Development, Characterization, and Biological Evaluation of a Self-Healing Hydrogel Patch Loaded with Ciprofloxacin for Wound Dressings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Solubility Study of Ciprofloxacin HCl in Polymer Solutions
2.2.2. Preparation of Ciprofloxacin Hydrogels
2.2.3. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR) Analysis
2.2.4. Characterization of CIP Hydrogel Patches
UV–Vis Spectroscopic Analysis
- Preparation of CIP calibration curve
- Drug Recovery of CIP from the CIP hydrogel patches
100%
Rheological Studies
- Viscosity
- Strain-sweep studies
- Frequency-sweep studies
- Three-interval thixotropy test (3ITT)
Morphology of CIP Hydrogel Patch
Swelling Behavior of CIP Hydrogel Patches
Self-Healing Analysis
Stability Studies of CIP Hydrogel Patch
2.2.5. Antimicrobial Study
Well-Diffusion Method
Determination of Minimum Inhibitory Concentration (MIC)
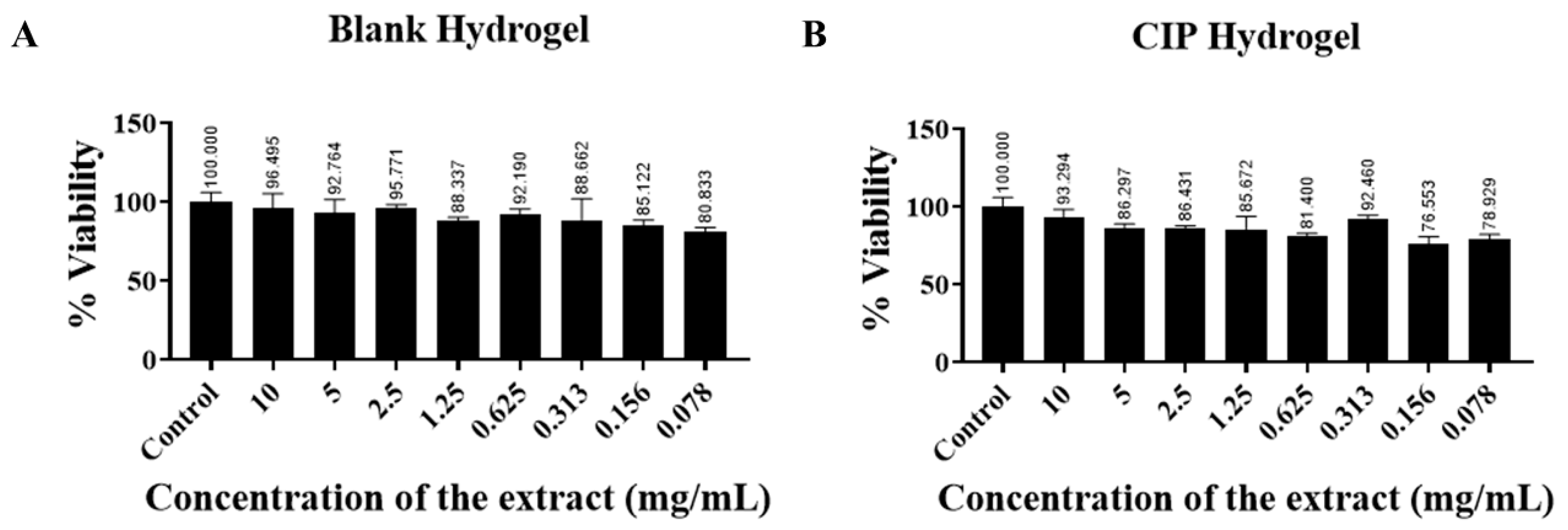
2.2.6. In Vitro Cytotoxicity Study
2.2.7. Bioadhesion Study of CIP Hydrogel Patch
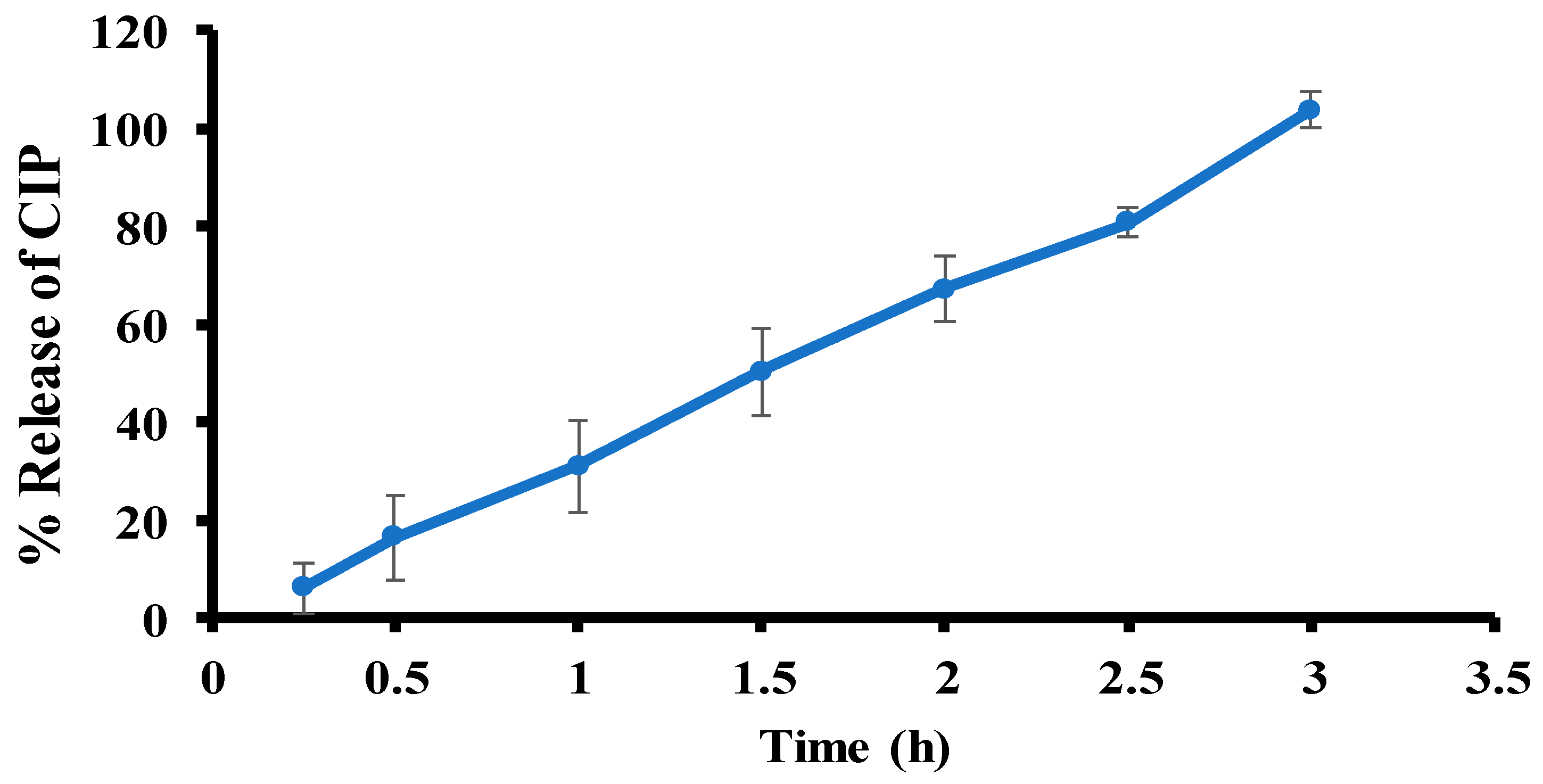
2.2.8. Ex Vivo Permeation
2.2.9. In Vivo Wound Healing Study—A Pilot Study
2.3. Statistical Analysis
3. Results and Discussion
3.1. Solubility of Ciprofloxacin HCl in the Polymer Solutions
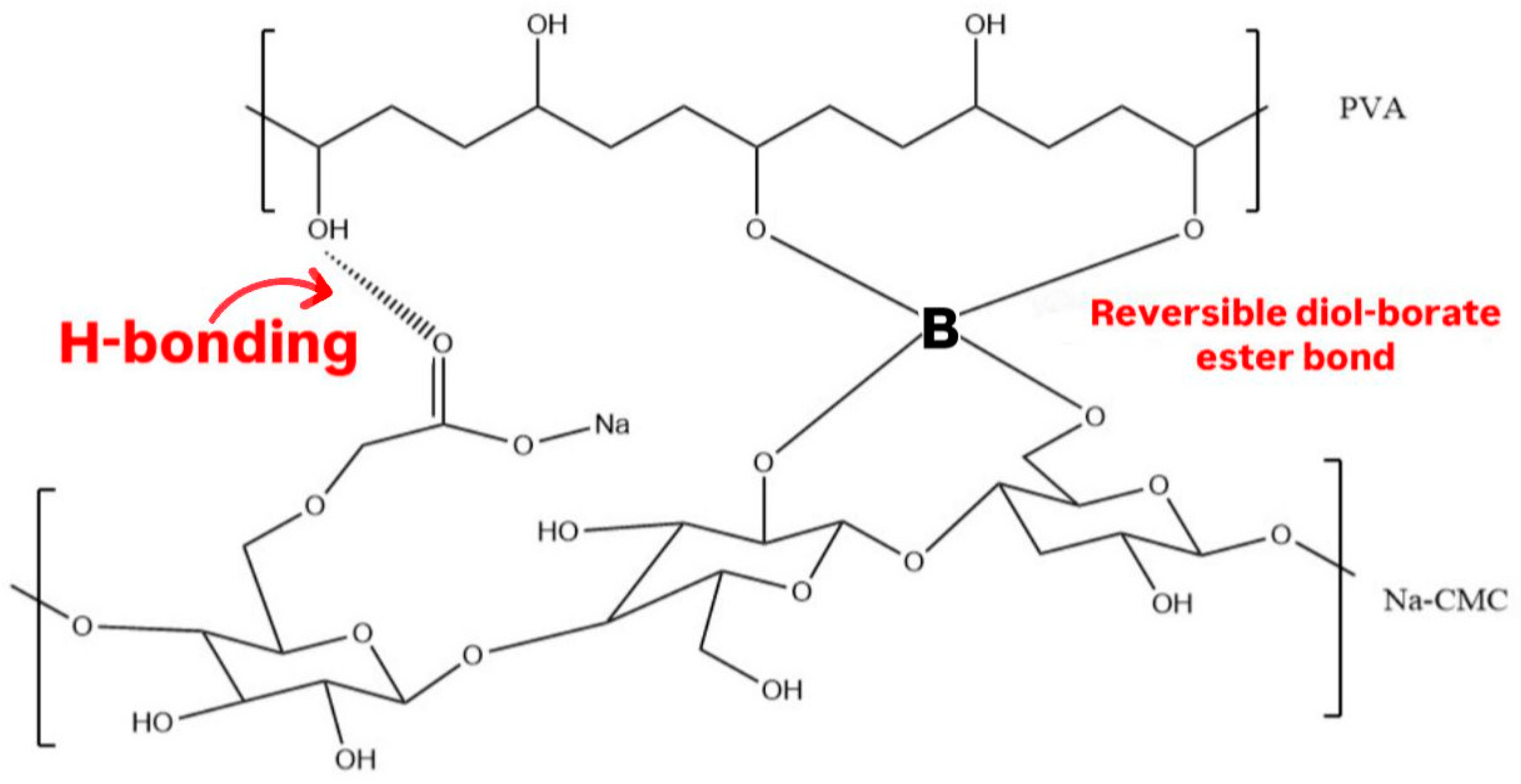
3.2. Ciprofloxacin Hydrogel Patches
3.3. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR) Analysis
3.4. Characterization of CIP Hydrogel Patches
3.4.1. Drug Recovery
3.4.2. Rheological Studies
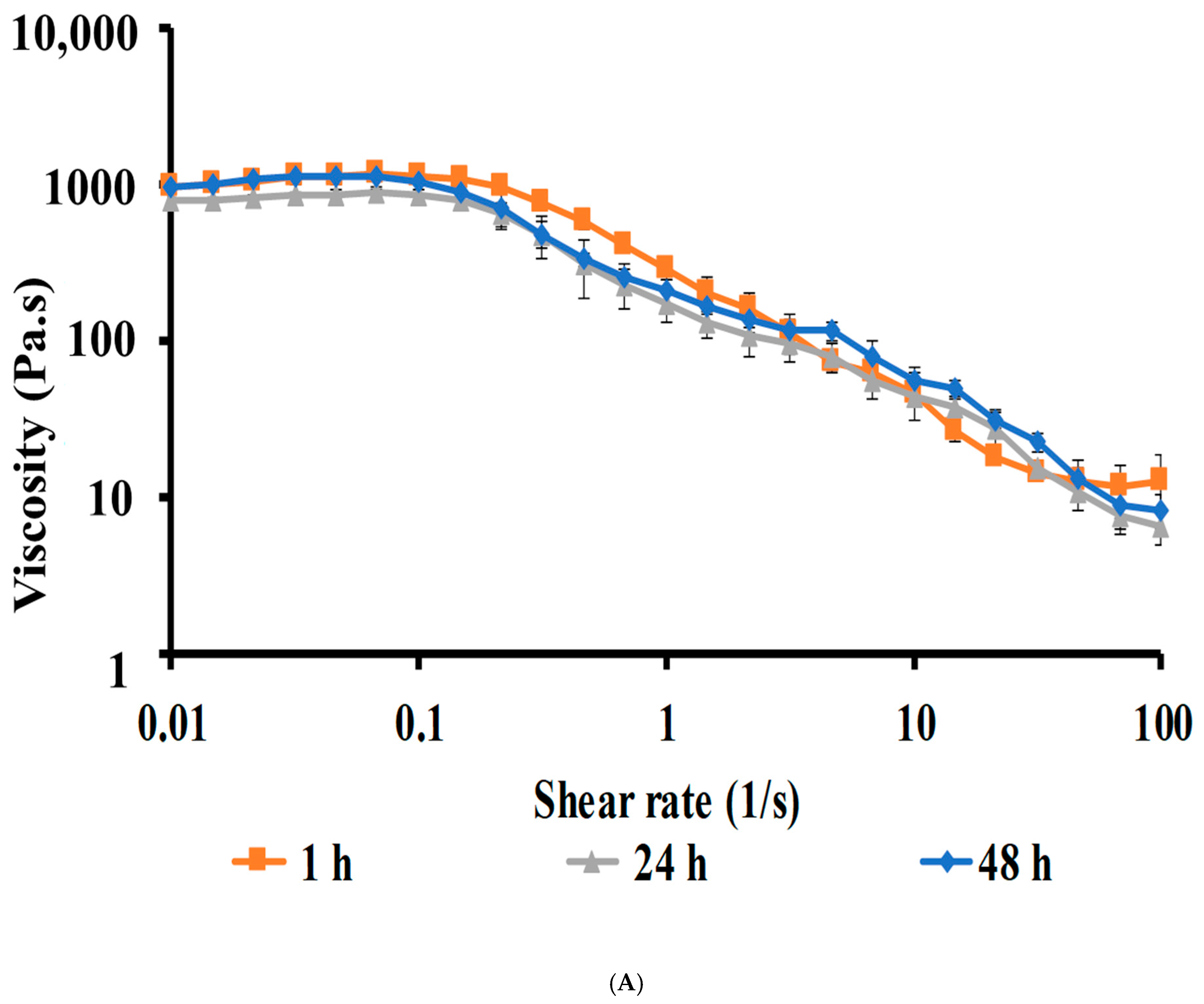
Viscosity of CIP Hydrogels
Strain-Sweep Studies
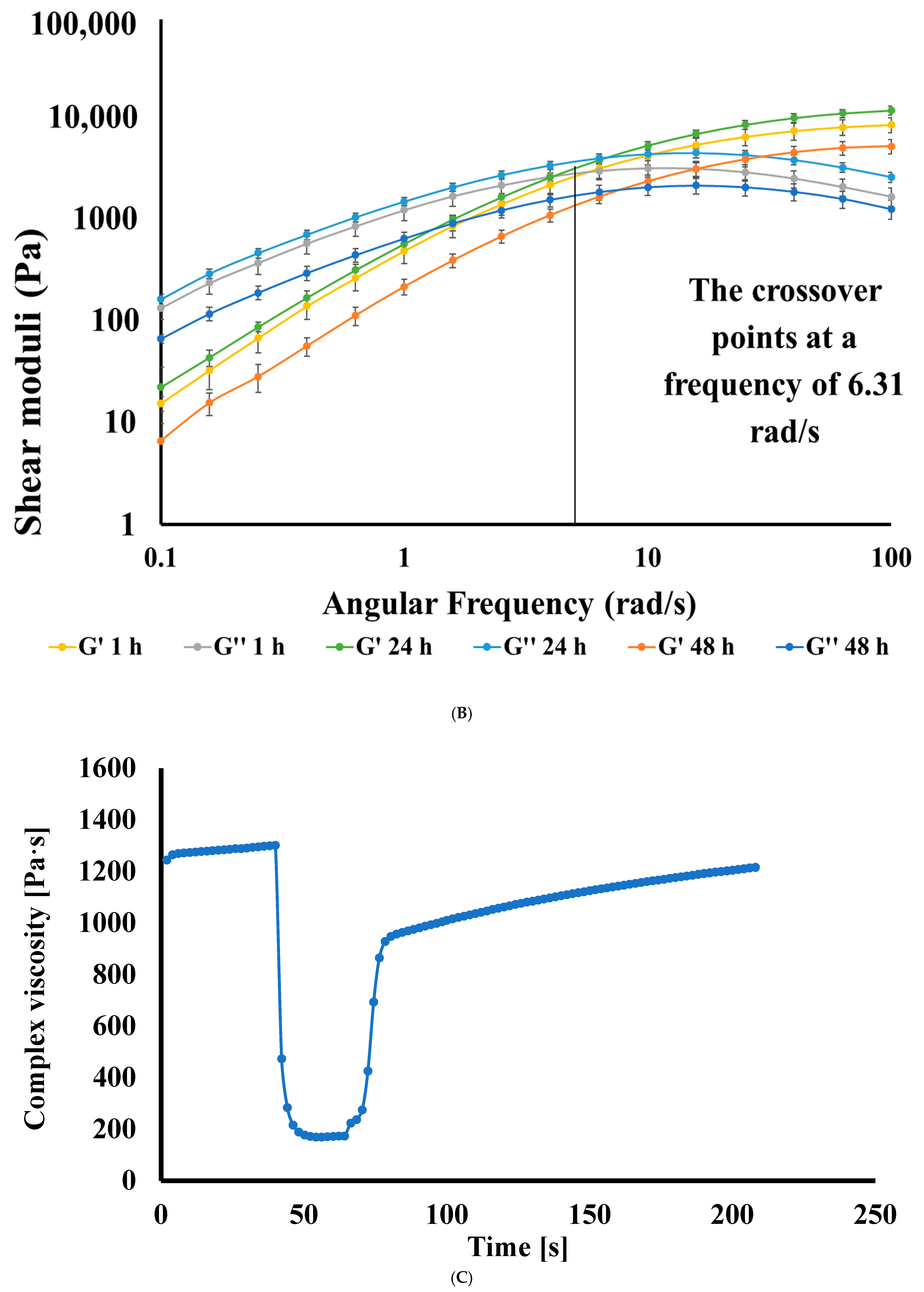
Frequency-Sweep Studies
Three-Interval Thixotropy Test (3ITT)
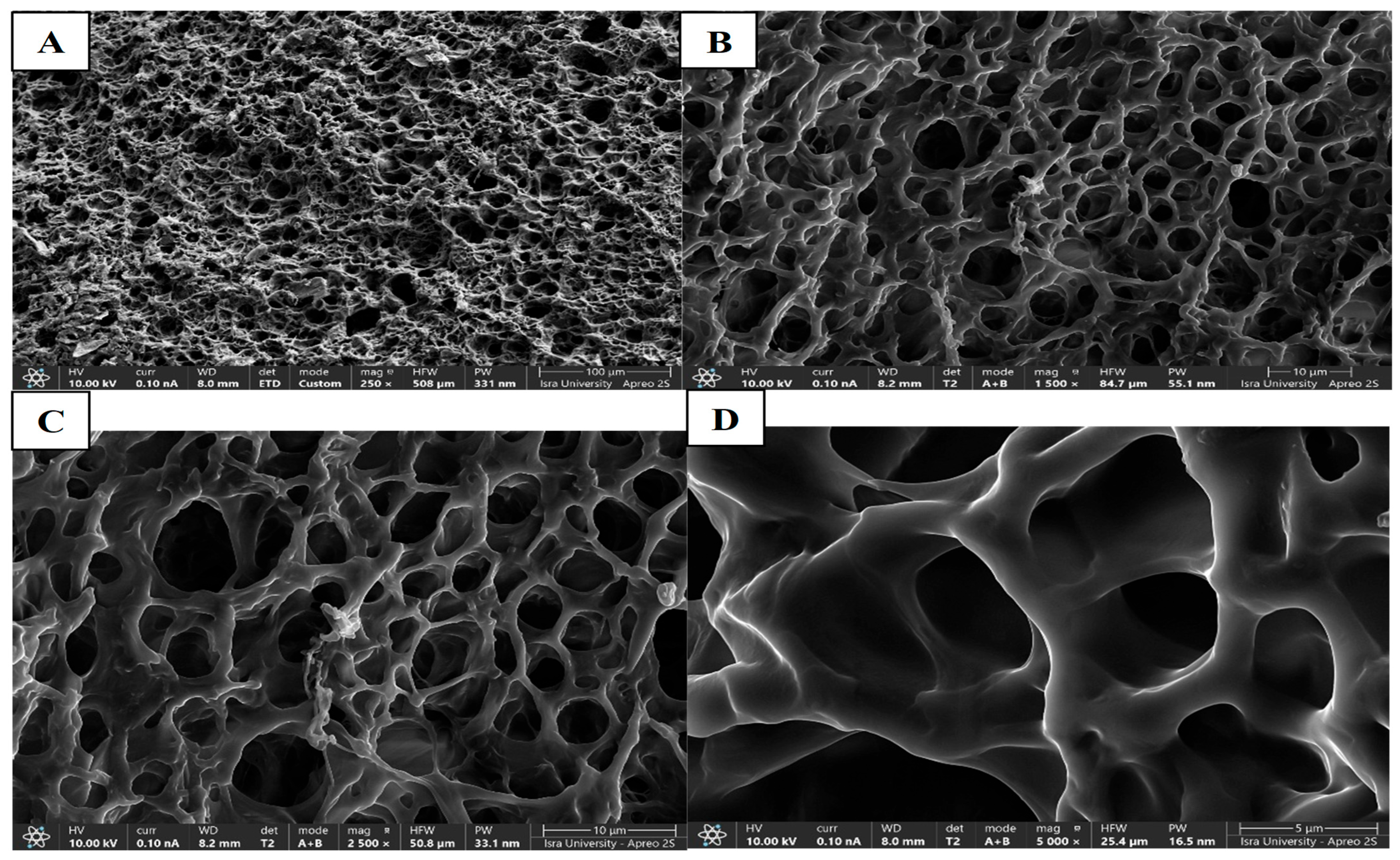
3.4.3. Morphology of CIP Hydrogel Patch
3.4.4. Swelling Behavior of CIP Hydrogel Patch
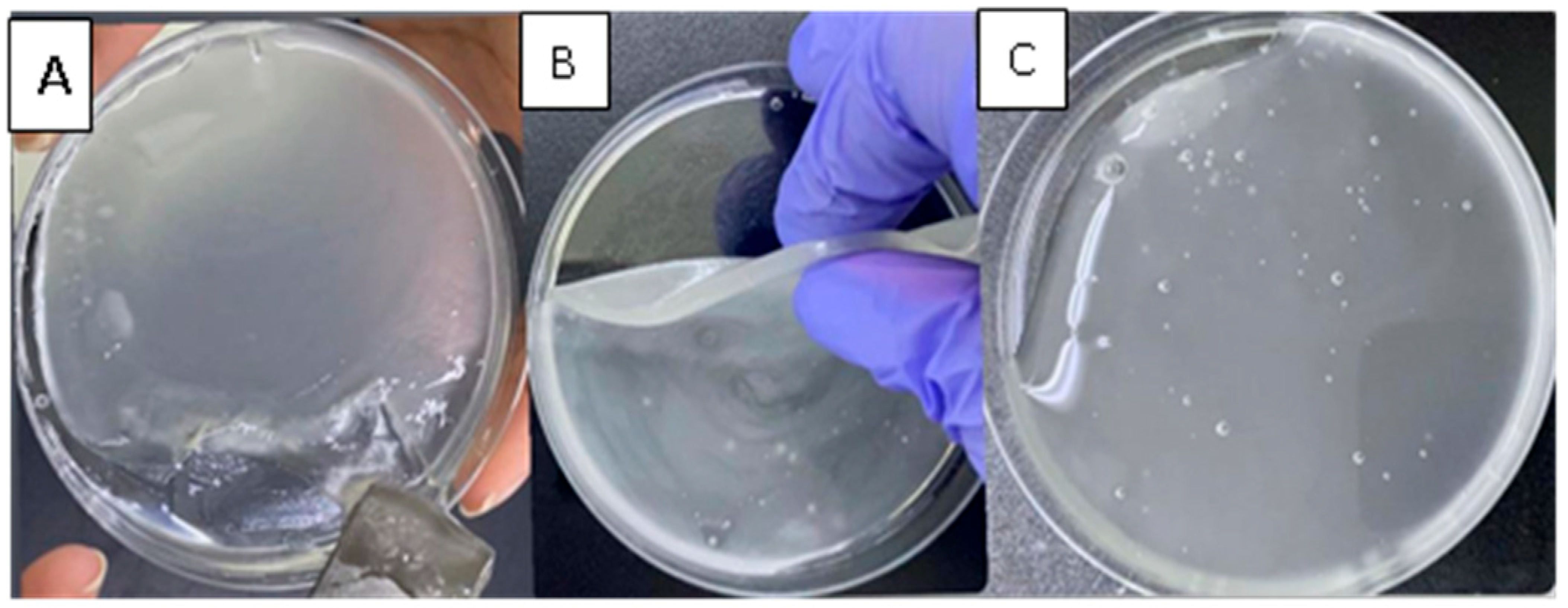
3.4.5. Self-Healing Ability
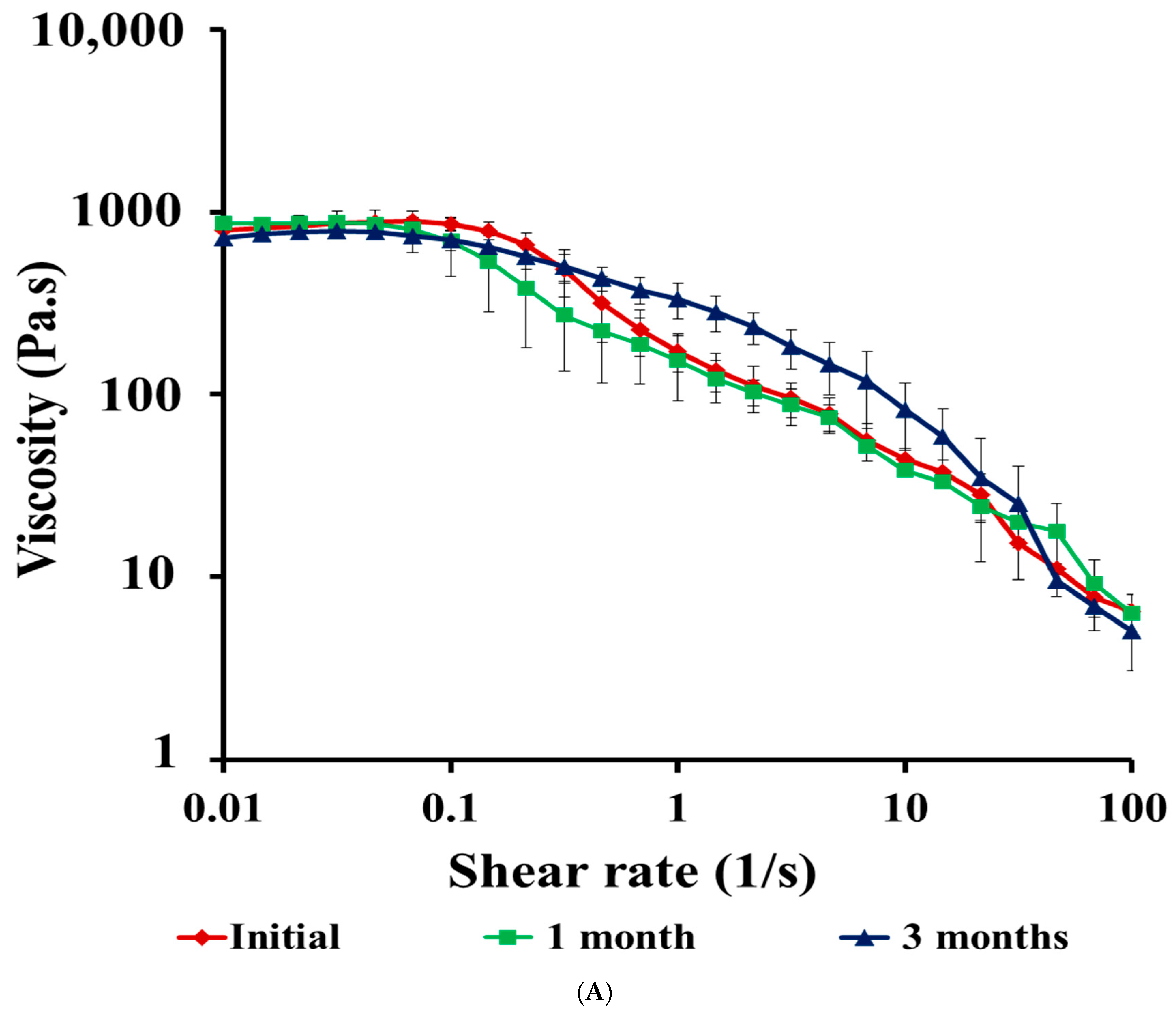
3.4.6. Stability Studies of CIP Hydrogel Patch
3.5. Antimicrobial Study
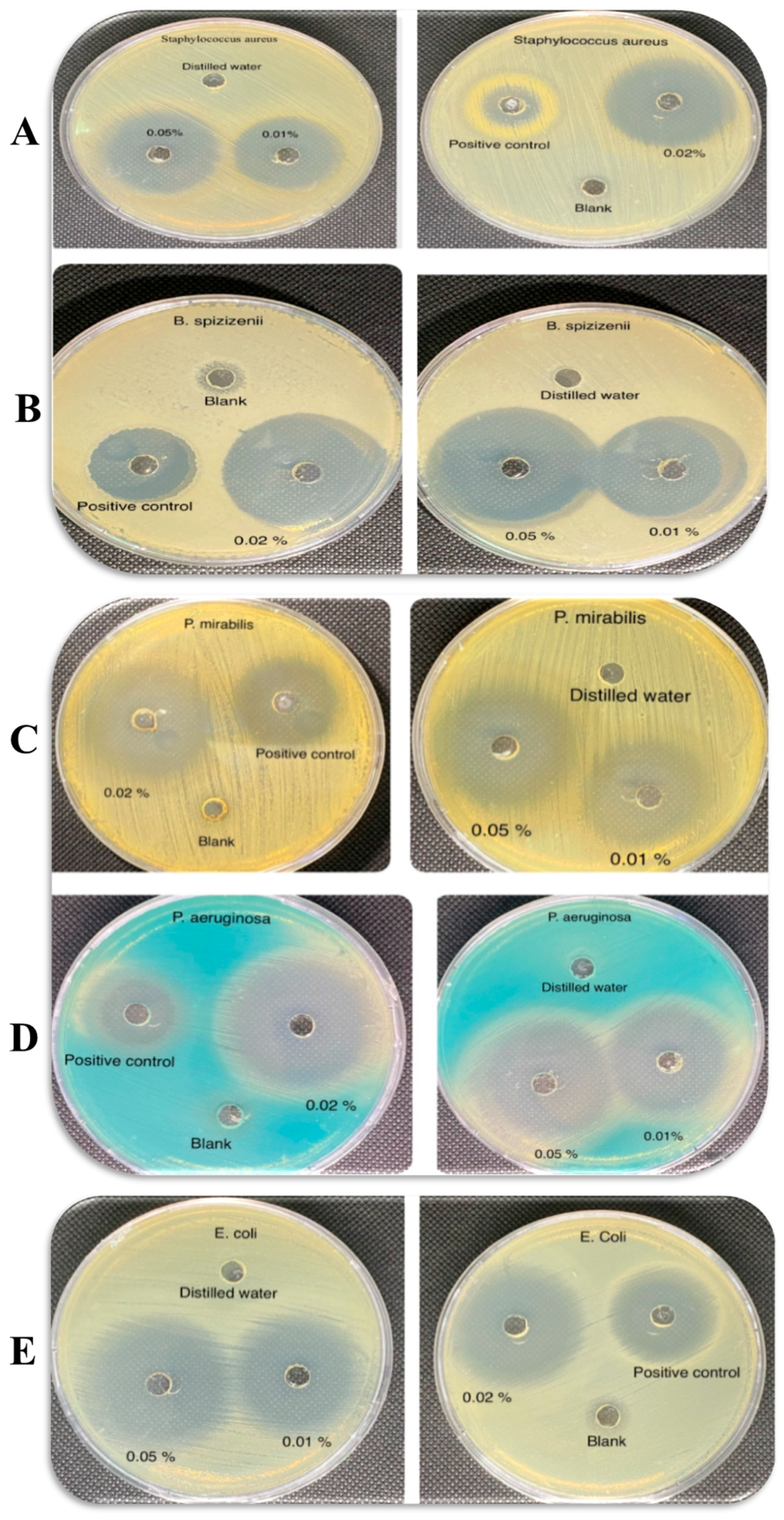
3.5.1. Well-Diffusion Test
3.5.2. Minimum Inhibitory Concentration
3.6. In Vitro Cytotoxicity Study
3.7. Bioadhesion of CIP Hydrogels
3.8. Ex Vivo Permeation
3.9. In Vivo Wound Healing Study—A Pilot Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKnight, G.; Shah, J.; Hargest, R. Physiology of the skin. Surgery 2022, 40, 8–12. [Google Scholar] [CrossRef]
- Eufrásio-da-Silva, T.; Erezuma, I.; Dolatshahi-Pirouz, A.; Orive, G. Enhancing regenerative medicine with self-healing hydrogels: A solution for tissue repair and advanced cyborganic healthcare devices. Biomater. Adv. 2024, 161, 213869. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of appropriate wound dressing for various wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef]
- Arabpour, Z.; Abedi, F.; Salehi, M.; Baharnoori, S.M.; Soleimani, M.; Djalilian, A.R. Hydrogel-Based Skin Regeneration. Int. J. Mol. Sci. 2024, 25, 1982. [Google Scholar] [CrossRef]
- Hurlow, J.J.; Humphreys, G.J.; Bowling, F.L.; McBain, A.J. Diabetic foot infection: A critical complication. Int. Wound J. 2018, 15, 814–821. [Google Scholar] [CrossRef]
- Jia, B.; Li, G.; Cao, E.; Luo, J.; Zhao, X.; Huang, H. Recent progress of antibacterial hydrogels in wound dressings. Mater. Today Bio. 2023, 19, 100582. [Google Scholar] [CrossRef]
- Rancan, F.; Contardi, M.; Jurisch, J.; Blume-Peytavi, U.; Vogt, A.; Bayer, I.S.; Schaudinn, C. Evaluation of drug delivery and efficacy of ciprofloxacin-loaded povidone foils and nanofiber mats in a wound-infection model based on ex vivo human skin. Pharmaceutics 2019, 11, 527. [Google Scholar] [CrossRef]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound dressings: A comprehensive review. Curr. Dermatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Obagi, Z.; Damiani, G.; Grada, A.; Falanga, V. Principles of wound dressings: A review. Surg. Technol. Int. 2019, 35, 50–57. [Google Scholar]
- Sharifi, S.; Saei, A.A.; Gharibi, H.; Mahmoud, N.N.; Harkins, S.; Dararatana, N.; Lisabeth, E.M.; Serpooshan, V.; Végvári, Á.; Moore, A.; et al. Mass Spectrometry, Structural Analysis, and Anti-Inflammatory Properties of Photo-Cross-Linked Human Albumin Hydrogels. ACS Appl. Bio Mater. 2022, 5, 2643–2663. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Ai, J.; Li, K.; Li, J.; Yu, F.; Ma, J. Super flexible, fatigue resistant, self-healing PVA/xylan/borax hydrogel with dual-crosslinked network. Int. J. Biol. Macromol. 2021, 172, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of hydrogels: From synthetic strategies, classification, and properties to biomedical applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research status of self-healing hydrogel for wound management: A review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Li, L.; Fei, X.; Xu, L.; Wang, Y.; Tian, J.; Li, Y. A novel hydrogel with self-healing property and bactericidal activity. J. Colloid Interface Sci. 2021, 584, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Craciun, A.M.; Morariu, S.; Marin, L. Self-healing chitosan hydrogels: Preparation and rheological characterization. Polymers 2022, 14, 2570. [Google Scholar] [CrossRef]
- Bucak, C.D.; Sahin, M.O. Super-flexible, moldable, injectable, self-healing PVA/B/CMC hydrogels synthesis and characterization, as potential water-retaining agent in agriculture. Polym. Bull. 2023, 80, 6591–6608. [Google Scholar] [CrossRef]
- Nasereddin, J.; Shakib, M. Ira: A free and open-source Fourier transform infrared (FTIR) data analysis widget for pharmaceutical applications. Anal. Lett. 2023, 56, 2637–2648. [Google Scholar] [CrossRef]
- Hamed, R.; Mahmoud, N.N.; Alnadi, S.H.; Alkilani, A.Z.; Hussein, G. Diclofenac diethylamine nanosystems-loaded bigels for topical delivery: Development, rheological characterization, and release studies. Drug Dev. Ind. Pharm. 2020, 46, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Abu Alata, W.a.; Abu-Sini, M.; Abulebdah, D.H.; Hammad, A.M.; Aburayya, R. Development and Comparative Evaluation of Ciprofloxacin Nanoemulsion-Loaded Bigels Prepared Using Different Ratios of Oleogel to Hydrogels. Gels 2023, 9, 592. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Xue, Y.; Jin, J.; Xu, Y.; Zeng, W.; Liu, J.; Xie, J. Injectable Hydrogel Delivery System with High Drug Loading for Prolonging Local Anesthesia. Adv. Sci. 2024, 11, 2309482. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Q.; Ning, D.; Dai, L.; Liu, K.; Ni, Y.; Chen, L.; Huang, L. Ultraflexible self-healing guar gum-glycerol hydrogel with injectable, antifreeze, and strain-sensitive properties. ACS Biomater. Sci. Eng. 2018, 4, 3397–3404. [Google Scholar] [CrossRef]
- Zhong, Y.; Seidi, F.; Li, C.; Wan, Z.; Jin, Y.; Song, J.; Xiao, H. Antimicrobial/biocompatible hydrogels dual-reinforced by cellulose as ultrastretchable and rapid self-healing wound dressing. Biomacromolecules 2021, 22, 1654–1663. [Google Scholar] [CrossRef]
- Abumansour, H.; Abusara, O.H.; Khalil, W.; Abul-Futouh, H.; Ibrahim, A.I.; Harb, M.K.; Abulebdah, D.H.; Ismail, W.H. Biological evaluation of levofloxacin and its thionated derivatives: Antioxidant activity, aldehyde dehydrogenase enzyme inhibition, and cytotoxicity on A549 cell line. Naunyn. Schmiedebergs. Arch. Pharmacol. 2024, 397, 6963–6973. [Google Scholar]
- Hamed, R.; Aburayya, R.; Alkilani, A.Z.; Hammad, A.M.; Abusara, O.H.; Abo-Zour, H. Thermo-Responsive Niosomal In Situ Gels for Topical Delivery of Prednisolone. AAPS PharmSciTech 2025, 26, 116. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.D.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef]
- Saeed, S.; Barkat, K.; Ashraf, M.U.; Shabbir, M.; Anjum, I.; Badshah, S.F.; Aamir, M.; Malik, N.S.; Tariq, A.; Ullah, R. Flexible Topical Hydrogel Patch Loaded with Antimicrobial Drug for Accelerated Wound Healing. Gels 2023, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Mansour, H.F. Comparative studies for ciprofloxacin hydrochloride pre-formed gels and thermally triggered (in situ) gels: In vitro and in vivo appraisal using a bacterial keratitis model in rabbits. Pharm. Dev. Technol. 2015, 20, 410–416. [Google Scholar] [CrossRef]
- Arefian, M.; Hojjati, M.; Tajzad, I.; Mokhtarzade, A.; Mazhar, M.; Jamavari, A. A review of Polyvinyl alcohol/Carboxymethyl cellulose (PVA/CMC) composites for various applications. J. Compos. Compd. 2020, 2, 69–76. [Google Scholar]
- Wang, C.; Shen, Z.; Hu, P.; Wang, T.; Zhang, X.; Liang, L.; Bai, J.; Qiu, L.; Lai, X.; Yang, X. Facile fabrication and characterization of high-performance Borax-PVA hydrogel. J. Solgel Sci. Technol. 2022, 101, 103–113. [Google Scholar] [CrossRef]
- Sahoo, S.; Chakraborti, C.K.; Behera, P.K. FTIR and Raman spectroscopic investigations of a controlled release Ciprofloxacin/Carbopol940 mucoadhesive suspension. Asian J. Pharm. Clin. Res 2012, 5, 125–130. [Google Scholar]
- Açik, G.; Kamaci, M.; Özata, B.; CANSOY, C.E.Ö. Effect of polyvinyl alcohol/chitosan blend ratios on morphological, optical, and thermal properties of electrospun nanofibers. Turk. J. Chem. 2019, 43, 137–149. [Google Scholar] [CrossRef]
- Yong-Sing, N.; Yun-Ming, L.; Cheng-Yong, H.; Abdullah, M.M.A.B.; Pakawanit, P.; Vizureanu, P.; Khalid, M.S.; Hui-Teng, N.; Yong-Jie, H.; Nabiałek, M. Improvements of flexural properties and thermal performance in thin geopolymer based on fly ash and ladle furnace slag using borax decahydrates. Materials 2022, 15, 4178. [Google Scholar] [CrossRef]
- Goel, N.; Sinha, N.; Kumar, B. Growth and properties of sodium tetraborate decahydrate single crystals. Mater. Res. Bull. 2013, 48, 1632–1636. [Google Scholar] [CrossRef]
- Alifah, N.; Palungan, J.; Ardayanti, K.; Ullah, M.; Nurkhasanah, A.N.; Mustopa, A.Z.; Lallo, S.; Agustina, R.; Yoo, J.-W.; Hasan, N. Development of Clindamycin-Releasing Polyvinyl Alcohol Hydrogel with Self-Healing Property for the Effective Treatment of Biofilm-Infected Wounds. Gels 2024, 10, 482. [Google Scholar] [CrossRef]
- Koysuren, O.; Karaman, M.; Dinc, H. Preparation and characterization of polyvinyl borate/polyvinyl alcohol (PVB/PVA) blend nanofibers. J. Appl. Polym. Sci. 2012, 124, 2736–2741. [Google Scholar] [CrossRef]
- Lv, A.; Ashworth, L.; Donnelly, R. Compounding with commercial drug products can cause errors. Int. J. Pharm. Compd. 2021. Available online: https://www.pharmacytimes.com/view/2006-11-6068 (accessed on 30 September 2025).
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Wang, L.L.; Chung, J.J.; Kim, Y.-H.; Atluri, P.; Burdick, J.A. Methods to assess shear-thinning hydrogels for application as injectable biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Seder, B.Y.; Bardaweel, S.K.; Qawass, H. Lipid-based formulations of microemulsion-loaded oleogels for the oral delivery of carvedilol. J. Dispers. Sci. Technol. 2023, 44, 708–718. [Google Scholar] [CrossRef]
- Hamed, R.; Magamseh, K.H.; Al-Shalabi, E.; Hammad, A.; Abu-Sini, M.; Abulebdah, D.H.; Tarawneh, O.; Sunoqrot, S. Green Hydrogels Prepared from Pectin Extracted from Orange Peels as a Potential Carrier for Dermal Delivery Systems. ACS Omega 2025, 10, 17182–17200. [Google Scholar] [CrossRef] [PubMed]
- Franck, A.; Germany, T. Viscoelasticity and Dynamic Mechanical Testing; TA Instruments: New Castle, DE, USA, 1993; AN004. [Google Scholar]
- Rezvan, G.; Pircheraghi, G.; Bagheri, R. Curcumin incorporated PVA-borax dual delivery hydrogels as potential wound dressing materials—Correlation between viscoelastic properties and curcumin release rate. J. Appl. Polym. Sci. 2018, 135, 46734. [Google Scholar] [CrossRef]
- Cuenot, S.; Gélébart, P.; Sinquin, C.; Colliec-Jouault, S.; Zykwinska, A. Mechanical relaxations of hydrogels governed by their physical or chemical crosslinks. J. Mech. Behav. Biomed. Mater. 2022, 133, 105343. [Google Scholar] [CrossRef]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Control. Release (JCR) 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Zhai, N.; Wang, B. Preparation of fast-swelling porous superabsorbent hydrogels with high saline water absorbency under pressure by foaming and post surface crosslinking. Sci. Rep. 2023, 13, 13815. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, W.; Jin, C. Protocol efficiently measuring the swelling rate of hydrogels. MethodsX 2020, 7, 100779. [Google Scholar] [CrossRef]
- Yu, P.; Wei, L.; Yang, Z.; Liu, X.; Ma, H.; Zhao, J.; Liu, L.; Wang, L.; Chen, R.; Cheng, Y. Hydrogel wound dressings accelerating healing process of wounds in movable parts. Int. J. Mol. Sci. 2024, 25, 6610. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Zheng, W.; Yang, G.; Jiang, X. Rapid fabrication of self-healing, conductive, and injectable gel as dressings for healing wounds in stretchable parts of the body. Adv. Funct. Mater. 2020, 30, 2002370. [Google Scholar] [CrossRef]
- Rinawa, K.; Maiti, S.; Sonnier, R.; Lopez Cuesta, J.-M. Dynamic rheological studies and applicability of time–temperature superposition principle for PA12/SEBS-g-MA blends. Polym. Bull. 2015, 72, 3305–3324. [Google Scholar] [CrossRef]
- Koosha, M.; Jahani, Y.; Mirzadeh, H. The effect of electron beam irradiation on dynamic shear rheological behavior of a poly (propylene-co-ethylene) heterophasic copolymer. Polym. Adv. Technol. 2011, 22, 2039–2043. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Scorzoni, L.; Sangalli-Leite, F.; de Lacorte Singulani, J.; Costa-Orlandi, C.B.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Searching new antifungals: The use of in vitro and in vivo methods for evaluation of natural compounds. J. Microbiol. Methods 2016, 123, 68–78. [Google Scholar] [CrossRef]
- Valan, A.S.; Kolli, S.; Eswaramoorthy, R.; Krithikadatta, J.; Sureshbabu, N.M. Comparison of antibacterial efficacy of triple antibiotic-loaded hydrogel versus modified triple antibiotic-loaded hydrogel as intracanal medicament against Enterococcus faecalis: An in vitro study. Eur. Endod. J. 2024, 9, 154. [Google Scholar] [CrossRef]
- Singaravelu, S.; Sankarapillai, J.; Chandrakumari, A.S.; Sinha, P. Effect of Azadirachta indica crude bark extracts concentrations against gram-positive and gram-negative bacterial pathogens. J. Pharm. Bioallied. Sci. 2019, 11, 33–37. [Google Scholar] [CrossRef]
- Masadeh, M.M.; Alzoubi, K.H.; Al-Azzam, S.I.; Khabour, O.F.; Al-Buhairan, A.M. Ciprofloxacin-induced antibacterial activity is atteneuated by pretreatment with antioxidant agents. Pathogens 2016, 5, 28. [Google Scholar] [CrossRef]
- Akhlaq, A.; Ashraf, M.; Omer, M.O.; Altaf, I. Synergistic antibacterial activity of carvacrol loaded chitosan nanoparticles with Topoisomerase inhibitors and genotoxicity evaluation. Saudi J. Biol. Sci. 2023, 30, 103765. [Google Scholar] [CrossRef]
- Masadeh, M.M.; Alzoubi, K.H.; Al-Azzam, S.I.; Al-Buhairan, A.M. Possible involvement of ROS generation in vorinostat pretreatment induced enhancement of the antibacterial activity of ciprofloxacin. Clin. Pharmacol. Adv. App. 2017, 9, 119–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kudłacik-Kramarczyk, S.; Drabczyk, A.; Głąb, M.; Alves-Lima, D.; Lin, H.; Douglas, T.E.L.; Kuciel, S.; Zagórska, A.; Tyliszczak, B. Investigations on the impact of the introduction of the Aloe vera into the hydrogel matrix on cytotoxic and hydrophilic properties of these systems considered as potential wound dressings. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111977. [Google Scholar] [CrossRef] [PubMed]
- Parente, M.; Ochoa Andrade, A.; Ares, G.; Russo, F.; Jiménez-Kairuz, Á. Bioadhesive hydrogels for cosmetic applications. Int. J. Cosmet. Sci. 2015, 37, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Škalko-Basnet, N. Potentials of chitosan-based delivery systems in wound therapy: Bioadhesion study. J. Funct. Biomater. 2012, 3, 37–48. [Google Scholar] [CrossRef]
- Chen, X.; Ji, N.; Li, F.; Qin, Y.; Wang, Y.; Xiong, L.; Sun, Q. Dual cross-linked starch–borax double network hydrogels with tough and self-healing properties. Foods 2022, 11, 1315. [Google Scholar] [CrossRef]
- Zou, C.-Y.; Lei, X.-X.; Hu, J.-J.; Jiang, Y.-L.; Li, Q.-J.; Song, Y.-T.; Zhang, Q.-Y.; Li-Ling, J.; Xie, H.-Q. Multi-crosslinking hydrogels with robust bio-adhesion and pro-coagulant activity for first-aid hemostasis and infected wound healing. Bioact. Mater. 2022, 16, 388–402. [Google Scholar] [CrossRef]
- Bu, Y.; Pandit, A. Cohesion mechanisms for bioadhesives. Bioact. Mater. 2022, 13, 105–118. [Google Scholar] [CrossRef]
- McCarron, P.A.; Murphy, D.J.; Little, C.; McDonald, J.; Kelly, O.J.; Jenkins, M.G. Preliminary clinical assessment of polyvinyl alcohol–tetrahydroxyborate hydrogels as potential topical formulations for local anesthesia of lacerations. Acad. Emerg. Med. 2011, 18, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.J.; Sankalia, M.G.; Loughlin, R.G.; Donnelly, R.F.; Jenkins, M.G.; McCarron, P.A. Physical characterisation and component release of poly (vinyl alcohol)–tetrahydroxyborate hydrogels and their applicability as potential topical drug delivery systems. Int. J. Pharm. 2012, 423, 326–334. [Google Scholar] [CrossRef]
- Moradifar, F.; Sepahdoost, N.; Tavakoli, P.; Mirzapoor, A. Multi-functional dressings for recovery and screenable treatment of wounds: A review. Heliyon 2025, 11, e41465. [Google Scholar] [CrossRef] [PubMed]
- Cam, M.E.; Yildiz, S.; Alenezi, H.; Cesur, S.; Ozcan, G.S.; Erdemir, G.; Edirisinghe, U.; Akakin, D.; Kuruca, D.S.; Kabasakal, L.; et al. Evaluation of burst release and sustained release of pioglitazone-loaded fibrous mats on diabetic wound healing: An in vitro and in vivo comparison study. J. R. Soc. Interface 2020, 17, 20190712. [Google Scholar] [CrossRef]
- Miranda-Calderon, L.; Yus, C.; Remirez de Ganuza, C.; Paesa, M.; Landa, G.; Tapia, E.; Pérez, E.; Perez, M.; Sebastian, V.; Irusta, S.; et al. Combinatorial wound dressings loaded with synergistic antibiotics in the treatment of chronic infected wounds. Chem. Eng. J. 2023, 476, 146679. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Health. 2022, 10, 1025633. [Google Scholar] [CrossRef]
- Chegini, Z.; Azizi, M.; Safaiee, M.; Kalhori, F.; Zare Shahraki, R.; Hemmati, J.; Hosseini, S.M.; Teimoori, A.; Shariati, A.; Arabestani, M.R. Wound healing properties and antibiofilm activity of hydrogel matrix containing nitric oxide, silver nanoparticles, and ciprofloxacin against Pseudomonas aeruginosa burn wound infection. Sci. Rep. 2025, 15, 26508. [Google Scholar] [CrossRef]
- Junker, J.P.; Kamel, R.A.; Caterson, E.; Eriksson, E. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.; Prosser, H.C.; Tan, J.T.; Vanags, L.Z.; Ng, M.K.; Bursill, C.A. Murine model of wound healing. J. Vis. Exp. 2013, 50265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, W.; Zhou, S.; Zhang, G.; He, J.; Li, Q. A novel model for cutaneous wound healing and scarring in the rat. Plast. Reconstr. Surg. 2019, 143, 468–477. [Google Scholar] [CrossRef] [PubMed]

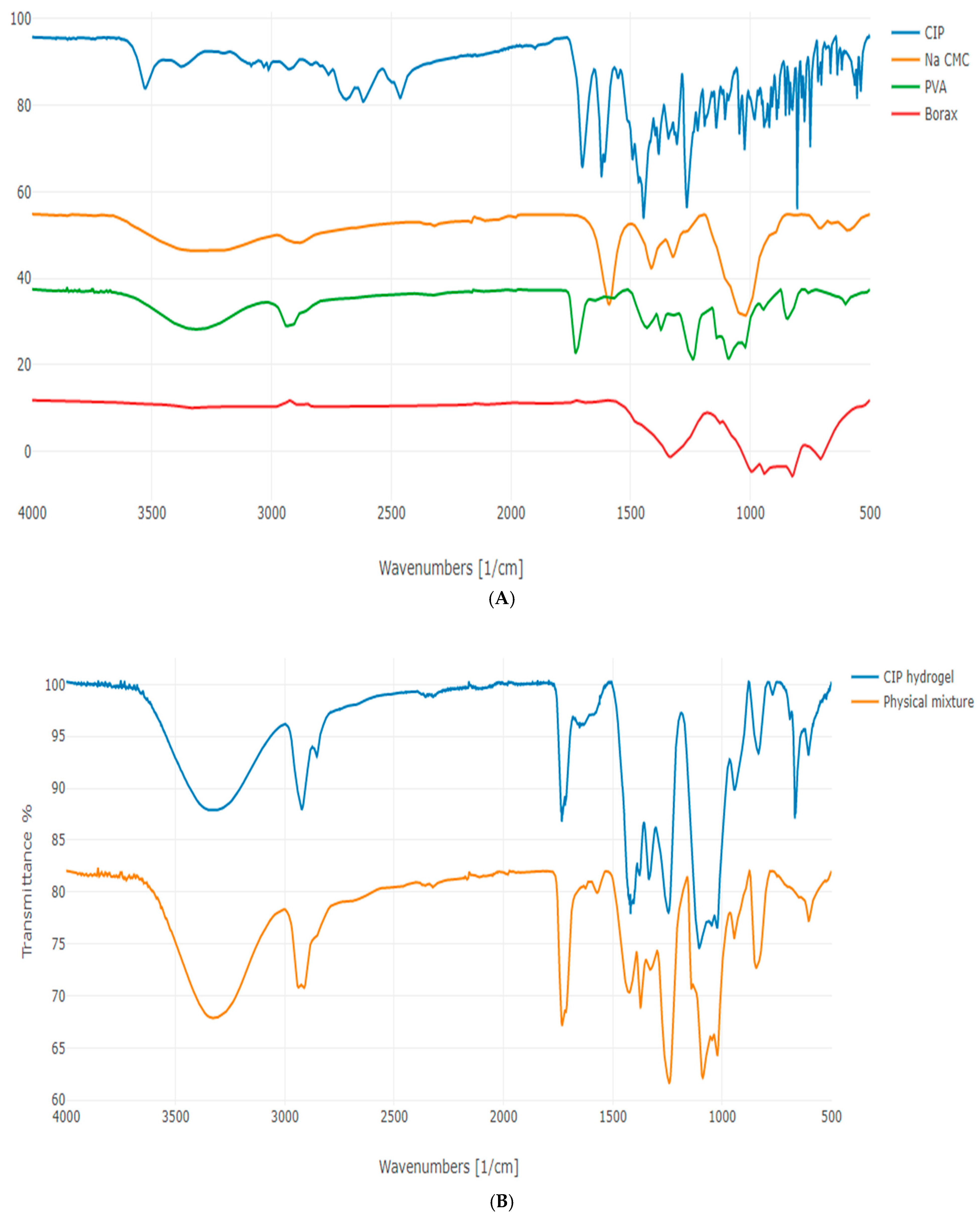



| Wavelength (cm−1) | Functional Group |
|---|---|
| 1419 and 1245 | B–O–R |
| 677 | B–O–B (bending) |
| 769 | C–H (bending) |
| 833 | B–O (vibration) |
| Time of Crosslinking (h) | LVR (%) | Applied Strain Within the LVR (%) | Critical Strain (γC, %) |
|---|---|---|---|
| 1 h | 0.01–2.51 | 0.1 | 2.51 |
| 24 h | 0.01–1.58 | 0.1 | 1.58 |
| 48 h | 0.01–1.58 | 0.1 | 1.58 |
| 1 month | 0.01–1.58 | 0.1 | 1.58 |
| 3 months | 0.01–1.00 | 0.1 | 1.00 |
| Zones of Inhibition (mm) | ||||||
|---|---|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria | |||||
| Tested Samples | Concentrations (μg/mL) | S. aureus | B. spizizenii | P. aeruginosa | P. mirabilis | E. coli |
| D.W (negative control) | - | NZ | NZ | NZ | NZ | NZ |
| Blank hydrogel patch (negative control) | - | NZ | NZ | NZ | NZ | NZ |
| CIP solution (positive control) | 5 | 16.3 ± 1.1 | 24.0 ± 0.0 | 17.8 ± 1.7 | 27.0 ± 0.0 | 24.6 ± 0.5 |
| CIP hydrogel patch | 570 | 33.3 ± 0.5 | 39.2 ± 0.3 | 36.3 ± 0.2 | 33.0 ± 0.0 | 33.6 ± 0.5 |
| CIP hydrogel patch | 250 | 31.0 ± 0.0 | 38.2 ± 0.3 | 34.1 ± 1.2 | 31.1 ± 0.2 | 30.6 ± 0.5 |
| CIP hydrogel patch | 110 | 29.3 ± 0.5 | 35.0 ± 0.0 | 31.0 ± 0.0 | 28.0 ± 0.0 | 29.8 ± 1.2 |
| CIP hydrogel patch | 50 | 26.3 ± 0.5 | 34.0 ± 1.4 | 28.6 ± 0.5 | 26.0 ± 0.2 | 27.3 ± 0.5 |
| CIP hydrogel patch | 28 | 24.0 ± 0.0 | 31.5 ± 0.7 | 25.6 ± 0.5 | 24.6 ± 0.5 | 25.3 ± 0.5 |
| CIP hydrogel patch | 14 | 19.6 ± 0.5 | 29.0 ± 0.0 | 24.0 ± 0.0 | 23.3 ± 0.5 | 23.8 ± 1.0 |
| CIP hydrogel patch | 7 | 17.3 ± 0.5 | 26.5 ± 0.7 | 21.0 ± 0.5 | 23.6 ± 0.5 | 22.6 ± 0.5 |
| CIP hydrogel patch | 3 | 13.6 ± 0.5 | 23.2 ± 0.3 | 17.3 ± 0.5 | 22.6 ± 0.5 | 21.3 ± 0.5 |
| CIP hydrogel patch | 1.7 | 10.0 ± 1.0 | 20.5 ± 0.7 | 13.1 ± 1.0 | 22.0 ± 0.0 | 20.3 ± 0.5 |
| CIP hydrogel patch | 0.85 | NZ | 18.7 ± 0.3 | NZ | 19.6 ± 0.5 | 19.5 ± 0.5 |
| CIP hydrogel patch | 0.4 | NZ | 14.0 ± 0 | NZ | NZ | 18.6 ± 0.5 |
| CIP hydrogel patch | 0.2 | NZ | 10.7 ± 1.0 | NZ | NZ | 15.1 ± 0.2 |
| CIP hydrogel patch | 0.1 | NZ | NZ | NZ | NZ | 12.8 ± 0.2 |
| CIP hydrogel patch | 0.05 | NZ | NZ | NZ | NZ | 9.8 ± 0.2 |
| CIP hydrogel patch | 0.02 | NZ | NZ | NZ | NZ | NZ |
| Groups | Day 1 | Day 4 | Day 9 |
|---|---|---|---|
| Negative control (Group 1) | 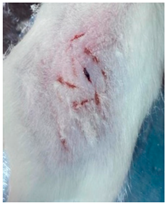 | 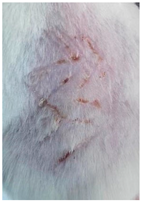 | 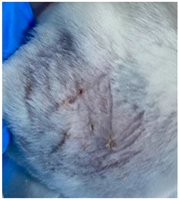 |
| CIP hydrogel patch (Group 2) | 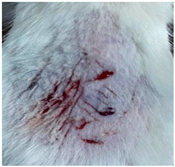 |  | 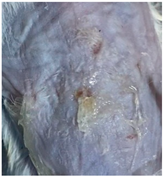 |
| Blank hydrogel patch (Group 3) | 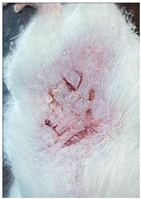 | 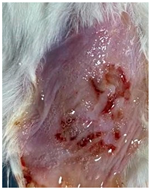 | 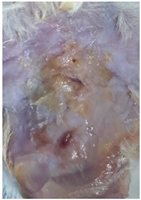 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Farhan, W.; Abusara, O.H.; Abu-Sini, M.; Hikmat, S.; Tarawneh, O.; Al-Kouz, S.; Hamed, R. Development, Characterization, and Biological Evaluation of a Self-Healing Hydrogel Patch Loaded with Ciprofloxacin for Wound Dressings. Polymers 2025, 17, 2686. https://doi.org/10.3390/polym17192686
Al-Farhan W, Abusara OH, Abu-Sini M, Hikmat S, Tarawneh O, Al-Kouz S, Hamed R. Development, Characterization, and Biological Evaluation of a Self-Healing Hydrogel Patch Loaded with Ciprofloxacin for Wound Dressings. Polymers. 2025; 17(19):2686. https://doi.org/10.3390/polym17192686
Chicago/Turabian StyleAl-Farhan, Wasan, Osama H. Abusara, Mohammad Abu-Sini, Suhair Hikmat, Ola Tarawneh, Sameer Al-Kouz, and Rania Hamed. 2025. "Development, Characterization, and Biological Evaluation of a Self-Healing Hydrogel Patch Loaded with Ciprofloxacin for Wound Dressings" Polymers 17, no. 19: 2686. https://doi.org/10.3390/polym17192686
APA StyleAl-Farhan, W., Abusara, O. H., Abu-Sini, M., Hikmat, S., Tarawneh, O., Al-Kouz, S., & Hamed, R. (2025). Development, Characterization, and Biological Evaluation of a Self-Healing Hydrogel Patch Loaded with Ciprofloxacin for Wound Dressings. Polymers, 17(19), 2686. https://doi.org/10.3390/polym17192686






