Development and Characterization of Effective Hemostatic Composites Based on Polyvinyl Alcohol/Kaolinite/Chitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Materials and Reagents
2.2. Preparation of Composite Sponge’s Hydrogel
2.3. Study of the Physicochemical Properties
2.4. Study of the Biomedical Properties
3. Results
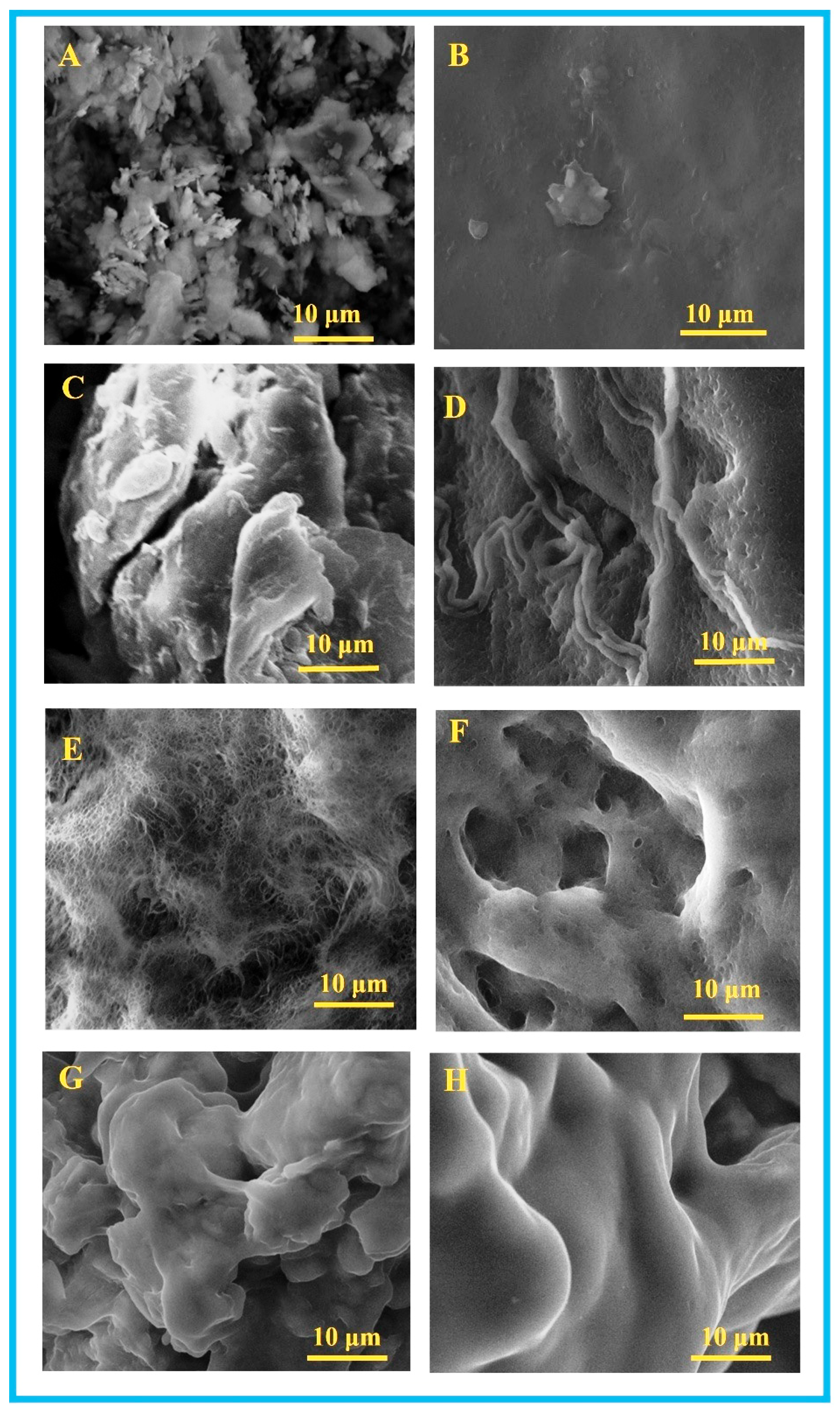
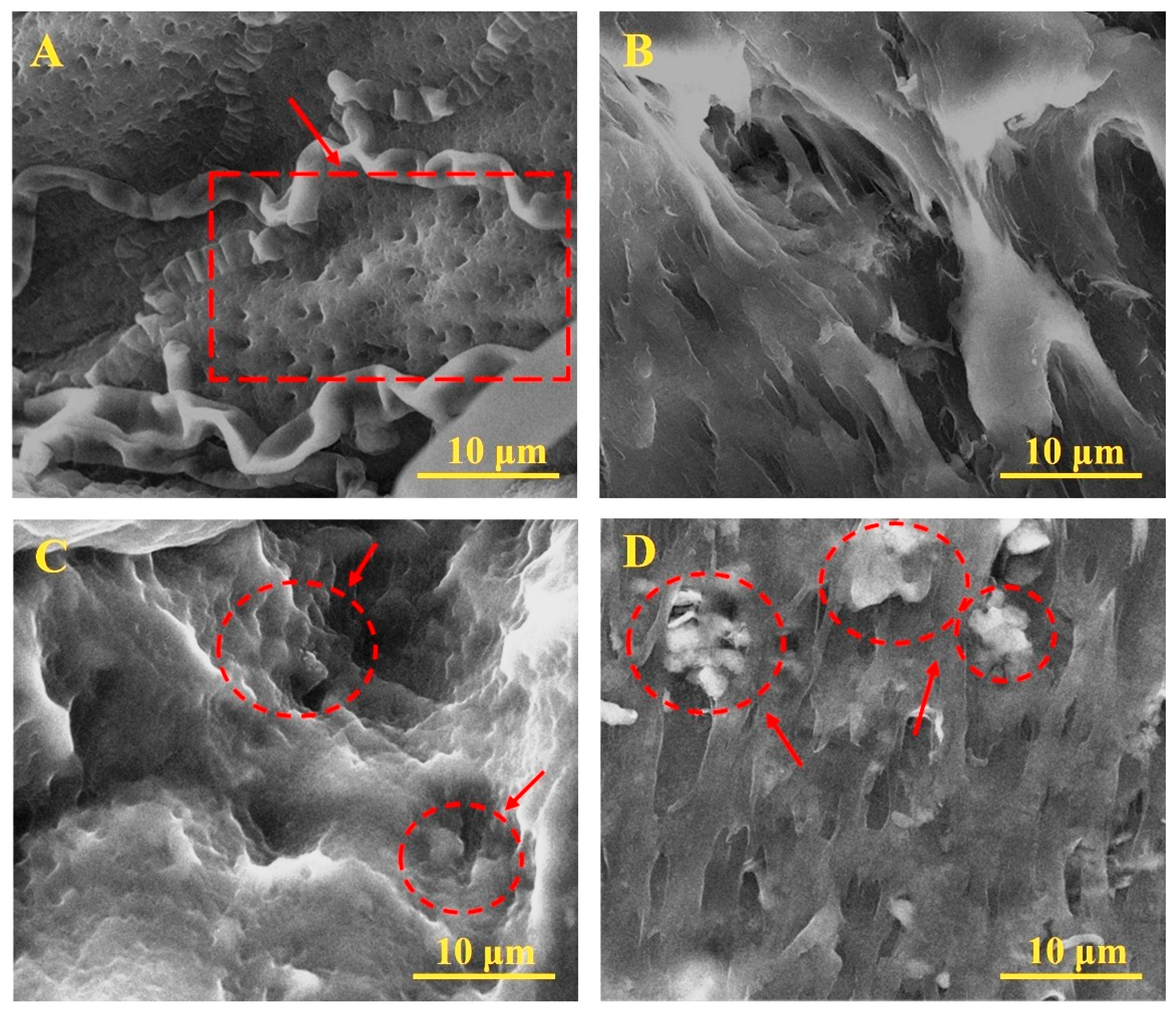
3.1. SEM Analysis of PVA/KAO/CS Composites
3.2. FTIR Analysis PVA/KAO/CS Hydrogels
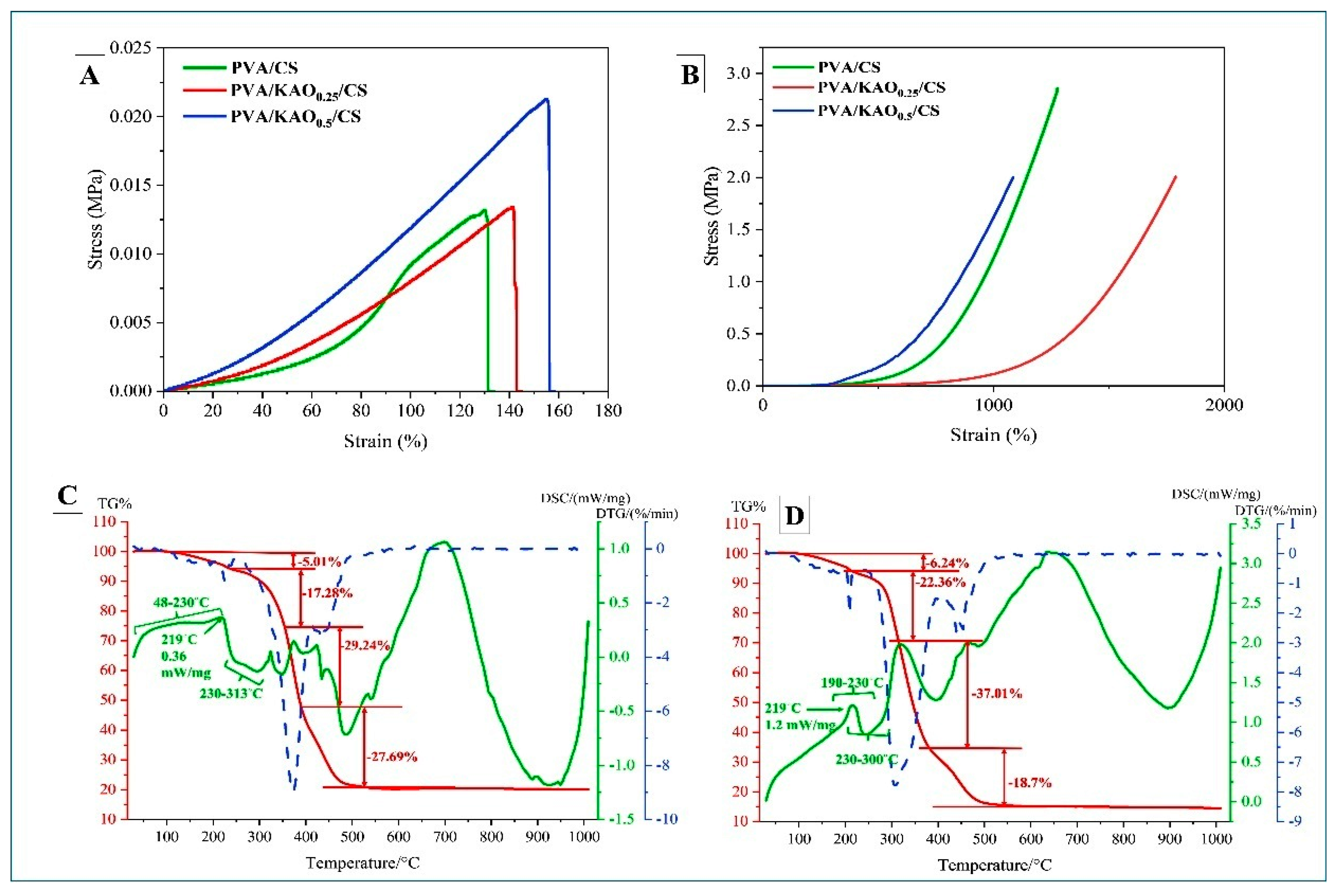
3.3. Mechanical Properties of PVA/KAO/CS Hydrogels
3.4. TGA of PVA/KAO/CS Hydrogels
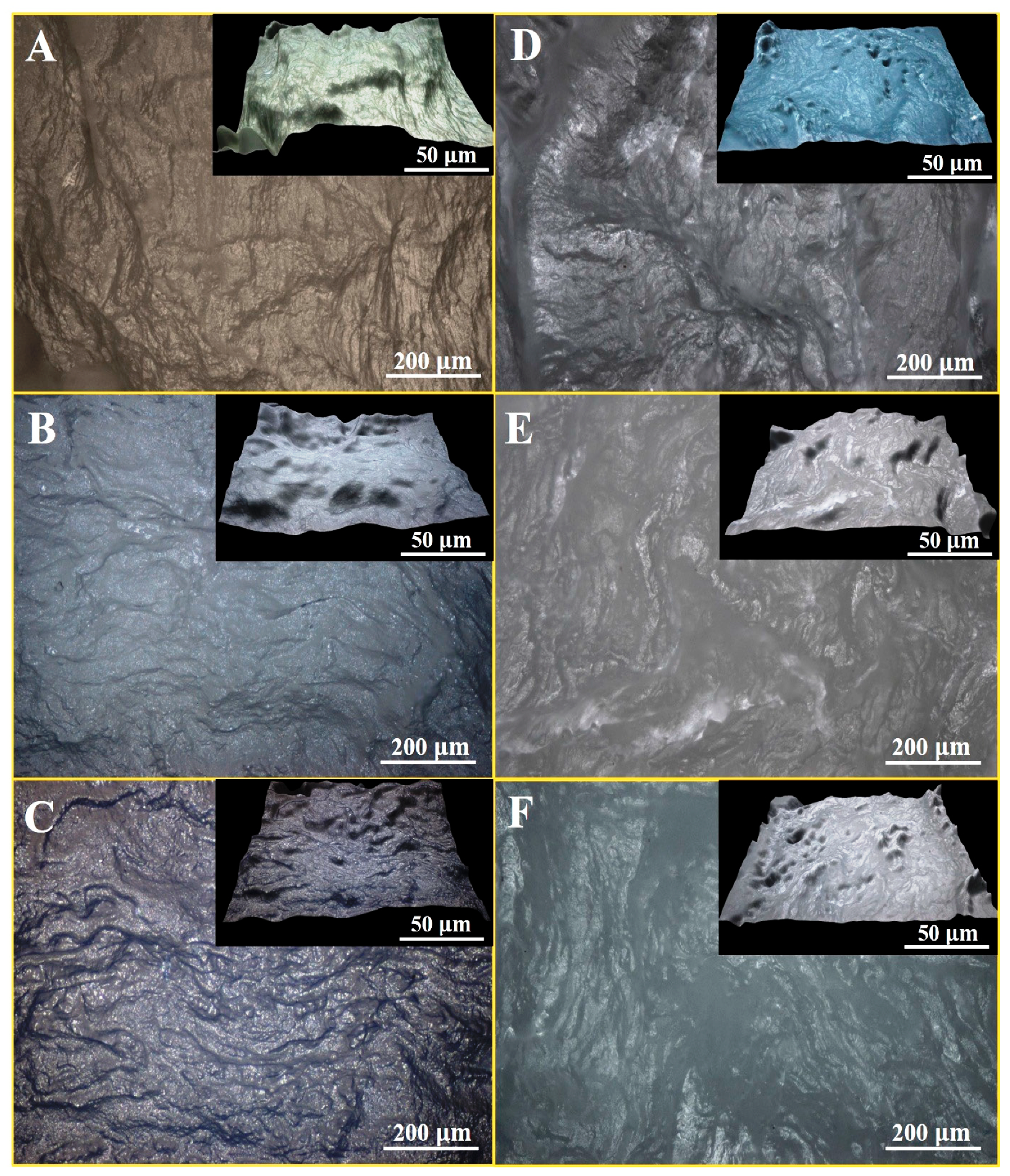
3.5. Optical Microscopy of PVA/KAO/CS Hydrogels
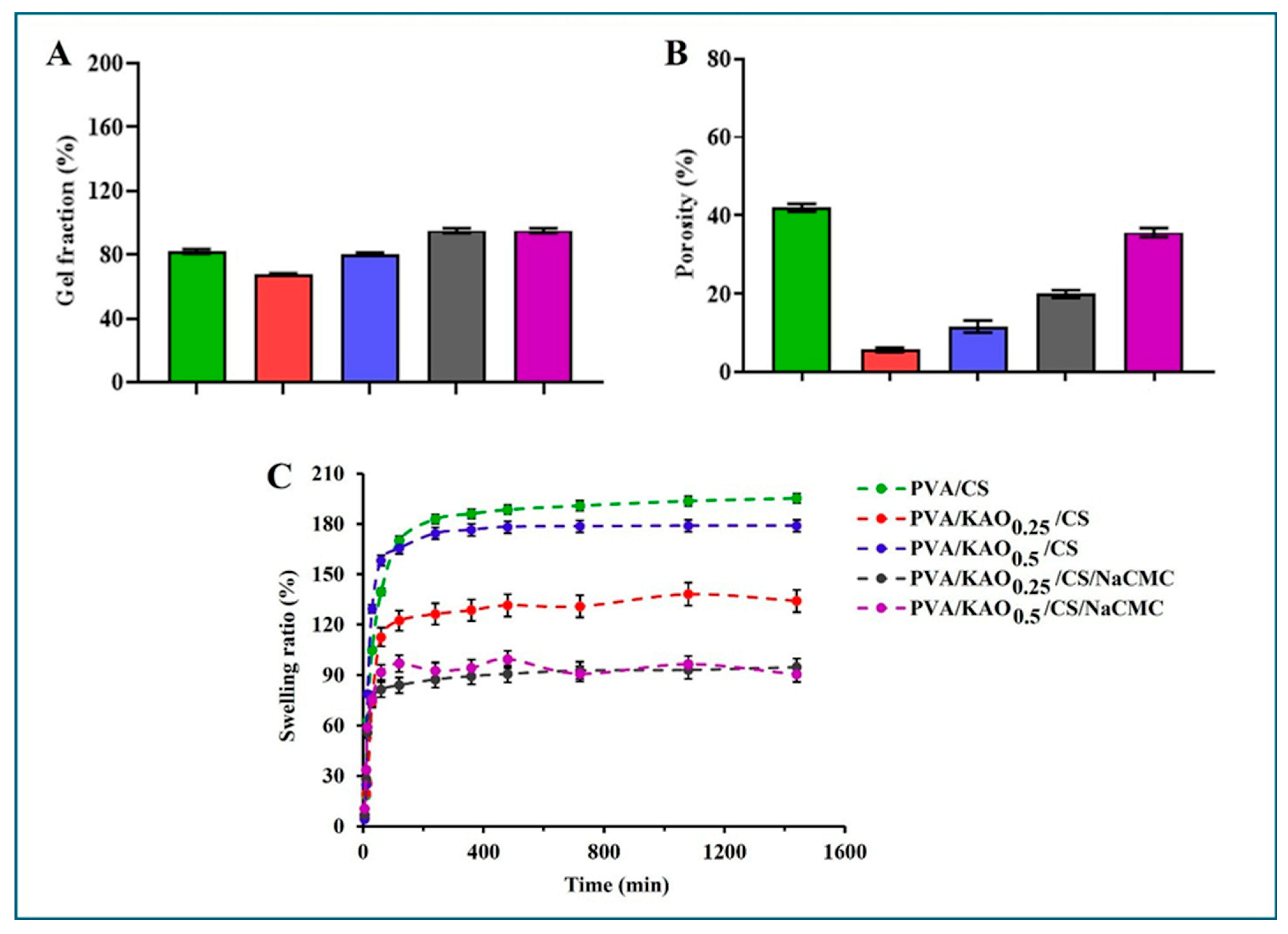
3.6. Gel Fraction, Porosity, Swelling Test of PVA/KAO/CS Hydrogels
3.7. Biomedical Evaluations of PVA/KAO/CS Hydrogels
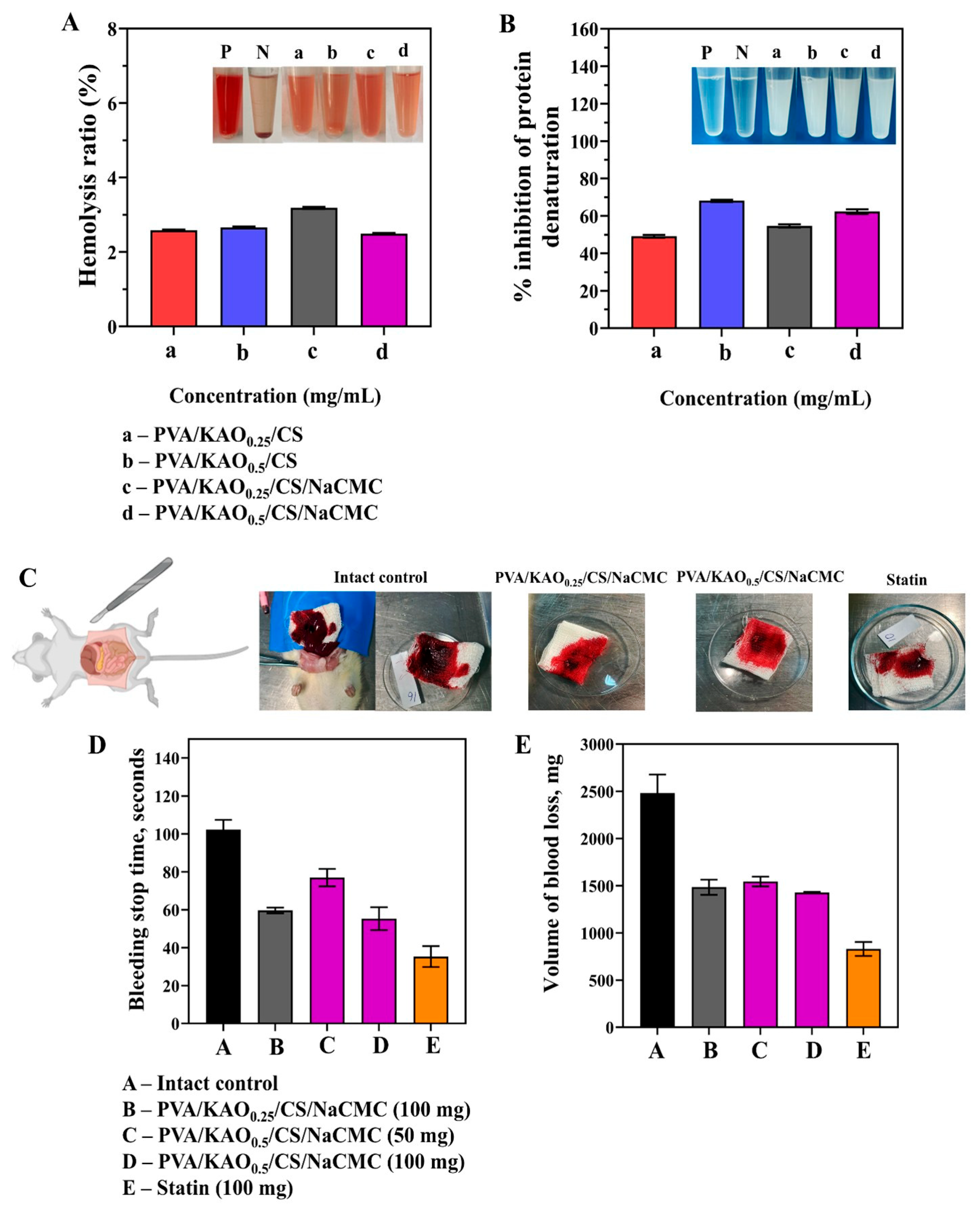
3.7.1. Hemolytic Activity, Inhibition of Protein Denaturation, and Hemostatic Activity
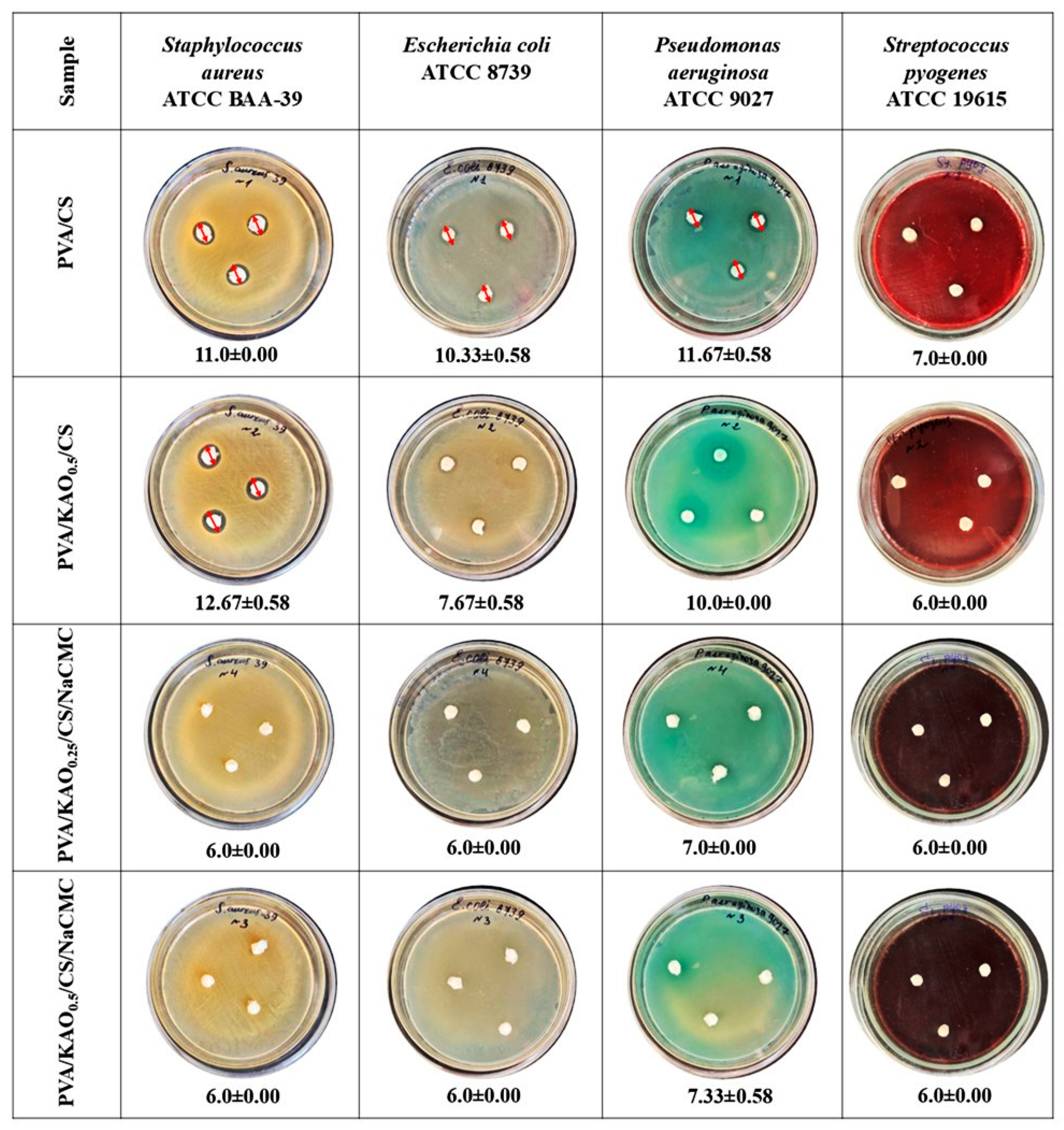
3.7.2. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVA | polyvinyl alcohol |
| KAO | kaolinite |
| CS | chitosan |
References
- Kassebaum, N.J.; Barber, R.M.; Dandona, L.; Hay, S.I.; Larson, H.J.; Lim, S.S.; Lopez, A.D.; Mokdad, A.H.; Naghavi, M.; Pinho, C.; et al. Global, Regional, and National Levels of Maternal Mortality, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1775–1812. [Google Scholar] [CrossRef] [PubMed]
- Curry, N.; Hopewell, S.; Dorée, C.; Hyde, C.; Brohi, K.; Stanworth, S. The Acute Management of Trauma Hemorrhage: A Systematic Review of Randomized Controlled Trials. Crit. Care 2011, 15, R92. [Google Scholar] [CrossRef] [PubMed]
- Faria, I.; Thivalapill, N.; Makin, J.; Puyana, J.C.; Raykar, N. Bleeding, Hemorrhagic Shock, and the Global Blood Supply. Crit. Care Clin. 2022, 38, 775–793. [Google Scholar] [CrossRef]
- Bonanno, F.G. Management of hemorrhagic shock according to the revised “physiological classification”—Update 2024. New Vis. Med. Med. Sci. 2024, 4, 136–181. [Google Scholar]
- Leaper, D.J.; Durani, P. Topical Antimicrobial Therapy of Chronic Wounds Healing by Secondary Intention Using Iodine Products. Int. Wound J. 2008, 5, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Galante, J.M.; Smith, C.A.; Sena, M.J.; Scherer, L.A.; Tharratt, R.S. Identification of Barriers to Adaptation of Battlefield Technologies into Civilian Trauma in California. Mil. Med. 2013, 178, 1227–1230. [Google Scholar] [CrossRef]
- Tackett, S.M.; Sugarman, R.; Kreuwel, H.T.C.; Alvarez, P.; Nasso, G. Hospital Economic Impact from Hemostatic Matrix Usage in Cardiac Surgery. J. Med. Econ. 2014, 17, 670–676. [Google Scholar] [CrossRef]
- Joshi, M.R.; Latham, J.; Okorogheye, G. Use of a Flowable Haemostat versus an Oxidised Regenerated Cellulose Agent in Primary Elective Cardiac Surgery: Economic Impact from a UK Healthcare Perspective. J. Cardiothorac. Surg. 2017, 12, 107. [Google Scholar] [CrossRef]
- June, A. Prehospital Combat Casualty Care The Starting Point of Battlefield Survival A Prehospital Trauma Registry for Tactical Combat Casualty Care. United States Army Med. Dep. J. 2011, 103. [Google Scholar] [CrossRef]
- Ghimire, S.; Sarkar, P.; Rigby, K.; Maan, A.; Mukherjee, S.; Crawford, K.E.; Mukhopadhyay, K. EMS Junctional Hemorrhage Control. Pharmaceutics 2023, 13, 1–27. [Google Scholar]
- Spronk, H.M.H.; Govers-Riemslag, J.W.P.; Ten Cate, H. The Blood Coagulation System as a Molecular Machine. BioEssays 2003, 25, 1220–1228. [Google Scholar] [CrossRef]
- Lind, S.; Marks, P.; Ewenstein, B. Blood: Principles and Practice of Hematology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Hoffman, M.; Monroe, D.M., 3rd. A cell-based model of hemostasis. Thromb Haemost 2001, 85, 958–96514. [Google Scholar]
- Dahlbäck, B. Blood Coagulation. Lancet 2000, 355, 1627–1632. [Google Scholar] [CrossRef]
- Ding, Y.; Qian, D.; Liang, J.; Li, Q.; Chen, J.; Jiang, Y. Clay-Based Hemostatic Agents: Fabrication, Mechanisms, and Evidence. Biomater. Adv. 2026, 178, 214473. [Google Scholar] [CrossRef]
- Yaya, A.; Tiburu, E.K.; Vickers, M.E.; Efavi, J.K.; Onwona-Agyeman, B.; Knowles, K.M. Characterisation and Identification of Local Kaolin Clay from Ghana: A Potential Material for Electroporcelain Insulator Fabrication. Appl. Clay Sci. 2017, 150, 125–130. [Google Scholar] [CrossRef]
- Flores Segura, J.C.; Reyes Cruz, V.E.; Pérez Bueno, J.d.J.; Lozada Ascencio, E.M.; Legorreta García, F. Characterization and Electrochemical Treatment of a Kaolin. Appl. Clay Sci. 2017, 146, 264–269. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; Setti, M.; El-Rahmany, M.M.; Iborra, C.V. Kaolinite in Pharmaceutics and Biomedicine. Int. J. Pharm. 2017, 533, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Zhang, Y.; Jiang, Y.; Hu, P.; Wang, H.; Zhang, Y. Nanoscale Zerovalent Iron-Incorporated Kaolinite for Hemostatic and Antibacterial Applications. Appl. Surf. Sci. 2023, 636, 157879. [Google Scholar] [CrossRef]
- Long, M.; Zhang, B.; Peng, S.; Liao, J.; Zhang, Y.; Wang, J.; Wang, M.; Qin, B.; Huang, J.; Huang, J.; et al. Interactions between Two-Dimensional Nanoclay and Blood Cells in Hemostasis. Mater. Sci. Eng. C 2019, 105, 110081. [Google Scholar] [CrossRef]
- Malette, W.G.; Quigley, H.J.; Gaines, R.D.; Johnson, N.D.; Rainer, W.G. Chitosan: A New Hemostatic. Ann. Thorac. Surg. 1983, 36, 55–58. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Zhou, S.; Zhang, D.; Zeng, L.; Zhou, C.; Liu, M. 3D Printing of Chitosan Hydrogel Reinforced with Tubular Nanoclay for Hemostasis and Infected Wound Healing. Bioact. Mater. 2025, 54, 404–422. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef]
- Chou, T.C.; Fu, E.; Wu, C.J.; Yeh, J.H. Chitosan Enhances Platelet Adhesion and Aggregation. Biochem. Biophys. Res. Commun. 2003, 302, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhang, L. Cellulose-Based Hydrogels: Present Status and Application Prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Halib, N.; Perrone, F.; Cemazar, M.; Dapas, B.; Farra, R.; Abrami, M.; Chiarappa, G.; Forte, G.; Zanconati, F.; Pozzato, G.; et al. Potential Applications of Nanocellulose-Containing Materials in the Biomedical Field. Materials 2017, 10, 977. [Google Scholar] [CrossRef]
- Saruchi; Kumar, V. Effective Degradation of Rhodamine B and Congo Red Dyes over Biosynthesized Silver Nanoparticles-Imbibed Carboxymethyl Cellulose Hydrogel. Polym. Bull. 2020, 77, 3349–3365. [Google Scholar] [CrossRef]
- Pettignano, A.; Charlot, A.; Fleury, E. Carboxyl-Functionalized Derivatives of Carboxymethyl Cellulose: Towards Advanced Biomedical Applications. Polym. Rev. 2019, 59, 510–560. [Google Scholar] [CrossRef]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key Advances of Carboxymethyl Cellulose in Tissue Engineering & 3D Bioprinting Applications. Carbohydr. Polym. 2021, 256, 117561. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Khalili, F.; Khosropour, N.; Aliabadi, H.A.M.; Radinekiyan, F.; Sukhtezari, S.; Maleki, A.; Madanchi, H.; Hamblin, M.R.; Mahdavi, M.; et al. Hybrid Bionanocomposite Containing Magnesium Hydroxide Nanoparticles Embedded in a Carboxymethyl Cellulose Hydrogel plus Silk Fibroin as a Scaffold for Wound Dressing Applications. ACS Appl. Mater. Interfaces 2021, 13, 33840–33849. [Google Scholar] [CrossRef]
- Diaz-Gomez, L.; Gonzalez-Prada, I.; Millan, R.; Da Silva-Candal, A.; Bugallo-Casal, A.; Campos, F.; Concheiro, A.; Alvarez-Lorenzo, C. 3D Printed Carboxymethyl Cellulose Scaffolds for Autologous Growth Factors Delivery in Wound Healing. Carbohydr. Polym. 2022, 278, 118924. [Google Scholar] [CrossRef]
- Ohta, S.; Mitsuhashi, K.; Chandel, A.K.S.; Qi, P.; Nakamura, N.; Nakamichi, A.; Yoshida, H.; Yamaguchi, G.; Hara, Y.; Sasaki, R.; et al. Silver-Loaded Carboxymethyl Cellulose Nonwoven Sheet with Controlled Counterions for Infected Wound Healing. Carbohydr. Polym. 2022, 286, 119289. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and Morphology of Freeze/Thawed PVA Hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Feng, W. PVA Hydrogel Properties for Biomedical Application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Nugent, M.J.D.; Higginbotham, C.L. Preparation of a Novel Freeze Thawed Poly(Vinyl Alcohol) Composite Hydrogel for Drug Delivery Applications. Eur. J. Pharm. Biopharm. 2007, 67, 377–386. [Google Scholar] [CrossRef]
- Stauffer, S.R.; Peppast, N.A. Poly(Vinyl Alcohol) Hydrogels Prepared by Freezing-Thawing Cyclic Processing. Polymer 1992, 33, 3932–3936. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.D.; Vélaz, I.; Peñas, F.J.; Zavala-Rivera, P.; Rosas-Durazo, A.J.; Maldonado-Arce, A.D.; Martínez-Barbosa, M.E. Effect of Freeze-Thawing Conditions for Preparation of Chitosan-Poly (Vinyl Alcohol) Hydrogels and Drug Release Studies. Carbohydr. Polym. 2018, 195, 476–485. [Google Scholar] [CrossRef]
- Gupta, S.; Goswami, S.; Sinha, A. A Combined Effect of Freeze-Thaw Cycles and Polymer Concentration on the Structure and Mechanical Properties of Transparent PVA Gels. Biomed. Mater. 2012, 7, 015006. [Google Scholar] [CrossRef]
- Butylina, S.; Geng, S.; Oksman, K. Properties of As-Prepared and Freeze-Dried Hydrogels Made from Poly(Vinyl Alcohol) and Cellulose Nanocrystals Using Freeze-Thaw Technique. Eur. Polym. J. 2016, 81, 386–396. [Google Scholar] [CrossRef]
- Mori, Y.; Tokura, H.; Yoshikawa, M. Properties of Hydrogels Synthesized by Freezing and Thawing Aqueous Polyvinyl Alcohol Solutions and Their Applications. J. Mater. Sci. 1997, 32, 491–496. [Google Scholar] [CrossRef]
- Elsabahy, M.; Hamad, M.A. Design and Preclinical Evaluation of Chitosan/Kaolin Nanocomposites with Enhanced Hemostatic Efficiency. Mar. Drugs 2021, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Sabet, M.M.; Omer, A.M.; Abbas, E.; Eid, A.I.; Mohy-Eldin, M.S.; Hassan, M.A. Hemostatic and Antibacterial PVA/Kaolin Composite Sponges Loaded with Penicillin–Streptomycin for Wound Dressing Applications. Sci. Rep. 2021, 11, 3428. [Google Scholar] [CrossRef]
- Al-Mofty, S.E.D.; Karaly, A.H.; Sarhan, W.A.; Azzazy, H.M.E. Multifunctional Hemostatic PVA/Chitosan Sponges Loaded with Hydroxyapatite and Ciprofloxacin. ACS Omega 2022, 7, 13210–13220. [Google Scholar] [CrossRef]
- Djumaev, A.; Tashmukhamedova, S. Physical and Chemical Properties of Polyvinyl Alcohol-Chemistry Manufacturing and Controls-Based Hydrogel Carrier Loaded with Herbal Hemostatic Agent for Application as Wound Dressings. Natl. J. Physiol. Pharm. Pharmacol. 2020, 10, 1. [Google Scholar] [CrossRef]
- Tamer, T.M.; Sabet, M.M.; Alhalili, Z.A.H.; Ismail, A.M.; Mohy-Eldin, M.S.; Hassan, M.A. Influence of Cedar Essential Oil on Physical and Biological Properties of Hemostatic, Antibacterial, and Antioxidant Polyvinyl Alcohol/Cedar Oil/Kaolin Composite Hydrogels. Pharmaceutics 2022, 14, 2649. [Google Scholar] [CrossRef]
- Tamer, T.M.; Alsehli, M.H.; Omer, A.M.; Afifi, T.H.; Sabet, M.M.; Mohy-Eldin, M.S.; Hassan, M.A. Development of Polyvinyl Alcohol/Kaolin Sponges Stimulated by Marjoram as Hemostatic, Antibacterial, and Antioxidant Dressings for Wound Healing Promotion. Int. J. Mol. Sci. 2021, 22, 13050. [Google Scholar] [CrossRef] [PubMed]
- Bekissanova, Z.; Railean, V.; Brzozowska, W.; Wojtczak, I.; Ospanova, A.; Buszewski, B.; Sprynskyy, M. Synthesis, Characterization of Silver/Kaolinite Nanocomposite and Studying Its Antibacterial Activity. Colloids Surfaces B Biointerfaces 2022, 220, 112908. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Nagahara, H.; Morooka, K.; Nakashima, Y.; Uranishi, Y.; Miyauchi, S.; Kurazume, R. 3D Image Reconstruction from Multi-Focus Microscopic Images. In Image and Video Technology; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2020; Volume 11994, pp. 73–85. [Google Scholar] [CrossRef]
- Sensing, R.; Sciences, S.I.; Imagery, M. Automatic 3D Reconstruction and Visualization of Microscopic Objects from a Monoscopic Multifocus Image Sequence Markus Niederöst. Archives 2003, XXXIV, 3–10. [Google Scholar]
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutierrez, M.C.; Rangel-Vargas, E.; Castro-Rosas, J.; Aguirre-Loredo, R.Y. Effect of Polyvinyl Alcohol on the Physicochemical Properties of Biodegradable Starch Films. Mater. Chem. Phys. 2020, 239, 122027. [Google Scholar] [CrossRef]
- Qi, X.; Hu, X.; Wei, W.; Yu, H.; Li, J.; Zhang, J.; Dong, W. Investigation of Salecan/Poly(Vinyl Alcohol) Hydrogels Prepared by Freeze/Thaw Method; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 118, ISBN 1439905584. [Google Scholar]
- Alimbek, A.; Bekissanova, Z.; Otegenova, B.; Jumagaziyeva, A.; Zhaksybay, B.B.; Zhumadilova, Y.; Ospanova, A. Synthesis and Antibacterial Evaluation of Chlorhexidine- and Triclosan-Impregnated Kaolinite Nanocomposites. Materials 2025, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.S.; Singh, B.K. Instrumental Characterization of Clay by FTIR, XRF, BET and, TPD-NH3. Bull. Mater. Sci. 2007, 30, 235–238. [Google Scholar] [CrossRef]
- Sahnoun, R.D.; Bouaziz, J. Sintering Characteristics of Kaolin in the Presence of Phosphoric Acid Binder. Ceram. Int. 2012, 38, 1–7. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Goswami, S.; Borah, P.; Saikia, M.; Barman, J.; Pramanik, N.B.; Haloi, D.J. Preparation, Characterization, and Antimicrobial Activity of Chitosan/Kaolin Clay Biocomposite Films. Macromol. Chem. Phys. 2023, 224, 2300008. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, C.; Wang, J.; Wang, M.; Wu, Y.; Ruan, Y. Determination of Degree of Substitution for N-Acylated Chitosan Using IR Spectra. Sci. China Ser. B Chem. 2001, 44, 216–224. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, Y.; Szeto, Y.-s.; Liao, L. A Novel Method to Prepare Chitosan/Montmorillonite Nanocomposites in the Presence of Hydroxy-Aluminum Oligomeric Cations. Compos. Sci. Technol. 2008, 68, 2917–2921. [Google Scholar] [CrossRef]
- Mohanasrinivasan, V.; Mishra, M.; Paliwal, J.S.; Singh, S.K.; Selvarajan, E.; Suganthi, V.; Subathra Devi, C. Studies on Heavy Metal Removal Efficiency and Antibacterial Activity of Chitosan Prepared from Shrimp Shell Waste. 3 Biotech 2014, 4, 167–175. [Google Scholar] [CrossRef]
- Badry, R.; Ezzat, H.A.; El-Khodary, S.; Morsy, M.; Elhaes, H.; Nada, N.; Ibrahim, M. Spectroscopic and Thermal Analyses for the Effect of Acetic Acid on the Plasticized Sodium Carboxymethyl Cellulose. J. Mol. Struct. 2021, 1224, 129013. [Google Scholar] [CrossRef]
- Singh, R.K.; Khatri, O.P. A Scanning Electron Microscope Based New Method for Determining Degree of Substitution of Sodium Carboxymethyl Cellulose. J. Microsc. 2012, 246, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Serhan, M.; Sprowls, M.; Jackemeyer, D.; Long, M.; Perez, I.D.; Maret, W.; Tao, N.; Forzani, E. Total Iron Measurement in Human Serum with a Smartphone. In Proceedings of the AIChE Annual Meeting, Orlando, FL, USA, 10–15 November 2019. [Google Scholar] [CrossRef]
- Zhao, S.; Qiu, S.; Zheng, Y.; Cheng, L.; Guo, Y. Synthesis and Characterization of Kaolin with Polystyrene via In-Situ Polymerization and Their Application on Polypropylene. Mater. Des. 2011, 32, 957–963. [Google Scholar] [CrossRef]
- Yahaya, L.E.; Adebowale, K.O.; Menon, A.R.R. Mechanical Properties of Organomodified Kaolin/Natural Rubber Vulcanizates. Appl. Clay Sci. 2009, 46, 283–288. [Google Scholar] [CrossRef]
- Lundin, J.G.; McGann, C.L.; Daniels, G.C.; Streifel, B.C.; Wynne, J.H. Hemostatic Kaolin-Polyurethane Foam Composites for Multifunctional Wound Dressing Applications. Mater. Sci. Eng. C 2017, 79, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A.P. Characterization of Poly(Vinyl Alcohol)/Poly(Ethylene Glycol) Hydrogels and PVA-Derived Hybrids by Small-Angle X-Ray Scattering and FTIR Spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Gupta, A.; Dhakate, S.R. Development of Structurally Stable Electrospun Carbon Nanofibers from Polyvinyl Alcohol. Mater. Res. Express 2017, 4, 045021. [Google Scholar] [CrossRef]
- Adeli-Sardou, M.; Yaghoobi, M.M.; Torkzadeh-Mahani, M.; Dodel, M. Controlled Release of Lawsone from Polycaprolactone/Gelatin Electrospun Nano Fibers for Skin Tissue Regeneration; Elsevier B.V: Amsterdam, The Netherlands, 2019; Volume 124, ISBN 9834316232. [Google Scholar]
- Fang, H.; Wang, J.; Li, L.; Xu, L.; Wu, Y.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. A Novel High-Strength Poly(Ionic Liquid)/PVA Hydrogel Dressing for Antibacterial Applications. Chem. Eng. J. 2019, 365, 153–164. [Google Scholar] [CrossRef]
- Zhou, M.; Lin, F.; Li, W.; Shi, L.; Li, Y.; Shan, G. Development of Nanosilver Doped Carboxymethyl Chitosan-Polyamideamine Alginate Composite Dressing for Wound Treatment. Int. J. Biol. Macromol. 2021, 166, 1335–1351. [Google Scholar] [CrossRef]
- He, J.; Liang, Y.; Shi, M.; Guo, B. Anti-Oxidant Electroactive and Antibacterial Nanofibrous Wound Dressings Based on Poly(ε-Caprolactone)/Quaternized Chitosan-Graft-Polyaniline for Full-Thickness Skin Wound Healing. Chem. Eng. J. 2020, 385, 123464. [Google Scholar] [CrossRef]
- Nelson Labs. Study Number 1237696-S01 ASTM Hemolysis (Direct Contact and Extract Methods) GLP Report; Nelson Labs: Salt Lake City, UT, USA, 2019; pp. 1–6. [Google Scholar]
- Sangeetha, G.; Vidhya, R. In Vitro Anti-Inflammatory Activity of Different Parts of Pedalium Murex (L.). Int. J. Herb. Med. 2016, 4, 31–36. [Google Scholar]
- Chacon, S.S.; García-Jaramillo, M.; Liu, S.Y.; Ahmed, M.; Kleber, M. Differential Capacity of Kaolinite and Birnessite to Protect Surface Associated Proteins against Thermal Degradation. Soil Biol. Biochem. 2018, 119, 101–109. [Google Scholar] [CrossRef]
- Sun, X.; Tang, Z.; Pan, M.; Wang, Z.; Yang, H.; Liu, H. Chitosan/Kaolin Composite Porous Microspheres with High Hemostatic Efficacy. Carbohydr. Polym. 2017, 177, 135–143. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Myung, S.; Jung, T.G.; Han, D.W.; Kim, B.; Lee, J.C. Extraction of γ-Chitosan from Insects and Fabrication of PVA/γ-Chitosan/Kaolin Nanofiber Wound Dressings with Hemostatic Properties. Discov. Nano 2024, 19, 77. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alimbek, A.; Otegenova, B.; Bekissanova, Z.; Savdenbekova, B.; Ibragimova, N.; Nemkayeva, R.; Sprynskyy, M.; Ospanova, A. Development and Characterization of Effective Hemostatic Composites Based on Polyvinyl Alcohol/Kaolinite/Chitosan. Polymers 2025, 17, 2637. https://doi.org/10.3390/polym17192637
Alimbek A, Otegenova B, Bekissanova Z, Savdenbekova B, Ibragimova N, Nemkayeva R, Sprynskyy M, Ospanova A. Development and Characterization of Effective Hemostatic Composites Based on Polyvinyl Alcohol/Kaolinite/Chitosan. Polymers. 2025; 17(19):2637. https://doi.org/10.3390/polym17192637
Chicago/Turabian StyleAlimbek, Aruzhan, Bayansulu Otegenova, Zhanar Bekissanova, Balzhan Savdenbekova, Nailya Ibragimova, Renata Nemkayeva, Myroslav Sprynskyy, and Alyiya Ospanova. 2025. "Development and Characterization of Effective Hemostatic Composites Based on Polyvinyl Alcohol/Kaolinite/Chitosan" Polymers 17, no. 19: 2637. https://doi.org/10.3390/polym17192637
APA StyleAlimbek, A., Otegenova, B., Bekissanova, Z., Savdenbekova, B., Ibragimova, N., Nemkayeva, R., Sprynskyy, M., & Ospanova, A. (2025). Development and Characterization of Effective Hemostatic Composites Based on Polyvinyl Alcohol/Kaolinite/Chitosan. Polymers, 17(19), 2637. https://doi.org/10.3390/polym17192637







