Transparent Polyurethane Elastomers with Excellent Foamability and Self-Healing Property via Molecular Design and Dynamic Covalent Bond Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods of Synthesis
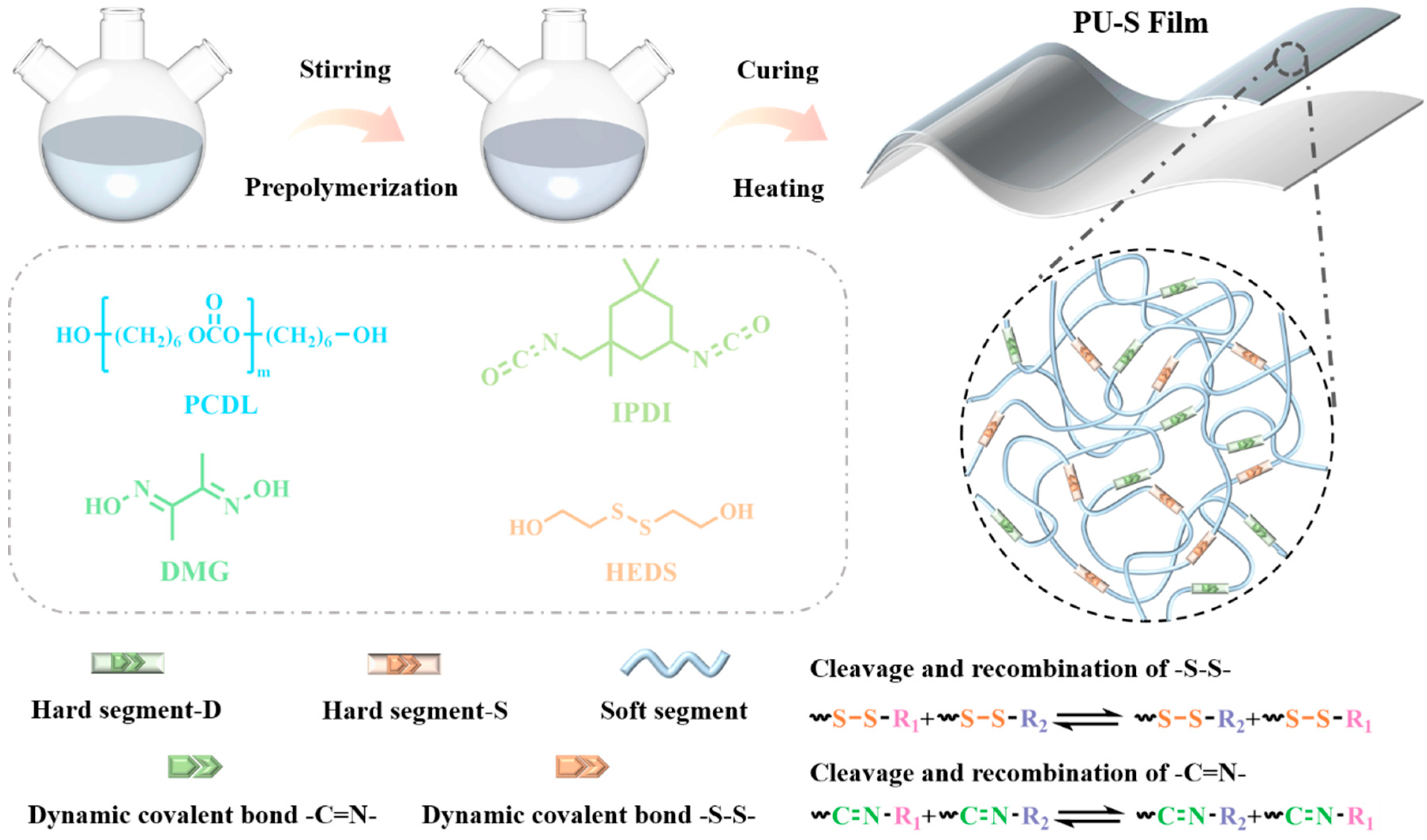
2.2.1. Synthesis of PU-S Elastomers
2.2.2. Preparation of PU-S Foams
2.3. Characterization
3. Results and Discussion
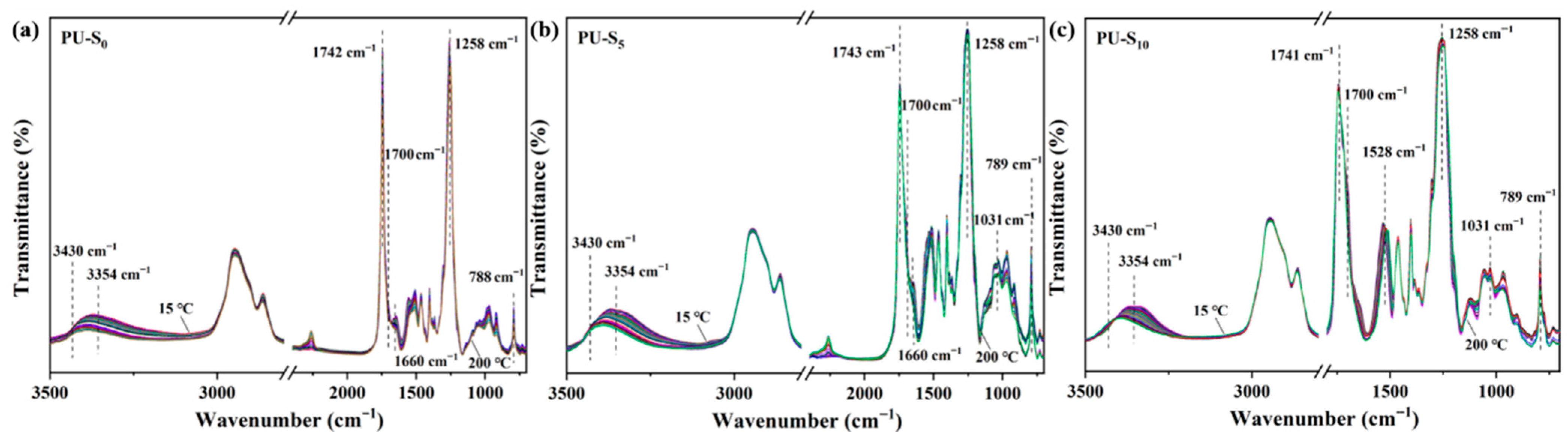
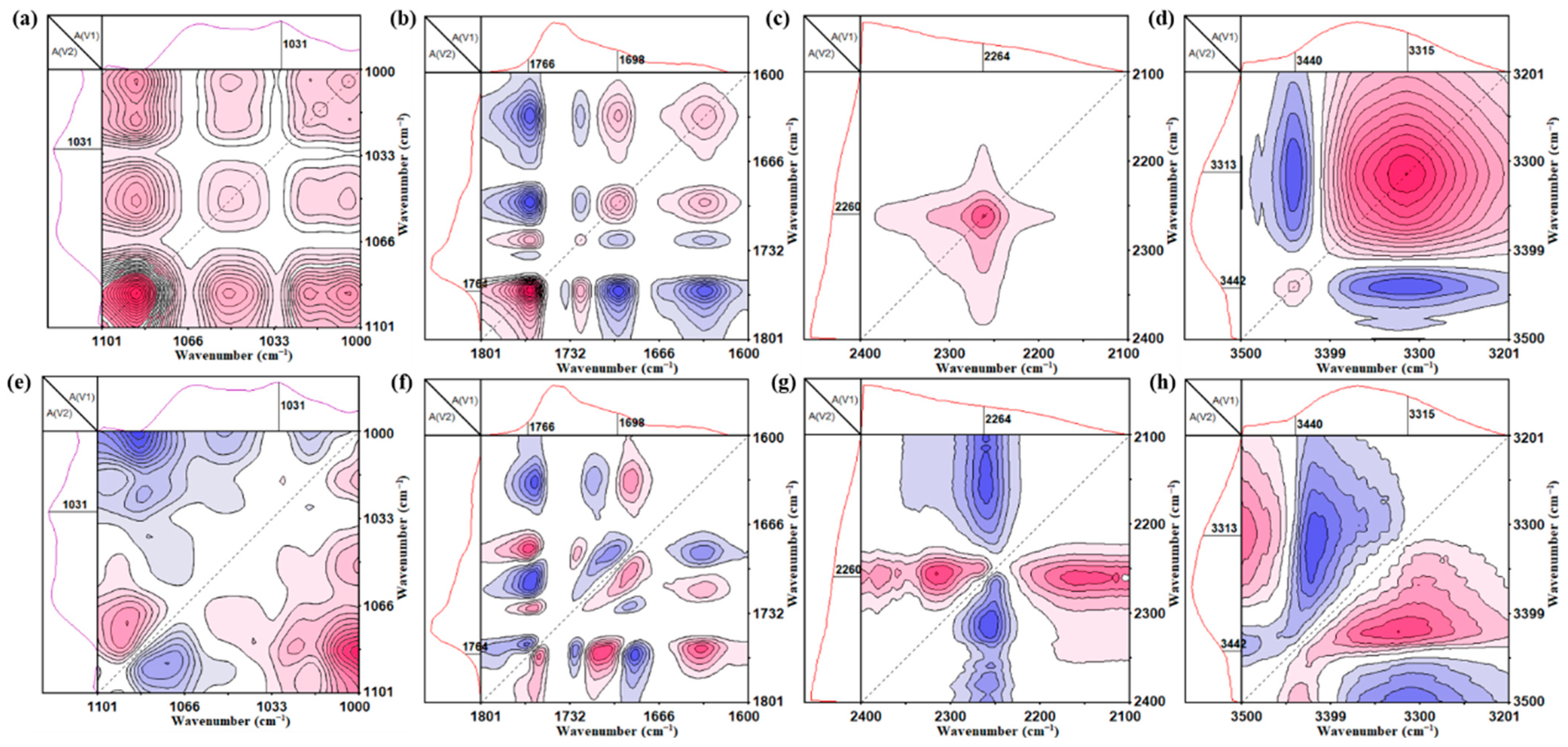
3.1. Functional Groups of Synthesized PU Films
3.2. Optical and Mechanical Properties of Synthesized PU Films
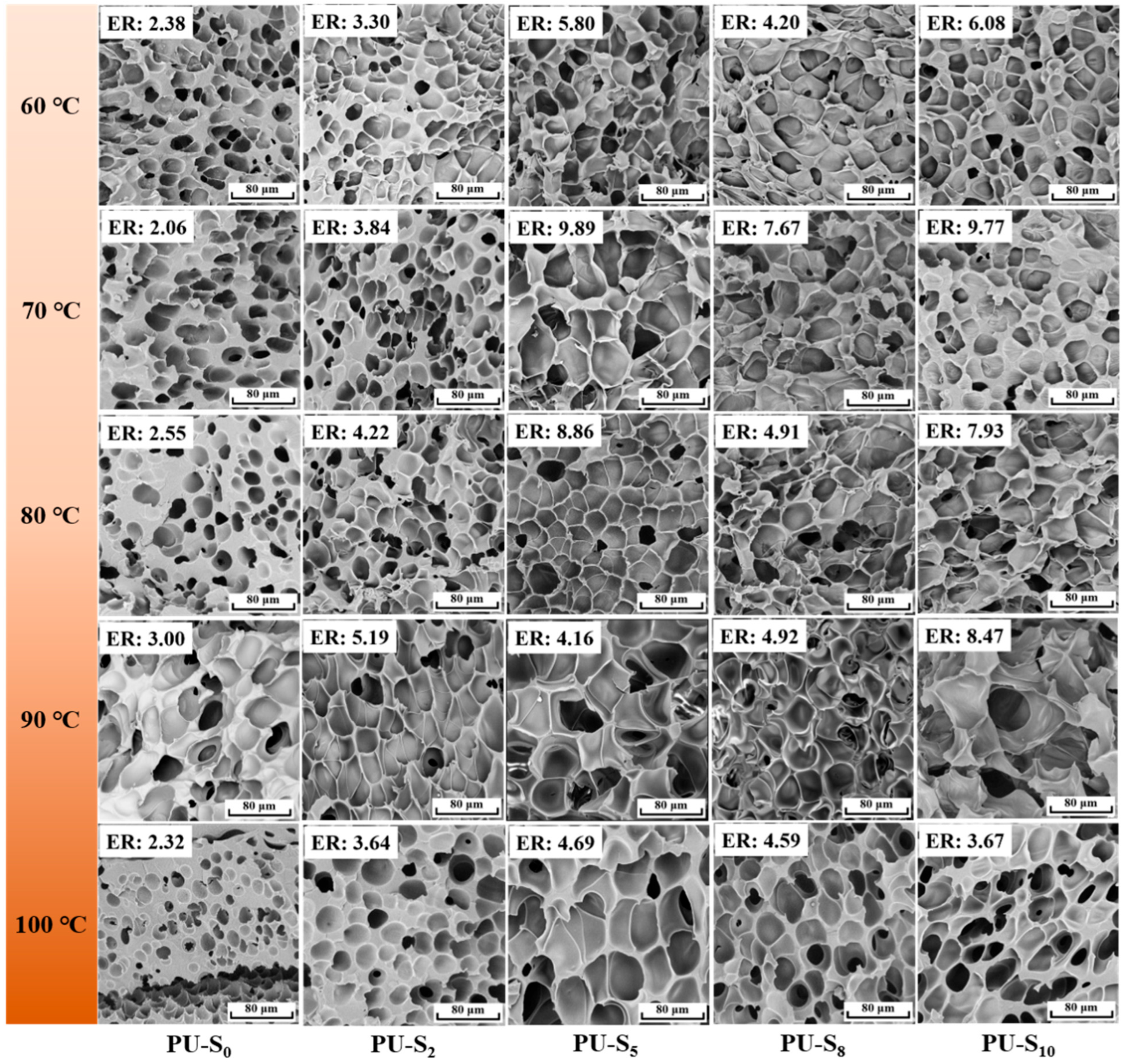
3.3. Foaming Behavior of Synthesized PU Films
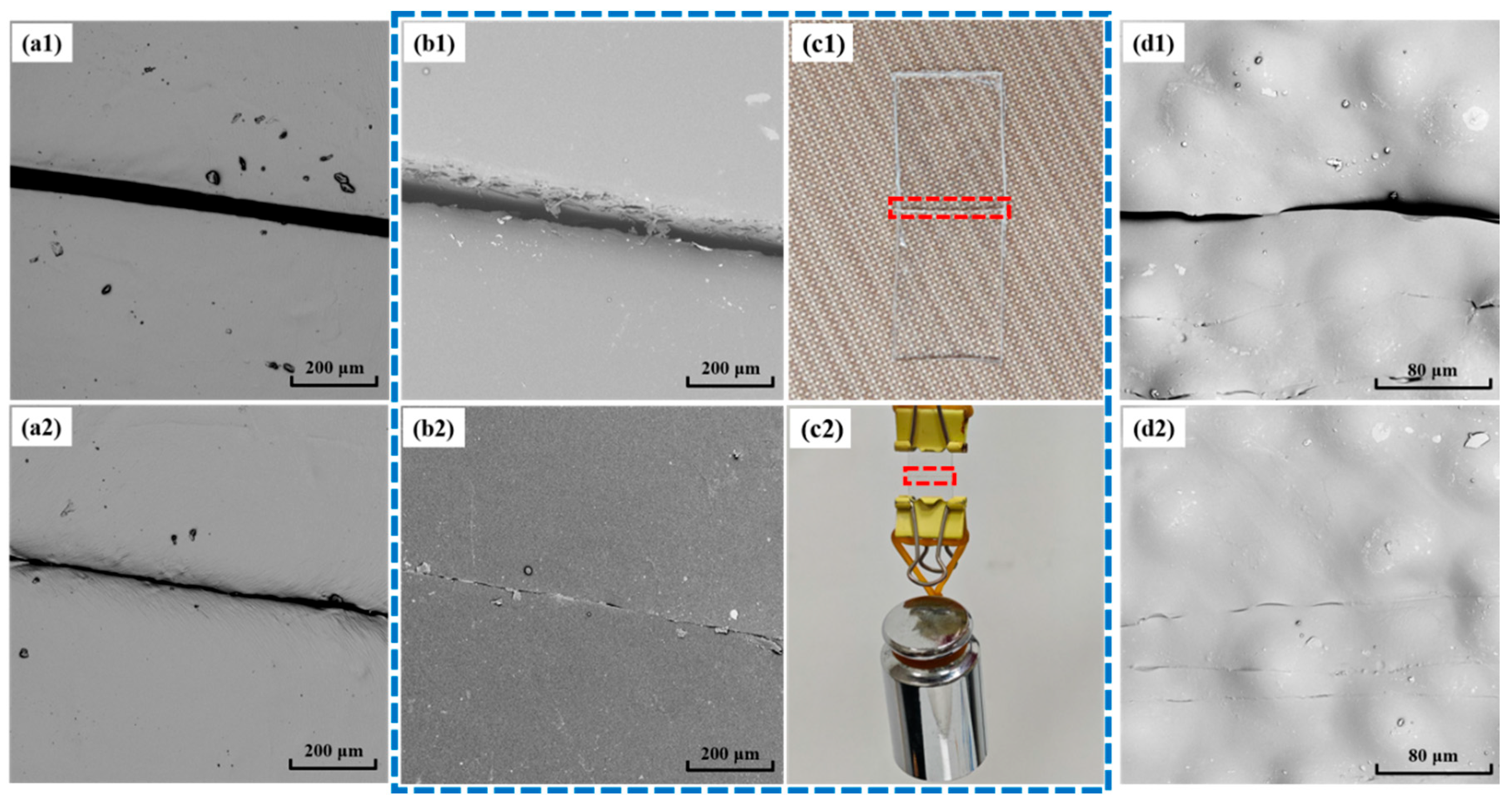
3.4. Self-Healing Performance of Synthesized PU Films and the Corresponding Foams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Xie, H.; Tian, X.; Sun, Y.; Shi, B.; Zhou, Y.; Sheng, D.; Liu, X.; Yang, Y. Hard, tough and fast self-healing thermoplastic polyurethane. Prog. Org. Coat. 2021, 159, 106409–106417. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, J.; Zhang, J.; Huang, Z.; Qi, D. Review of research on thermoplastic self-healing polyurethanes. React. Funct. Polym. 2024, 199, 105886–105897. [Google Scholar] [CrossRef]
- Jin, C.; Sinawang, G.; Osaki, M.; Zheng, Y.; Yamaguchi, H.; Harada, A.; Takashima, Y. Self-healing thermoplastic polyurethane linked via host-guest interactions. Polymers 2020, 12, 1393. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Wang, K.; Wang, Y.H. High performance of thermoplastic polyurethane-graphene oxide self-healing composite film. Coatings 2021, 11, 128. [Google Scholar] [CrossRef]
- Zhong, W.; Zhou, X.; Zhang, M.; Cui, W.; Mi, H.Y.; Dong, B.; Liu, C.; Shen, C. Wrinkled thermoplastic polyamide elastomer foams with enhanced mechanical properties fabricated by dynamic supercritical CO2 foaming. Polym. Eng. Sci. 2025, 65, 834–845. [Google Scholar] [CrossRef]
- Jing, T.; Heng, X.; Jingqing, T.; Haozhe, L.; Li, L.; Pingyun, L.; Xiaode, G. Construction of a strong, fast self-healing adhesive for propellants based on the synergy of weak hydrogen bond array reorganization and disulfide exchange reactions. Polymer 2023, 265, 125590–125600. [Google Scholar] [CrossRef]
- Yang, R.; Hu, J.; Li, W.; Zhang, X. A super strong polyurethane elastomer with efficient self-healing performance enabled by partially mixing reversible hard domains. Eur. Polym. J. 2024, 213, 113123–113133. [Google Scholar] [CrossRef]
- Yen, H.C.; Mao, H.I.; Chang, Y.Y.; Chan, T.H.; Lin, W.H.; Chen, C.W. Influence of Branch Side-Chains on the Foaming Properties of Thermoplastic Polyether Ester Elastomers in Supercritical CO2. J. Appl. Polym. Sci. 2025, 142, 56828–56838. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, J.; Cao, P.; Gong, J.; Tang, Z.; Zhou, K.; Luo, H.; Zhang, X.; Wang, T.; Chen, S. Strengthening-Durable Trade-Off and Self-Healing, Recyclable Shape Memory Polyurethanes Enabled by Dynamic Boron–Urethane Bonds. Macromol. Rapid Commun. 2024, 45, 2400277–2400286. [Google Scholar] [CrossRef]
- Eom, Y.; Kim, S.-M.; Lee, M.; Jeon, H.; Park, J.; Lee, E.S.; Hwang, S.Y.; Park, J.; Oh, D.X. Mechano-responsive hydrogen-bonding array of thermoplastic polyurethane elastomer captures both strength and self-healing. Nat. Commun. 2021, 12, 621–631. [Google Scholar] [CrossRef]
- Yang, C.; Chen, N.; Liu, X.; Wang, Q.; Zhang, C. Coupling selective laser sintering and supercritical CO2 foaming for 3D printed porous polyvinylidene fluoride with improved piezoelectric performance. RSC Adv. 2021, 11, 20662–20669. [Google Scholar] [CrossRef]
- Huang, J.; Jin, B.; Zhang, X.; Wang, S.; Wu, B.; Gong, P.; Park, C.B.; Li, G. High energy absorption efficiency and biodegradable polymer based microcellular materials via environmental-friendly CO2 foaming for disposable cushioning packaging. Polymer 2024, 292, 126612–126625. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Q.; Gong, J.; Shen, H.; Luo, H.; Chen, S.; Zhang, X.; Zhang, N.; Pei, X.; Wang, T. Dynamic non-covalent bonds powering enhanced temporary shape retention temperature and mechanical robustness in shape memory polyurethane. ACS Appl. Mater. Interfaces 2024, 16, 64031–64041. [Google Scholar] [CrossRef]
- Ma, Z.; Li, H.; Jing, X.; Liu, Y.; Mi, H.-Y. Recent advancements in self-healing composite elastomers for flexible strain sensors: Materials, healing systems, and features. Sens. Actuators A. 2021, 329, 112800–112817. [Google Scholar] [CrossRef]
- Ritzen, L.; Montano, V.; Garcia, S.J. 3D printing of a self-healing thermoplastic polyurethane through FDM: From polymer slab to mechanical assessment. Polymers 2021, 13, 305. [Google Scholar] [CrossRef]
- Aiswarya, S.; Awasthi, P.; Banerjee, S.S. Self-healing thermoplastic elastomeric materials: Challenges, opportunities and new approaches. Eur. Polym. J. 2022, 181, 111658–111675. [Google Scholar] [CrossRef]
- Wu, H.; Liu, X.; Sheng, D.; Zhou, Y.; Xu, S.; Xie, H.; Tian, X.; Sun, Y.; Shi, B.; Yang, Y. High performance and near body temperature induced self-healing thermoplastic polyurethane based on dynamic disulfide and hydrogen bonds. Polymer 2021, 214, 123261–123270. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Yang, Q.; Gong, P.; Park, C.B.; Li, G. Sustainable polyester vitrimer capable of fast self-healing and multiple shape-programming via efficient synthesis and configuration processing. J. Mater. Chem. A 2023, 11, 10912–10926. [Google Scholar] [CrossRef]
- Jin, S.; Jeon, H.; Kim, S.M.; Lee, M.; Park, C.; Joo, Y.; Seo, J.; Oh, D.X.; Park, J. Self-healable spray paint coatings based on polyurethanes with thermal stability: Effects of disulfides and diisocyanates. Prog. Org. Coat. 2025, 198, 108931–108941. [Google Scholar] [CrossRef]
- Zhou, L.; Xiang, S.; Wang, C.; Zhang, H.; Zhang, K.; Chen, M. Programming degradation and closed-loop recycling of ultra-stable bio-based thermosetting polyurethane relied on double-locked covalent adaptable networks. Chem. Eng. J. 2024, 496, 153901–153911. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Wu, J.; Huang, Z.; Shen, W.; Yin, X.; Sun, Y.; Lin, X.; Lin, W.; Yi, G. Tough, self-healing, and recyclable cross-linked polyurethane elastomers via synergistic effect of dual dynamic covalent bonds. J. Appl. Polym. Sci. 2024, 141, 54876–54891. [Google Scholar] [CrossRef]
- Grignard, B.; Thomassin, J.-M.; Gennen, S.; Poussard, L.; Bonnaud, L.; Raquez, J.-M.; Dubois, P.; Tran, M.-P.; Park, C.; Jerome, C. CO2-blown microcellular non-isocyanate polyurethane (NIPU) foams: From bio-and CO2-sourced monomers to potentially thermal insulating materials. Green Chem. 2016, 18, 2206–2215. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, Z.; Zhang, J.; Tang, J.; Wu, P.; Wang, Y.; Zhao, Y.; Leng, Y. Electrical and thermal and self-healing properties of graphene-thermopolyurethane flexible conductive films. Nanomaterials 2020, 10, 753. [Google Scholar] [CrossRef]
- Zheng, W.; Deng, W.; Xiong, S.; Jin, Z.; Hu, J.; Zhao, Y.; Chen, X.; Bi, S.; Yu, P. Synergistic Inherent and Dynamic Cross-Links for Self-Healable Polydimethylsiloxane Elastomer Foams. ACS Appl. Polym. Mater. 2025, 7, 3945–3953. [Google Scholar] [CrossRef]
- Patrick, J.; Sottos, N.; White, S. Microvascular based self-healing polymeric foam. Polymer. 2012, 53, 4231–4240. [Google Scholar] [CrossRef]
- Rashid, K.; Fatima, N.; Haq, E.U.; Shaheen, N.; Ju, M. Feasibility assessment of microwave-cured lightweight aggregate concrete by mineral encapsulated self-healing. J. Build. Eng. 2023, 76, 107084–107097. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Hailstone, R.; Lewis, C.L. Thermoplastic blend exhibiting shape memory-assisted self-healing functionality. ACS Appl. Mater. Interfaces 2020, 12, 46733–46742. [Google Scholar] [CrossRef]
- Feng, L.; He, X.; Zhang, Y.; Qu, D.; Chai, C. Triple roles of thermoplastic polyurethane in toughening, accelerating and enhancing self-healing performance of thermo-reversible epoxy resins. J. Polym. Environ. 2021, 29, 829–836. [Google Scholar] [CrossRef]
- Ma, H.; Gong, P.; Li, G.; Park, C.B. Highly efficient electromagnetic wave absorption nanocomposite foam fabricated via low-dimension cell wall stretching and designed via nanoparticle Monte Carlo modeling. Compos. Sci. Technol. 2023, 244, 110274–110285. [Google Scholar] [CrossRef]
- Subuki, I.; Akhbar, S.; Nor Wahid, F.K. Influence of thermoplastic zein in PCL/TZ and HAp bio composite via solid state supercritical CO2 foaming. Sci. Res. J. 2020, 17, 178–190. [Google Scholar] [CrossRef]
- Ma, H.; Xie, Z.; Liu, Y.; Zhang, Q.; Gong, P.; Meng, F.; Niu, Y.; Park, C.B.; Li, G. Improved dielectric and electromagnetic interference shielding performance of materials by hybrid filler network design in three-dimensional nanocomposite films. Mater. Des. 2023, 226, 111666–111676. [Google Scholar] [CrossRef]
- Shou, T.; Zhai, M.; Wu, Y.; Wu, S.; Hu, S.; Zhao, X.; Zhang, L. Bio-Based, Recyclable and Self-Healing Polyurethane Composites with High Energy Dissipation and Shape Memory. Macromol. Rapid. Commun. 2022, 43, 2200486–2200495. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cao, Z.; Zhao, Q.; Mei, S.; Zhang, Y.; Zhao, W.; Li, X.; Zhang, X.; Cui, Z.; Fu, P. Self-healing and shape memory reconfigurable poly (urethane-urea-amide) elastomers containing multiple dynamic bonds for improving performance of 4D printout. Chem. Eng. J. 2024, 485, 149933–149945. [Google Scholar] [CrossRef]
- Zhao, X.; Shou, T.; Liang, R.; Hu, S.; Yu, P.; Zhang, L. Bio-based thermoplastic polyurethane derived from polylactic acid with high-damping performance. Ind. Crops. Prod. 2020, 154, 112619–112626. [Google Scholar] [CrossRef]
- Hu, J.; Mo, R.; Jiang, X.; Sheng, X.; Zhang, X. Towards mechanical robust yet self-healing polyurethane elastomers via combination of dynamic main chain and dangling quadruple hydrogen bonds. Polymer 2019, 183, 121912–121922. [Google Scholar] [CrossRef]
- Griggs, T.; Ahmed, J.; Majd, H.; Edirisinghe, M.; Chen, B. A bio-based thermoplastic polyurethane with triple self-healing action for wearable technology and smart textiles. Mater. Adv. 2024, 5, 6210–6221. [Google Scholar] [CrossRef]
- Li, P.; Lan, B.; Zhang, X.; Lei, S.; Yang, Q.; Gong, P.; Park, C.B.; Li, G. Facile in situ construction of a covalent adaptable network polyester vitrimer with advanced performance in repairability, foamability and recyclability. Green Chem. 2022, 24, 5490–5501. [Google Scholar] [CrossRef]
- Okechukwu, V.O.; Mavumengwana, V.; Hümmelgen, I.A.; Mamo, M.A. Concomitant in situ FTIR and impedance measurements to address the 2-methylcyclopentanone vapor-sensing mechanism in MnO2–polymer nanocomposites. ACS Omega 2019, 4, 8324–8333. [Google Scholar] [CrossRef]
- Cheng, B.X.; Zhang, J.L.; Jiang, Y.; Wang, S.; Zhao, H. High toughness, multi-dynamic self-healing polyurethane for outstanding energy harvesting and sensing. ACS Appl. Mater. Interfaces. 2023, 15, 58806–58814. [Google Scholar] [CrossRef]
- Ying, W.B.; Yu, Z.; Kim, D.H.; Lee, K.J.; Hu, H.; Liu, Y.; Kong, Z.; Wang, K.; Shang, J.; Zhang, R. Waterproof, highly tough, and fast self-healing polyurethane for durable electronic skin. ACS Appl. Mater. Interfaces. 2020, 12, 11072–11083. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Cui, J.; Shi, Z.; Yan, F.; Han, Y.; Li, Z.; Zhu, Z. A Stretchable and Self-Healing Dual-Functional Wearable Sensor Enabled by Wet-Spun Conductive Thermoplastic Nanocomposite Fibers. Analytica. 2023, 4, 336–346. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, W.; Liu, S.; Shi, X.; Xi, A.; Zhang, F. Dynamic semi IPNs with duple dynamic linkers: Self-healing, reprocessing, welding, and shape memory behaviors. Polymers 2021, 13, 1679. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, X.; He, X.; Yao, J.; Tan, R.; Chen, P.; Yao, B.; Zhou, J.; Yao, Z. Multifunctional shape memory composites for joule heating, self-healing, and highly efficient microwave absorption. Adv. Funct. Mater. 2023, 33, 2211352–2211363. [Google Scholar] [CrossRef]
- Li, Z.; Yu, R.; Guo, B. Shape-memory and self-healing polymers based on dynamic covalent bonds and dynamic noncovalent interactions: Synthesis, mechanism, and application. ACS Appl. Bio. Mater. 2021, 4, 5926–5943. [Google Scholar] [CrossRef]
- Xin-ling, W.; Kai, Z.; Jian, C.; Xiao-peng, C.; Hai-liang, N. Preparation and Properties of Shape Memory Polyurethane Composites with Self-Healing Capability. Plast. Sci. Technol. 2024, 52, 148229–148242. [Google Scholar]
- An, H.; Ma, Q.; Zhang, F.; Zhai, C.; Sun, J.; Tang, Y.; Wang, W. Insight into microstructure evolution during starch retrogradation by infrared and Raman spectroscopy combined with two-dimensional correlation spectroscopy analysis. Food Hydrocoll. 2024, 146, 109174–109184. [Google Scholar] [CrossRef]
- Lan, G.; Zhou, Y.; Shen, H.; Tang, H.; Li, Y. Formation mechanism of highly dispersed semi-embedded ruthenium nanoparticles in porous carbon matrix determined by in situ temperature-programmed infrared spectroscopy. Chin. J. Catal. 2018, 39, 146–156. [Google Scholar] [CrossRef]
- Huang, H.; Yi, D.; Lu, Y.; Wu, X.; Bai, Y.; Meng, X.; Shi, L. Study on the adsorption behavior and mechanism of dimethyl sulfide on silver modified bentonite by in situ FTIR and temperature-programmed desorption. Chem. Eng. J. 2013, 225, 447–455. [Google Scholar] [CrossRef]
- Kim, S.M.; Jeon, H.; Shin, S.H.; Park, S.A.; Jegal, J.; Hwang, S.Y.; Oh, D.X.; Park, J. Superior toughness and fast self-healing at room temperature engineered by transparent elastomers. Adv. Mater. 2018, 30, 1705145–1705152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z.; Zhang, J.; Ding, L.; Pan, L.; Huang, C.; Zheng, X.; Zeng, C.; Lin, C. Novel polyurethane with high self-healing efficiency for functional energetic composites. Polym. Test. 2019, 76, 82–89. [Google Scholar] [CrossRef]
- Gong, P.; Wang, G.; Tran, M.-P.; Buahom, P.; Zhai, S.; Li, G.; Park, C.B. Advanced bimodal polystyrene/multi-walled carbon nanotube nanocomposite foams for thermal insulation. Carbon 2017, 120, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Wang, W.; Gong, P.; Yang, Q.; Park, C.B.; Li, G. Ultra-fast degradable PBAT/PBS foams of high performance in compression and thermal insulation made from environment-friendly supercritical foaming. J. Supercrit. Fluids 2022, 181, 105512–105520. [Google Scholar] [CrossRef]
- Ghosh, T.; Karak, N. Biobased multifunctional macroglycol containing smart thermoplastic hyperbranched polyurethane elastomer with intrinsic self-healing attribute. ACS Sustain. Chem. Eng. 2018, 6, 4370–4381. [Google Scholar] [CrossRef]
- Chen, T.; Fang, L.; Lu, C.; Xu, Z. Effects of blended reversible epoxy domains on structures and properties of self-healing/shape-memory thermoplastic polyurethane. Macromol. Mater. Eng. 2020, 305, 1900578. [Google Scholar] [CrossRef]
- Jasmee, S.; Omar, G.; Masripan, N.; Kamarolzaman, A.A.; Ashikin, A.; Ani, F.C. Hydrophobicity performance of polyethylene terephthalate (PET) and thermoplastic polyurethane (TPU) with thermal effect. Mater. Res. Express. 2018, 5, 096304. [Google Scholar] [CrossRef]
- Li, X.; Yu, R.; He, Y.; Zhang, Y.; Yang, X.; Zhao, X.; Huang, W. Self-healing polyurethane elastomers based on a disulfide bond by digital light processing 3D printing. ACS Macro. Lett. 2019, 8, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Y.; Zhao, X.; Yang, X.; Yu, R.; Zhang, Y.; Huang, W. A High Strength but Fast Fracture-Self-Healing Thermoplastic Elastomer. Macromol. Rapid Commun. 2021, 42, 2100135. [Google Scholar] [CrossRef]
- Xia, L.; Tu, H.; Zeng, W.; Yang, X.; Zhou, M.; Li, L.; Guo, X. A room-temperature self-healing elastomer with ultra-high strength and toughness fabricated via optimized hierarchical hydrogen-bonding interactions. J. Mater. Chem. A 2022, 10, 4344–4354. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, L.; Chen, A.; Wu, F. Improvement of mechanical property for PLA/TPU blend by adding PLA-TPU copolymers prepared via in situ ring-opening polymerization. Polymers 2022, 14, 1530. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Yang, B.; Wang, L.; Sun, H. A colorless, transparent and self-healing polyurethane elastomer modulated by dynamic disulfide and hydrogen bonds. New J. Chem. 2020, 44, 5746–5754. [Google Scholar] [CrossRef]
- Ma, H.; Fashandi, M.; Rejeb, Z.B.; Ming, X.; Liu, Y.; Gong, P.; Li, G.; Park, C.B. Efficient electromagnetic wave absorption and thermal infrared stealth in PVTMS@ MWCNT nano-aerogel via abundant nano-sized cavities and attenuation interfaces. Nano-Micro. Lett. 2024, 16, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Ritzoulis, C.; Chen, J. Extensional and shear rheology of a food hydrocolloid. Food Hydrocoll. 2018, 74, 296–306. [Google Scholar] [CrossRef]
- Bi, H.; Ren, Z.; Ye, G.; Sun, H.; Guo, R.; Jia, X.; Xu, M. Fabrication of cellulose nanocrystal reinforced thermoplastic polyurethane/polycaprolactone blends for three-dimension printing self-healing nanocomposites. Cellulose 2020, 27, 8011–8026. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, R.; Linghu, M.; Liu, X.; Lei, L.; Yang, Q.; Gong, P.; Li, G. Transparent Polyurethane Elastomers with Excellent Foamability and Self-Healing Property via Molecular Design and Dynamic Covalent Bond Regulation. Polymers 2025, 17, 2639. https://doi.org/10.3390/polym17192639
Zhu R, Linghu M, Liu X, Lei L, Yang Q, Gong P, Li G. Transparent Polyurethane Elastomers with Excellent Foamability and Self-Healing Property via Molecular Design and Dynamic Covalent Bond Regulation. Polymers. 2025; 17(19):2639. https://doi.org/10.3390/polym17192639
Chicago/Turabian StyleZhu, Rongli, Mingxi Linghu, Xueliang Liu, Liang Lei, Qi Yang, Pengjian Gong, and Guangxian Li. 2025. "Transparent Polyurethane Elastomers with Excellent Foamability and Self-Healing Property via Molecular Design and Dynamic Covalent Bond Regulation" Polymers 17, no. 19: 2639. https://doi.org/10.3390/polym17192639
APA StyleZhu, R., Linghu, M., Liu, X., Lei, L., Yang, Q., Gong, P., & Li, G. (2025). Transparent Polyurethane Elastomers with Excellent Foamability and Self-Healing Property via Molecular Design and Dynamic Covalent Bond Regulation. Polymers, 17(19), 2639. https://doi.org/10.3390/polym17192639




