 2567-2021; Test Method for Properties of Resin Casting Body. Standards Press of China: Beijing, China, 2021.
- Pérez-Martín, H.; Mackenzie, P.; Baidak, A.; Brádaigh, C.M.Ó.; Ray, D. Crystallinity studies of PEKK and carbon fibre/PEKK composites: A review. Compos. Part B—Eng. 2021, 223, 109127. [Google Scholar] [CrossRef]
- Dai, G.; Zhan, L.; Guan, C.; Huang, M. The effect of cooling rate on crystallization behavior and tensile properties of CF/PEEK composites. J. Polym. Eng. 2021, 41, 423–430. [Google Scholar] [CrossRef]
- Lee, I.G.; Kim, D.H.; Jung, K.H.; Kim, H.J.; Kim, H.S. Effect of the cooling rate on the mechanical properties of glass fiber reinforced thermoplastic composites. Compos. Struct. 2017, 177, 28–37. [Google Scholar] [CrossRef]
- Zhang, J.D.; An, P.; Yu, K.; Liu, G.; Huang, J.; Gu, Y.; Yao, J.; Liu, H.; Chen, C.; Zhang, C.; et al. The effect of cooling rates on crystallization and low-velocity impact behaviour of carbon fibre reinforced poly(aryl ether ketone) composites. Compos. Part B—Eng. 2023, 254, 110569. [Google Scholar] [CrossRef]
- Gao, S.L.; Kim, J.K. Cooling rate influences in carbon fibre/PEEK composites. Part II: Interlaminar fracture toughness. Compos. Part A—Appl. S. 2001, 32, 763–774. [Google Scholar] [CrossRef]



| Isothermal Temperature (°C) | PAEK | ||||||
|---|---|---|---|---|---|---|---|
(°C) | (°C) | (J/g) | (°C) | (J/g) | (%) | ||
| 260 | 155.3 | 271.5 | 6.0 | 314.9 | 24.7 | 23.6 | 19:81 |
| 270 | 154.6 | 281.8 | 8.9 | 316.4 | 23.8 | 25.2 | 27:73 |
| 275 | 154.3 | 285.7 | 8.3 | 316.5 | 18.8 | 20.8 | 31:69 |
| 280 | 153.8 | 290.8 | 10.3 | 318.0 | 22.1 | 24.9 | 32:68 |
| 285 | 146.5 | 295.5 | 9.9 | 319.4 | 16.7 | 20.5 | 37:63 |
| PEEK | |||||||
| 290 | 150.5 | 300.9 | 7.4 | 343.7 | 36.4 | 33.7 | 17:83 |
| 300 | 150.5 | 310.4 | 7.4 | 344.6 | 31.7 | 30.1 | 19:81 |
| 305 | 150.3 | 313.7 | 9.3 | 344.9 | 35.5 | 34.5 | 20:80 |
| 310 | 150.1 | 319.8 | 9.9 | 346.1 | 31.0 | 31.5 | 24:76 |
| 315 | 149.7 | 324.4 | 10.4 | 347.7 | 31.0 | 31.8 | 25:75 |
| Isothermal Temperature (°C) | PAEK | |||
|---|---|---|---|---|
| RSS | ||||
| 270 | 0.73 | 0.07920 | 0.50754 | 0.06194 |
| 275 | 0.69 | 0.07471 | 0.06312 | 0.01008 |
| 280 | 0.68 | 0.01384 | 0.02374 | 0.02864 |
| 285 | 0.63 | 0.00201 | 0.01110 | 0.07235 |
| PEEK | ||||
| 300 | 0.81 | 5.06146 | 0.92508 | 0.37935 |
| 305 | 0.80 | 1.92739 | 0.32118 | 0.00395 |
| 310 | 0.76 | 0.19232 | 0.10777 | 0.00404 |
| 315 | 0.75 | 0.02511 | 0.04199 | 0.00864 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yu, K.; Luo, Y.; Li, W.; Zhong, X.; Liu, G.; Bao, J.; Chen, C. Isothermal Crystallization Kinetics and Their Effect on the Molding Process and Mechanical Properties of PAEK and PEEK. Polymers 2025, 17, 2713. https://doi.org/10.3390/polym17192713
Zhang J, Yu K, Luo Y, Li W, Zhong X, Liu G, Bao J, Chen C. Isothermal Crystallization Kinetics and Their Effect on the Molding Process and Mechanical Properties of PAEK and PEEK. Polymers. 2025; 17(19):2713. https://doi.org/10.3390/polym17192713
Chicago/Turabian StyleZhang, Jindong, Kun Yu, Yunfeng Luo, Weidong Li, Xiangyu Zhong, Gang Liu, Jianwen Bao, and Chunhai Chen. 2025. "Isothermal Crystallization Kinetics and Their Effect on the Molding Process and Mechanical Properties of PAEK and PEEK" Polymers 17, no. 19: 2713. https://doi.org/10.3390/polym17192713
APA StyleZhang, J., Yu, K., Luo, Y., Li, W., Zhong, X., Liu, G., Bao, J., & Chen, C. (2025). Isothermal Crystallization Kinetics and Their Effect on the Molding Process and Mechanical Properties of PAEK and PEEK. Polymers, 17(19), 2713. https://doi.org/10.3390/polym17192713








