Plasticizer-Enabled Solvent-Free Curing of Self-Healing Binder System for Energetic Materials
Abstract
1. Introduction
2. Experimental
2.1. Materials
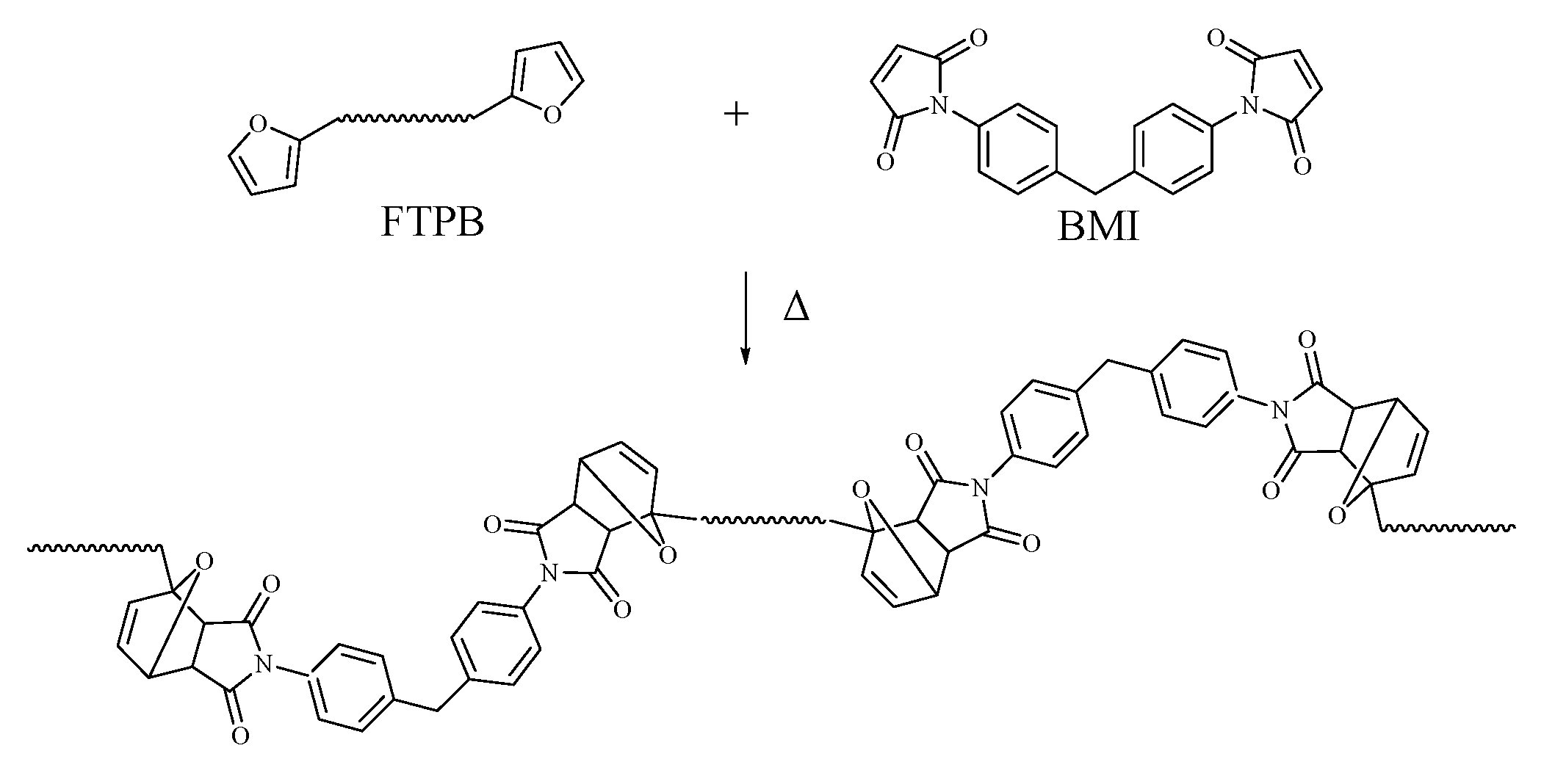
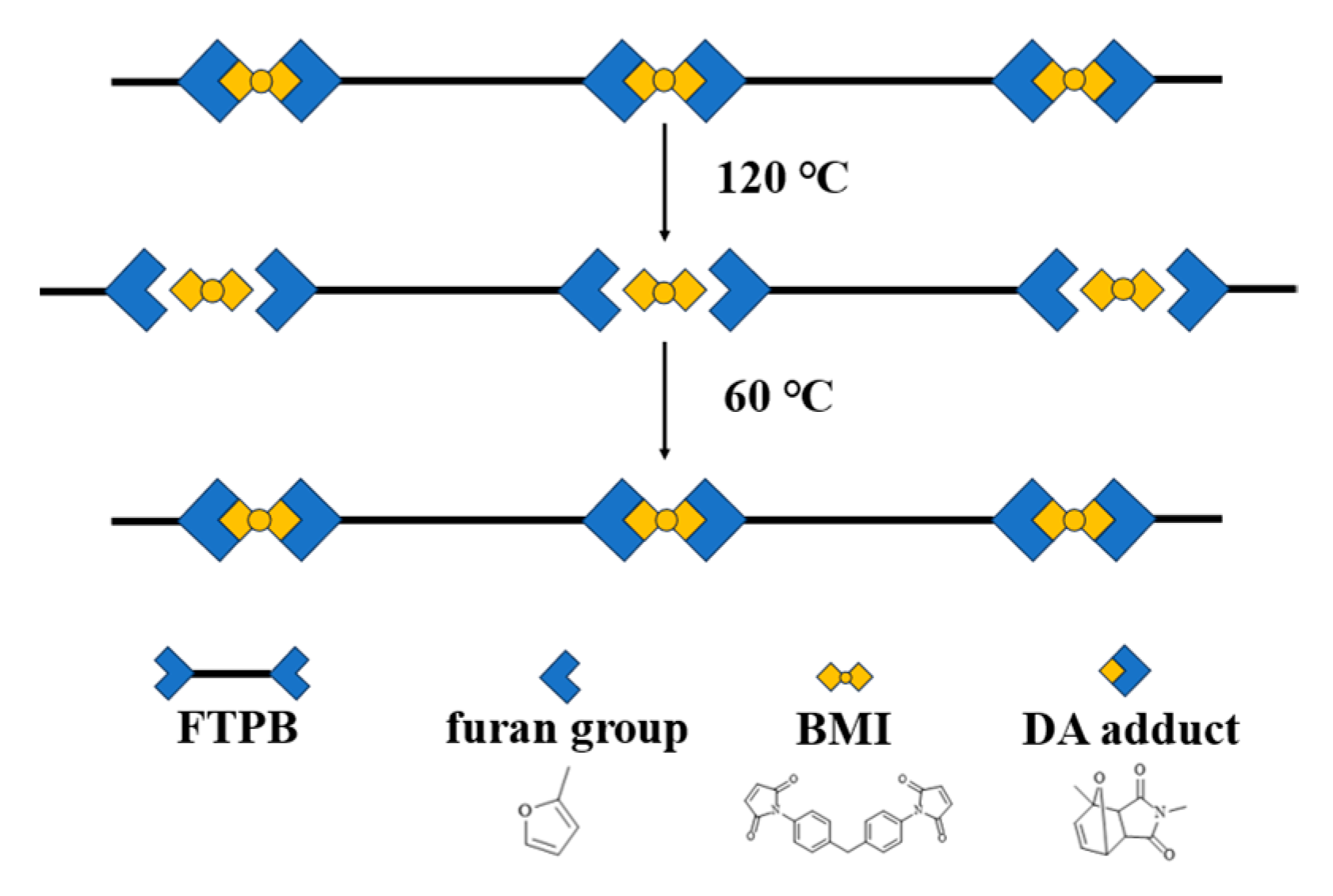
2.2. Synthesis of FTPB Binder
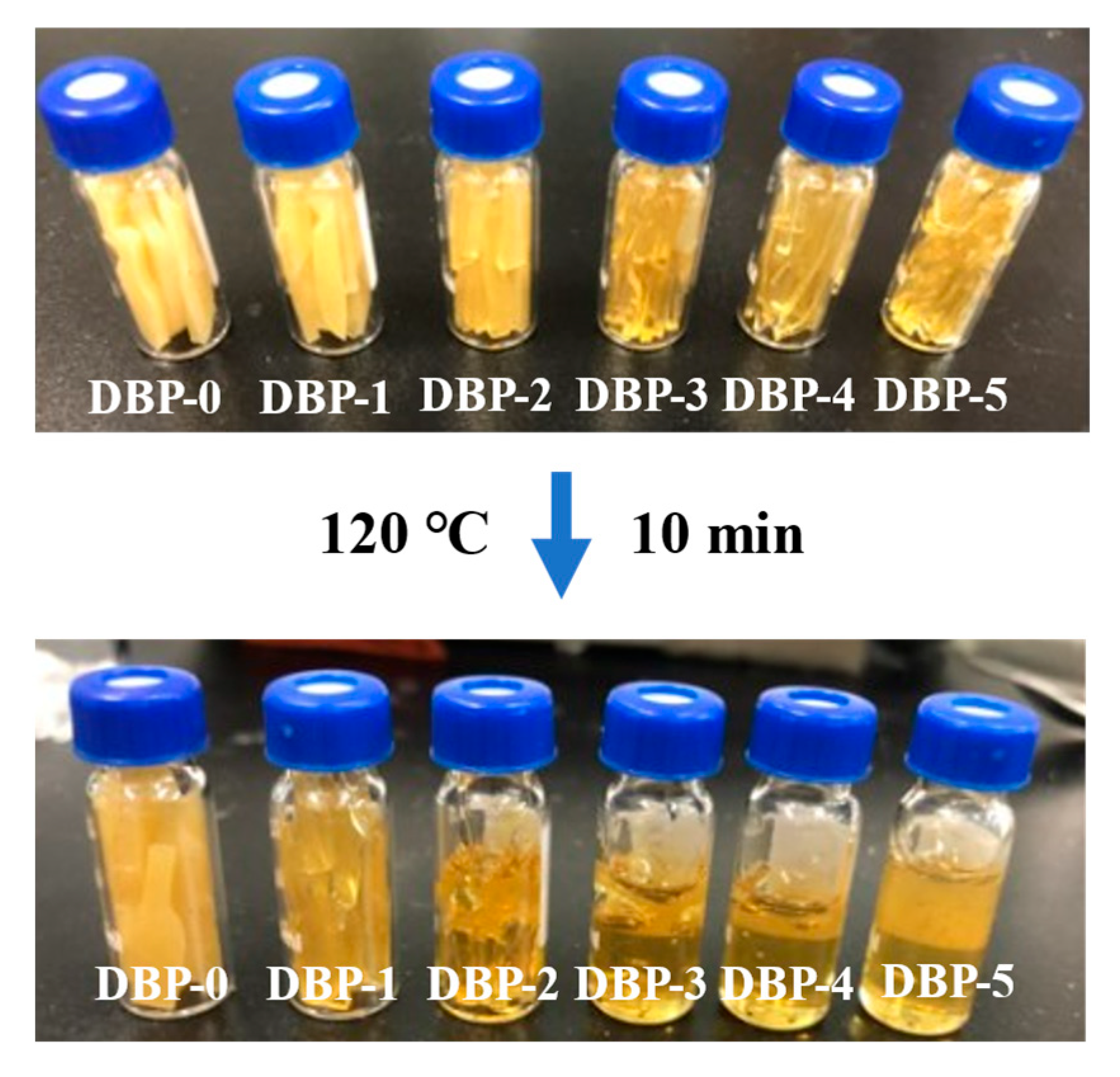
2.3. Solvent-Free Curing of FTPB
2.4. Preparation of Self-Healing Solid Propellant (SHSP)
2.5. Characterization Methods
3. Results and Discussion
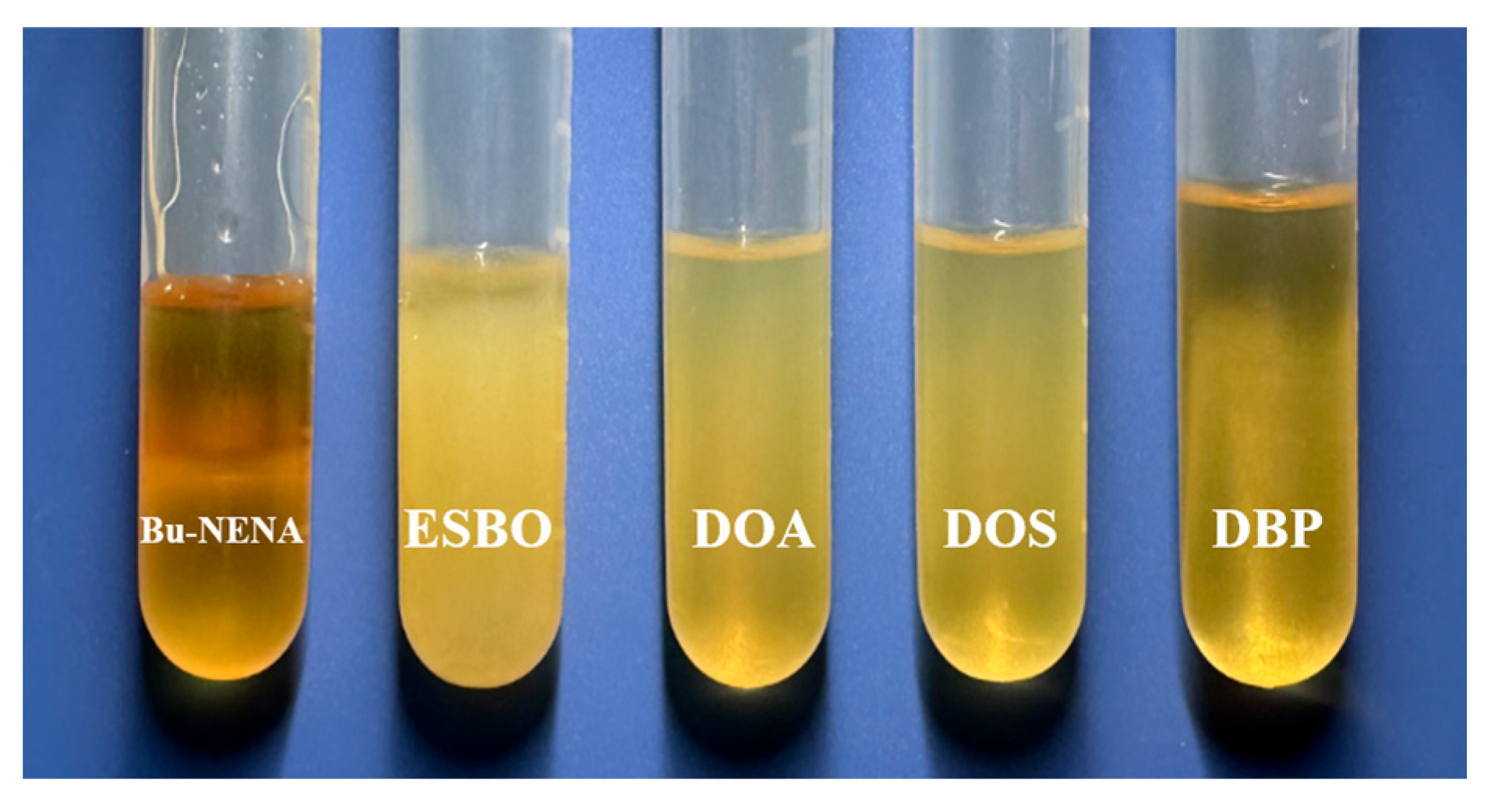
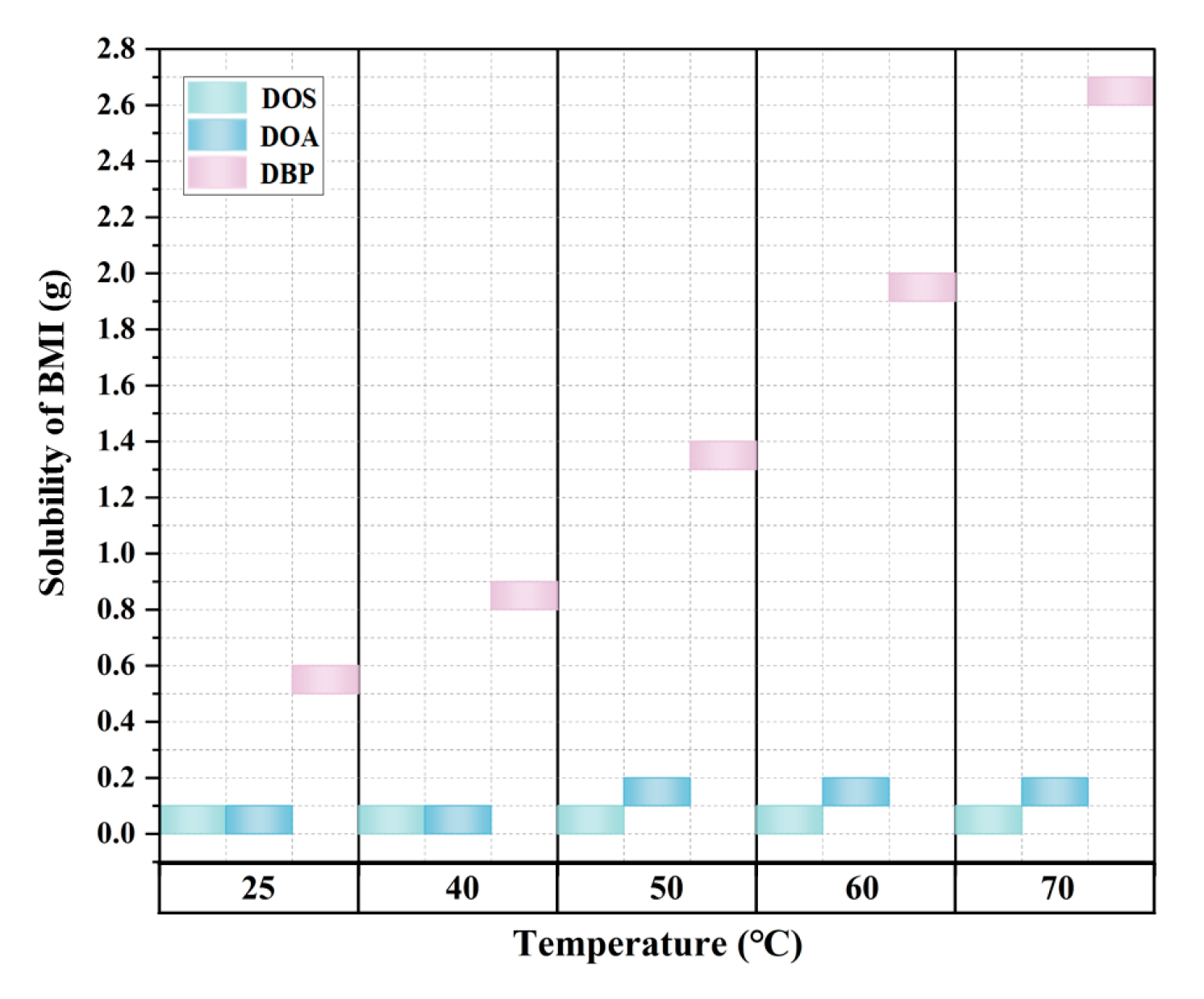
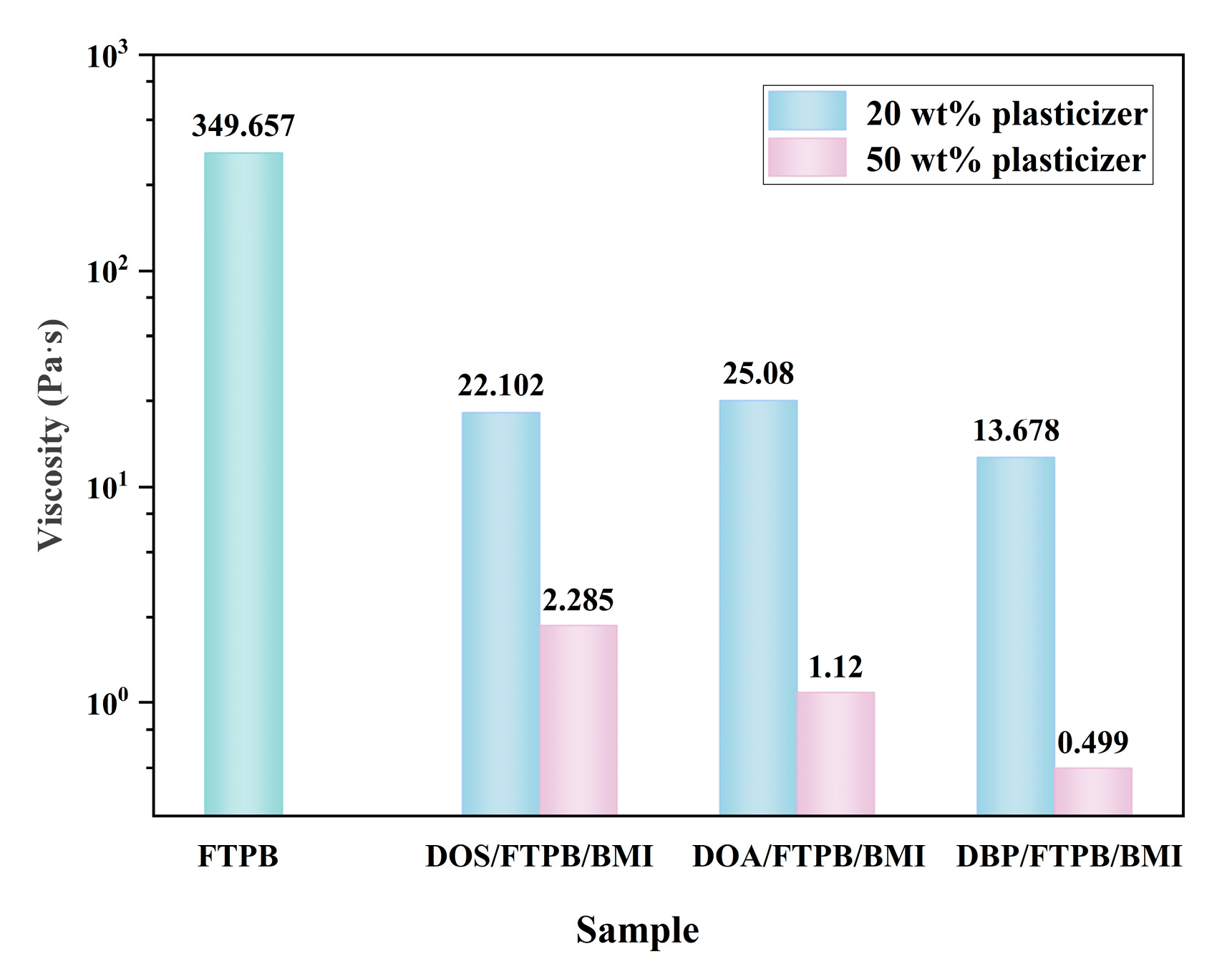
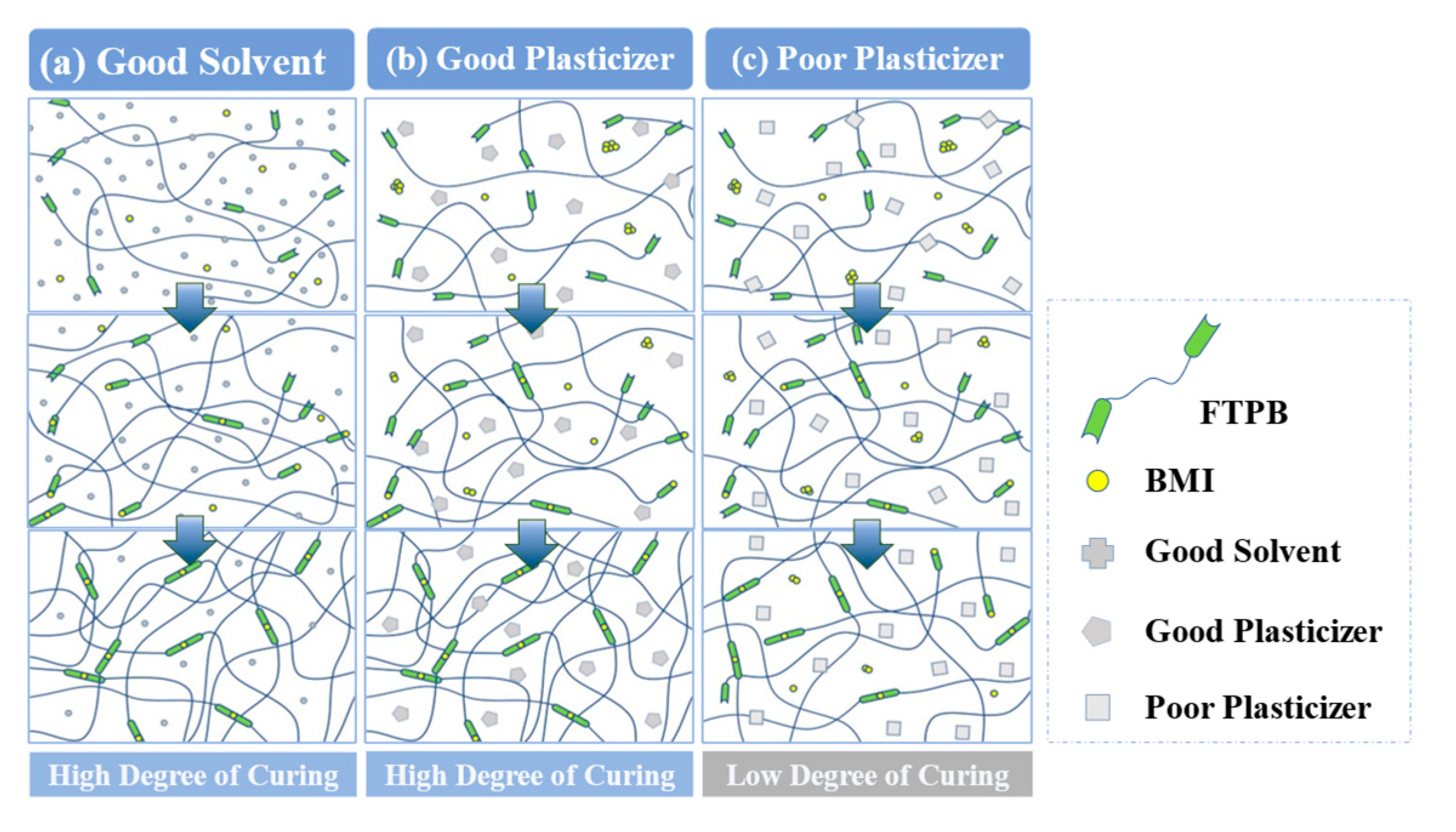
3.1. Selection of Plasticizer
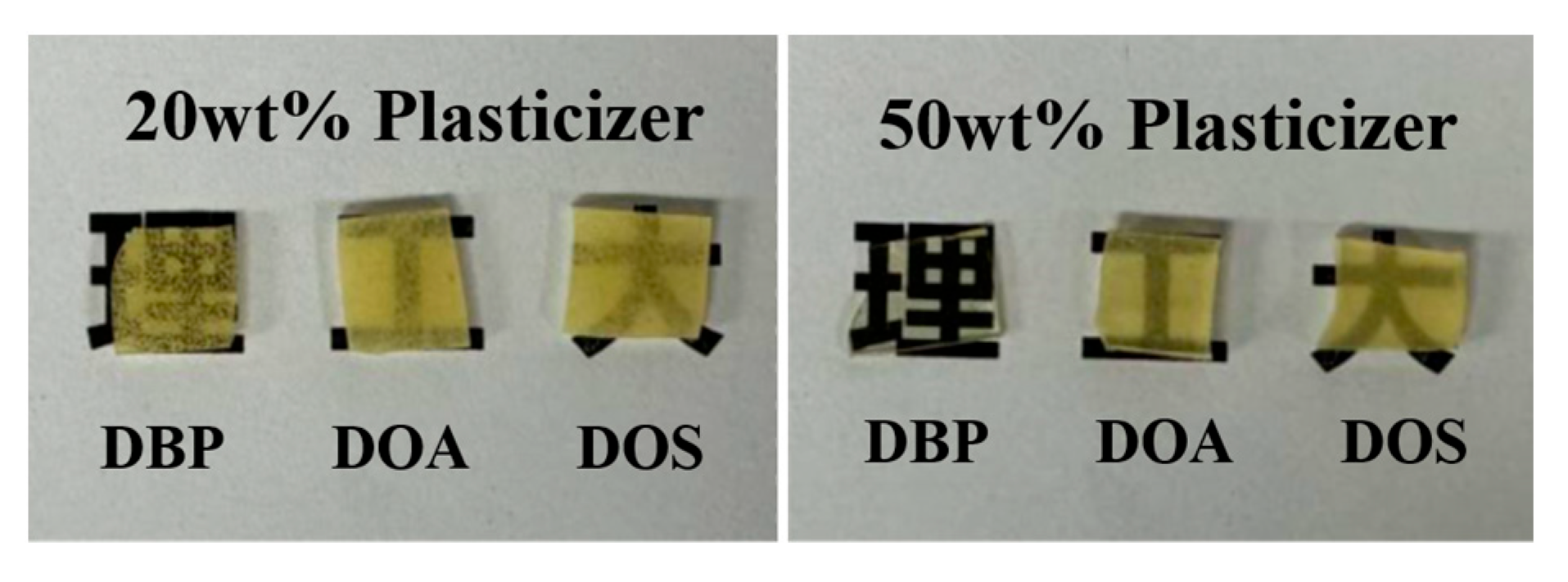
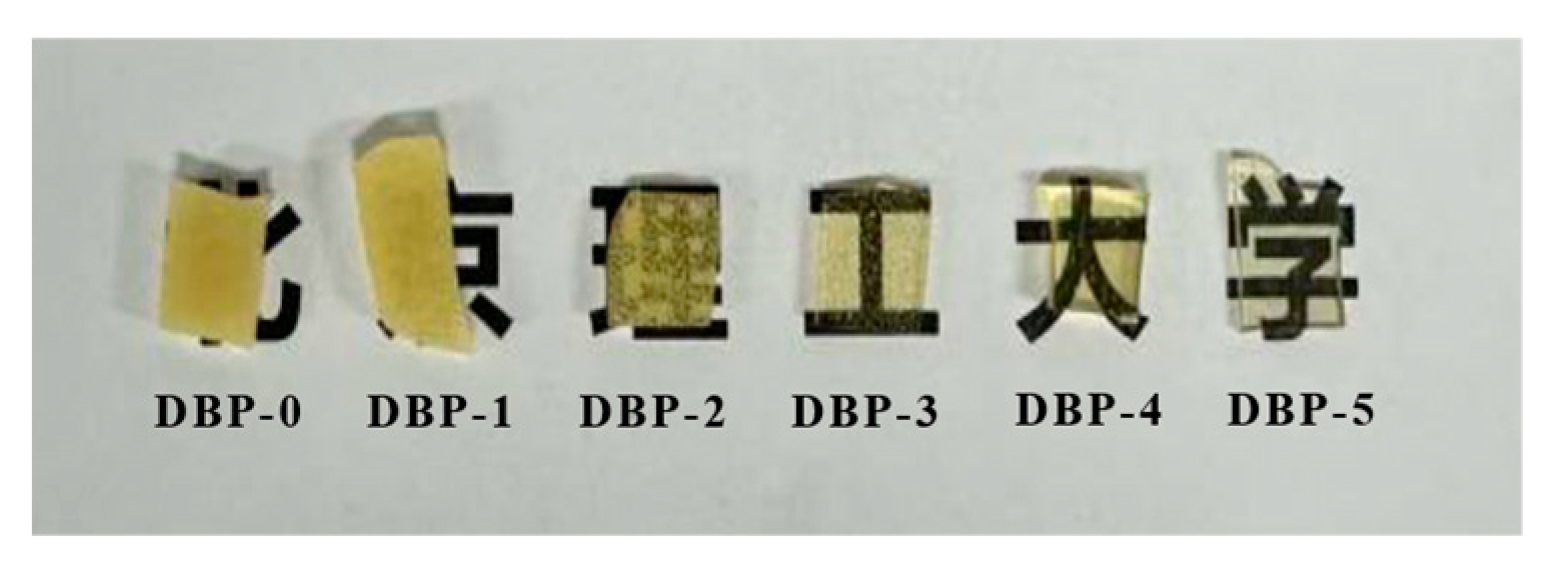
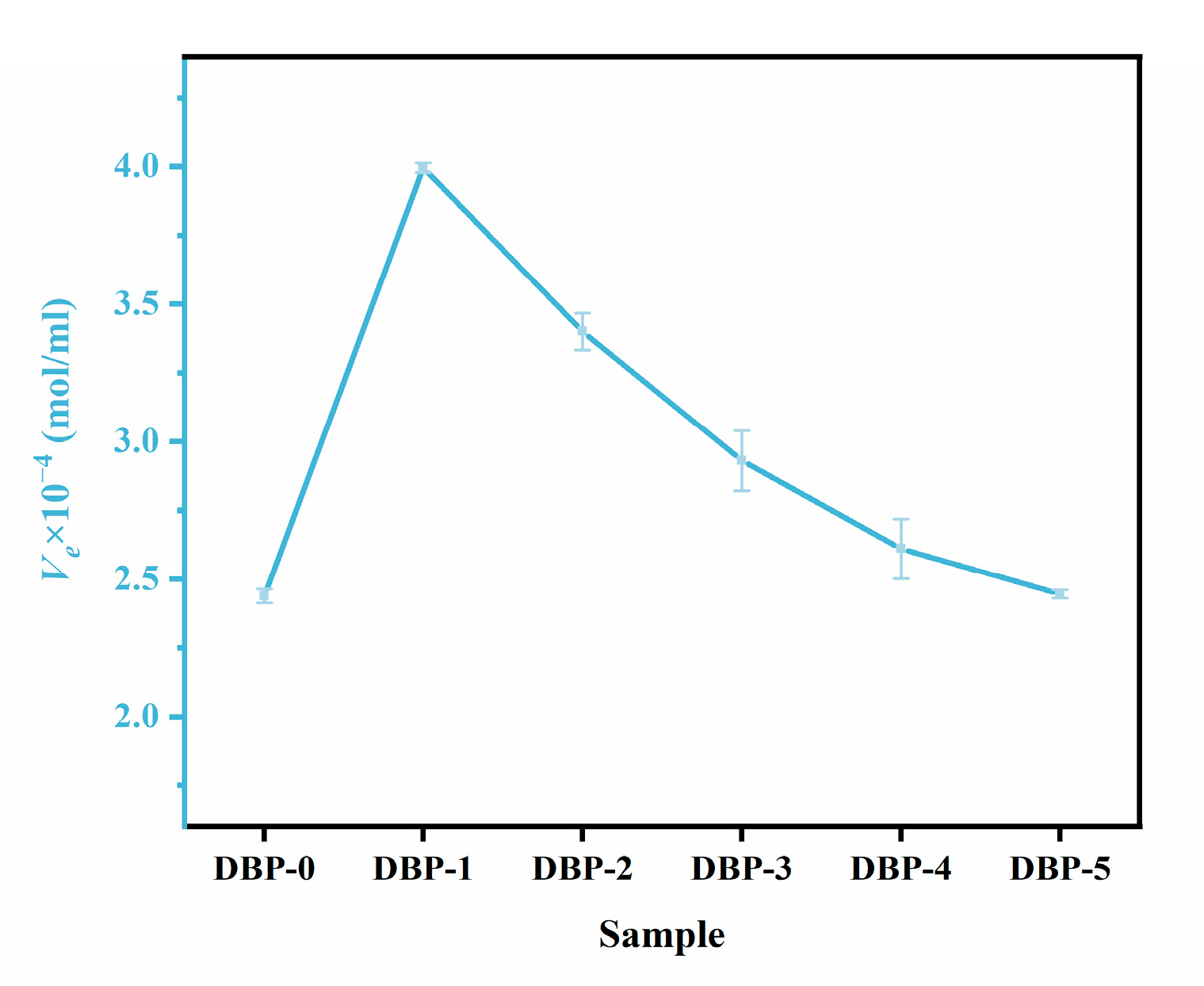
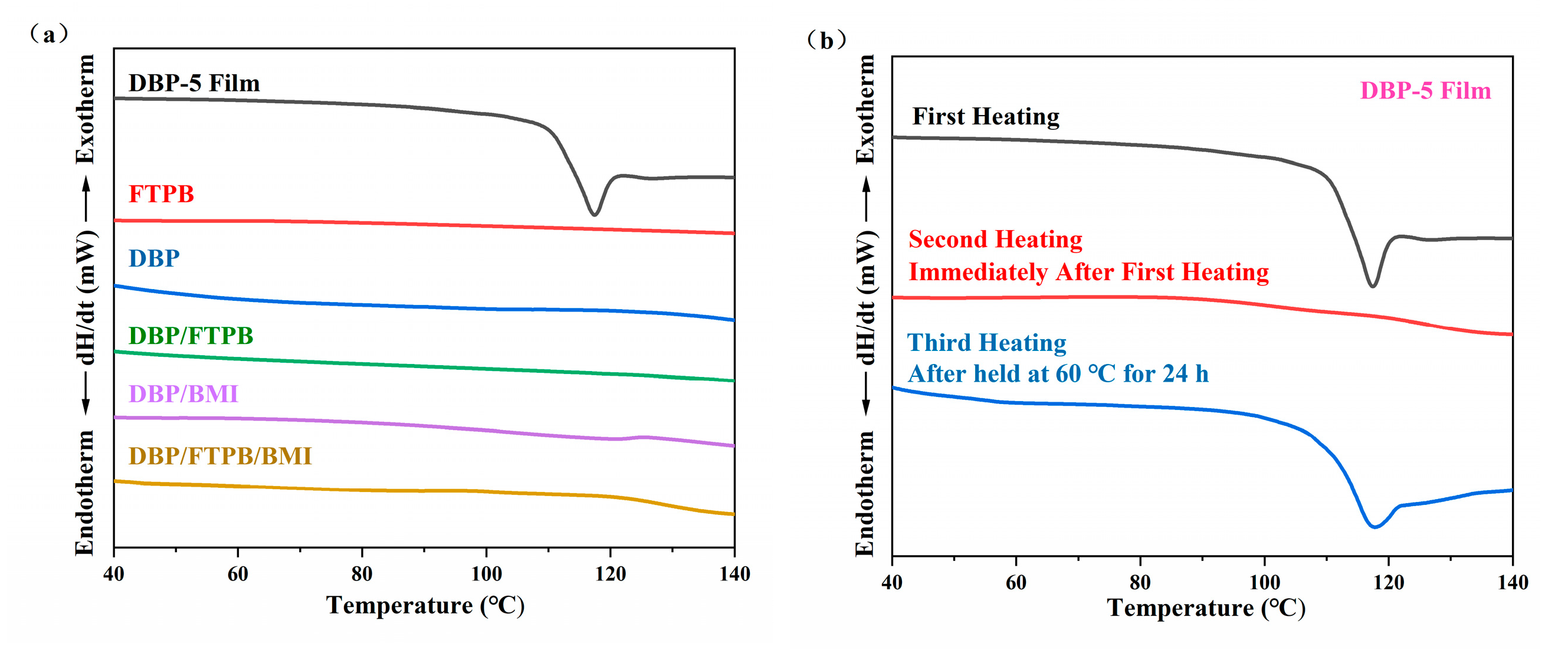
3.2. Properties of DBP-Plasticized FTPB Films
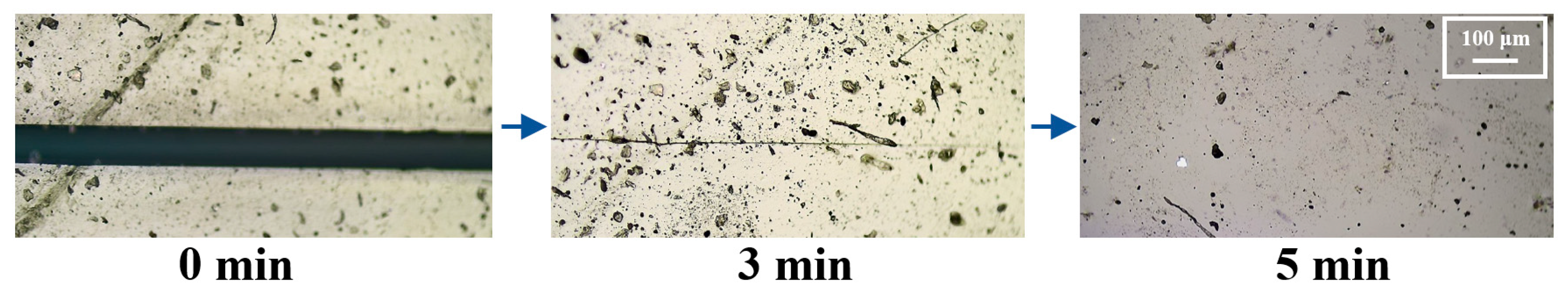
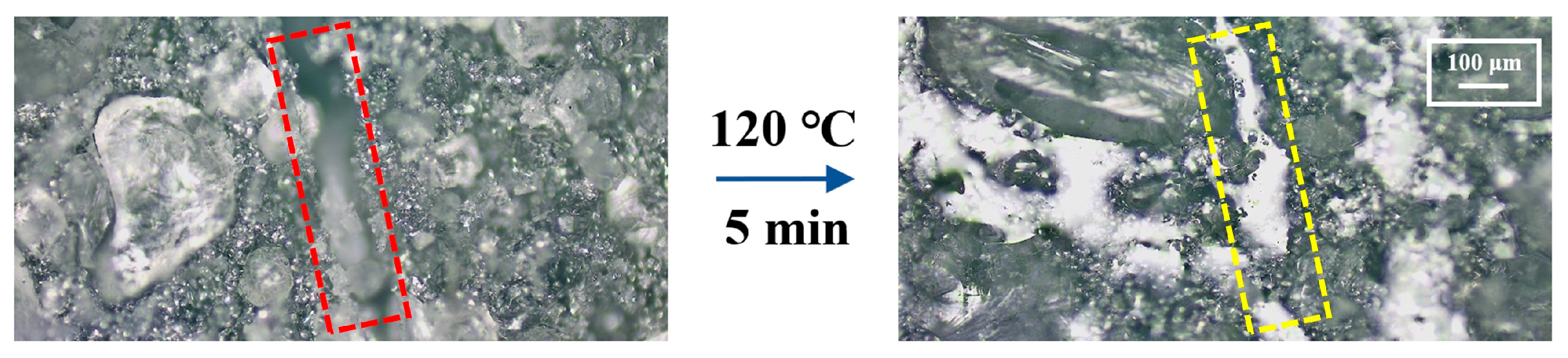
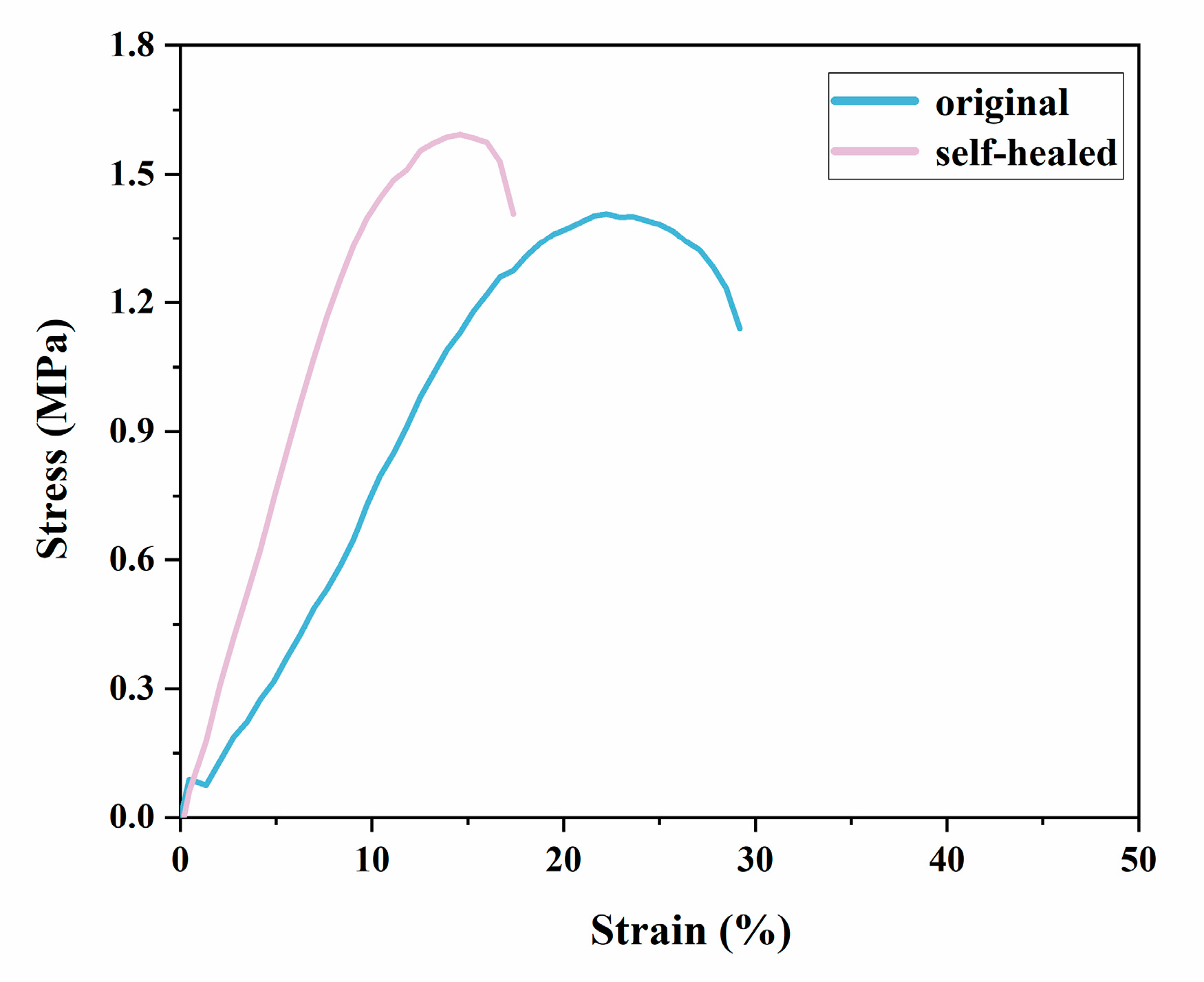
3.3. Performance of SHSP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Awaja, F.; Zhang, S.; Tripathi, M.; Nikiforov, A.; Pugno, N. Cracks, Microcracks and Fracture in Polymer Structures: Formation, Detection, Autonomic Repair. Prog. Mater. Sci. 2016, 83, 536–573. [Google Scholar] [CrossRef]
- Song, H.S.; Rumon, M.M.H.; Rahman Khan, M.M.; Jeong, J.H. Toward Intelligent Materials with the Promise of Self-Healing Hydrogels in Flexible Devices. Polymers 2025, 17, 542. [Google Scholar] [CrossRef]
- Diesendruck, C.E.; Sottos, N.R.; Moore, J.S.; White, S.R. Biomimetic Self-Healing. Angew. Chem. Int. Ed. 2015, 54, 10428–10447. [Google Scholar] [CrossRef]
- Birjandi Nejad, H.; Garrison, K.L.; Mather, P.T. Comparative Analysis of Shape Memory-based Self-healing Coatings. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1415–1426. [Google Scholar] [CrossRef]
- Nevejans, S.; Ballard, N.; Fernández, M.; Reck, B.; García, S.J.; Asua, J.M. The Challenges of Obtaining Mechanical Strength in Self-Healing Polymers Containing Dynamic Covalent Bonds. Polymer 2019, 179, 121670. [Google Scholar] [CrossRef]
- Zeimaran, E.; Pourshahrestani, S.; Kadri, N.A.; Kong, D.; Shirazi, S.F.S.; Naveen, S.V.; Murugan, S.S.; Kumaravel, T.S.; Salamatinia, B. Self-Healing Polyester Urethane Supramolecular Elastomers Reinforced with Cellulose Nanocrystals for Biomedical Applications. Macromol. Biosci. 2019, 19, 1900176. [Google Scholar] [CrossRef]
- Venkateswaran, M.R.; Khosravi, A.; Zarepour, A.; Iravani, S.; Zarrabi, A. Self-Healing Materials in Biomedicine and the Circular Economy. Environ. Sci. Nano 2024, 11, 2771–2802. [Google Scholar] [CrossRef]
- Kooij, H.M.; Susa, A.; García, S.J.; Zwaag, S.; Sprakel, J. Imaging the Molecular Motions of Autonomous Repair in a Self-Healing Polymer. Adv. Mater. 2017, 29, 1701017. [Google Scholar] [CrossRef]
- Mashkoor, F.; Lee, S.J.; Yi, H.; Noh, S.M.; Jeong, C. Self-Healing Materials for Electronics Applications. Int. J. Mol. Sci. 2022, 23, 622. [Google Scholar] [CrossRef] [PubMed]
- Kothari, J.; Iroh, J.O. Self-Healing Poly(Urea Formaldehyde) Microcapsules: Synthesis and Characterization. Polymers 2023, 15, 1668. [Google Scholar] [CrossRef]
- Zhang, M.; Cong, R.; Luo, Y. Room Temperature Self-Healing Epoxy Waterborne Polyurethane Containing Microcapsules with Waterborne Polyurethane Shells and Mercaptan Cores. Mater. Adv. 2022, 3, 7015–7027. [Google Scholar] [CrossRef]
- Almutairi, M.D.; He, F.; Alshammari, Y.L.; Alnahdi, S.S.; Khan, M.A. Analysis of the Self-Healing Capability of Thermoplastic Elastomer Capsules in a Polymeric Beam Structure Based on Strain Energy Release Behaviour during Crack Growth. Polymers 2023, 15, 3384. [Google Scholar] [CrossRef] [PubMed]
- Tanasi, P.; Hernández Santana, M.; Carretero-González, J.; Verdejo, R.; López-Manchado, M.A. Thermo-Reversible Crosslinked Natural Rubber: A Diels-Alder Route for Reuse and Self-Healing Properties in Elastomers. Polymer 2019, 175, 15–24. [Google Scholar] [CrossRef]
- Raut, S.K.; Behera, P.K.; Pal, T.S.; Mondal, P.; Naskar, K.; Singha, N.K. Self-Healable Hydrophobic Polymer Material Having Urethane Linkages via a Non-Isocyanate Route and Dynamic Diels–Alder ‘Click’ Reaction. Chem. Commun. 2021, 57, 1149–1152. [Google Scholar] [CrossRef]
- Imamaki, Y.; Sugane, K.; Shibata, M. Preparation and Properties of Self-Healing Disulfide Bond-Containing Thermoset Polyimide Resins Produced by the Thiol-Maleimide Reaction. Polym. Bull. 2024, 81, 6363–6379. [Google Scholar] [CrossRef]
- Ogawa, Y.; Sugane, K.; Shibata, M. Self-Healing Amine- and Carboxy-Cured Bio-Based Epoxy Vitrimers Driven by the Disulfide Metathesis Reaction. Polym. Bull. 2025, 82, 625–647. [Google Scholar] [CrossRef]
- Ussama, W.; Shibata, M. Self-Healing Polyester Networks Prepared from Poly(Butylene Succinate-Co-Butylene Itaconate) and Thiol-Terminated Polyether Containing Disulfide Linkages. Polymer 2022, 244, 124668. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; Cramer, C.J.; Hillmyer, M.A.; Dichtel, W.R. Mechanically Activated, Catalyst-Free Polyhydroxyurethane Vitrimers. J. Am. Chem. Soc. 2015, 137, 14019–14022. [Google Scholar] [CrossRef]
- Felsenthal, L.M.; Kim, S.; Dichtel, W.R. Robust Self-Healing Adhesives Based on Dynamic Urethane Exchange Reactions. ACS Appl. Mater. Interfaces 2024, 16, 57687–57694. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, M.; Park, S.; Park, S.A.; Jeon, H.; Koo, J.M.; Park, S.B.; Kim, H.J.; Eom, Y.; Lee, E.S.; et al. Molecular Dynamics Interpretation of Hydrogen Bonds for Colorless, Water-Resistant, Tough, and Self-Healable Elastomers. J. Mater. Chem. A 2023, 11, 22737–22748. [Google Scholar] [CrossRef]
- Tahir, N.A.; Zubir, S.A.; Shuib, R.K. Harnessing Thiol–Isocyanate Click Chemistry and Hydrogen Bonding for Tunable Self-Healing Polyurethanes Elastomers. J. Polym. Sci. 2025, 63, 3786–3798. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, S.R.; Lee, D.S. Sorbitol as a Chain Extender of Polyurethane Prepolymers to Prepare Self-Healable and Robust Polyhydroxyurethane Elastomers. Molecules 2018, 23, 2515. [Google Scholar] [CrossRef] [PubMed]
- Auepattana-Aumrung, K.; Crespy, D. Self-Healing and Anticorrosion Coatings Based on Responsive Polymers with Metal Coordination Bonds. Chem. Eng. J. 2023, 452, 139055. [Google Scholar] [CrossRef]
- Pignanelli, J.; Billet, B.; Straeten, M.; Prado, M.; Schlingman, K.; Ahamed, M.J.; Rondeau-Gagné, S. Imine and Metal–Ligand Dynamic Bonds in Soft Polymers for Autonomous Self-Healing Capacitive-Based Pressure Sensors. Soft Matter 2019, 15, 7654–7662. [Google Scholar] [CrossRef]
- Bleay, S.M.; Loader, C.B.; Hawyes, V.J.; Humberstone, L.; Curtis, P.T. A Smart Repair System for Polymer Matrix Composites. Compos. Part Appl. Sci. Manuf. 2001, 32, 1767–1776. [Google Scholar] [CrossRef]
- Aguirresarobe, R.H.; Nevejans, S.; Reck, B.; Irusta, L.; Sardon, H.; Asua, J.M.; Ballard, N. Healable and Self-Healing Polyurethanes Using Dynamic Chemistry. Prog. Polym. Sci. 2021, 114, 101362. [Google Scholar] [CrossRef]
- Ghanbari, R.; Serajian, A.; Ataei, S.; Nazarzadeh Zare, E. Intrinsic Self-Healing Polyurethanes: Advances, Applications, and Future Prospects. Eur. Polym. J. 2024, 221, 113566. [Google Scholar] [CrossRef]
- Irzhak, V.I.; Uflyand, I.E.; Dzhardimalieva, G.I. Self-Healing of Polymers and Polymer Composites. Polymers 2022, 14, 5404. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Sun, A.; Jing, M.; Liu, X.; Wei, L.; Wu, K.; Fu, Q. A Self-Reinforcing and Self-Healing Elastomer with High Strength, Unprecedented Toughness and Room-Temperature Reparability. Mater. Horiz. 2021, 8, 267–275. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Sun, A.; Wei, L.; Li, R.; He, H.; Yang, S.; Wang, S.; Niu, Y.; Li, Y. Exploring Piperazine for Intrinsic Weather-Proof, Robust and Self-Healable Poly(Urethane Urea) toward Surface and Tire Protection. Polymer 2021, 227, 123829. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.; Wang, J.; Hao, Y.; Hao, G.; Xiao, L.; Chen, J.; Zhou, B.; Fu, J.; Jiang, W. Parthenocissus-Inspired, Strongly Adhesive, Efficiently Self-Healing Polymers for Energetic Adhesive Applications. J. Mater. Chem. A 2021, 9, 16076–16085. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.; Zhou, X.; Xiao, L.; Hao, G.; Luo, J.; Zong, H.; Wang, Y.; Jiang, W. Simultaneous Configuration of High Room-Temperature Self-Healing Efficiencies and Tough Mechanical Properties of Energetic Adhesives for Energetic Composites. ACS Appl. Polym. Mater. 2023, 5, 3005–3014. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, Y.; Na, Q.; Guo, T.; Zhang, M.; Qi, Z.; Liu, N.; Yang, F.; Luo, Y.; Yang, W. Preparation and Characterization of Self-Healing Furan-Terminated Polybutadiene (FTPB) Based on Diels–Alder Reaction. RSC Adv. 2021, 11, 32369–32375. [Google Scholar] [CrossRef]
- Santoso, H.; Salma, S.A.; Yuliati, F.; Afifa, S.C.N.; Sumarno, S. Polyurethanes Synthesized from Diisocyanate Trimers and Polyethylene Glycol Undergo Crosslinking via Thermally Reversible Diels–Alder Reactions. Macromol. Res. 2025, 33, 1015–1028. [Google Scholar] [CrossRef]
- Platonova, E.; Ponomareva, P.; Lokiaeva, Z.; Pavlov, A.; Nelyub, V.; Polezhaev, A. New Building Blocks for Self-Healing Polymers. Polymers 2022, 14, 5394. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, J.; Xia, M.; Li, G.; Luo, Y. Performance and Kinetics Study of Self-Repairing Hydroxyl-Terminated Polybutadiene Binders Based on the Diels–Alder Reaction. Polymers 2017, 9, 200. [Google Scholar] [CrossRef]
- Jamarani, R.; Erythropel, H.C.; Nicell, J.A.; Leask, R.L.; Marić, M. How Green Is Your Plasticizer? Polymers 2018, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Brazel, C. The Plasticizer Market: An Assessment of Traditional Plasticizers and Research Trends to Meet New Challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Zhao, Y.; Tenhaeff, W.E. Thermally and Oxidatively Stable Polymer Electrolyte for Lithium Batteries Enabled by Phthalate Plasticization. ACS Appl. Polym. Mater. 2020, 2, 80–90. [Google Scholar] [CrossRef]
- Zuo, X.; Yi, P.; Chen, Q.; Wu, M.; Zhang, L.; Pan, B.; Xing, B. Inter-Molecular Interactions of Phthalic Acid Esters and Multi-Stage Sorption Revealed by Experimental Investigations and Computation Simulations. Chem. Eng. J. 2022, 431, 134018. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Zhang, C.; Li, Z.; Chi, Q. Improved Heat Resistance and Electrical Properties of Epoxy Resins by Introduction of Bismaleimide. J. Electron. Mater. 2023, 52, 1865–1874. [Google Scholar] [CrossRef]
- Gong, P.; Zhao, Y.; Li, K.; Tian, H.; Li, C. Effect of Plasticizer Content on the Structure and Properties of SPI/MA-g-PBAT Blend Films. J. Polym. Environ. 2022, 30, 562–568. [Google Scholar] [CrossRef]
- Garbarczyk, M.; Grinberg, F.; Nestle, N.; Kuhn, W. A Novel Approach to the Determination of the Crosslink Density in Rubber Materials with the Dipolar Correlation Effect in Low Magnetic Fields. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2207–2216. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Giannetti, A.; Lima, G.M.R.; Orozco, F.; Picchioni, F.; Mattoli, V.; Bose, R.K.; Pucci, A. Thermally Switchable Electrically Conductive Thermoset rGO/PK Self-Healing Composites. Polymers 2021, 13, 339. [Google Scholar] [CrossRef]
- Raut, S.K.; Mondal, P.; Parameswaran, B.; Sarkar, S.; Dey, P.; Gilbert, R.; Bhadra, S.; Naskar, K.; Nair, S.; Singha, N.K. Self-Healable Ultrahydrophobic Modified Bio-Based Elastomer Using Diels-Alder ‘Click Chemistry’. Eur. Polym. J. 2021, 146, 110204. [Google Scholar] [CrossRef]
- Yang, T.; Lin, C.; Huang, M.; Ying, P.; Zhang, P.; Wu, J.; Wang, T.; Kovalev, A.; Myshkin, N.; Levchenko, V. Self-Healing and Recyclable Polyurethane/Nanocellulose Elastomer Based on the Diels–Alder Reaction. Polymers 2024, 16, 2029. [Google Scholar] [CrossRef]
- Tian, W.; Xiong, F.; Han, H.; Li, W. Recyclable and Self-Healing Polyurethane Composite Film Based on Disulfide and DA Bond Modulation Strategies for Marine Anticorrosion Applications. Prog. Org. Coat. 2025, 209, 109550. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, M.; Yang, R.; Wang, B.; Yang, W.; Yang, F.; Xia, M.; Luo, Y. Polybutadiene Derived Allophanate as the Crosslinker for Diels–Alder Type Self-Healing Polyurethane Networks. J. Appl. Polym. Sci. 2022, 139, e53150. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Hou, X.; Yao, Q.; Chen, H.; Wei, Z.; Zhao, Y.; Geng, Z.; Yang, F.; Xia, M.; Luo, Y. Plasticizer-Enabled Solvent-Free Curing of Self-Healing Binder System for Energetic Materials. Polymers 2025, 17, 2635. https://doi.org/10.3390/polym17192635
Zhang M, Hou X, Yao Q, Chen H, Wei Z, Zhao Y, Geng Z, Yang F, Xia M, Luo Y. Plasticizer-Enabled Solvent-Free Curing of Self-Healing Binder System for Energetic Materials. Polymers. 2025; 17(19):2635. https://doi.org/10.3390/polym17192635
Chicago/Turabian StyleZhang, Minghao, Xudong Hou, Qifa Yao, Hanyu Chen, Zuting Wei, Yue Zhao, Zhishuai Geng, Fanzhi Yang, Min Xia, and Yunjun Luo. 2025. "Plasticizer-Enabled Solvent-Free Curing of Self-Healing Binder System for Energetic Materials" Polymers 17, no. 19: 2635. https://doi.org/10.3390/polym17192635
APA StyleZhang, M., Hou, X., Yao, Q., Chen, H., Wei, Z., Zhao, Y., Geng, Z., Yang, F., Xia, M., & Luo, Y. (2025). Plasticizer-Enabled Solvent-Free Curing of Self-Healing Binder System for Energetic Materials. Polymers, 17(19), 2635. https://doi.org/10.3390/polym17192635







