Synthesis of Linear Modified Siloxane-Based Thickeners and Study of Their Phase Behavior and Thickening Mechanism in Supercritical Carbon Dioxide
Abstract
1. Introduction
2. Chemicals and Methods
2.1. Chemicals
2.2. Synthesis of Linear Modified Silicone-Based Supercritical CO2 Thickener
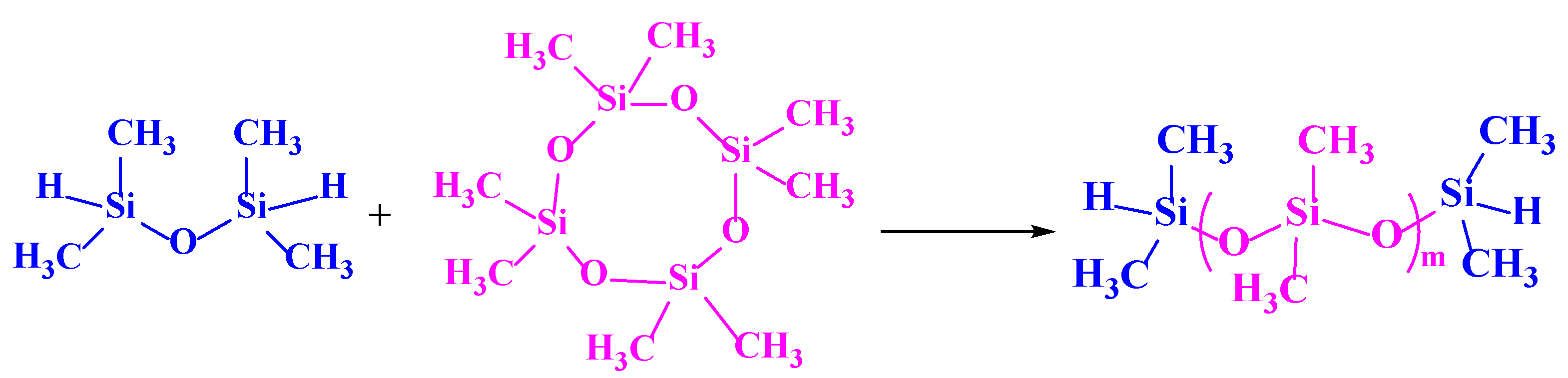
2.2.1. Preparation of Silicon Hydrogen-Terminated Linear Polysiloxane
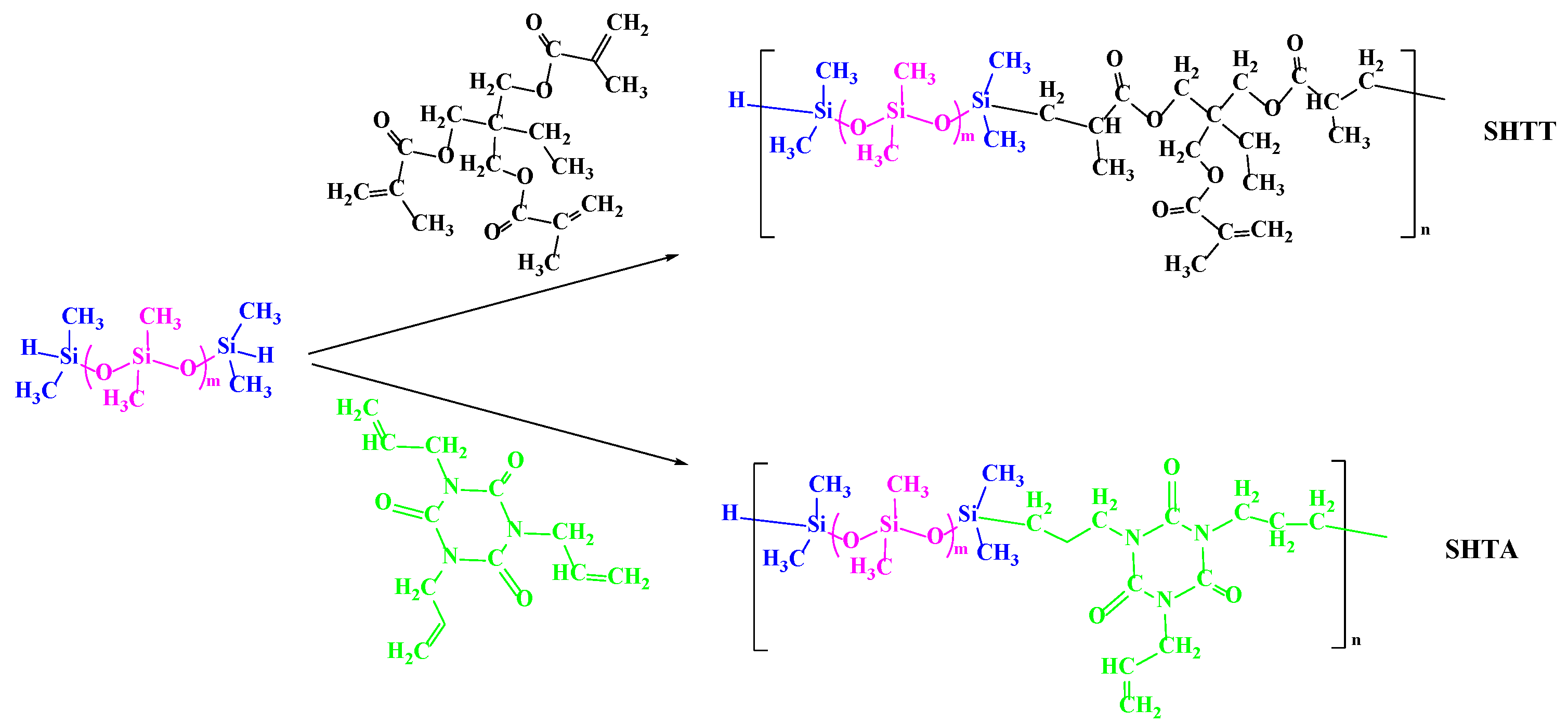
2.2.2. Synthesis of Supercritical CO2 Copolymer Thickeners
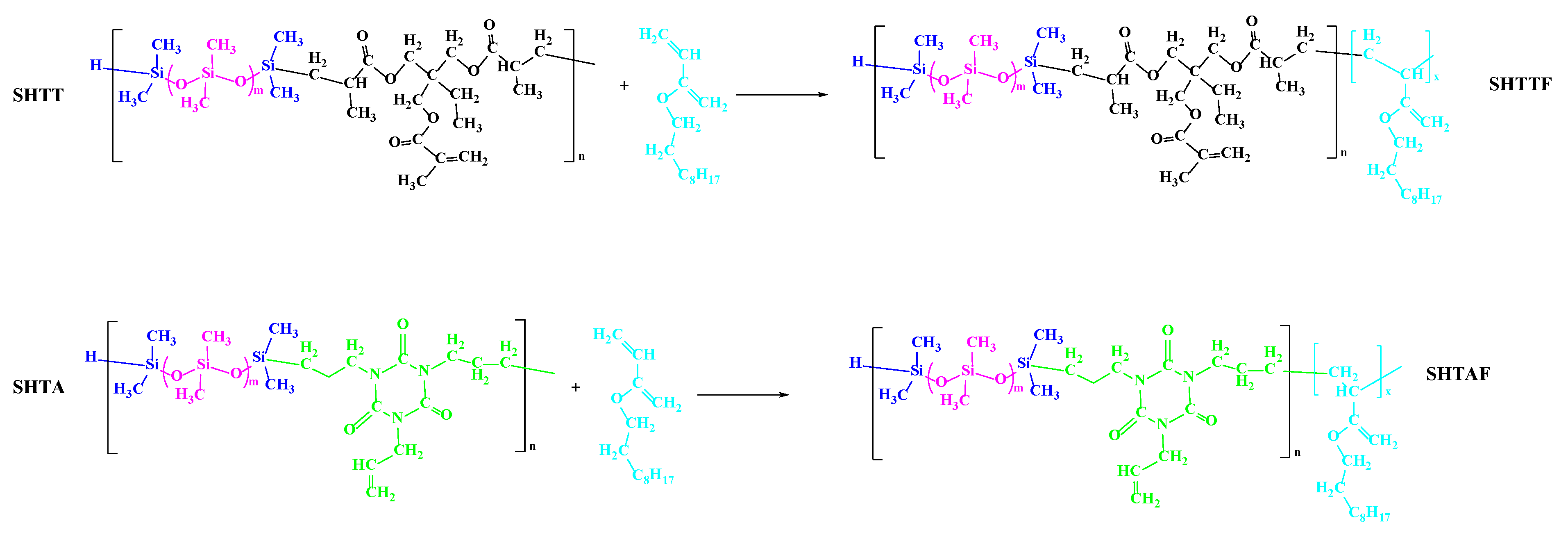
2.2.3. Modification of SHTT and SHTA
2.3. Characterization
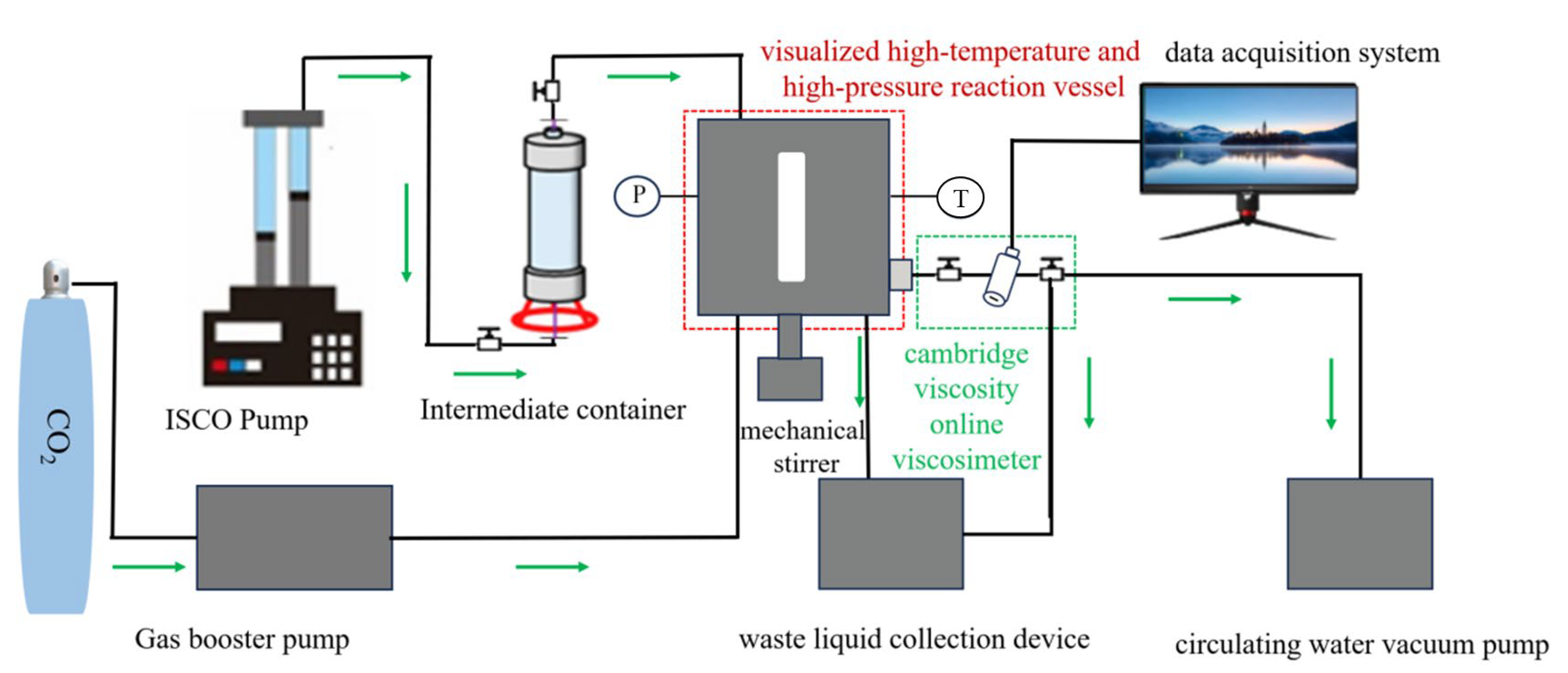
2.4. Solubility and Thickening Properties of Copolymers and Modified Copolymers
- (1)
- Accurately weigh the required thickener and add it to the intermediate container.
- (2)
- Seal the visual reaction vessel and heat it to the measurement temperature.
- (3)
- Inject CO2 to 0.5 MPa and then reduce the pressure. Repeat this cycle 5 times to expel the air inside the visual reaction vessel.
- (4)
- Use an ISCO pump to inject the weighed thickener in the intermediate container into the visual reaction vessel.
- (5)
- Use a CO2 gas booster pump to pressurize CO2 to the pressure required for the experiment. The physical property parameters of CO2 were referenced from the software REFPROP (version 9.1).
- (6)
- Start mechanical stirring at the bottom of the visual reaction vessel (stirring speed range: 0–1350 rpm). Stir at a constant speed for at least 40 min to promote the dissolution of the thickener and form a transparent and homogeneous solution. (The dissolution of the thickener in supercritical CO2 was observed through the front viewing window using an external LED light source placed behind the rear viewing window. High solubility of the thickener was indicated when the mixed fluid inside the visual reaction vessel became clear (the reference object was clearly visible).)
- (7)
- A circulating water vacuum pump was used to evacuate the Cambridge online viscometer.
- (8)
- Open the intake valve of the online viscometer to allow the completely dissolved thickener solution from step (6) to enter. Meanwhile, manually and slowly adjust the volume of the visual reaction vessel to maintain a stable system pressure. Use a data acquisition system to obtain viscosity data.
- (9)
- After the test is completed, direct the system into the waste liquid collection device. Simultaneously, clean the equipment and wait for the next experiment.
2.5. Molecular Simulation Study
- (1)
- A model was constructed based on the molecular structures presented in Table 1. Force field charges were assigned, and the configuration with the lowest energy was selected for geometric optimization using the Forcite module.
- (2)
- The model obtained from the initial geometric optimization was subjected to annealing using the Forcite module. The system went through 10 annealing cycles, starting from 313.15 K, ramping up to 500 K, and then returning to 313.15 K to reach system equilibrium.
- (3)
- Under the NPT ensemble, the desired temperature and pressure conditions (305.15 K, 10 MPa) were set. The Andersen thermostat and Berendsen barostat were employed, respectively. The time step was set to 1 fs, and the total simulation duration was 500 ps. In the aforementioned molecular dynamics process, one frame was output every 5 ps, and the data from the last 100 ps were used for subsequent data analysis.
2.5.1. Cohesive Energy Density (CED)
2.5.2. Interaction Energy
2.5.3. Radial Distribution Function (RDF)
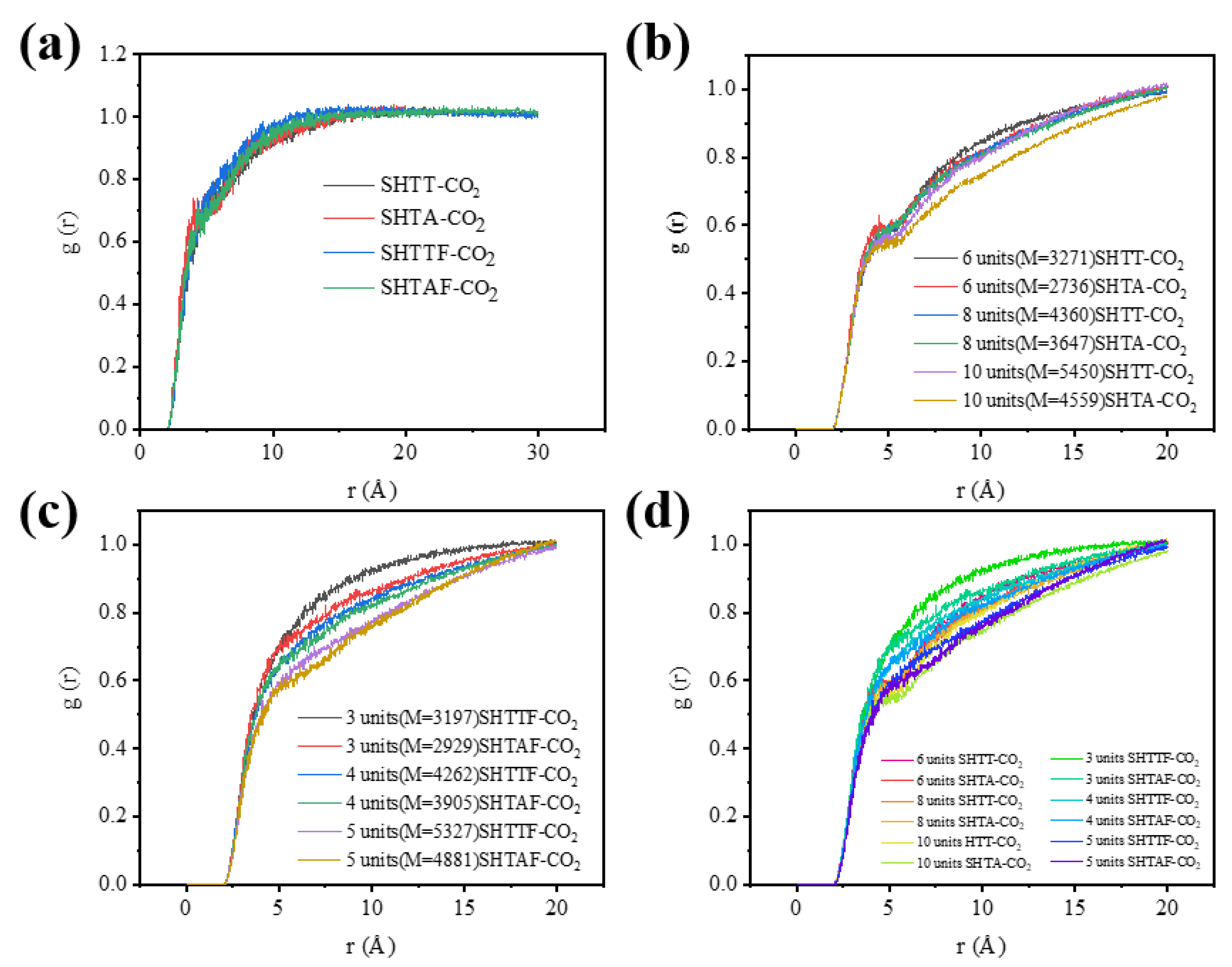
3. Results and Discussion
3.1. Characterization of Supercritical CO2 Thickener
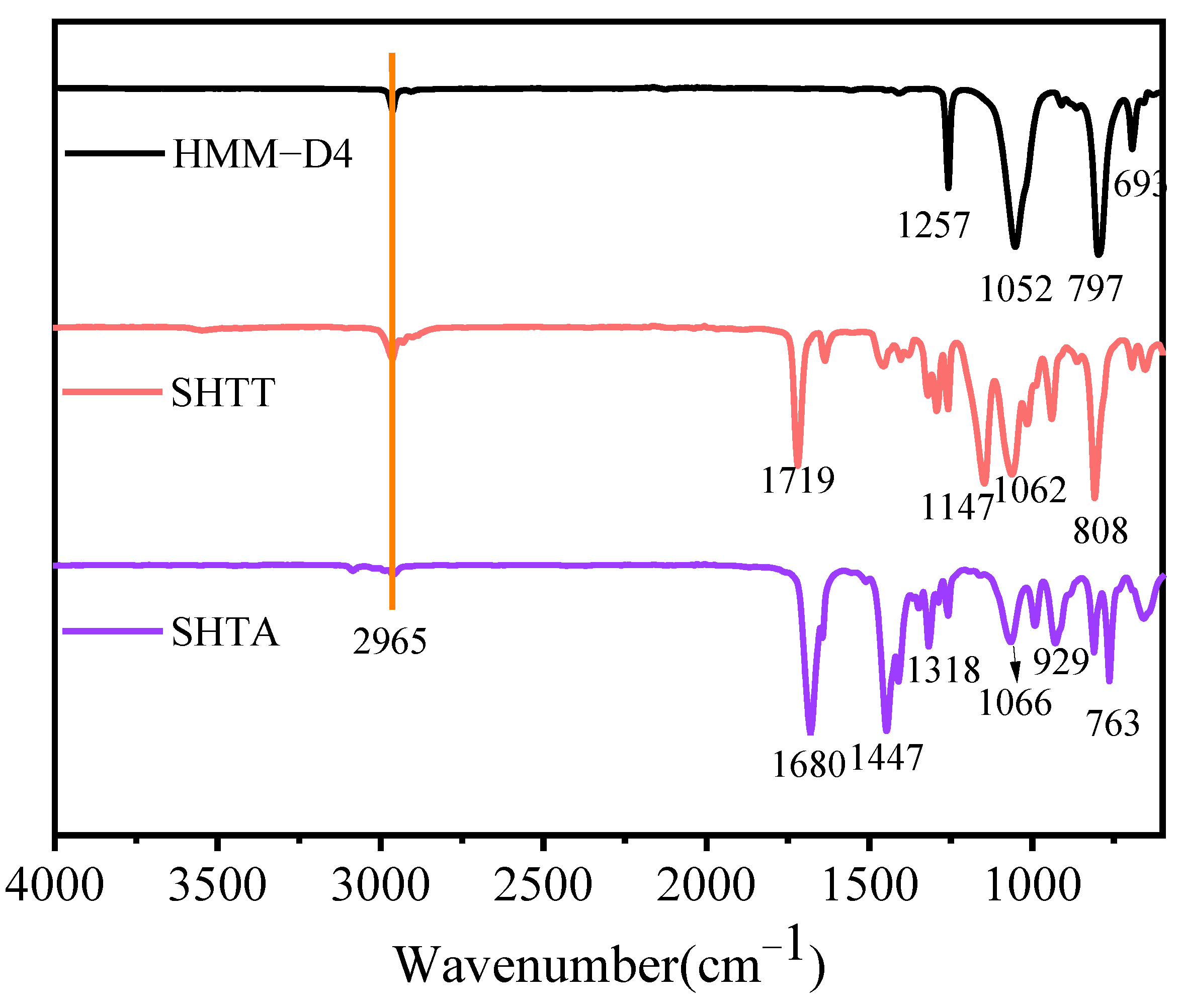
3.1.1. FT-IR
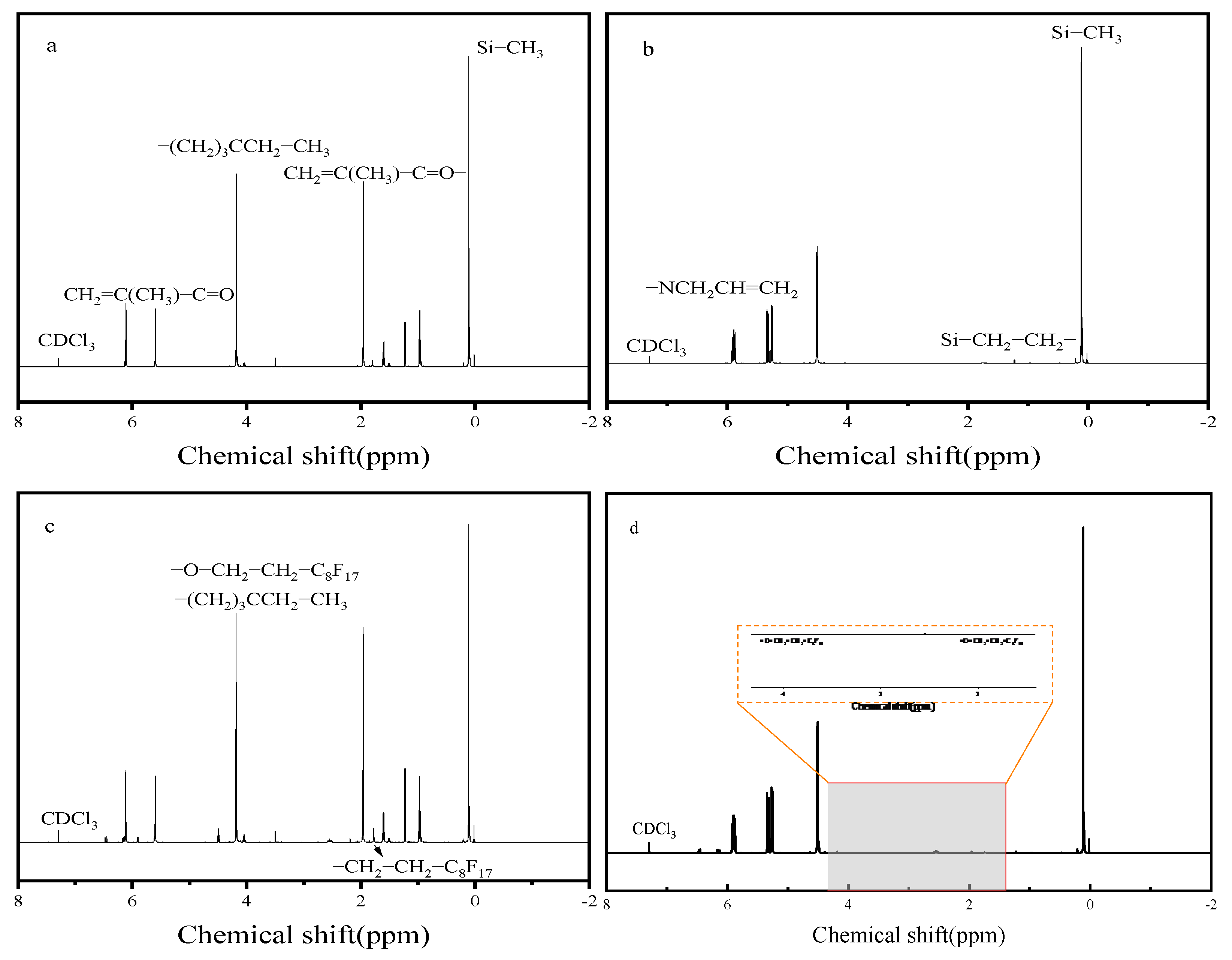
3.1.2. 1H NMR
3.2. Phase Behavior
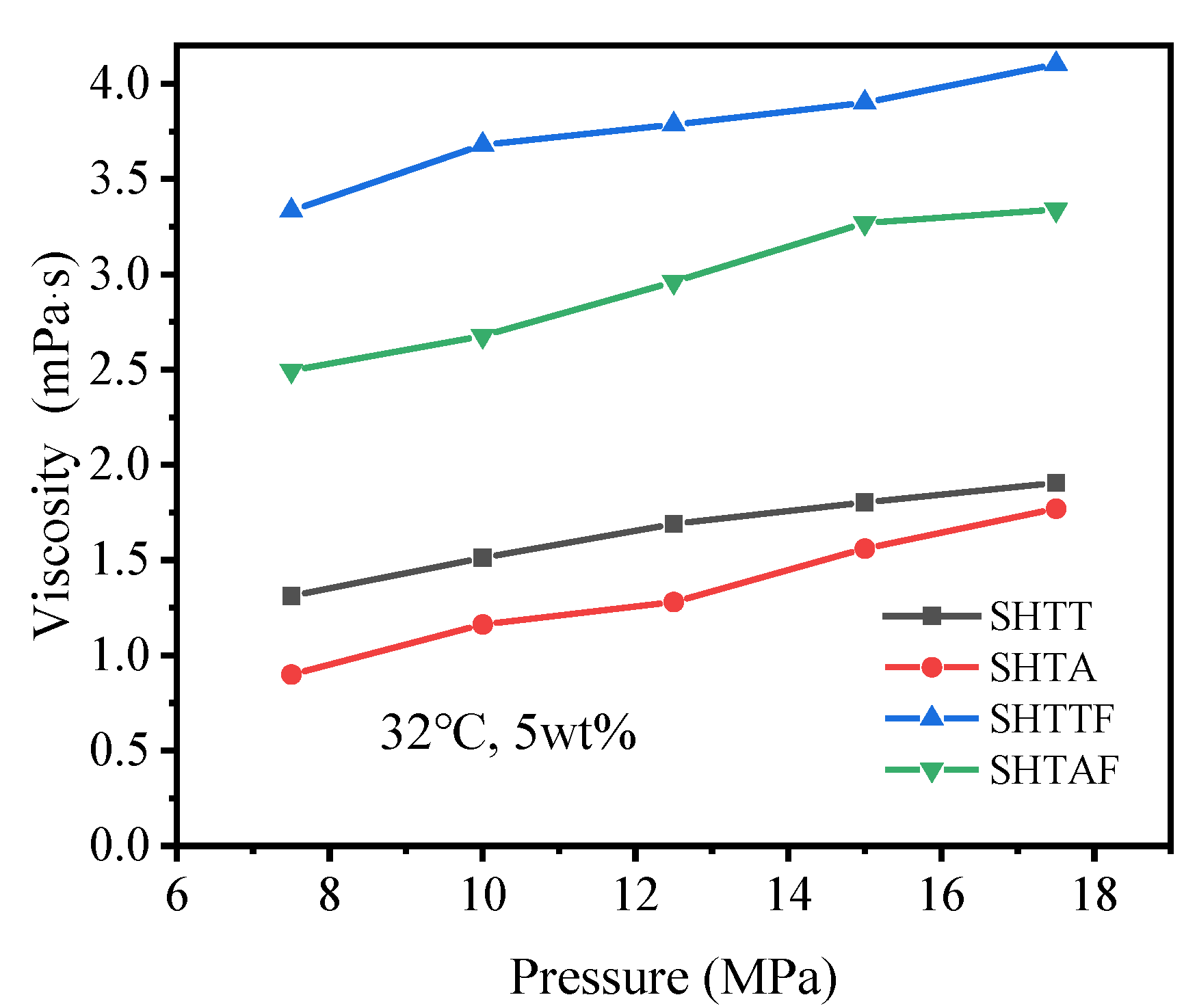
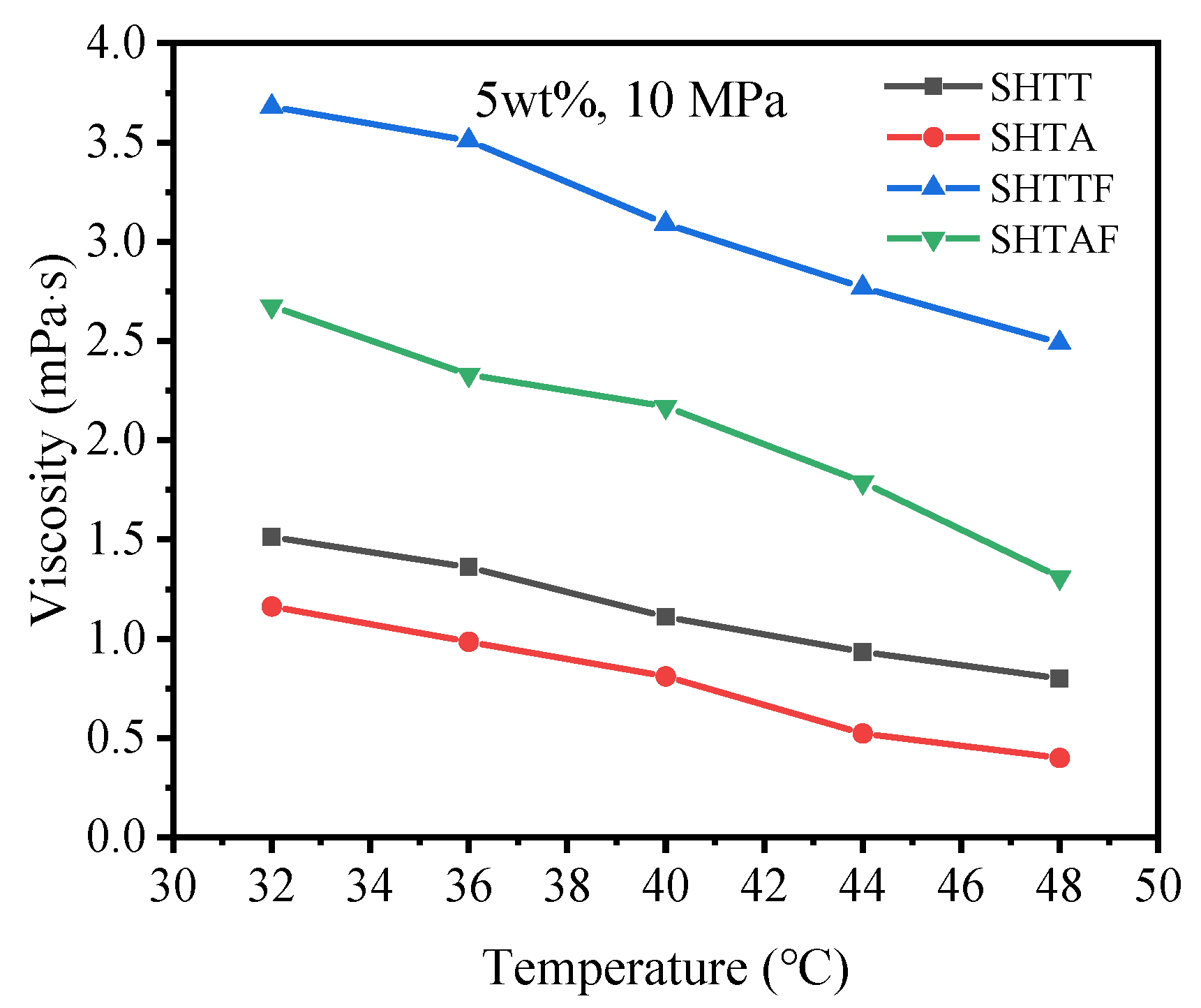
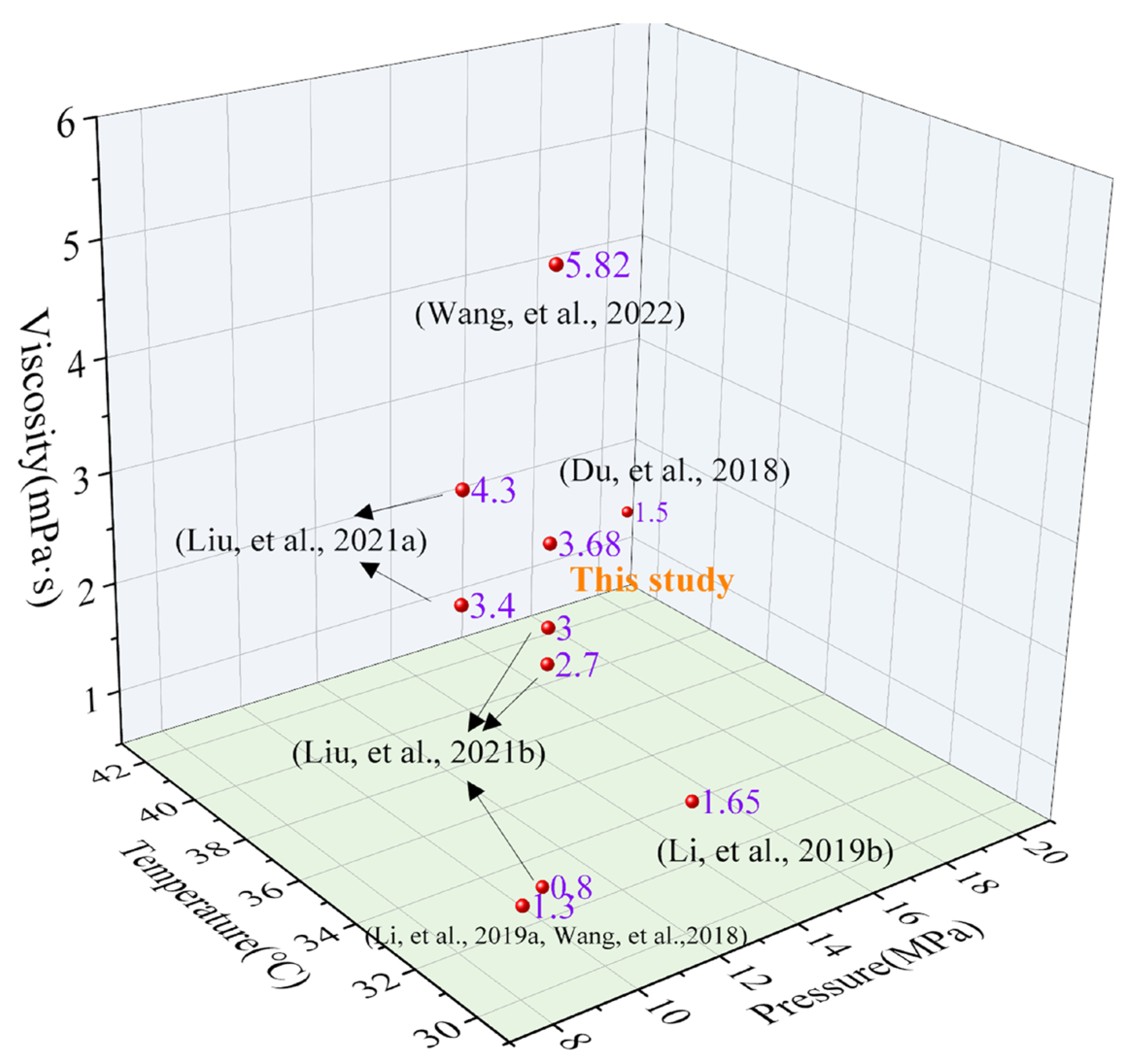
3.3. Thickening Ability
3.4. Thickening Mechanisms
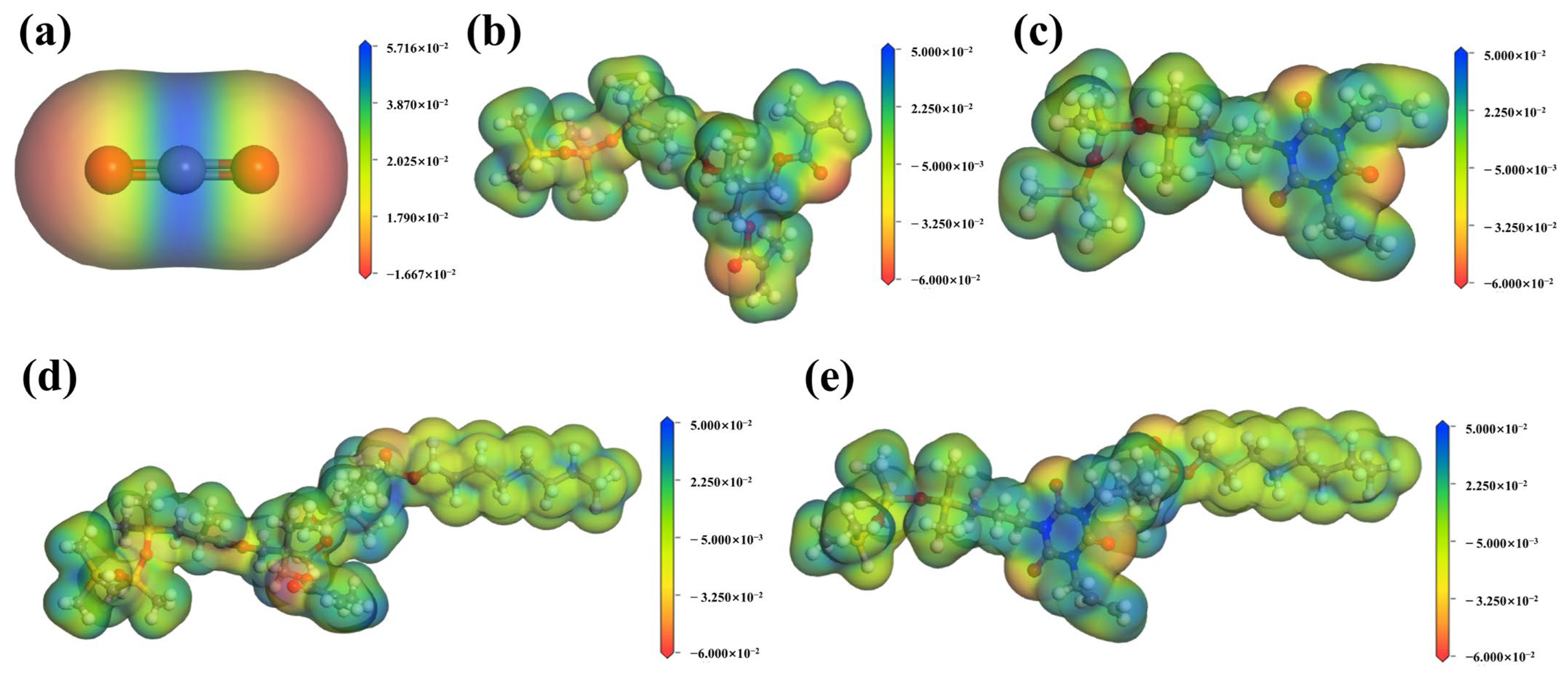
3.4.1. Molecular Electrostatic Potential Map
3.4.2. CED and Δ
3.4.3. Intermolecular Interaction Energy
3.4.4. Radial Distribution Function (RDF)
4. Conclusions
- (1)
- Four modified silicone-based thickeners for supercritical CO2 fracturing fluids were successfully synthesized. FT-IR and NMR characterization confirmed the successful incorporation of CO2-philic groups into thickener molecular structures, achieving the synthesis of fluorinated silicone thickeners.
- (2)
- The fluorinated silicone thickeners demonstrated exceptional thickening performance and favorable solubility. At 5 wt% concentration under 10 MPa and 32 °C, they formed homogeneous mixtures with CO2, elevating the viscosity of CO2 to 3.68 mPa·s. Systematic experiments established positive correlations between CO2 viscosity and both thickener concentration and pressure, while revealing an inverse relationship with temperature. The limitation of this study is that it only investigated the thickener concentrations within the range of 0~5 wt%.
- (3)
- Integrating experimental results with molecular simulations, the thickening mechanism was elucidated: The introduction of fluorinated compounds with carbon dioxide-affinitive moieties significantly enhances the dissolution of thickening functional-modified silicone segments in CO2, thereby substantially increasing the viscosity of CO2 fracturing fluids.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCUS | CO2 capture, utilization, and storage |
| FT-IR | fourier transform infrared spectrum |
| 1H NMR | 1H nuclear magnetic resonance spectroscopy |
| ESP | electrostatic potential |
| HMM | tetramethyldisiloxane |
| D4 | octamethylcyclotetrasiloxane |
| MD | molecular dynamics |
| CED | cohesive energy density |
| E | the energy of the substance |
| V | the molar volume of the substance |
| δ | the solubility parameter |
| Einter | the total intermolecular energy of the system |
| Evan | the energy from van der Waals interactions |
| Eelect | the energy from electrostatic interactions |
| Eint | the polymer–CO2 interaction energy |
| Epolymer+CO2 | the total energy of the polymer–CO2 system |
| Epolymer | the energy of the polymer |
| ECO2 | the energy of CO2, kJ/mol |
| RDF | radial distribution function |
| the radial distribution function value | |
| the number of species A and B (molecules or atoms) in the system | |
| the distance interval width | |
| the number of B particles (or A particles) found within the distance range r~r + Δr from a central A particle (or B particle) | |
| the density of system |
References
- Li, J.; Pu, W.; Du, D.; Wu, T.; Shen, C.; Liao, Z. Molecular dynamics simulation of solvation behavior and thickening properties of modified siloxanes in supercritical carbon dioxide under different pressure and temperature. J. Mol. Liq. 2024, 414, 126082. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Q.; Zou, X.; Fan, J.; Wang, S.; Zhu, Y. Research progress on and outlook of direct CO2 thickeners for enhanced oil recovery. RSC Adv. 2025, 15, 714–731. [Google Scholar] [CrossRef]
- Prasad, S.K.; Sangwai, J.S.; Byun, H.-S. A review of the supercritical CO2 fluid applications for improved oil and gas production and associated carbon storage. J. CO2 Util. 2023, 72, 102479. [Google Scholar] [CrossRef]
- Dai, C.; Liu, P.; Gao, M.; Liu, Z.; Liu, C.; Wu, Y.; Wang, X.; Liu, S.; Zhao, M.; Yan, H. Preparation and thickening mechanism of copolymer fluorinated thickeners in supercritical CO2. J. Mol. Liq. 2022, 360, 119563. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, T.; Li, J.; Pang, Q.; Yang, H.; Liu, G.; Huang, H.; Zhu, Y. Dynamics simulation of the effect of cosolvent on the solubility and tackifying behavior of PDMS tackifier in supercritical CO2 fracturing fluid. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130985. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Liang, S.; Zhao, S. Systematic Review of Solubility, Thickening Properties and Mechanisms of Thickener for Supercritical Carbon Dioxide. Nanomaterials 2024, 14, 996. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Qi, S.; Lu, W.; Guo, S.; Wang, Z.; Yu, X.; Zhang, Y. Experimental Research on Supercritical Carbon Dioxide Fracturing of Sedimentary Rock: A Critical Review. Acta Geol. Sin.–Engl. Ed. 2023, 97, 925–945. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, A. Supercritical Carbon Dioxide Utilization for Hydraulic Fracturing of Shale Reservoir, and Geo-Storage: A Review. Energy Fuels 2023, 37, 14604–14621. [Google Scholar] [CrossRef]
- Pal, N.; Zhang, X.; Ali, M.; Mandal, A.; Hoteit, H. Carbon dioxide thickening: A review of technological aspects, advances and challenges for oilfield application. Fuel 2022, 315, 122947. [Google Scholar] [CrossRef]
- Li, N.; Zhang, H.; Ren, X.; Wang, J.; Yu, J.; Jiang, C.; Zhang, H.; Li, Y. Development status of supercritical carbon dioxide thickeners in oil and gas production: A review and prospects. Gas Sci. Eng. 2024, 125, 205312. [Google Scholar] [CrossRef]
- Zhou, M.; Ni, R.; Zhao, Y.; Huang, J.; Deng, X. Research progress on supercritical CO2 thickeners. Soft Matter 2021, 17, 5107–5115. [Google Scholar] [CrossRef] [PubMed]
- Gandomkar, A.; Reza Nasriani, H.; Enick, R.M.; Torabi, F. The effect of CO2-philic thickeners on gravity drainage mechanism in gas invaded zone. Fuel 2023, 331, 125760. [Google Scholar] [CrossRef]
- Sun, W.; Wang, H.; Zha, Y.; Yu, J.; Zhang, J.; Ge, Y.; Sun, B.; Zhang, Y.; Gao, C. Experimental and microscopic investigations of the performance of copolymer thickeners in supercritical CO2. Chem. Eng. Sci. 2020, 226, 115857. [Google Scholar] [CrossRef]
- Kilic, S.; Enick, R.M.; Beckman, E.J. Fluoroacrylate-aromatic acrylate copolymers for viscosity enhancement of carbon dioxide. J. Supercrit. Fluids 2019, 146, 38–46. [Google Scholar] [CrossRef]
- Zhao, M.; Yan, R.; Li, Y.; Wu, Y.; Dai, C.; Yan, H.; Liu, Z.; Cheng, Y.; Guo, X. Study on the thickening behavior and mechanism of supercritical CO2 by modified polysiloxane. Fuel 2022, 323, 124358. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Li, D.; Liang, L. SiO2 synergistic modification of siloxane thickener to improve the viscosity of supercritical CO2 fracturing fluid. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 6602–6617. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Liang, L. Preparation and Performance of Supercritical Carbon Dioxide Thickener. Polymers 2021, 13, 78. [Google Scholar] [CrossRef]
- Wang, X.; Liang, S.; Zhang, Q.; Wang, T.; Zhang, X. Molecular Dynamics Simulation on Thickening and Solubility Properties of Novel Thickener in Supercritical Carbon Dioxide. Molecules 2024, 29, 2529. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Liang, L.; Zeng, Y. Achieving solubility alteration with functionalized polydimethylsiloxane for improving the viscosity of supercritical CO2 fracturing fluids. RSC Adv. 2021, 11, 17197–17205. [Google Scholar] [CrossRef]
- Chang, K.-S.; Chung, Y.-C.; Yang, T.-H.; Lue, S.J.; Tung, K.-L.; Lin, Y.-F. Free volume and alcohol transport properties of PDMS membranes: Insights of nano-structure and interfacial affinity from molecular modeling. J. Membr. Sci. 2012, 417–418, 119–130. [Google Scholar] [CrossRef]
- Hu, D.; Sun, S.; Yuan, P.-Q.; Zhao, L.; Liu, T. Exploration of CO2-Philicity of Poly(vinyl acetate-co-alkyl vinyl ether) through Molecular Modeling and Dissolution Behavior Measurement. J. Phys. Chem. B 2015, 119, 12490–12501. [Google Scholar] [CrossRef]
- Tang, M.; Fang, X.; Li, B.; Xu, M.; Wang, H.; Cai, S. Hydrophobic Modification of Wood Using Tetramethylcyclotetrasiloxane. Polymers 2022, 14, 2077. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Sonawane, N.; Patil, V.; Mawale, R.; Pawar, N. Designing efficient UV-curable flame-retardant and antimicrobial wood coating: Phosphorodiamidate reactive diluent with epoxy acrylate. J. Polym. Res. 2021, 28, 376. [Google Scholar] [CrossRef]
- Huang, M.; Peng, Z.-h.; Zhang, W.; Xiang, Y.; Cao, F. Influence of β-Si3N4 whiskers on sintering and crystallization of fused silica ceramics. J. Am. Ceram. Soc. 2023, 106, 967–976. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wang, X.; Yu, H.; Li, Q.; Wang, F.; Bai, H.; Kobina, F. An application of thickener to increase viscosity of liquid CO2 and the assessment of the reservoir geological damage and CO2 utilization. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 368–377. [Google Scholar]
- Dai, C.; Wang, T.; Zhao, M.; Sun, X.; Gao, M.; Xu, Z.; Guan, B.; Liu, P. Impairment mechanism of thickened supercritical carbon dioxide fracturing fluid in tight sandstone gas reservoirs. Fuel 2018, 211, 60–66. [Google Scholar] [CrossRef]
- Du, M.; Sun, X.; Dai, C.; Li, H.; Wang, T.; Xu, Z.; Zhao, M.; Guan, B.; Liu, P. Laboratory experiment on a toluene-polydimethyl silicone thickened supercritical carbon dioxide fracturing fluid. J. Pet. Sci. Eng. 2018, 166, 369–374. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wang, F.; Li, Q.; Kobina, F.; Bai, H.; Yuan, L. Effect of a Modified Silicone as a Thickener on Rheology of Liquid CO2 and Its Fracturing Capacity. Polymers 2019, 11, 540. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Dong, W.; Li, Q.; Wang, F.; Bai, H.; Zhang, R.; Owusu, A.B. Effect of different factors on the yield of epoxy-terminated polydimethylsiloxane and evaluation of CO2 thickening. RSC Adv. 2018, 8, 39787–39796. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Owusu, A.B. A modified Ester-branched thickener for rheology and wettability during CO2 fracturing for improved fracturing property. Environ. Sci. Pollut. Res. 2019, 26, 20787–20797. [Google Scholar] [CrossRef]
- Pu, W.; Li, J.; Du, D.; Zhao, J.; Wu, T.; Xiong, Y.; Chen, P.; Jiang, R. Research progress of thickener for supercritical carbon dioxide fracturing fluid. Petroleum, 2025; in press. [Google Scholar] [CrossRef]
- Nelson, P.N. A theoretical study of the interactions between carbon dioxide and some Group(III) trihalides: Implications in carbon dioxide sequestration. J. Mol. Struct. 2021, 1223, 129212. [Google Scholar] [CrossRef]
- Sun, W.; Sun, B.; Li, Y.; Fan, H.; Gao, Y.; Sun, H.; Li, G. Microcosmic understanding on thickening capability of copolymers in supercritical carbon dioxide: The key role of π–π stacking. RSC Adv. 2017, 7, 34567–34573. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Zou, Y.; Wang, F.; Chen, B.; Guan, L.; Wu, Y. Models for the solubility calculation of a CO2/polymer system: A review. Mater. Today Commun. 2020, 25, 101277. [Google Scholar] [CrossRef]
- Wang, J.; Ding, W.; Zhang, B.; Jin, H. Polycyclic aromatic hydrocarbons dissolution in supercritical carbon dioxide by molecular dynamics simulation. J. Mol. Liq. 2023, 391, 123358. [Google Scholar] [CrossRef]
- Sun, B.; Sun, W.; Wang, H.; Li, Y.; Fan, H.; Li, H.; Chen, X. Molecular simulation aided design of copolymer thickeners for supercritical CO2 as non-aqueous fracturing fluid. J. CO2 Util. 2018, 28, 107–116. [Google Scholar] [CrossRef]
- Tan, J.; Wang, Z.; Chen, S.; Hu, H. Progress and Outlook of Supercritical CO2–Heavy Oil Viscosity Reduction Technology: A Minireview. Energy Fuels 2023, 37, 11567–11583. [Google Scholar] [CrossRef]

| Name | Molecular Structure |
|---|---|
| CO2 |  |
| SHTT |  |
| SHTA | 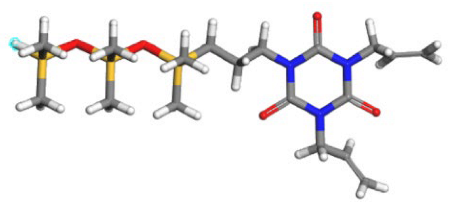 |
| SHTTF | 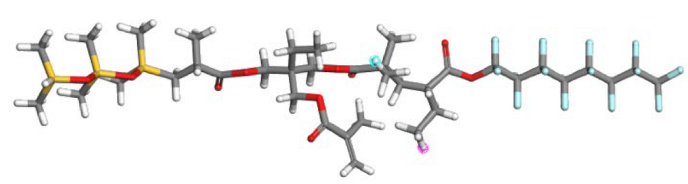 |
| SHTAF |  |
| System | Temperature (°C)/Pressure (MPa) | Dissolution Pressure (MPa) | |||
|---|---|---|---|---|---|
| 25 °C, 7.5 MPa | 32 °C, 7.5 MPa | 32 °C, 10 MPa | 32 °C, 7.5 MPa | ||
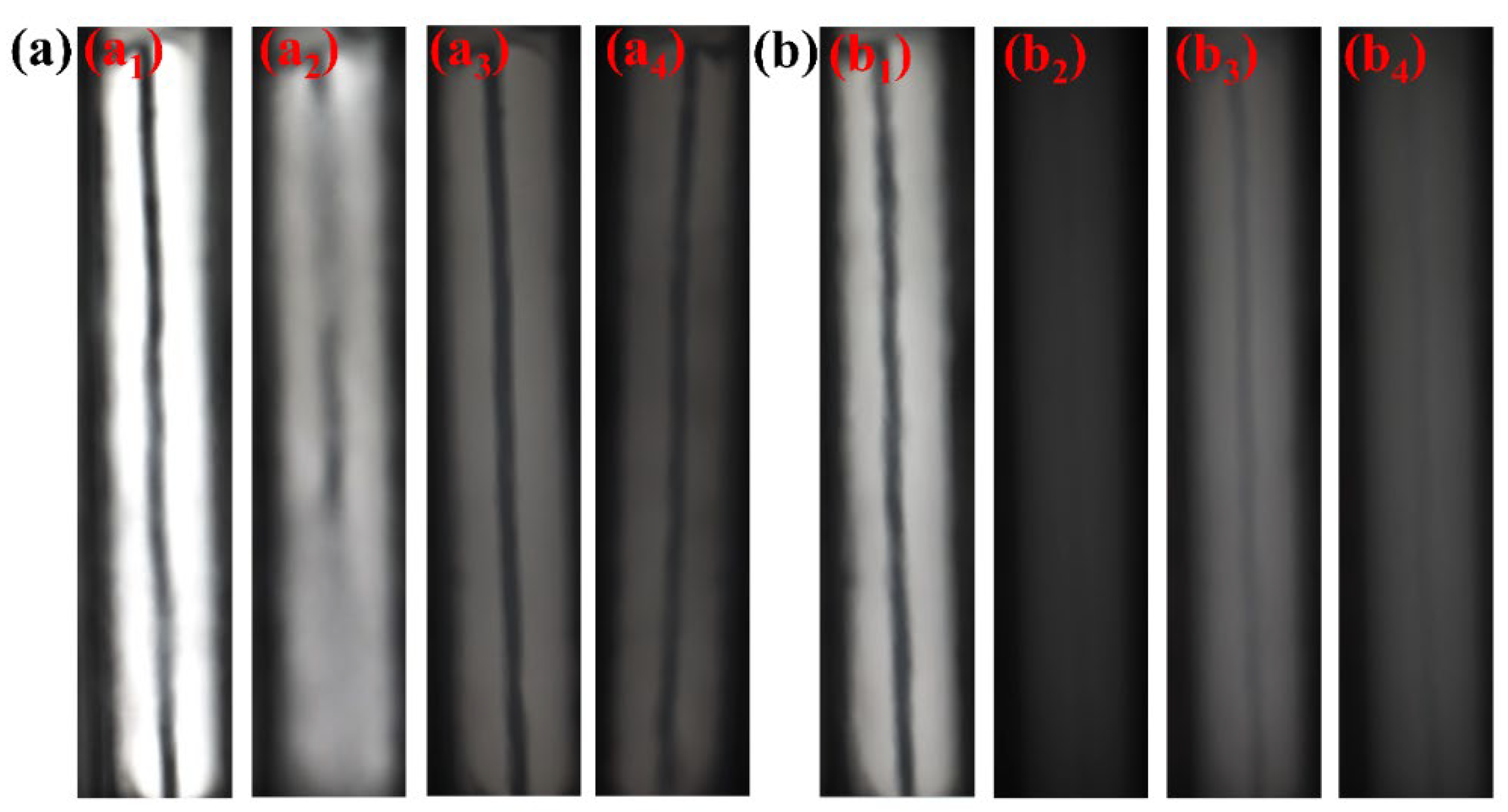
| SHTT-CO2 | a1 | a2 | a3 | a4 | 9.2 |
| Clarified | Turbid, reference object not visible | Clear and transparent | Turbid, reference object visible | ||
| SHTTF-CO2 | b1 | b2 | b3 | b4 | 8.6 |
| Clarified | Turbid, reference object not visible | Clear and transparent | Turbid, reference object visible | ||
| Temperature (°C)/Pressure (MPa) | System | SHTT-CO2 | SHTA-CO2 | SHTTF-CO2 | SHTAF-CO2 | |
|---|---|---|---|---|---|---|
| Content (wt%) | Viscosity (mPa·s) | |||||
| 32 °C, 10 MPa | 0 | 0.06335 (REFPROP) | ||||
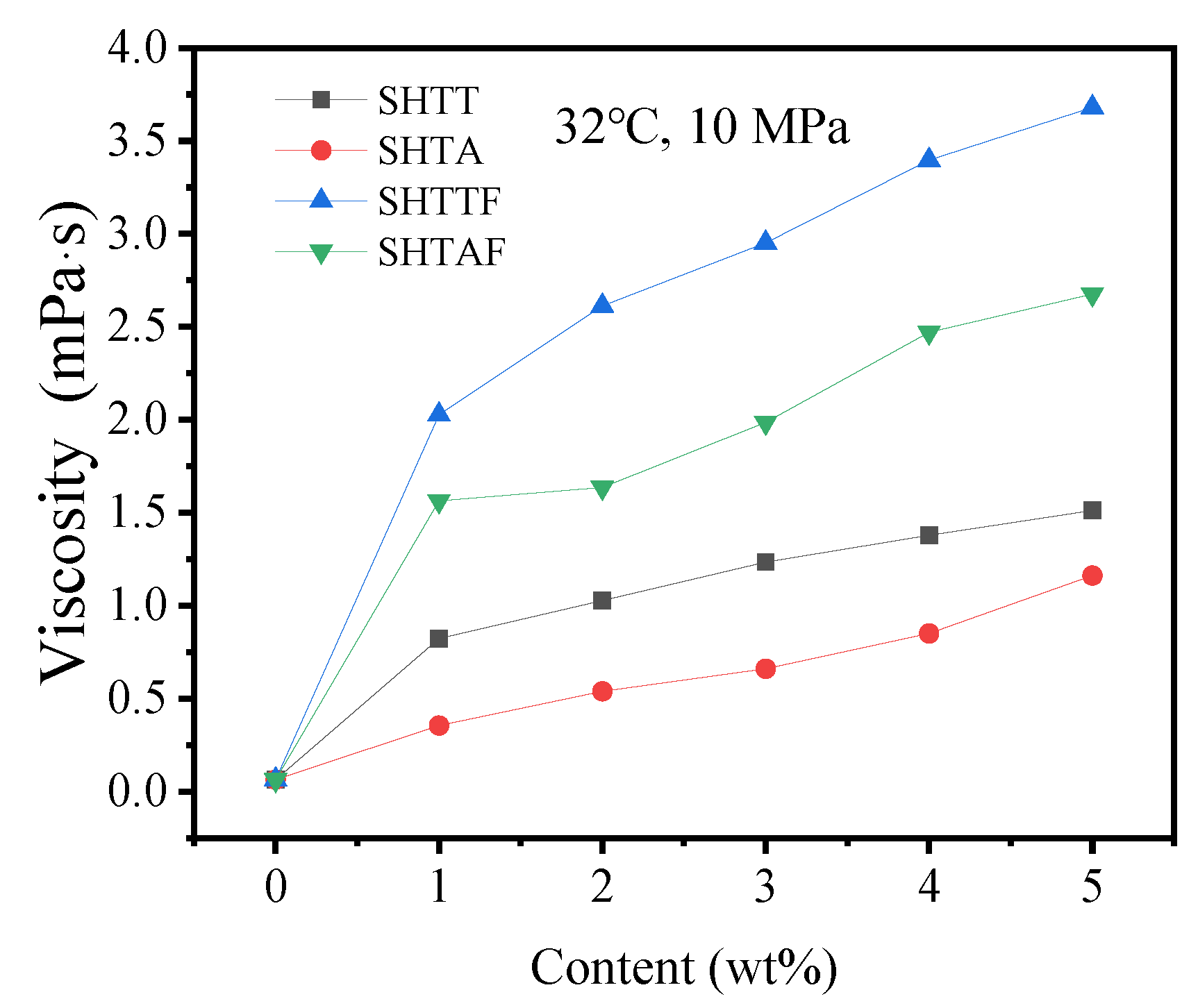
| 1 | 0.823 | 0.355 | 2.026 | 1.564 | ||
| 2 | 1.027 | 0.539 | 2.612 | 1.636 | ||
| 3 | 1.234 | 0.66 | 2.951 | 1.986 | ||
| 4 | 1.378 | 0.85 | 3.396 | 2.47 | ||
| 5 | 1.512 | 1.162 | 3.68 | 2.676 | ||
| Temperature (°C)/Content (wt%) | System | SHTT-CO2 | SHTA-CO2 | SHTTF-CO2 | SHTAF-CO2 | |
|---|---|---|---|---|---|---|
| Pressure (MPa) | Viscosity (mPa·s) | |||||
| 32 °C, 5 wt% | 7.5 | 1.312 | 0.9 | 3.334 | 2.495 | |
| 10 | 1.512 | 1.162 | 3.68 | 2.676 | ||
| 12.5 | 1.69 | 1.28 | 3.785 | 2.96 | ||
| 15 | 1.803 | 1.56 | 3.9 | 3.27 | ||
| 17.5 | 1.905 | 1.77 | 4.102 | 3.34 | ||
| Pressure (MPa)/Content (wt%) | System | SHTT-CO2 | SHTA-CO2 | SHTTF-CO2 | SHTAF-CO2 | |
|---|---|---|---|---|---|---|
| Temperature (°C) | Viscosity (mPa·s) | |||||
| 10 MPa, 5 wt% | 32 | 1.512 | 1.162 | 3.68 | 2.676 | |
| 36 | 1.36 | 0.985 | 3.51 | 2.33 | ||
| 40 | 1.11 | 0.81 | 3.09 | 2.17 | ||
| 44 | 0.934 | 0.523 | 2.77 | 1.79 | ||
| 48 | 0.8 | 0.4 | 2.49 | 1.31 | ||
| Thickener | Concentration (wt%) | Cosolvent (wt%) | Experimental Condition | Viscosity (mPa·s) | Ref. |
|---|---|---|---|---|---|
| HBD-1 | 5 | no | 32 °C/8 MPa | 3.4 | [17] |
| HBD-2 | 5 | no | 32 °C/8 MPa | 4.3 | |
| SHTTF | 5 | no | 32 °C/10 MPa | 3.68 | This study |
| HS-1 | 5 | no | 32 °C/10 MPa | 0.8 | [19] |
| HS-2 | 5 | no | 32 °C/10 MPa | 2.7 | |
| HS-3 | 5 | no | 32 °C/10 MPa | 3.0 | |
| PDMS (350 mPa·s) | 5 | 10 wt% toluene | 42 °C/20 MPa | 1.5 | [27] |
| SiO2/HBD-2 | 5 wt% HBD-2 + 1 wt% SiO2 | no | 32 °C/10 MPa | 5.82 | [16] |
| EEPDMS | 3 | 9 wt% toluene | 30 °C/8 MPa | 1.3 | [28,29] |
| Ester-branched polydimethylsiloxane | 2.5 | 7.5 wt% n-hexane | 30 °C/12 MPa | 1.65 | [30] |
| System | Evan/(J/m3) | Eelect/(J/m3) | Eother/(J/m3) | CED/(J/m3) | δ/(J/m3)1/2 | Δδ/(J/m3)1/2 |
|---|---|---|---|---|---|---|
| CO2 | 7.958 × 107 | 5.629 × 107 | 3.033 × 106 | 1.389 × 108 | 11.777 | 0 |
| SHTT-CO2 | 1.365 × 108 | 9.910 × 107 | 5.454 × 106 | 2.411 × 108 | 15.512 | 3.735 |
| SHTA-CO2 | 1.369 × 108 | 9.938 × 107 | 5.413 × 106 | 2.417 × 108 | 15.507 | 3.730 |
| SHTTF-CO2 | 7.202 × 107 | 4.727 × 107 | 2.803 × 106 | 1.221 × 108 | 11.036 | 0.741 |
| SHTAF-CO2 | 6.082 × 107 | 3.986 × 107 | 2.305 × 106 | 1.030 × 108 | 10.119 | 1.658 |
| System | /(kcal/mol) | /(kcal/mol) | /(kcal/mol) | Eint/(kcal/mol) |
|---|---|---|---|---|
| SHTT-CO2 | −2449.962372 | −1406.236231 | −846.918705 | −196.807436 |
| SHTA-CO2 | −2205.395479 | −1181.816535 | −847.726976 | −175.851968 |
| SHTTF-CO2 | −1635.494087 | −390.389668 | −1028.260001 | −216.844418 |
| SHTAF-CO2 | −1473.056476 | −292.508835 | −977.561245 | −202.986396 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Xiong, Y.; Du, D.; Jiang, R.; Li, J. Synthesis of Linear Modified Siloxane-Based Thickeners and Study of Their Phase Behavior and Thickening Mechanism in Supercritical Carbon Dioxide. Polymers 2025, 17, 2640. https://doi.org/10.3390/polym17192640
Chen P, Xiong Y, Du D, Jiang R, Li J. Synthesis of Linear Modified Siloxane-Based Thickeners and Study of Their Phase Behavior and Thickening Mechanism in Supercritical Carbon Dioxide. Polymers. 2025; 17(19):2640. https://doi.org/10.3390/polym17192640
Chicago/Turabian StyleChen, Pengfei, Ying Xiong, Daijun Du, Rui Jiang, and Jintao Li. 2025. "Synthesis of Linear Modified Siloxane-Based Thickeners and Study of Their Phase Behavior and Thickening Mechanism in Supercritical Carbon Dioxide" Polymers 17, no. 19: 2640. https://doi.org/10.3390/polym17192640
APA StyleChen, P., Xiong, Y., Du, D., Jiang, R., & Li, J. (2025). Synthesis of Linear Modified Siloxane-Based Thickeners and Study of Their Phase Behavior and Thickening Mechanism in Supercritical Carbon Dioxide. Polymers, 17(19), 2640. https://doi.org/10.3390/polym17192640






