Comparison of Different Aliphatic Polyester-Based Microparticles as Protein Delivery Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis and Characterization of Polymers
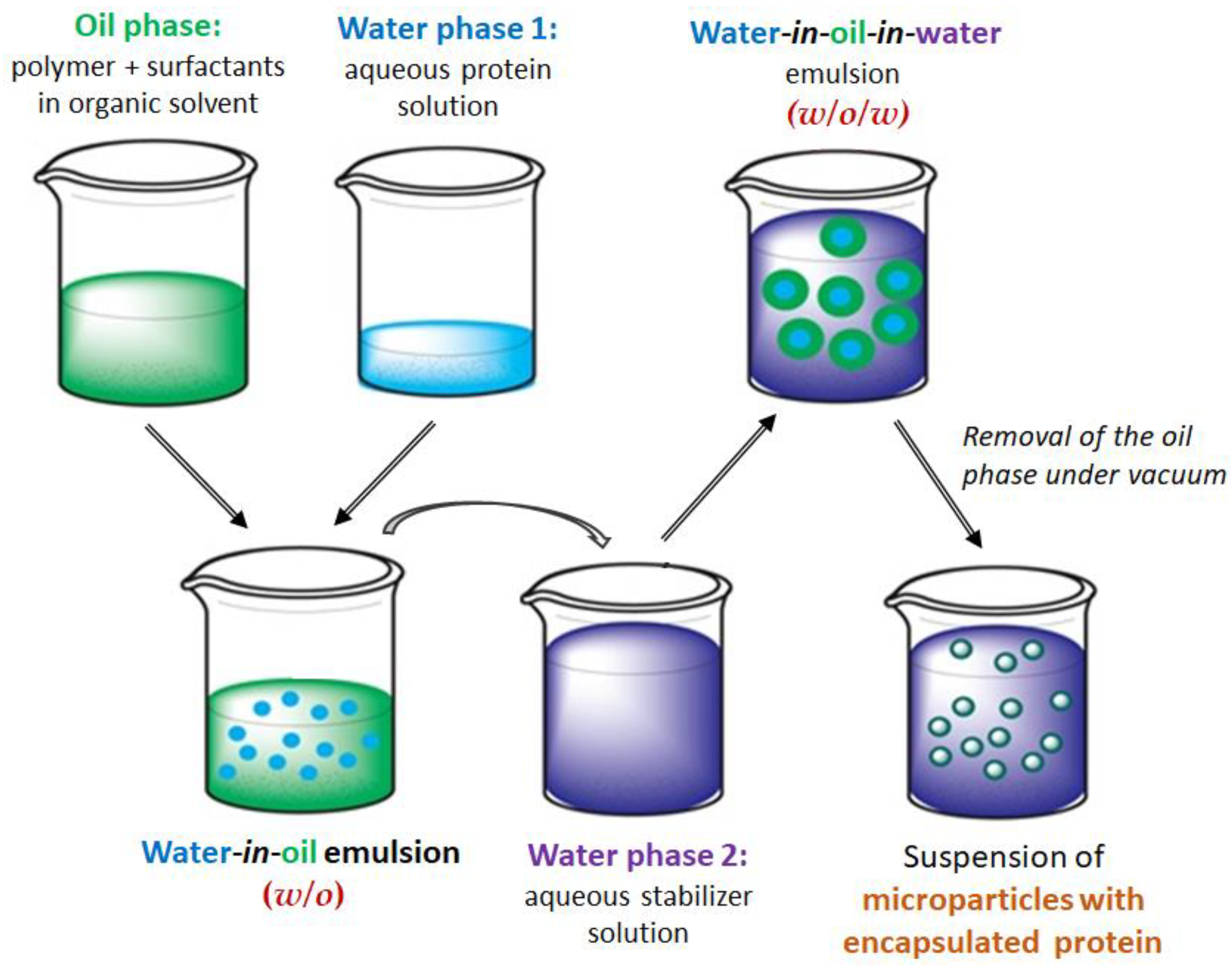
2.2.2. Preparation of Protein-Encapsulated Microparticles
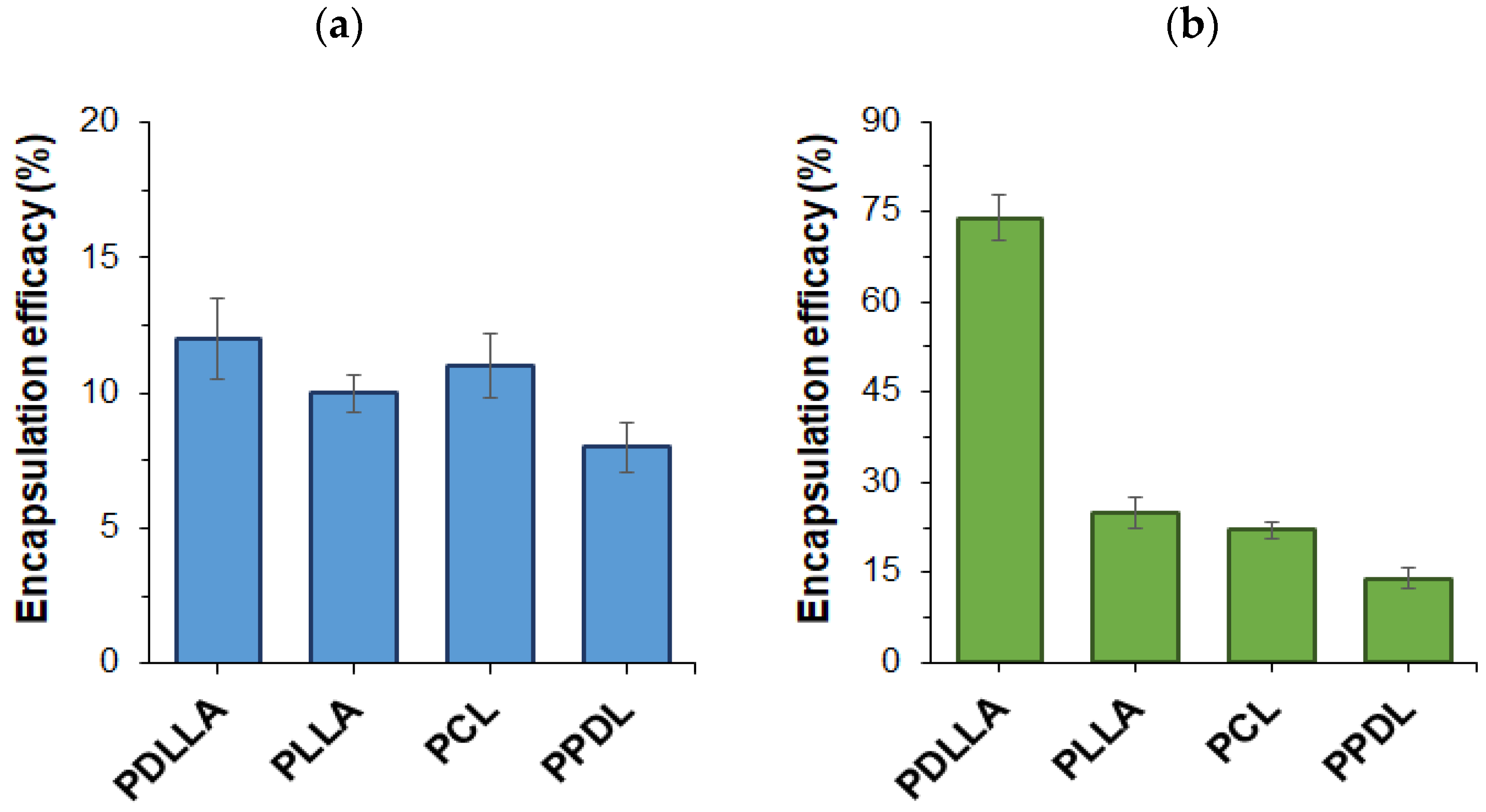
2.2.3. Determination of Protein Encapsulation Efficiency
2.2.4. Characterization of Microparticles
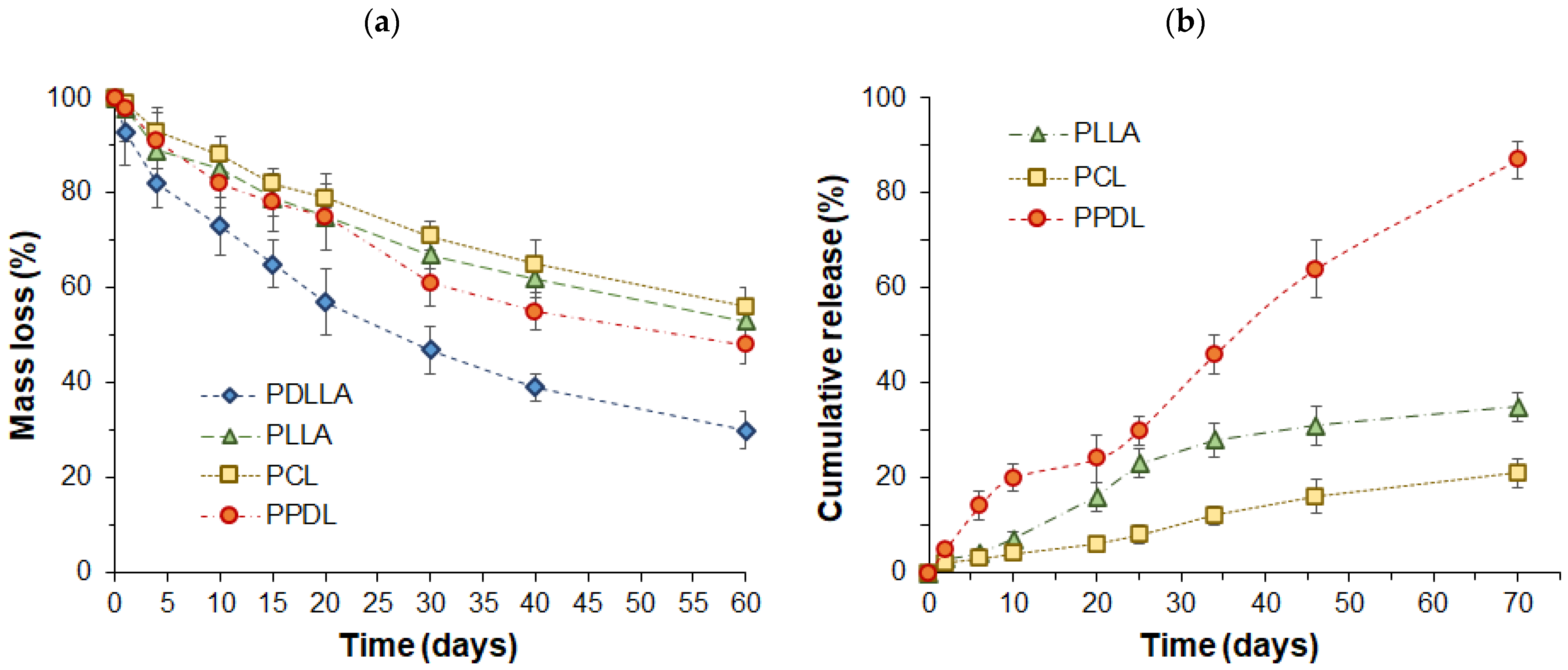
2.2.5. Polymer Degradation Study
2.2.6. Protein Release Study
2.2.7. Enzyme Activity Assay
2.2.8. Data Processing and Statistics
3. Results and Discussion
3.1. Polymers and Their Characteristics
| Polymer Structure | Physicochemical Properties | |||
|---|---|---|---|---|
| Hydrophobicity | Crystallinity | Degradation | ||
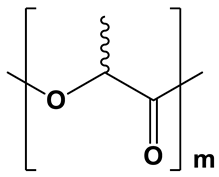 | Moderately hydrophobic; water contact angle =68–76° [38,39,40] | Amorphous; XRD: the region 2θ = 15.0–30.0° contains only a pronounced “amorphous halo” [15] | Relatively fast degradation; 3–6 months [41] | |
| PDLLA | ||||
| PLLA | 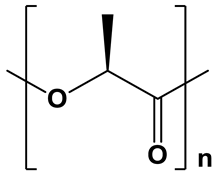 | Hydrophobic; water contact angle =74–85° [42,43,44] | Semicrystalline; degree of crystallinity up to 40% (50%, thermal treatment) [45,46,47,48]; XRD: two sharp signals at 2θ = 16.6° and 19.0° [15] | Moderate degradation; From 6 months to 2 years, depending on the crystallinity degree and molecular weight [41,49] |
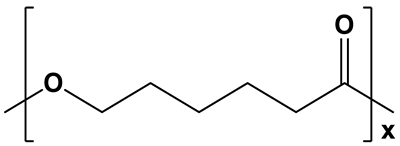 | Highly hydrophobic; water contact angle =78–95° [50,51,52] | Semicrystalline;degree of crystallinity from 39 to 69% [53,54,55]; XRD: two sharp signals at 2θ = 21.3° and 23.6° [15,55] | Long degradation; from 1 to 3 years [53,56] | |
| PCL | ||||
| PPDL | 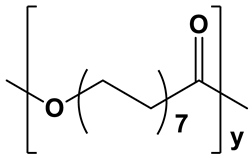 | Extremely hydrophobic; water contact angle ≥93° [57] | Semicrystalline; degree of crystallinity from 54 to 74% [58,59,60,61]; XRD: two sharp signals at 2θ = 21.4° and 23.9° [15] | Long degradation;up to several years [58,61,62] |
3.2. Preparation and Characterization of Protein-Loaded Microparticles
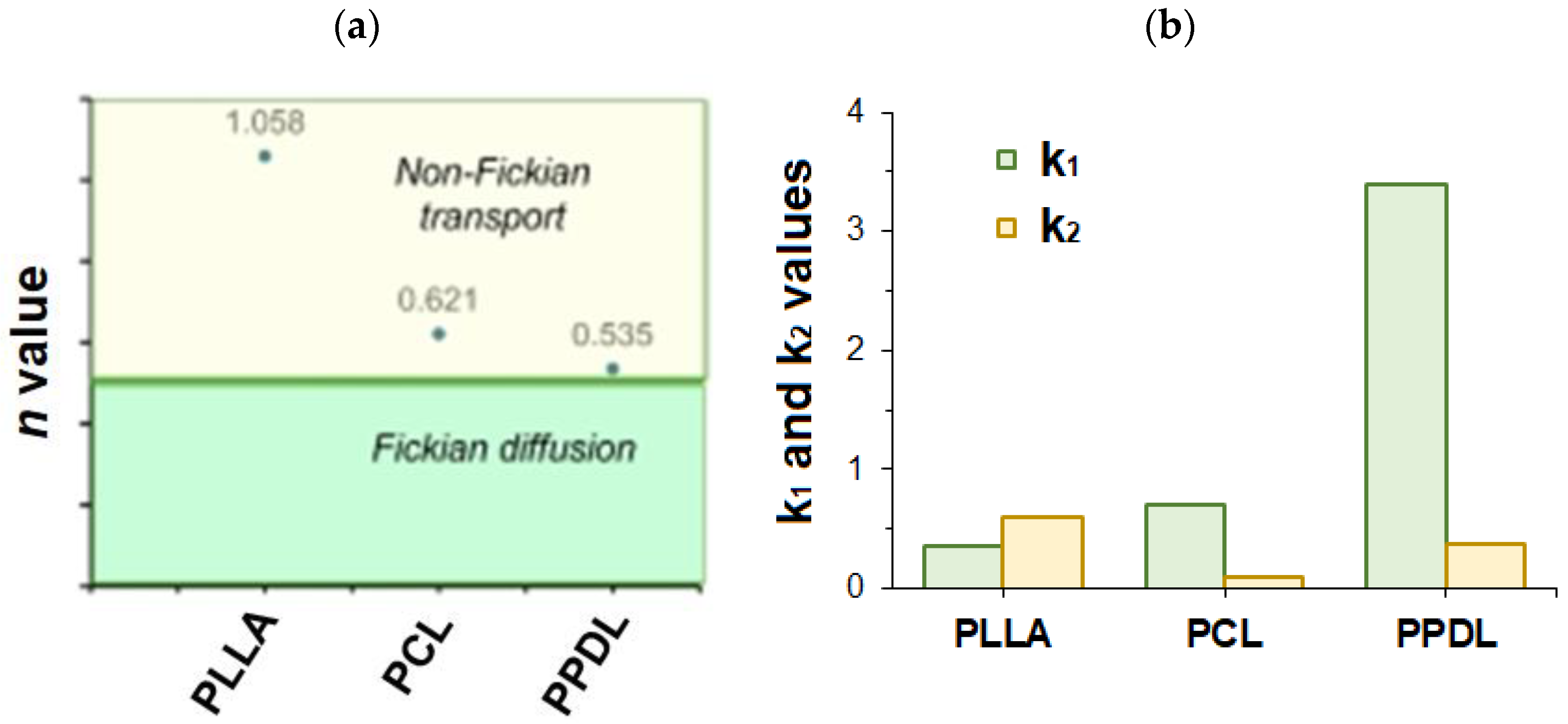
3.3. Particle Biodegradation and Protein Release
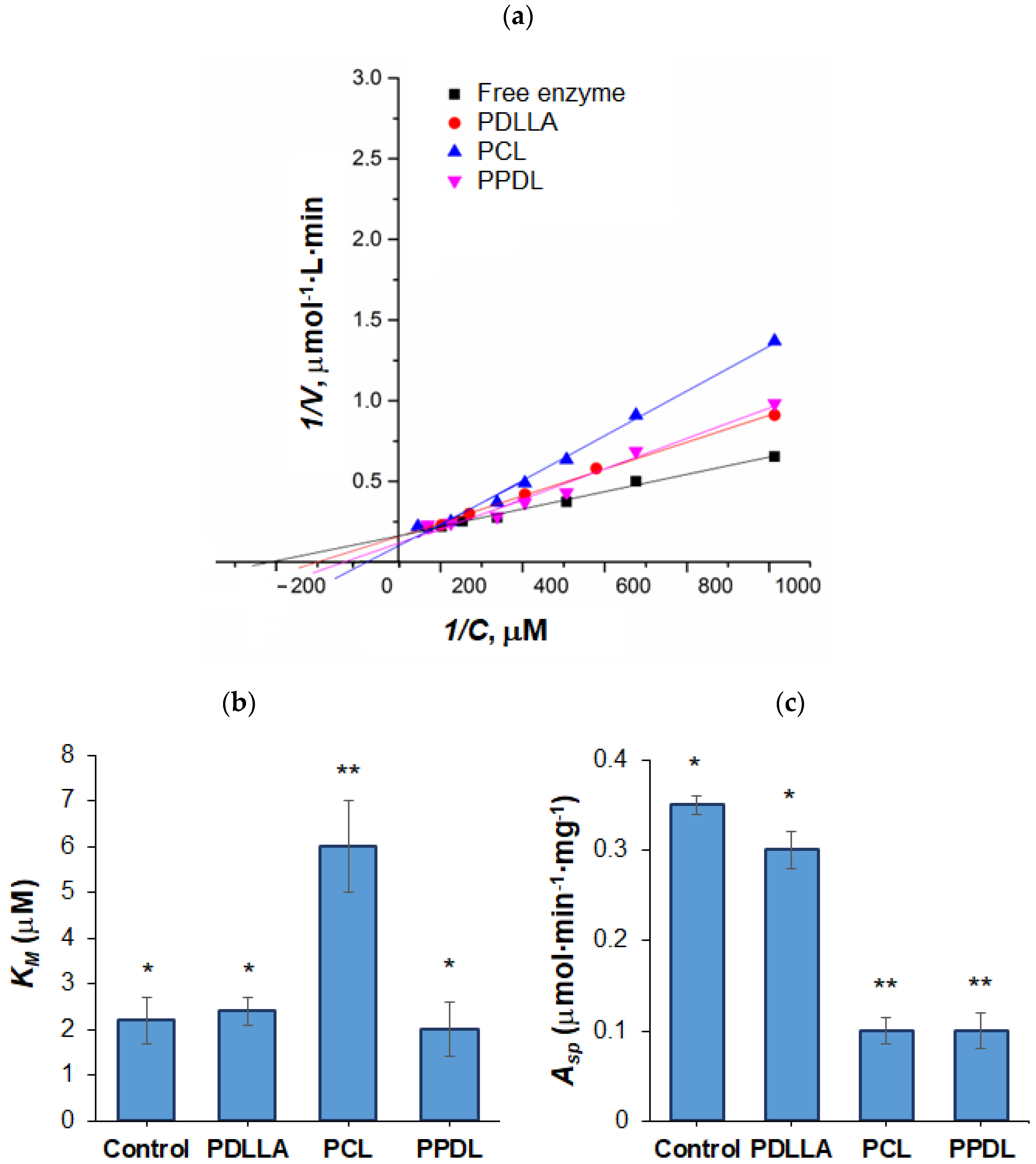
3.4. Enzyme Encapsulation and Activity Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDLLA | Poly(D,L-lactide) |
| PLLA | Poly(L-lactide) |
| PCL | Poly(ε-caprolactone) |
| PPDL | Poly(ω-pentadecalactone) |
| BSA | Bovine serum albumin |
| ACHT | α-Chymotrypsin |
| SEM | Scanning electron microscopy |
| DLS | Dynamic light scattering |
| SEC | Size-exclusion chromatography |
| EE | Encapsulation efficacy |
| DL | Drug loading |
| DMAB | Didodecyldimethylammonium bromide |
| ROP | Ring-opening polymerization |
References
- Sundar, D.S.; Antoniraj, M.G.; Kumar, C.S.; Mohapatra, S.S.; Houreld, N.N.; Ruckmani, K. Recent Trends of Biocompatible and Biodegradable Nanoparticles in Drug Delivery: A Review. Curr. Med. Chem. 2016, 23, 3730–3751. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Safaei, M.; Zarei, K.; Moradi, M.; Yazdani Nezhad, H. A Review on Biomedical Application of Polysaccharide-Based Hydrogels with a Focus on Drug Delivery Systems. Polymers 2022, 14, 5432. [Google Scholar] [CrossRef]
- Korzhikova-Vlakh, E.G.; Zashikhina, N.N.; Stulova, E.G.; Dzhuzha, A.Y.; Korzhikov-Vlakh, V.A. Effect of L- or D,L-leucine content on self-assembly and properties of its amphiphilic copolymers with L-lysine. Mendeleev Commun. 2024, 34, 365–368. [Google Scholar] [CrossRef]
- Osorno, L.L.; Brandley, A.N.; Maldonado, D.E.; Yiantsos, A.; Mosley, R.J.; Byrne, M.E. Review of contemporary self-assembled systems for the controlled delivery of therapeutics in medicine. Nanomaterials 2021, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Do, H.D.; Le Thi, H.; Le Thi, T.H.; Nguyen, H.N.; Bui, V.K.; Thi, M.N.H.; Ha, P.T. Folate-modified, curcumin and paclitaxel co-loaded PLA-TPGS nanoparticles: Preparation, optimization and in vitro cytotoxicity assays. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025004. [Google Scholar] [CrossRef]
- Imperiale, J.C.; Acosta, G.B.; Sosnik, A. Polymer-based carriers for ophthalmic drug delivery. J. Control. Release 2018, 285, 106–141. [Google Scholar] [CrossRef]
- Sudareva, N.N.; Suvorova, O.M.; Tarasenko, I.I.; Saprykina, N.N.; Smirnova, N.V.; Petunov, S.G.; Radilov, A.S.; Timin, A.S.; Korzhikova-Vlakh, E.G.; Vilesov, A.D. Hybrid systems for oral delivery of a therapeutic neuropeptide. Mendeleev Commun. 2020, 30, 25–27. [Google Scholar] [CrossRef]
- Ahmadzada, T.; Reid, G.; McKenzie, D.R. Fundamentals of siRNA and miRNA therapeutics and a review of targeted nanoparticle delivery systems in breast cancer. Biophys. Rev. 2018, 10, 69–86. [Google Scholar] [CrossRef]
- He, C.; Tang, Z.; Tian, H.; Chen, X. Co-delivery of chemotherapeutics and proteins for synergistic therapy. Adv. Drug Deliv. Rev. 2016, 98, 64–76. [Google Scholar] [CrossRef]
- Patel, A.; Patel, M.; Yang, X.; Mitra, A.K. Recent advances in protein and Peptide drug delivery: A special emphasis on polymeric nanoparticles. Protein Pept. Lett. 2014, 21, 1102–1120. [Google Scholar] [CrossRef]
- Pilipenko, I.; Korovkina, O.; Gubina, N.; Ekimova, V.; Ishutinova, A.; Korzhikova-Vlakh, E.; Tennikova, T.; Korzhikov-Vlakh, V. Random Copolymers of Lysine and Isoleucine for Efficient mRNA Delivery. Int. J. Mol. Sci. 2022, 23, 5363. [Google Scholar] [CrossRef]
- Sasada, S.; Kurihara, H.; Kinoshita, T.; Yoshida, M.; Honda, N.; Shimoi, T.; Shimomura, A.; Yunokawa, M.; Yonemori, K.; Shimizu, C.; et al. 64Cu-DOTA-trastuzumab PET imaging for HER2-specific primary lesions of breast cancer. Ann. Oncol. 2017, 28, 2028–2029. [Google Scholar] [CrossRef]
- van der Graaf, P.H. Clinical Significance of Therapeutic Peptide and Protein Drug Interactions: A Call to Action. Clin. Pharmacol. Ther. 2023, 113, 1173–1174. [Google Scholar] [CrossRef]
- Massi, L.; Najer, A.; Chapman, R.; Spicer, C.D.; Nele, V.; Che, J.; Booth, M.A.; Doutch, J.J.; Stevens, M.M. Tuneable peptide cross-linked nanogels for enzyme-triggered protein delivery. J. Mater. Chem. B 2020, 8, 8894–8907. [Google Scholar] [CrossRef] [PubMed]
- Korzhikov, V.; Averianov, I.; Litvinchuk, E.; Tennikova, T.B. Polyester-based microparticles of different hydrophobicity: The patterns of lipophilic drug entrapment and release. J. Microencapsul. 2016, 33, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, B.; Liu, X.; Pan, Z.; Liu, C.; Ma, H.; Liu, X.; Liu, L.; Jiang, C. The dosage effects of dexamethasone on osteogenic activity andbiocompatibility of poly(lactic-co-glycolic acid)/hydroxyapatite nanofibers. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Sinitsyna, E.; Bagaeva, I.; Gandalipov, E.; Fedotova, E.; Korzhikov-Vlakh, V.; Tennikova, T.; Korzhikova-Vlakh, E. Nanomedicines Bearing an Alkylating Cytostatic Drug from the Group of 1,3,5-Triazine Derivatives: Development and Characterization. Pharmaceutics 2022, 14, 2506. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, H.; Luo, Y.; Chen, Y.; Wang, M.; Wu, C.; Hu, P. Recent Applications of PLGA in Drug Delivery Systems. Polymers 2024, 16, 2606. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef]
- Chen, E.M.; Quijano, A.R.; Seo, Y.-E.; Jackson, C.; Josowitz, A.D.; Noorbakhsh, S.; Merlettini, A.; Sundaram, R.K.; Focarete, M.L.; Jiang, Z.; et al. Biodegradable PEG-poly(ω-pentadecalactone-co-p-dioxanone) nanoparticles for enhanced and sustained drug delivery to treat brain tumors. Biomaterials 2018, 178, 193–203. [Google Scholar] [CrossRef]
- Gurler, E.B.; Ergul, N.M.; Ozbek, B.; Ekren, N.; Oktar, F.N.; Haskoylu, M.E.; Oner, E.T.; Eroglu, M.S.; Ozbeyli, D.; Korkut, V.; et al. Encapsulated melatonin in polycaprolactone (PCL) microparticles as a promising graft material. Mater. Sci. Eng. C 2019, 100, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Bufalini, C.; Campardelli, R. Versatile Emulsion-Based Encapsulation System Production Processes: A Review. Processes 2025, 13, 1409. [Google Scholar] [CrossRef]
- Iqbal, M.; Zafar, N.; Fessi, H.; Elaissari, A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Tobío, M.; Gref, R.; Sánchez, A.; Langer, R.; Alonso, M.J. Stealth PLA-PEG Nanoparticles as Protein Carriers for Nasal Administration. Pharm. Res. 1998, 15, 270–275. [Google Scholar] [CrossRef]
- Allahyari, M.; Mohit, E. Peptide/protein vaccine delivery system based on PLGA particles. Hum. Vaccin. Immunother. 2016, 12, 806–828. [Google Scholar] [CrossRef]
- Huang, L.; Wang, S.; Yin, Z. Study in the stabilization of proteins encapsulated in PLGA delivery system: Effects of additives on protein encapsulation, release, and stability. J. Drug Deliv. Sci. Technol. 2022, 73, 103436. [Google Scholar] [CrossRef]
- Ruirui, Z.; He, J.; Xu, X.; Li, S.; Peng, H.; Deng, Z.; Huang, Y. PLGA-based drug delivery system for combined therapy of cancer: Research progress. Mater. Res. Express 2021, 8, 122002. [Google Scholar] [CrossRef]
- Gaur, M.; Maurya, S.; Akhtar, M.S.; Yadav, A.B. Synthesis and Evaluation of BSA-Loaded PLGA–Chitosan Composite Nanoparticles for the Protein-Based Drug Delivery System. ACS Omega 2023, 8, 18751–18759. [Google Scholar] [CrossRef]
- Bilati, U.; Allémann, E.; Doelker, E. Nanoprecipitation versus emulsion-based techniques for the encapsulation of proteins into biodegradable nanoparticles and process-related stability issues. AAPS PharmSciTech 2005, 6, E594–E604. [Google Scholar] [CrossRef]
- Shi, S.; Hickey, A.J. PLGA Microparticles in Respirable Sizes Enhance an In Vitro T Cell Response to Recombinant Mycobacterium Tuberculosis Antigen TB10.4-Ag85B. Pharm. Res. 2010, 27, 350–360. [Google Scholar] [CrossRef]
- Sakhabeev, R.G.; Polyakov, D.S.; Sinitsyna, E.S.; Korzhikov-Vlakh, V.A.; Bagaeva, I.O.; Korzhikova-Vlakh, E.G.; Ses, T.P.; Tereshina, V.S.; Shavlovsky, M.M. Features of the Humoral Immune Response When Using Protein Immobilized on the Surface of Nano- and Microparticles Based on Poly(Lactic Acid). J. Evol. Biochem. Physiol. 2024, 60, 446–475. [Google Scholar] [CrossRef]
- Sakhabeev, R.; Polyakov, D.; Korzhikov-Vlakh, V.; Sinitsyna, E.; Platonova, G.; Korzhikova-Vlakh, E.; Shavlovsky, M. Effect of the size of polymer particles bearing protein antigen on the in vivo T-cellular immune response. Cell. Ther. Transplant. 2024, 13, 42–48. [Google Scholar] [CrossRef]
- Korzhikov, V.A.; Gusevskaya, K.V.; Litvinchuk, E.N.; Vlakh, E.G.; Tennikova, T.B. Enzyme-mediated ring-opening polymerization of pentadecalactone to obtain biodegradable polymer for fabrication of scaffolds for bone tissue engineering. Int. J. Polym. Sci. 2013, 2013, 476748. [Google Scholar] [CrossRef]
- Sriyai, M.; Chaiwon, T.; Molloy, R.; Meepowpan, P.; Punyodom, W. Efficiency of liquid tin(II) n-alkoxide initiators in the ring-opening polymerization of L-lactide: Kinetic studies by non-isothermal differential scanning calorimetry. RSC Adv. 2020, 10, 43566–43578. [Google Scholar] [CrossRef]
- Hevilla, V.; Sonseca, A.; Echeverría, C.; Muñoz-Bonilla, A.; Fernández-García, M. Enzymatic Synthesis of Polyesters and Their Bioapplications: Recent Advances and Perspectives. Macromol. Biosci. 2021, 21, 2100156. [Google Scholar] [CrossRef]
- Silva, E.; de Vasconcellos, L.M.R.; Rodrigues, B.V.M.; dos Santos, D.M.; Campana-Filho, S.P.; Marciano, F.R.; Webster, T.J.; Lobo, A.O. PDLLA honeycomb-like scaffolds with a high loading of superhydrophilic graphene/multi-walled carbon nanotubes promote osteoblast in vitro functions and guided in vivo bone regeneration. Mater. Sci. Eng. C 2017, 73, 31–39. [Google Scholar] [CrossRef]
- Korzhikov-Vlakh, V.; Sinitsyna, E.; Arkhipov, K.; Levit, M.; Korzhikova-Vlakh, E.; Tennikova, T. Improvement in Biological Performance of Poly(Lactic Acid)-Based Materials via Single-Point Surface Modification with Glycopolymer. Surfaces 2024, 7, 1008–1028. [Google Scholar] [CrossRef]
- Korzhikov-Vlakh, V.; Mikhailova, A.; Sinitsyna, E.; Korzhikova-Vlakh, E.; Tennikova, T. Gradient Functionalization of Poly(lactic acid)-Based Materials with Polylysine for Spatially Controlled Cell Adhesion. Polymers 2024, 16, 2888. [Google Scholar] [CrossRef]
- Low, Y.J.; Andriyana, A.; Ang, B.C.; Zainal Abidin, N.I. Bioresorbable and degradable behaviors of <scp>PGA</scp>: Current state and future prospects. Polym. Eng. Sci. 2020, 60, 2657–2675. [Google Scholar]
- Shi, J.; Zhang, J.; Zhang, Y.; Zhang, L.; Yang, Y.-B.; Manor, O.; You, J. Crystallinity Dependence of PLLA Hydrophilic Modification during Alkali Hydrolysis. Polymers 2022, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chang, K.; Qi, L.; Li, X.; Gao, W.; Gao, Q. Surface Modification of Poly(l-lactic acid) through Stereocomplexation with Enantiomeric Poly(d-lactic acid) and Its Copolymer. Polymers 2021, 13, 1757. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zhu, Q.; Cao, D.; Liu, T.; Zhu, K.R.; Chang, K.; Gao, Q. Preparation and Properties of Stereocomplex of Poly(lactic acid) and Its Amphiphilic Copolymers Containing Glucose Groups. Polymers 2020, 12, 760. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Zhao, H. Enzymatic ring-opening polymerization (ROP) of polylactones: Roles of non-aqueous solvents. J. Chem. Technol. Biotechnol. 2018, 93, 9–19. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef]
- Tang, Z.G.; Black, R.A.; Curran, J.M.; Hunt, J.A.; Rhodes, N.P.; Williams, D.F. Surface properties and biocompatibility of solvent-cast poly[ε-caprolactone] films. Biomaterials 2004, 25, 4741–4748. [Google Scholar] [CrossRef]
- Ishaug-Riley, S. Human articular chondrocyte adhesion and proliferation on synthetic biodegradable polymer films. Biomaterials 1999, 20, 2245–2256. [Google Scholar] [CrossRef]
- Ouyang, H.W.; Goh, J.C.H.; Mo, X.M.; Teoh, S.H.; Lee, E.H. Characterization of anterior cruciate ligament cells and bone marrow stromal cells on various biodegradable polymeric films. Mater. Sci. Eng. C 2002, 20, 63–69. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Castilla-Cortázar, I.; Vidaurre, A.; Marí, B.; Campillo-Fernández, A.J. Morphology, Crystallinity, and Molecular Weight of Poly(ε-caprolactone)/Graphene Oxide Hybrids. Polymers 2019, 11, 1099. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Saar, J.S.; Shi, Y.; Lienkamp, K. Bioinspired All-Polyester Diblock Copolymers Made from Poly(pentadecalactone) and Poly(3-hydroxycinnamate): Synthesis and Polymer Film Properties. Macromol. Chem. Phys. 2020, 221, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Etxeberria, A.; Varga, A.L.; Sarasua, J.-R. Synthesis and characterization of ω-pentadecalactone-co-ε-decalactone copolymers: Evaluation of thermal, mechanical and biodegradation properties. Polymer 2015, 81, 12–22. [Google Scholar] [CrossRef]
- Letizia Focarete, M.; Scandola, M.; Kumar, A.; Gross, R.A. Physical characterization of poly(ω-pentadecalactone) synthesized by lipase-catalyzed ring-opening polymerization. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 1721–1729. [Google Scholar] [CrossRef]
- Ceccorulli, G.; Scandola, M.; Kumar, A.; Kalra, B.; Gross, R.A. Cocrystallization of Random Copolymers of ω-Pentadecalactone and ε-Caprolactone Synthesized by Lipase Catalysis. Biomacromolecules 2005, 6, 902–907. [Google Scholar] [CrossRef]
- Tsutsuba, T.; Sawanaka, Y.; Suzuki, M.; Inagaki, K.; Arai, K.; Okaniwa, S.; Torii, J.; Tachibana, Y.; Kasuya, K. Evaluation of the effect of the number of methylene units in poly(ω-hydroxyalkanoate)s on their biodegradability. Polym. Degrad. Stab. 2024, 222, 110701. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Z.; Zhang, S.; Liu, C.; Gross, R.A.; Kyriakides, T.R.; Saltzman, W.M. Biodegradation, biocompatibility, and drug delivery in poly(ω-pentadecalactone-co-p-dioxanone) copolyesters. Biomaterials 2011, 32, 6646–6654. [Google Scholar] [CrossRef]
- Wang, M.; Yan, W.; Zhou, Y.; Fan, L.; Liu, Y.; Li, J. Progress in the application of lecithins in water-in-oil emulsions. Trends Food Sci. Technol. 2021, 118, 388–398. [Google Scholar] [CrossRef]
- Weete, J.D.; Betageri, S.; Griffith, G.L. Improvement of lecithin as an emulsifier for water-in-oil emulsions by thermalization. J. Am. Oil Chem. Soc. 1994, 71, 731–737. [Google Scholar] [CrossRef]
- Youan, B.B.C.; Benoit, M.A.; Baras, B.; Gillard, J. Protein-loaded poly(epsilon-caprolactone) microparticles. I. Optimization of the preparation by (water-in-oil)-in water emulsion solvent evaporation. J. Microencapsul. 1999, 16, 587–599. [Google Scholar]
- Conway, B.R.; Alpar, H.O. Co-encapsulation of Proteins into Polylactide Microspheres. Pharm. Pharmacol. Commun. 1996, 2, 173–176. [Google Scholar]

| Polymer | Yield, % | Mna | Ða | DP b | η, dL/g c |
|---|---|---|---|---|---|
| PDLLA | 68 | 8300 | 1.8 | 115 | 0.24 |
| PLLA | 66 | 8700 | 1.5 | 120 | 0.20 |
| PCL | 92 | 10,600 | 2.4 | 108 | 0.35 |
| PPDL | 88 | 19,000 | 1.9 | 85 | 0.90 |
| Organic (Oil) Phase | (μm) a | |||
|---|---|---|---|---|
| Polymer | Solvent System | Surfactant System | SEM | DLS |
| PDLLA | Dichloromethane Dichloromethane | Lutrol F68 + DMAB Lutrol F68 + DMAB + lecithin | 4.0 ± 1.9 | 2.8 ± 1.3 |
| 3.1 ± 1.1 | 2.3 ± 0.8 | |||
| PLLA | Dichloromethane Dichloromethane | Lutrol F68 + DMAB Lutrol F68 + DMAB + lecithin | 3.0 ± 1.2 | 2.8 ± 0.9 |
| 1.8 ± 0.5 | 1.0 ± 0.4 | |||
| Dichloromethane/acetone | Lutrol F68 + SDS | 2.1 ± 0.6 | 1.8 ± 0.5 | |
| PCL | Dichloromethane Dichloromethane | Lutrol F68 + DMAB Lutrol F68 + DMAB + lecithin | 3.0 ± 1.3 | 2.6 ± 0.8 |
| 1.7 ± 0.6 | 2.0 ± 0.7 | |||
| PPDL | Dichloromethane Dichloromethane | Lutrol F68 + DMAB Lutrol F68 + DMAB + lecithin | 9.2 ± 3.9 | 10.1 ± 4.3 |
| 0.9 ± 0.4 | 0.8 ± 0.3 | |||
| Drug Release Model | Polymer Used for Microparticle Preparation | |||
|---|---|---|---|---|
| PLLA | PCL | PPDL | ||
| Zero order F = k0 · t | * | 0.9464 k0 = 0.624 | 0.9931 k0 = 0.321 | 0.9926 k0 = 1.298 |
| ** | 0.9891 k0 = 0.778 | 0.9594 k0 = 0.337 | 0.9276 k0 = 1.433 | |
| First order F = 100 · [1 − Exp(−k1 · t)] | * | 0.9679 k1 = 0.008 | 0.9942 k1 = 0.004 | 0.9756 k1 = 0.020 |
| ** | 0.9866 k1 = 0.008 | 0.9615 k1 = 0.003 | 0.9450 k1 = 0.017 | |
| Higuchi F = kH · t0.5 | * | 0.9713 kH = 4.174 | 0.9616 kH = 2.084 | 0.9584 kH = 8.414 |
| ** | 0.9304 kH = 2.835 | 0.9974 kH = 1.307 | 0.9824 kH = 5.577 | |
| Korsmeyer-Peppas F = kKP · tn | * | 0.9940 kKP = 0.440 n = 1.219 | 0.9905 kKP = 0.989 n = 0.630 | 0.9885 kKP = 4.984 n = 0.557 |
| ** | 0.9905 kKP = 0.662 n = 1.058 | 0.9977 kKP = 1.239 n = 0.521 | 0.9824 kKP = 5.112 n = 0.535 | |
| Hixson-Crowell F = 100 · [1 − (1 − kHC · t)3] | * | 0.9921 kHC = 0.003 | 0.9850 kHC = 0.001 | 0.9746 kHC = 0.005 |
| ** | 0.9875 kHC = 0.003 | 0.9608 kHC = 0.001 | 0.9394 kHC = 0.005 | |
| Hopfenberg F = 100 · [1 − (1 − kHB · t)n] | * | 0.9679 kHB = 7.0 × 10−6 n = 1111 | 0.9942 kHB = 2.4 × 10−5 n = 145 | 0.9924 kHB = 0.012 n = 1.227 |
| ** | 0.9924 kHB = 0.20 n = 0.38 | 0.9615 kHB = 2.0 × 10−5 n = 175 | 0.9449 kHB = 2.8 × 10−5 n = 611 | |
| Baker-Lonsdale 3/2 · [1 − (1 − F/100)2/3] − F/100 = kBL · t | * | 0.9694 KBL = 3.3 × 10−4 | 0.9588 KBL = 7.6 × 10−5 | 0.9384 KBL = 1.5 × 10−3 |
| ** | 0.9280 KBL = 1.4 × 10−4 | 0.9973 KBL = 2.9 × 10−5 | 0.9827 KBL = 5.7 × 10−4 | |
| Peppas-Sahlin F = k1 · tm + k2 · t(2∗m) | * | 0.9935 k1 = 0.290 k2 = 0.545 m = 0.553 | 0.9875 k1 = 0.621 k2 = 0.060 m = 0.638 | 0.9809 k1 = 3.349 k2 = 0.302 m = 0.573 |
| ** | 0.9929 k1 = 0.800 k2 = 0.094 m = 0.148 | 0.9985 k1 = 1.059 k2 = 0.254 m = 0.030 | 0.9999 k1 = 2.665 k2 = 0.072 m = 0.030 | |
| Weibull F = 100 · {1 − Exp[−((t − Ti)β)/α]} | * | 0.9812 α = 42.564 β = 0.714 Ti = 1.517 | 0.9942 α = 285.520 β = 0.992 Ti = 1.651 | 0.9925 α = 13742.281 β = 2.287 Ti = 18.138 |
| ** | 0.9925 α = 644.845 β = 1.501 Ti = 3.204 | 0.9975 α = 80.992 β = 0.532 Ti = 2.1 × 10−7 | 0.9968 α = 5.794 β = 0.641 Ti = 1.801 | |
| Gompertz F = 100 · Exp{−α · Exp[−β · log(t)]} | * | 0.9893 α = 6.865 β = 1.053 | 0.9895 α = 8.882 β = 0.937 | 0.9623 α = 68.730 β = 3.013 |
| ** | 0.9801 α = 6.973 β = 1.014 | 0.9946 α = 4.544 β = 0.360 | 0.9924 α = 3.433 β = 0.702 | |
| Polymer | EE (%) | DL (μg/mg) | , μm (SEM) |
|---|---|---|---|
| PDLLA | 61 ± 6 | 244 ± 25 | 2.5 ± 0.8 |
| PLLA a | 30 ± 4 | 120 ± 17 | 1.5 ± 0.5 |
| PCL | 25 ± 2 | 99 ± 8 | 3.5 ± 1.4 |
| PPDL | 5 ± 1 | 18 ± 5 | 1.1 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzhikov-Vlakh, V.; Sinitsyna, E.; Stepanova, M.; Korzhikova-Vlakh, E.; Tennikova, T. Comparison of Different Aliphatic Polyester-Based Microparticles as Protein Delivery Systems. Polymers 2025, 17, 2676. https://doi.org/10.3390/polym17192676
Korzhikov-Vlakh V, Sinitsyna E, Stepanova M, Korzhikova-Vlakh E, Tennikova T. Comparison of Different Aliphatic Polyester-Based Microparticles as Protein Delivery Systems. Polymers. 2025; 17(19):2676. https://doi.org/10.3390/polym17192676
Chicago/Turabian StyleKorzhikov-Vlakh, Viktor, Ekaterina Sinitsyna, Mariia Stepanova, Evgenia Korzhikova-Vlakh, and Tatiana Tennikova. 2025. "Comparison of Different Aliphatic Polyester-Based Microparticles as Protein Delivery Systems" Polymers 17, no. 19: 2676. https://doi.org/10.3390/polym17192676
APA StyleKorzhikov-Vlakh, V., Sinitsyna, E., Stepanova, M., Korzhikova-Vlakh, E., & Tennikova, T. (2025). Comparison of Different Aliphatic Polyester-Based Microparticles as Protein Delivery Systems. Polymers, 17(19), 2676. https://doi.org/10.3390/polym17192676











