Unveiling the Role of Fluorination in Suppressing Dark Current and Enhancing Photocurrent to Enable Thick-Film Near-Infrared Organic Photodetectors
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurements
2.2. Materials
2.3. Fabrication of OPDs
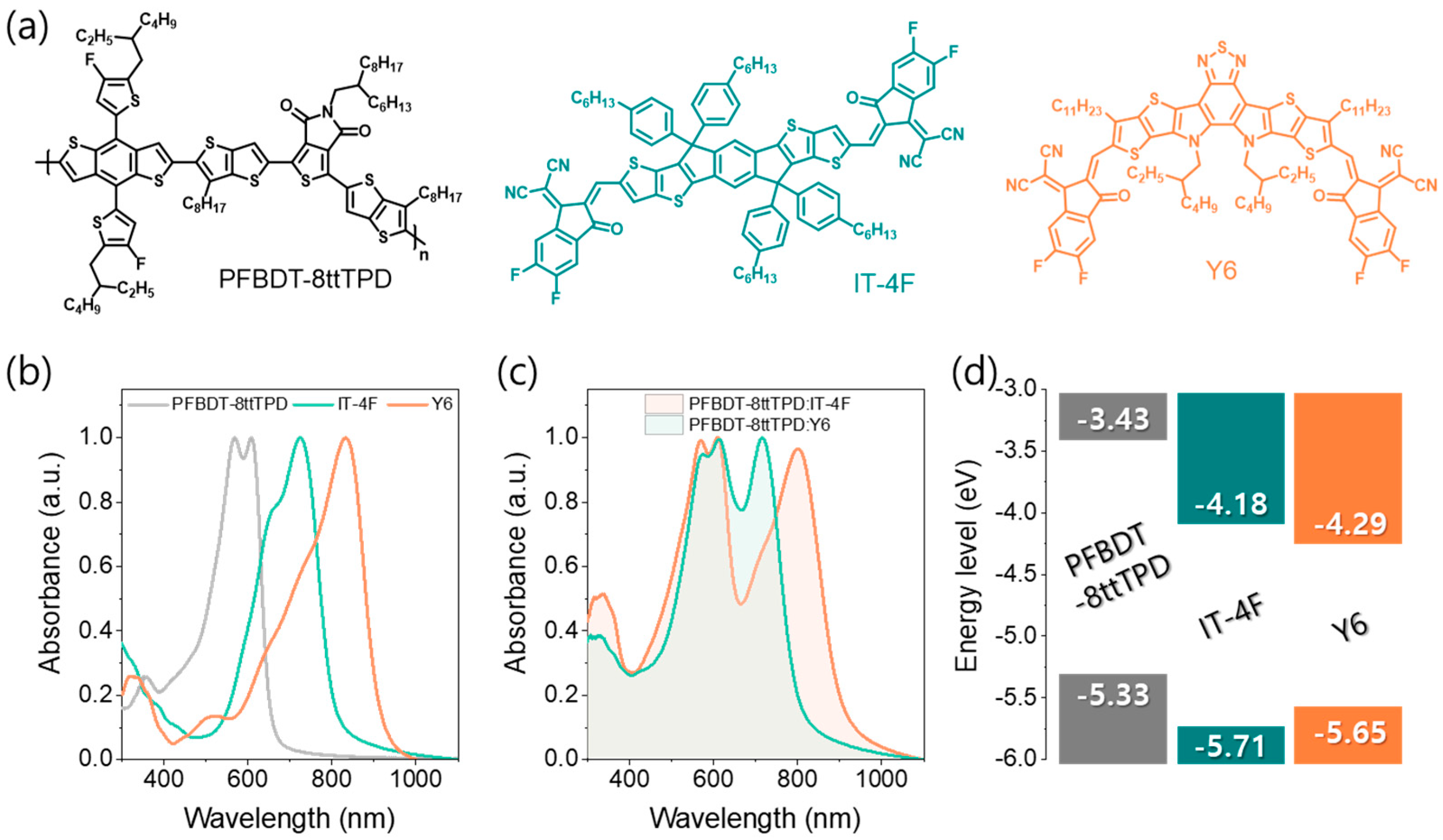
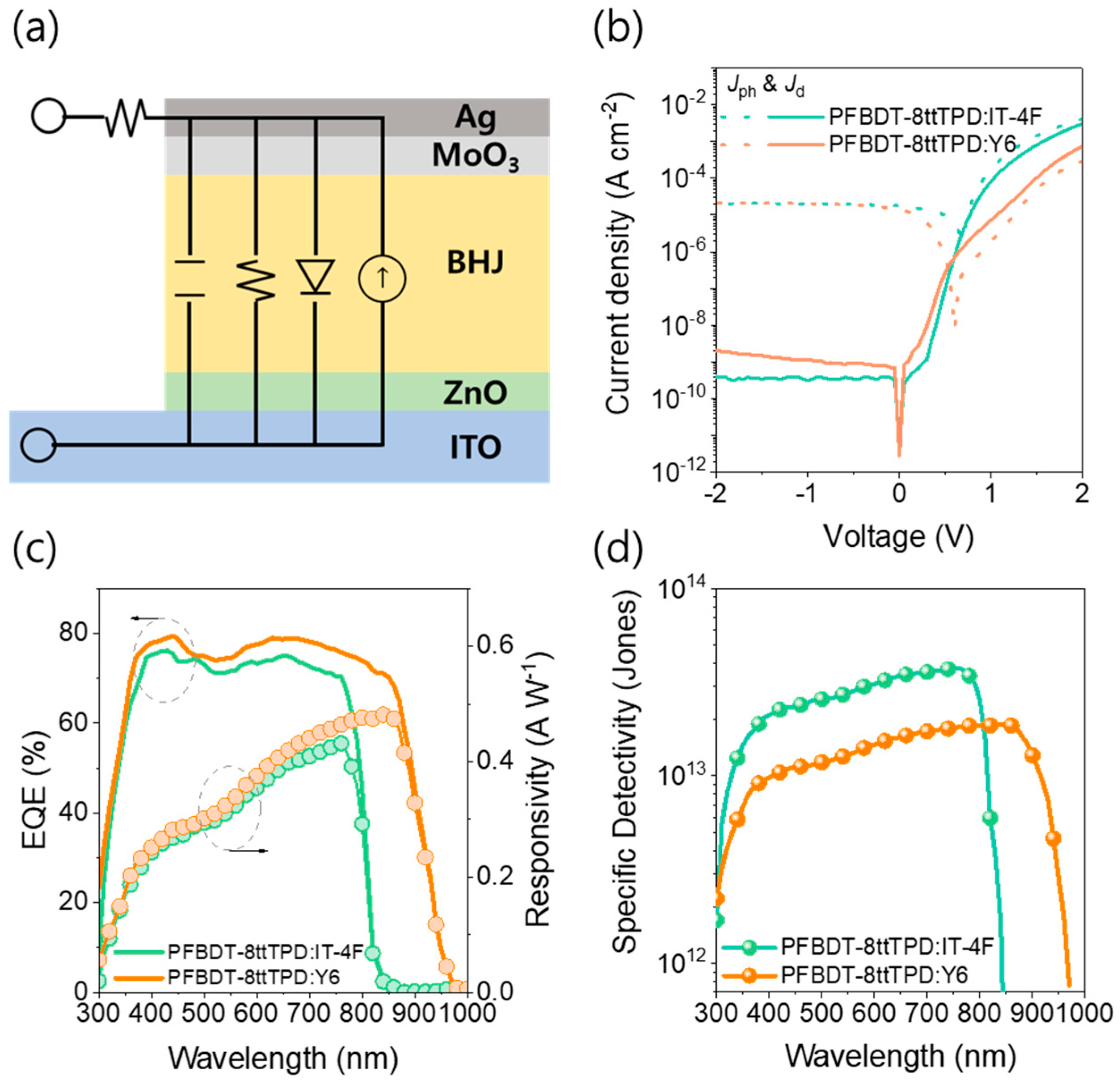
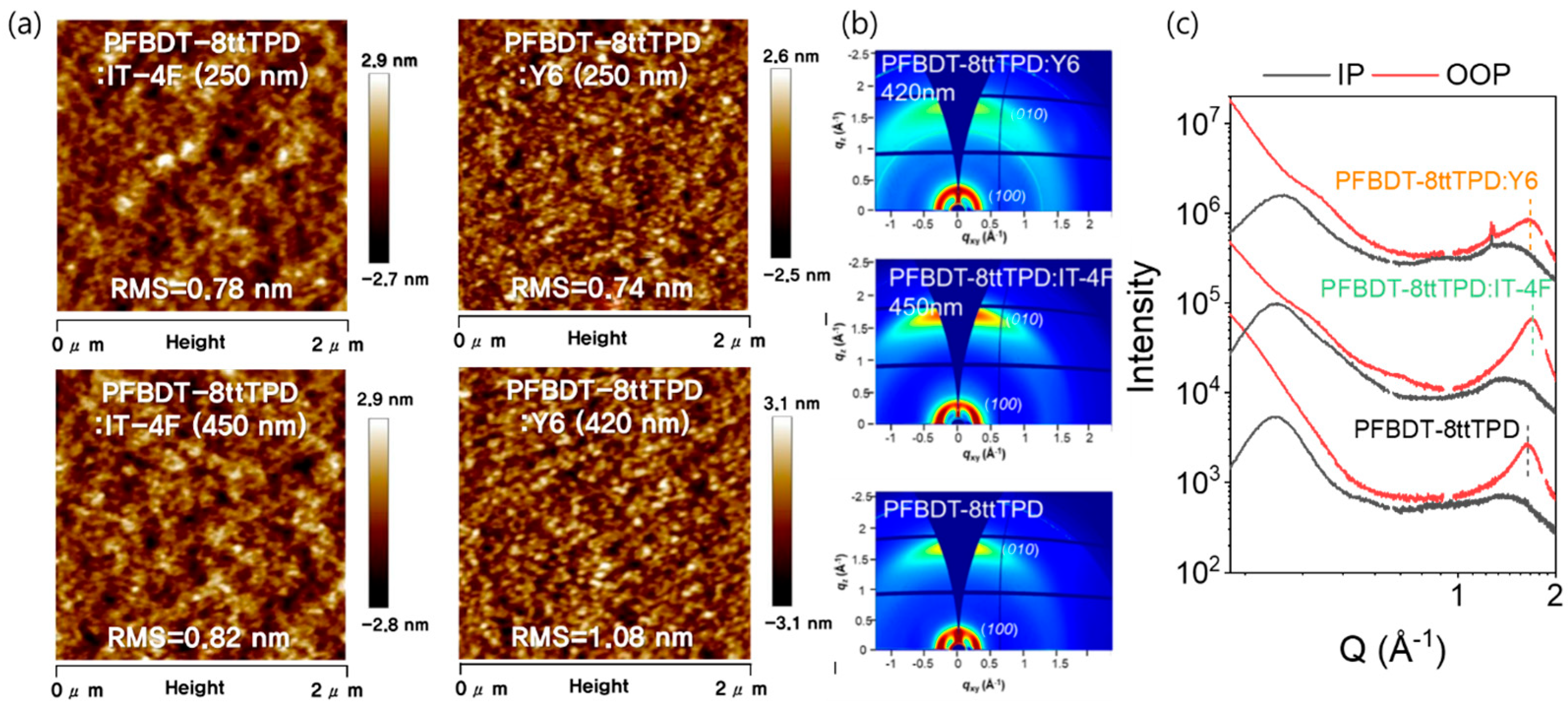
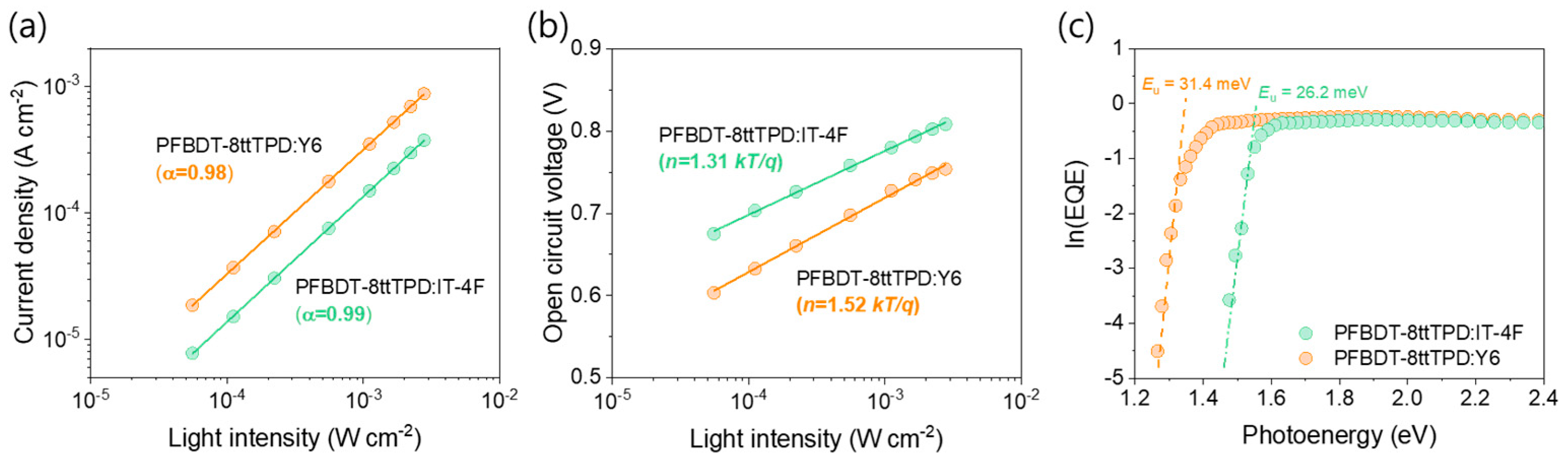
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Azzellino, G.; Grimoldi, A.; Binda, M.; Caironi, M.; Natali, D.; Sampietro, M. Fully Inkjet-Printed Organic Photodetectors with High Quantum Yield. Adv. Mater. 2013, 25, 6829–6833. [Google Scholar] [CrossRef]
- Pace, G.; Grimoldi, A.; Natali, D.; Sampietro, M.; Coughlin, J.E.; Bazan, G.C.; Caironi, M. All-Organic and Fully-Printed Semitransparent Photodetectors Based on Narrow Bandgap Conjugated Molecules. Adv. Mater. 2014, 26, 6773–6777. [Google Scholar] [CrossRef]
- Pierre, A.; Deckman, I.; Lechêne, P.B.; Arias, A.C. High Detectivity All-Printed Organic Photodiodes. Adv. Mater. 2015, 27, 6411–6417. [Google Scholar] [CrossRef]
- Simone, G.; Dyson, M.J.; Meskers, S.C.J.; Janssen, R.A.J.; Gelinck, G.H. Organic Photodetectors and their Application in Large Area and Flexible Image Sensors: The Role of Dark Current. Adv. Funct. Mater. 2020, 30, 1904205. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Fukuda, K.; Yokota, T.; Someya, T. A Highly Responsive Organic Image Sensor Based on a Two-Terminal Organic Photodetector with Photomultiplication. Adv. Mater. 2019, 31, 1903687. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nakajima, A.; Yoshimi, M.; Sawada, T.; Fukuda, S.; Suezaki, T.; Ichikawa, M.; Koi, Y.; Goto, M.; Meguro, T.; et al. A high efficiency thin film silicon solar cell and module. Sol. Energy 2004, 77, 939–949. [Google Scholar] [CrossRef]
- Armin, A.; Hambsch, M.; Kim, I.K.; Burn, P.L.; Meredith, P.; Namdas, E.B. Thick junction broadband organic photodiodes. Laser Photonics Rev. 2014, 8, 924–932. [Google Scholar] [CrossRef]
- Baierl, D.; Pancheri, L.; Schmidt, M.; Stoppa, D.; Betta, G.-F.D.; Scarpa, G.; Lugli, P. A hybrid CMOS-imager with a solution-processable polymer as photoactive layer. Nat. Commun. 2012, 3, 1175. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Parvez, K.; Li, R.; Dong, R.; Feng, X.; Müllen, K. Transparent Conductive Electrodes from Graphene/PEDOT:PSS Hybrid Inks for Ultrathin Organic Photodetectors. Adv. Mater. 2015, 27, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Simone, G.; Di Carlo Rasi, D.; de Vries, X.; Heintges, G.H.L.; Meskers, S.C.J.; Janssen, R.A.J.; Gelinck, G.H. Near-Infrared Tandem Organic Photodiodes for Future Application in Artificial Retinal Implants. Adv. Mater. 2018, 30, 1804678. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhang, L.; Zheng, J.; Li, J.; Lei, S.; Wu, Z.; Yang, D.; Ma, D.; Chen, J. A Simple Fused-Ring Acceptor toward High-Sensitivity Binary Near-Infrared Photodetector. Adv. Opt. Mater. 2022, 10, 2200371. [Google Scholar] [CrossRef]
- Ha, J.-W.; Eun, H.J.; Park, B.; Ahn, H.; Hwang, D.R.; Shim, Y.S.; Heo, J.; Lee, C.; Yoon, S.C.; Kim, J.H.; et al. Effect of Cyano Substitution on Non-Fullerene Acceptor for Near-Infrared Organic Photodetectors above 1000 nm. Adv. Funct. Mater. 2023, 33, 2211486. [Google Scholar] [CrossRef]
- Huang, J.; Lee, J.; Vollbrecht, J.; Brus, V.V.; Dixon, A.L.; Cao, D.X.; Zhu, Z.; Du, Z.; Wang, H.; Cho, K.; et al. A High-Performance Solution-Processed Organic Photodetector for Near-Infrared Sensing. Adv. Mater. 2020, 32, 1906027. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yin, B.; Hu, G.; Cao, Y.; Lu, S.; Chen, Y.; He, Y.; Yang, X.; Huang, B.; Li, J.; et al. Sensitive short-wavelength infrared photodetection with a quinoidal ultralow band-gap n-type organic semiconductor. Chem 2024, 10, 1425–1444. [Google Scholar] [CrossRef]
- Yin, B.; Zhou, X.; Li, Y.; Hu, G.; Wei, W.; Yang, M.; Jeong, S.; Deng, W.; Wu, B.; Cao, Y.; et al. Sensitive Organic Photodetectors With Spectral Response up to 1.3 µm Using a Quinoidal Molecular Semiconductor. Adv. Mater. 2024, 36, 2310811. [Google Scholar] [CrossRef]
- Mikhnenko, O.V.; Blom, P.W.M.; Nguyen, T.-Q. Exciton diffusion in organic semiconductors. Energy Environ. Sci. 2015, 8, 1867–1888. [Google Scholar] [CrossRef]
- Firdaus, Y.; Le Corre, V.M.; Karuthedath, S.; Liu, W.; Markina, A.; Huang, W.; Chattopadhyay, S.; Nahid, M.M.; Nugraha, M.I.; Lin, Y.; et al. Long-range exciton diffusion in molecular non-fullerene acceptors. Nat. Commun. 2020, 11, 5220. [Google Scholar] [CrossRef]
- Stübinger, T.; Brütting, W. Exciton diffusion and optical interference in organic donor–acceptor photovoltaic cells. J. Appl. Phys. 2001, 90, 3632–3641. [Google Scholar] [CrossRef]
- Najafov, H.; Lee, B.; Zhou, Q.; Feldman, L.C.; Podzorov, V. Observation of long-range exciton diffusion in highly ordered organic semiconductors. Nat. Mater. 2010, 9, 938–943. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.; Wang, W.; An, Q.; Wang, J.; Sun, Q.; Zhang, M. Trap-Assisted Photomultiplication Polymer Photodetectors Obtaining an External Quantum Efficiency of 37 500%. ACS Appl. Mater. Interfaces 2015, 7, 5890–5897. [Google Scholar] [CrossRef]
- Zeiske, S.; Sandberg, O.J.; Zarrabi, N.; Li, W.; Meredith, P.; Armin, A. Direct observation of trap-assisted recombination in organic photovoltaic devices. Nat. Commun. 2021, 12, 3603. [Google Scholar] [CrossRef]
- Ma, X.; Janssen, R.A.J.; Gelinck, G.H. Trap-Assisted Charge Generation and Recombination in State-of-the-Art Organic Photodetectors. Adv. Mater. Technol. 2023, 8, 2300234. [Google Scholar] [CrossRef]
- Sandberg, O.J.; Kaiser, C.; Zeiske, S.; Zarrabi, N.; Gielen, S.; Maes, W.; Vandewal, K.; Meredith, P.; Armin, A. Mid-gap trap state-mediated dark current in organic photodiodes. Nat. Photonics 2023, 17, 368–374. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhong, Z.; Zhong, W.; Zhang, J.; Ying, L.; Yu, G.; Huang, F.; Cao, Y. High-detectivity organic photodetectors based on a thick-film photoactive layer using a conjugated polymer containing a naphtho[1,2-c:5,6-c]bis[1,2,5]thiadiazole unit. J. Mater. Chem. C 2019, 7, 6070–6076. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Liu, X.; Miao, J.; Han, Y.; Liu, J.; Wang, L. An n-Type All-Fused-Ring Molecule with Photoresponse to 1000 nm for Highly Sensitive Near-Infrared Photodetector. Adv. Mater. 2023, 35, 2211714. [Google Scholar] [CrossRef]
- Wang, W.; Miao, X.; Cai, G.; Ding, L.; Li, Y.; Li, T.; Zhu, Y.; Tao, L.; Jia, Y.; Liang, Y.; et al. Enhancing Transition Dipole Moments of Heterocyclic Semiconductors via Rational Nitrogen-Substitution for Sensitive Near Infrared Detection. Adv. Mater. 2022, 34, 2201600. [Google Scholar] [CrossRef]
- Lee, J.; Ko, S.-J.; Lee, H.; Huang, J.; Zhu, Z.; Seifrid, M.; Vollbrecht, J.; Brus, V.V.; Karki, A.; Wang, H.; et al. Side-Chain Engineering of Nonfullerene Acceptors for Near-Infrared Organic Photodetectors and Photovoltaics. ACS Energy Lett. 2019, 4, 1401–1409. [Google Scholar] [CrossRef]
- Lee, U.-H.; Park, B.; Rhee, S.; Ha, J.-W.; Whang, D.R.; Eun, H.J.; Kim, J.H.; Shim, Y.; Heo, J.; Lee, C.; et al. Achieving Highly Sensitive Near-Infrared Organic Photodetectors using Asymmetric Non-Fullerene Acceptor. Adv. Opt. Mater. 2023, 11, 2300312. [Google Scholar] [CrossRef]
- Babics, M.; Bristow, H.; Zhang, W.; Wadsworth, A.; Neophytou, M.; Gasparini, N.; McCulloch, I. Non-fullerene-based organic photodetectors for infrared communication. J. Mater. Chem. C 2021, 9, 2375–2380. [Google Scholar] [CrossRef]
- Park, J.B.; Ha, J.-W.; Yoon, S.C.; Lee, C.; Jung, I.H.; Hwang, D.-H. Visible-Light-Responsive High-Detectivity Organic Photodetectors with a 1 μm Thick Active Layer. ACS Appl. Mater. Interfaces 2018, 10, 38294–38301. [Google Scholar] [CrossRef]
- Ha, J.-W.; Song, C.E.; Kim, H.S.; Ryu, D.H.; Shin, W.S.; Hwang, D.-H. Highly Efficient and Photostable Ternary Organic Solar Cells Enabled by the Combination of Non-Fullerene and Fullerene Acceptors with Thienopyrrolodione-based Polymer Donors. ACS Appl. Mater. Interfaces 2020, 12, 51699–51708. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted polymer solar cells integrated with a low-temperature-annealed sol-gel-derived ZnO Film as an electron transport layer. Adv. Mater. 2011, 23, 1679. [Google Scholar] [CrossRef]
- Ha, J.-W.; Eom, S.H.; Cha, B.K.; Song, S.; Eun, H.J.; Kim, J.H.; Park, J.M.; Kim, B.; Park, B.; Ko, S.-J.; et al. Organic X-Ray Image Sensors Using a Medium Bandgap Polymer Donor with Low Dark Current. Energy Environ. Mater. 2024, 7, e12750. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, J.; Deng, W.; Luo, M.; Xie, Y.; Liang, Q.; Zou, Y.; He, Z.; Wu, H.; Cao, Y. High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder. Nat. Photonics 2020, 14, 300–305. [Google Scholar] [CrossRef]
- Venkateshvaran, D.; Nikolka, M.; Sadhanala, A.; Lemaur, V.; Zelazny, M.; Kepa, M.; Hurhangee, M.; Kronemeijer, A.J.; Pecunia, V.; Nasrallah, I.; et al. Approaching disorder-free transport in high-mobility conjugated polymers. Nature 2014, 515, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Chantana, J.; Kawano, Y.; Nishimura, T.; Minemoto, T. Urbach energy of Cu(In,Ga)Se2 and Cu(In,Ga)(S,Se)2 absorbers prepared by various methods: Indicator of their quality. Mater. Today Commun. 2019, 21, 100652. [Google Scholar] [CrossRef]
- Kielar, M.; Dhez, O.; Pecastaings, G.; Curutchet, A.; Hirsch, L. Long-Term Stable Organic Photodetectors with Ultra Low Dark Currents for High Detectivity Applications. Sci. Rep. 2016, 6, 39201. [Google Scholar] [CrossRef]
- Jang, W.; Rasool, S.; Kim, B.G.; Kim, J.; Yoon, J.; Manzhos, S.; Lee, H.K.; Jeon, I.; Wang, D.H. Superior Noise Suppression, Response Time, and Device Stability of Non-Fullerene System over Fullerene Counterpart in Organic Photodiode. Adv. Funct. Mater. 2020, 30, 2001402. [Google Scholar] [CrossRef]
- Leem, D.-S.; Lee, K.-H.; Li, N.; Park, B.W.; Choi, T.; Ro, T.; Kwon, O.K.; Kwon, Y.-N.; Ng, T.N.; Kim, S. Highly Responsive and Thermally Reliable Near-Infrared Organic Photodiodes Utilizing Naphthalocyanine Molecules Tuned with Axial Ligands. Adv. Opt. Mater. 2021, 9, 2001682. [Google Scholar] [CrossRef]
- Deckman, I.; Lechêne, P.B.; Pierre, A.; Arias, A.C. All-printed full-color pixel organic photodiode array with a single active layer. Org. Elerctron. 2018, 56, 139–145. [Google Scholar] [CrossRef]
- Jeong, W.; Kang, J.; Lim, S.Y.; Ahn, H.; Kim, H.M.; Won, J.H.; Jung, I.H. Spontaneously Induced Hierarchical Structure by Surface Energy in Novel Conjugated Polymer-Based Ultrafast-Response Organic Photodetectors. Adv. Opt. Mater. 2022, 10, 2102607. [Google Scholar] [CrossRef]

| Thickness [nm] | Jd [A cm−2] | Jph [A cm−2] c | R [A W−1] | D* [Jones] | |
|---|---|---|---|---|---|
| PFDBT−8ttTPD :IT−4F a | 250 | 8.74 × 10−9 | 2.18 × 10−5 | 0.43 | 2.52 × 1012 |
| 350 | 7.43 × 10−10 | 2.00 × 10−5 | 0.43 | 2.77 × 1013 | |
| 450 | 4.08 × 10−10 | 2.06 × 10−5 | 0.43 | 3.78 × 1013 | |
| PFBDT−8ttTPD :Y6 b | 250 | 4.79 × 10−8 | 2.12 × 10−5 | 0.44 | 3.60 × 1012 |
| 450 | 2.03 × 10−9 | 1.86 × 10−5 | 0.48 | 1.89 × 1013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Kwak, S.L.; Ha, J.-W.; Hwang, D.-H. Unveiling the Role of Fluorination in Suppressing Dark Current and Enhancing Photocurrent to Enable Thick-Film Near-Infrared Organic Photodetectors. Polymers 2025, 17, 2663. https://doi.org/10.3390/polym17192663
Bai Y, Kwak SL, Ha J-W, Hwang D-H. Unveiling the Role of Fluorination in Suppressing Dark Current and Enhancing Photocurrent to Enable Thick-Film Near-Infrared Organic Photodetectors. Polymers. 2025; 17(19):2663. https://doi.org/10.3390/polym17192663
Chicago/Turabian StyleBai, Yongqi, Seon Lee Kwak, Jong-Woon Ha, and Do-Hoon Hwang. 2025. "Unveiling the Role of Fluorination in Suppressing Dark Current and Enhancing Photocurrent to Enable Thick-Film Near-Infrared Organic Photodetectors" Polymers 17, no. 19: 2663. https://doi.org/10.3390/polym17192663
APA StyleBai, Y., Kwak, S. L., Ha, J.-W., & Hwang, D.-H. (2025). Unveiling the Role of Fluorination in Suppressing Dark Current and Enhancing Photocurrent to Enable Thick-Film Near-Infrared Organic Photodetectors. Polymers, 17(19), 2663. https://doi.org/10.3390/polym17192663







