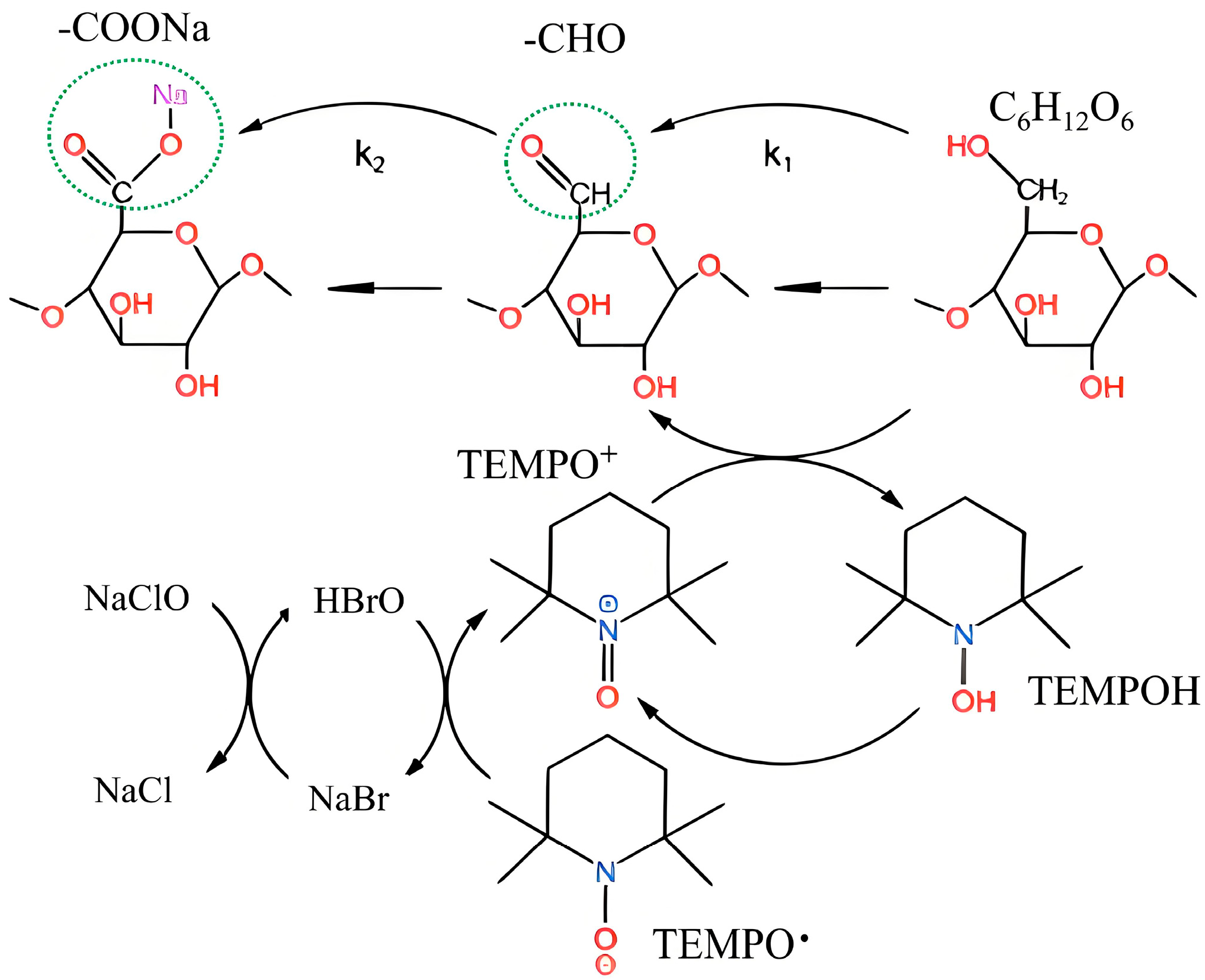
3.1. Analysis of Apparent Oxidation Process
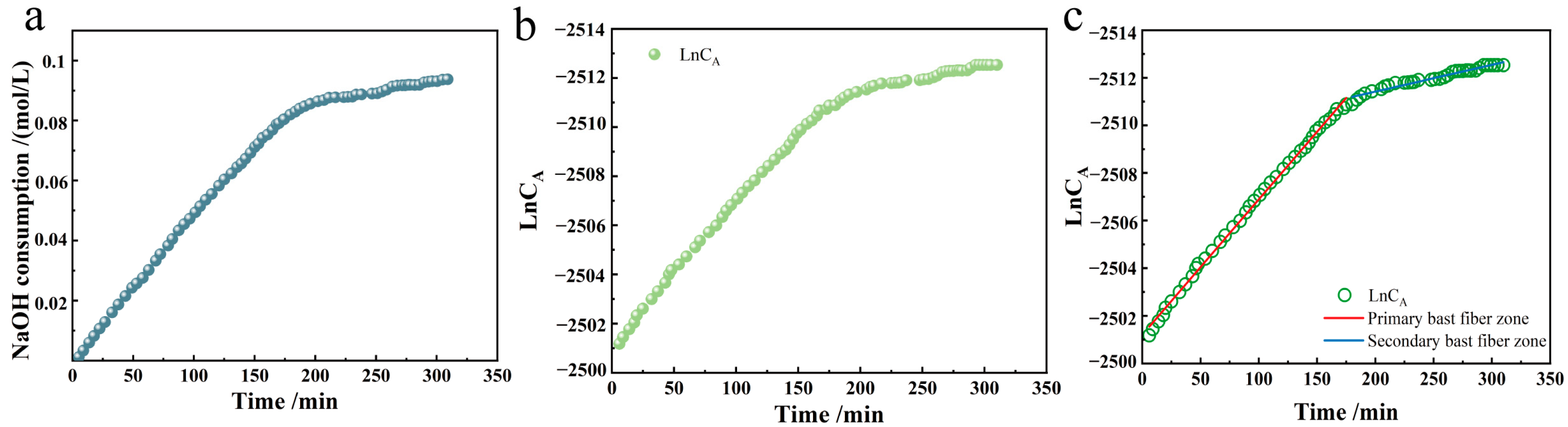
We constructed the kinetic consumption profile of NaOH during TEMPO/NaBr/NaClO-mediated selective oxidation of industrial hemp staple fibers, with real-time titration data presented as cumulative NaOH consumption (mmol) versus reaction time (min) in
Figure 3a. The experimental conditions were as follows: 750 mL H
2O, 0.21 mmol/L TEMPO, 3.24 mmol/L NaBr, 109.26 mmol/L NaClO (7.0% effective chlorine content), pH 10, temperature 20 °C, reaction time 5 h.
The Ln-t image and its reaction rate graph were made according to the primary reaction rate equation, as shown in
Figure 3b,c.
From the reaction rate curve, it can be seen that the chemical reaction rate remained relatively stable at the reaction time of 0–180 min. The reaction rate slightly decreased after 180 min, but the chemical reaction continued. This result aligns with the data on the oxidation of other natural celluloses using the TEMPO/NaBr/NaClO method [
14]. The structural characterization of industrial hemp fibers reveals that the stem bast fibers are primarily localized in the middle and outer cortical layers, exhibiting a porous microstructure with loose fibrillar packing. This anatomical feature facilitates reagent penetration during TEMPO-mediated oxidation. The primary bast fiber has higher secondary wall thickness and cell wall thickness than the secondary bast fiber, which accounts for about 92–95% of the fiber composition [
15,
16,
17]. In contrast, secondary bast fibers lie closer to the pith, where the junction region reaction struggles to proceed, resulting in a lamellar spatial structure. The microfiber angle of the S1 layer of hemp varies between 70–90°, the microfiber angle of the outer layer of the S2 layer varies between 25–30°, and the microfiber angle of the inner layer of the S2 layer varies around 0–5° [
18]. Carrying out the oxidation reaction becomes challenging due to the significant variation in the angle of arrangement of the microfibers in each layer. In this paper, we refer to the initial reaction period as the oxidation of primary bast fibers and the later stages as the oxidation of cannabinoid secondary bast fibers. In addition, xylan in hemp fibers was mainly concentrated in the primary wall of the thick-walled bast tissue, differing from its distribution in the secondary wall of other dicotyledonous plants (where galactans primarily accumulated in thin-walled cells) [
17]. This suggests that hemicellulose participated in both reaction segments [
19,
20]. Most of them degrade into water-soluble substances [
21]. Lignin in hemp fibers primarily accumulates in the intercellular layer and primary wall [
18], and its removal depends on oxidant concentration and oxidation time.
The reaction rate curves for the oxidation of industrial hemp staple fibers by the TEMPO system shown in
Figure 3 above were fitted to obtain the corresponding reaction rate constants and fitting coefficients, the values of which are shown in
Table 1.
The coefficient of determination R2 removes the effect of the degree of dispersion of the raw data, so the high coefficients of determination of the primary and secondary bast fibers demonstrate that the reaction in both regions conforms to the first-order kinetic equations, with rate constants of k1 = 1.17 min−1 in the primary bast region and k2 = 0.18 min−1 in the secondary bast region. By analyzing the reaction rate constants of these two curves, it can be seen that the reaction rate of the TEMPO/NaBr/NaClO system for oxidation of the primary bast zone of industrial hemp staple fibers is 6.5 times higher than that of the secondary bast zone. This difference in reaction rates highlights the different reactivity and kinetics of the oxidation process of hemp primary and secondary bast fibers.
The observed plateau in the oxidation kinetics and the attainable degree of conversion are not a limitation of the process, but rather a direct manifestation of the intrinsic structural heterogeneity of industrial hemp staple fibers. This heterogeneous material comprises both readily oxidizable primary bast fibers and more recalcitrant secondary bast fibers. The applied mild oxidation conditions are strategically designed to selectively functionalize the accessible domains while preserving the integrity of the cellulose polymer chains. The successful application of kinetic deconvolution to model these distinct reaction phases provides a valuable framework for assessing and exploiting the differential reactivity within such complex lignocellulosic feedstocks.
The application of the pseudo-first-order model is predicated on the assumption that the oxidation of the aldehyde intermediate (Equation (11)) is significantly faster than the initial oxidation of the alcohol to the aldehyde (Equation (9)), i.e., k2≫k1 [
11,
13]. This foundational assumption, widely supported in previous studies of the TEMPO/NaBr/NaClO system [
6,
10,
11] ensures that the aldehyde concentration remains negligible throughout the reaction.
3.2. Effect of TEMPO Usage
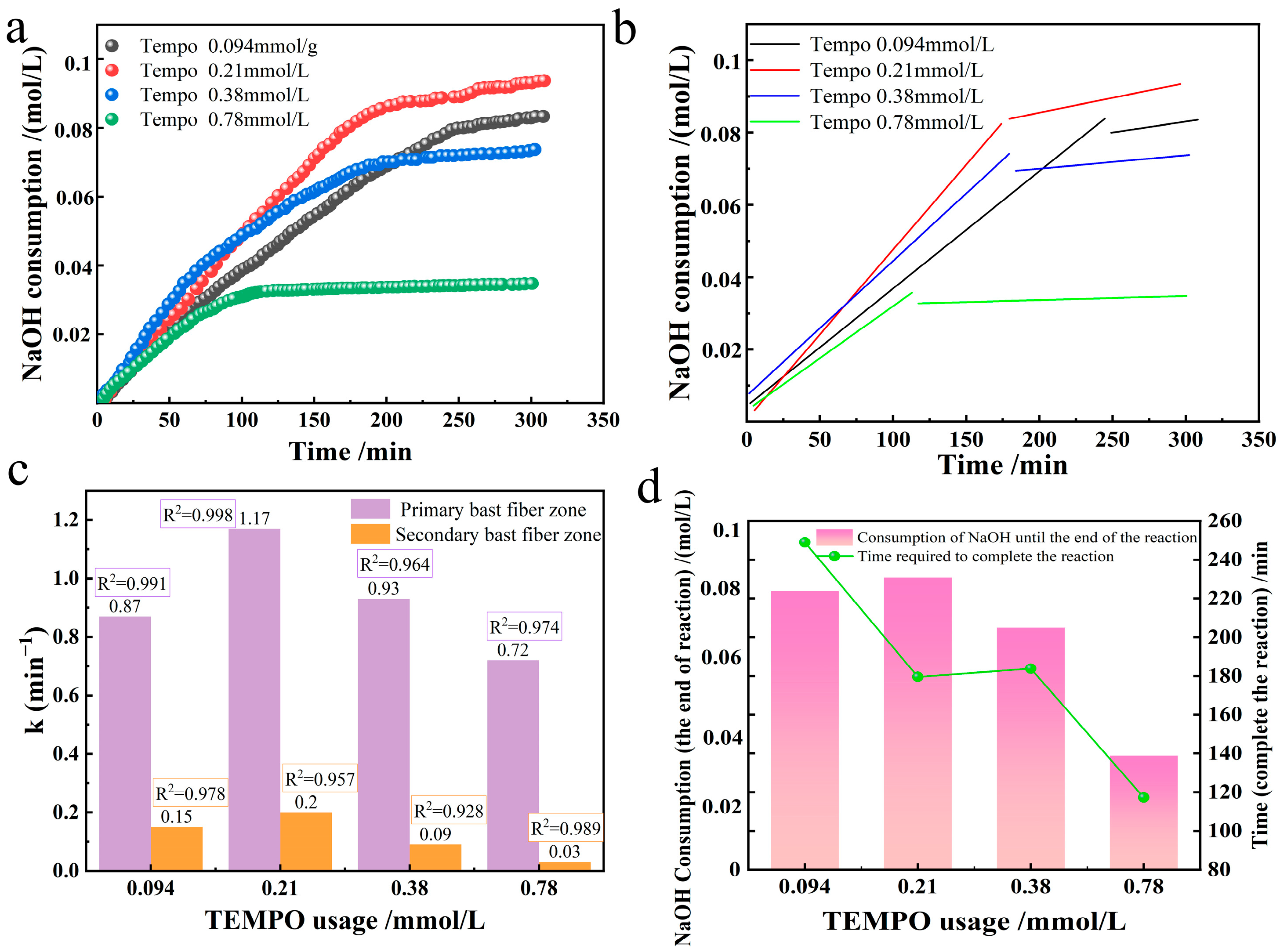
In the TEMPO system, the amount of TEMPO was varied to observe the effect on the oxidation system, selectively oxidizing industrial hemp staple fibers produced. Under the alkaline environment of room temperature 20 °C and pH = 10, 3.24 mmol/L NaBr, 109.26 mmol/L NaClO, and 10 g industrial hemp staple fibers were dissolved in 750 mL deionized water. We tested four TEMPO usage groups (0.094, 0.21, 0.38, 0.78 mmol/L), corresponding to solution concentrations of 0.0015%, 0.003%, 0.006%, and 0.012%, to compare changes in reaction rate constants. We measured NaOH consumption during the preparation of oxidized industrial hemp staple fibers across these TEMPO usages, performed linear fitting, and present the results in
Figure 4.
The usage of TEMPO catalyst has a profound non-monotonic effect on the oxidation kinetics of industrial hemp fibers, which was quantified by the apparent first-order rate constant k. As shown in
Figure 4c, the k of the primary phloem fibers exhibits a distinct volcanic trend. With the increase of TEMPO concentration, k first rises sharply and then drops sharply. The observed initial positive correlation (0–0.094 mmol/L TEMPO) is consistent with the classical catalytic behavior. Increasing the catalyst load will enhance the steady-state concentration of the active oxidant (TEMPO
+), thereby accelerating the rate-determining step: the oxidation of primary alcohols to aldehydes Equation (8). The increase in the proportion of cumulative NaOH consumption at the completion of the reaction confirmed this (a dual-axis plot
Figure 4d), indicating an improvement in conversion efficiency within this dose range.
However, the decrease in k after exceeding the optimal dose (0.21–0.78 mmol/L TEMPO) indicates that there are more complex interactions in the reaction system. This reverse relationship indicates that an excessive TEMPO begins to inhibit the process it was supposed to catalyze. We believe that this inhibition stems from the variable-speed competition mechanism, which is at the core of our hypothesis. Excessively high TEMPO may disrupt the critical equilibrium between catalytic substances (TEMPO/TEMPO+/TEMPOH). Specifically, high [TEMPO] can transfer the equilibrium of the regeneration reaction (Equation (7), TEMPO + TEMPO + 2TEMPO•) to the reactants, effectively isolating TEMPO+ and hindering its efficient oxidation of cellulose substrates. This creates a competitive environment in which TEMPO molecules interfere with each other, ultimately suppressing the overall catalytic throughput.
Three-dimensional comprehensive analysis shows that in the TEMPO variable group, the trend of oxidation reaction rate constant and NaOH consumption at the end of the reaction is consistent. Under the premise that the concentration of BrO
− remained unchanged, with the increase of TEMPO concentration, the oxidation reaction rate and the generation of glucuronic acid increased, and the reaction time became shorter, indicating that the reaction rate of aldehyde-related k
1 accelerated. With the acceleration of reaction rate k
1, which means that the generation rate of BrO
− accelerates, NaClO becomes less and less in the reaction, and the time required to the end of the reaction is related to the consumption rate of NaClO. TEMPO is competitive with polysaccharides against secondary oxides, and a shorter reaction time can reduce the damage to fibers and slightly increase the selective oxidation yield [
22,
23]. When the TEMPO usage increased from 0.094 mmol/L to 0.21 mmol/L, the reaction rate k
1 of the aldehyde group increased by about a quarter. The consumption of TEMPO
+ is divided into two routes: the oxidation kinetic rate equation building part Equation (8)/Equation (9). In Equation (8), TEMPO
+ reacts with TEMPOH to form 2 TEMPO•, which corresponds to Equation (6)/Equation (7) to form 2 TEMPO
+, one of which continues back into the reaction cycle in Equation (8). The reaction of TEMPO
+ with TEMPOH produces only one effective TEMPO• involved in the subsequent reaction. In Equation (9), TEMPO
+ is converted to TEMPOH by reaction with C
6-OH. According to the established mechanism [
7,
8,
9], the order of occurrence of these two reactions is first the reaction with the cellulose hydroxyl (Equation (9)) to generate TEMPOH, followed by the disproportionation reaction (Equation (8)). The rate of reaction Equation (9) is exactly increased by about 50% when the amount of TEMPO is doubled, these results indicate that the formation of aldehyde groups by C
6-OH in oxidized cellulose is the key rate-limiting reaction in a series of TEMPO-related reactions.
Notably, the observed near-linear increase in carboxyl group formation (
Figure 4b) during the initial phase at optimal TEMPO usage (0.21 mmol/L) deviates from a perfect first-order decay curve. This apparent zero-order kinetic characteristic suggests that within this specific concentration window, the rate of aldehyde formation is not limited by the concentration of cellulose hydroxyls, but rather by the steady-state concentration of the active oxidant (TEMPO
+), which is maintained by the dynamic balance between its generation (from NaClO/NaBr) and consumption (reduction to TEMPOH). This observed plateau in rate provides strong experimental support for the proposed ’variable-speed competition mechanism’ between TEMPO and NaClO, wherein an optimal equilibrium exists. When the amount of TEMPO was greater than 0.21 mmol/L, the rate constant of the oxidation reaction and the amount of NaOH consumed to the end of the reaction did not increase but decreased, therefore, when the amount of TEMPO was greater than 0.21 mmol/L, the selective oxidation reaction would be inhibited by an excessive amount of TEMPO. The rate constant of the reaction was the smallest when the TEMPO usage of 0.78 mmol/L was used, the time taken to the end of the reaction was the shortest, and the least amount of glucuronic acid was produced. Excess TEMPO hinders the conversion of TEMPOH, resulting in a slowing of the reaction rate k
1 for the generation of aldehyde groups. At this time, the consumption rate of OCl
− is faster than that under the condition that TEMPO is less than 0.21 mmol/L, and the reaction rate k
2 of carboxyl group formation is slowed down with the formation of more Cl
−.
Researchers must account for the number of independent variables when comparing multiple regression equations with differing predictor counts. During the experiment, the measurement period of the instrument used impacted the sample measurement interval, resulting in a different number of samples in each group of data. We use coefficient-corrected measurements as final results to ensure experimental accuracy. As can be seen from the data in
Figure 4c, the primary and secondary bast fibers have high coefficients of determination R
2, suggesting that the response of these two regions at different TEMPO usages is consistent with the first-order kinetic equation.
3.3. Effect of NaBr Usage
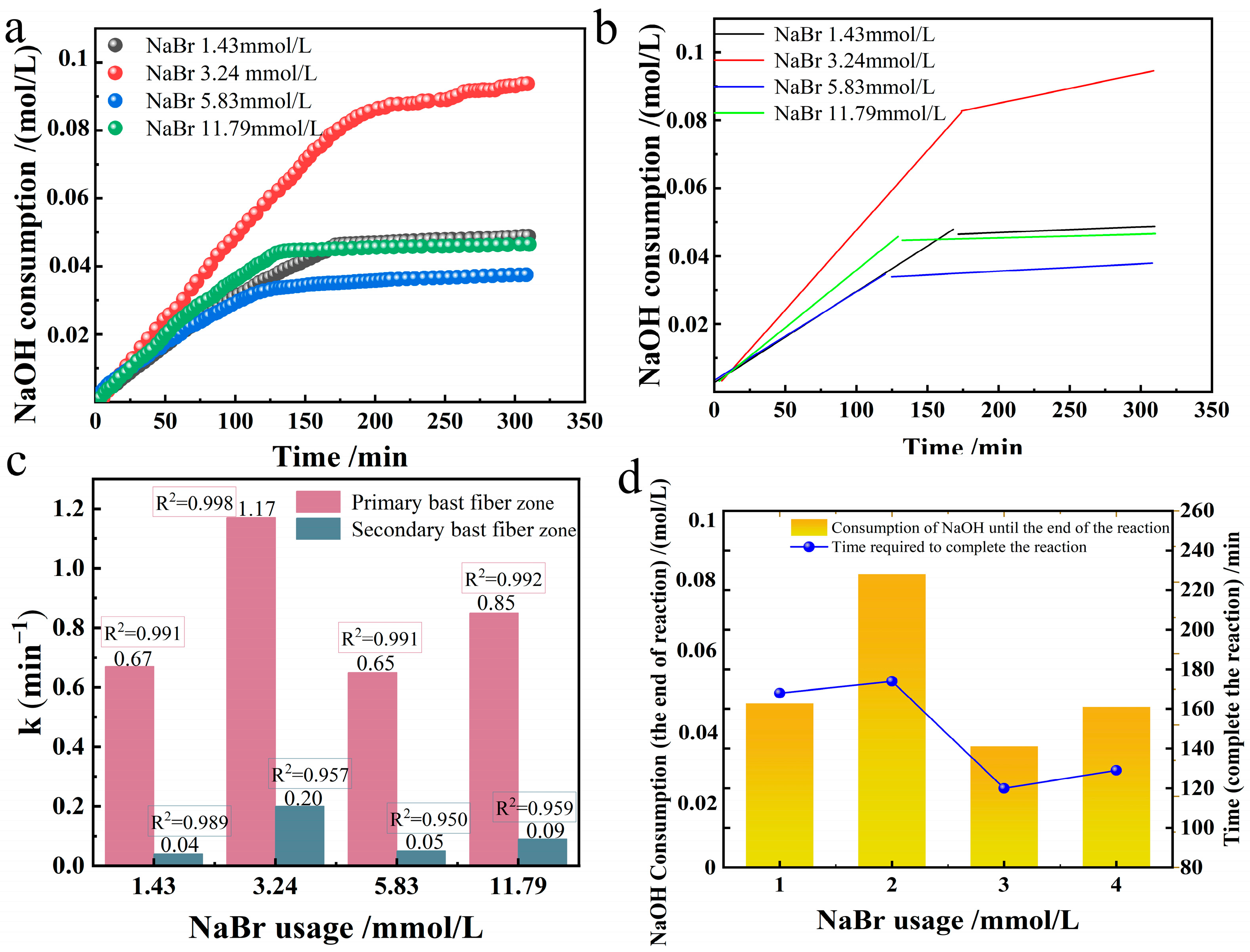
We investigated how NaBr usage affects the selective oxidation of industrial hemp fibers in the TEMPO/NaBr/NaClO system. In an alkaline environment at room temperature 20 °C and pH = 10, 0.21 mmol/L of TEMPO, 109.26 mmol/L of NaClO, and 10 g of industrial hemp staple fiber were taken and dissolved in 750 mL of deionized water. Four groups of different NaBr usages (1.43, 3.24, 5.83, 11.79 mmol/L) corresponding to solution gradient configurations (0.015%, 0.03%, 0.06%, 0.12%) were set, respectively, to compare the change of reaction rate constant. We plotted NaOH consumption for oxidized hemp fibers across different NaBr usages and performed linear fitting, as shown in
Figure 5.
The relationship between the reaction rate constants of primary and secondary bast fibers for the preparation of oxidized industrial hemp staple fibers at different NaBr usages is shown in
Figure 5c. When the amount of NaBr is within the range of (0, 3.24], there is a positive correlation between the amount of NaBr and the reaction rate constant of oxidation. The larger the amount of NaBr, the larger the oxidation reaction rate constant. The peak value of the oxidation reaction rate constant is consistent with the data of the oxidized viscose fibers in this system [
10]. The NaBr usage was negatively correlated with the reaction rate constant of oxidation when the NaBr usage was in the range of [3.24, 5.83], and the larger the NaBr usage, the more diminutive the oxidation reaction rate constant, and this proportionality was in agreement with the results of the study of oxidation of cotton fibers by this system [
11]. When the usage of NaBr was within the range of [5.83, 11.79], the reaction rate constant increased slightly with the increase of the usage of NaBr, which was observed for the first time.
A two-dimensional plot simultaneously showing reaction time and NaOH consumption was drawn, as shown in
Figure 5d. When the NaBr usage was in the range of (0, 3.24], there was a positive correlation between the NaBr usage and the amount of NaOH consumed up to the end of the reaction, i.e., the more NaBr usage was used, the more glucuronic acid was produced, and the higher the selective oxidative conversion rate was; When the amount of NaBr was within the range of [3.24, 5.83], the amount of NaBr was negatively correlated with the consumption of NaOH to the end of the reaction, that is, the more NaBr was used, the less glucuronic acid was generated, and the lower the conversion rate of selective oxidation was. When the NaBr usage was in the range of [5.83, 11.79], there was a slight recovery in NaOH consumption to the end of the reaction. Therefore, when the usage of NaBr is 3.24 mmol/L, the selective oxidation conversion rate is the highest, and the selective oxidation conversion rate increases nearly twice as much as when the usage of NaBr is 1.43 mmol/L. When the usage of NaBr is 11.79 mmol/L, the selective oxidation conversion is similar to that when the usage of NaBr is 1.43 mmol/L. From the dimension of time required until the end of the reaction, the time needed for the NaBr usage of 1.43 mmol/L and 3.24 mmol/L groups to the end of the reaction is around 170 min. The amount of NaBr used was 5.83 mmol/L and 11.79 mmol/L, and the time required for the reaction to end was about 130 min for both groups. However, when the usage of NaBr was between 3.24 mmol/L and 5.83 mmol/L, the time to the end of the reaction was significantly decreased by 23.5%.
In the three-dimensional analysis, the trends of the oxidation reaction rate constants, the amount of NaOH consumed until the end of the reaction, and the time required until the end of the reaction under different NaBr usage conditions were in the same direction. In 1995, De Nooy observed that hypochlorite delayed acid formation, presumably due to the influence of HOCI/OCI
− and HOBr/OBr
− equilibria [
6]. This experiment shows that there is a dynamic equilibrium between OCI
− and BrO
−. Since the concentration of BrO
− and OCI
− in equilibrium depends on the concentration base of Br
−, the oxidation rate of OCl
− to Br
− is much higher than the reduction rate of OBr
− when the amount of NaBr is small, and the reaction rate also increases with the increase of NaBr. OCl
− and OBr
− concentrations tend to the equilibrium maximum concentrations. During this period, NaClO, which did not participate in the reaction, accelerated the rate of non-selective oxidation and depolymerization, resulting in less glucuronic acid being produced [
22,
23]. When the optimal value of NaBr is 3.24 mmol/L, it oxidizes rapidly with NaClO, and the cellulose is less damaged, which lays the foundation for long-term, high-rate, and highly selective oxidation conversion. The time required to the end of the reaction was similar for the first two groups, consistent with the conclusion that NaClO usage correlates with reaction time in the TEMPO group analysis. When NaBr is greater than 3.24 mmol/L, the concentration of BrO
− is equal to or greater than the concentration of OCl
−. After the reaction of Br
− with HClO, the excess remaining Br
− as a product will hinder the conversion of TEMPO, inhibiting the reaction rate k
1 for the formation of aldehyde groups. At the same time, a large amount of Br
− is converted to BrO
− by HOCl and accompanied by the production of Cl
−, which leads to a decrease in reactants and an increase in products in reactions that generate carboxyl groups. The k
1 and k
2 reactions, which are directly related to the formation of uronic acid, are simultaneously hindered. This reduction in the oxidation rate constant, the consumption of NaOH, and the reaction time to completion is very unfavorable for the reaction.
According to the method “Effects of different TEMPO usage on oxidation rate constant, selective oxidation rate and time to the end of reaction”, the effects of different NaBr usages on oxidation rate constant, selective oxidation conversion rate, and time to the end of reaction were analyzed, the high coefficients of determination for primary and secondary bast fibers indicate that the responses of different NaBr usage groups to both regions are by the first-order kinetic equation.
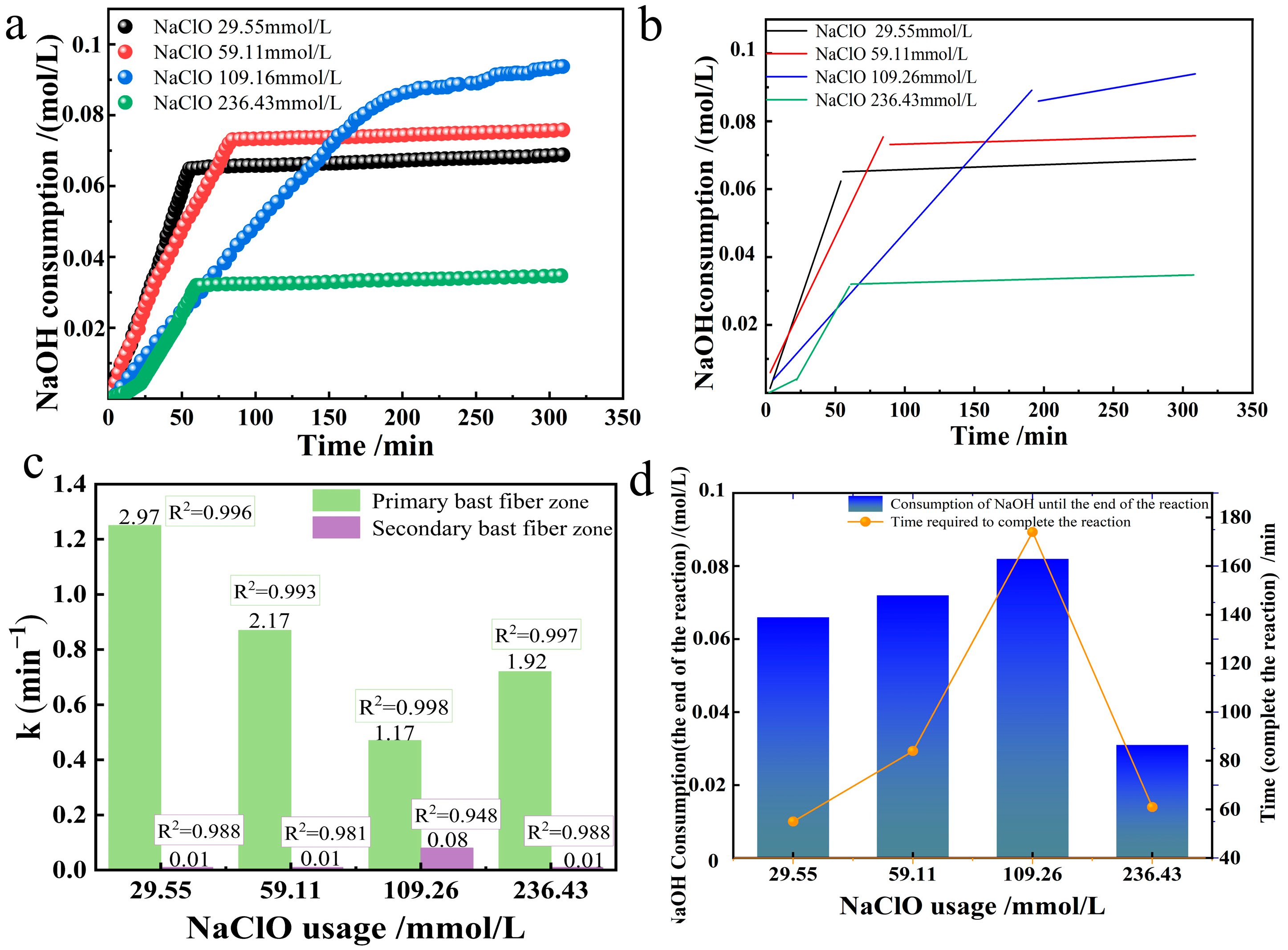
3.4. Effect of NaClO Usage
The effect of selective oxidation of industrial hemp staple fibers produced by the oxidation system was observed by varying the amount of NaClO in the TEMPO/NaBr/NaClO system. Under the alkaline environment of room temperature 20 °C and pH = 10, 0.21 mmol/L TEMPO, 3.24 mmol/L NaBr, and 10 g industrial hemp staple fibers were dissolved in 750 mL deionized water, four groups of different NaClO usages (29.55, 59.11, 109.26, 236.43 mmol/L) corresponding to the solution gradient ratio configurations (0.15%, 0.3%, 0.6%, 1.2%) were added to observe the changes in the oxidation reaction rate constant, as shown in
Figure 6c. The NaOH consumption of oxidized industrial hemp staple fibers at different NaClO usages and their linear fitting plots were plotted as shown in
Figure 6a,b.
The relationship between the reaction rate constants of primary and secondary bast fibers when oxidizing industrial hemp staple fibers at different NaClO usages is shown in
Figure 6c. The amount of NaClO in the primary bast fibers was negatively correlated with the oxidation rate constant when the NaClO usage was in the range of (0, 109.26], and the larger the NaClO usage, the smaller the oxidation rate constant; in the range of [109.26, 236.43], the reaction rate constant increased slightly with the increase in the amount of NaClO.
A two-dimensional plot simultaneously showing reaction time and NaOH consumption was drawn, as shown in
Figure 6d. When the amount of NaClO is within the range of (0, 109.26], the concentration of NaClO is positively correlated with the consumption of NaOH to the end of the reaction; that is, the more the amount of NaClO, the more glucuronic acid will be generated, and the higher the selective oxidation conversion rate will be. This rule is used as a process regulation in the preparation of cellulose nanocrystals [
19]. At the NaClO usage of [109.26, 236.43], the NaClO concentration was negatively correlated with the amount of NaOH consumed up to the end of the reaction, i.e., the higher the NaClO usage, the less glucuronic acid was produced, and the lower the selective oxidative conversion rate. At the amount of NaClO of 236.43 mmol/L, the initial slope of the first linear fit is relatively low, and then turned up, probably due to the large amount of NaClO added, causing the solution pH to be too high and inhibiting the reaction. From the dimension of time required to the end of the reaction, when the amount of NaClO is within the range of (0, 109.26], the amount of NaClO is positively correlated with the time required to the end of the reaction; that is, the more NaClO is used, the longer the reaction time will be. When the amount of NaClO was 109.26 mmol/L, the time required to the end of the reaction was 174 min, after which the amount of NaClO was increased to 236.43 mmol/L g, and the time required to the end of the reaction was shortened, which was close to the time required to the end of the reaction for the amounts of NaClO 29.55 and 59.11 mmol/L.
Three-dimensional comprehensive analysis shows that when the amount of NaClO is (0, 109.26], the higher the total amount of NaClO, the higher the selective conversion rate and the higher the consumption of NaOH at the end of the reaction. Since Cl− cannot be converted into additional ClO−, this means that the reaction will eventually terminate as the ClO− is consumed, suggesting that the selective oxidation fails to proceed adequately when the amount of NaClO is low. With the increase in the amount of NaClO, the reaction speed gradually decreases. Considering that there are two formation routes of Cl−, which is twice that of Br−, it means that while the equilibrium concentration of OBr− and OCl− increases, more and more Cl− prevents the conversion of Br− to BrO−.
The effects of different NaClO usages on the oxidation rate constants, selective oxidation rates, and time to the end of the reaction were analyzed by referring to the method of “Effects of different TEMPO usages on the oxidation rate constants, selective oxidation rates, and time to the end of the reaction”. The high coefficients of determination for primary and secondary bast fibers indicate that the reactions in these two regions are consistent with the first-order kinetic equation at different NaClO usages.
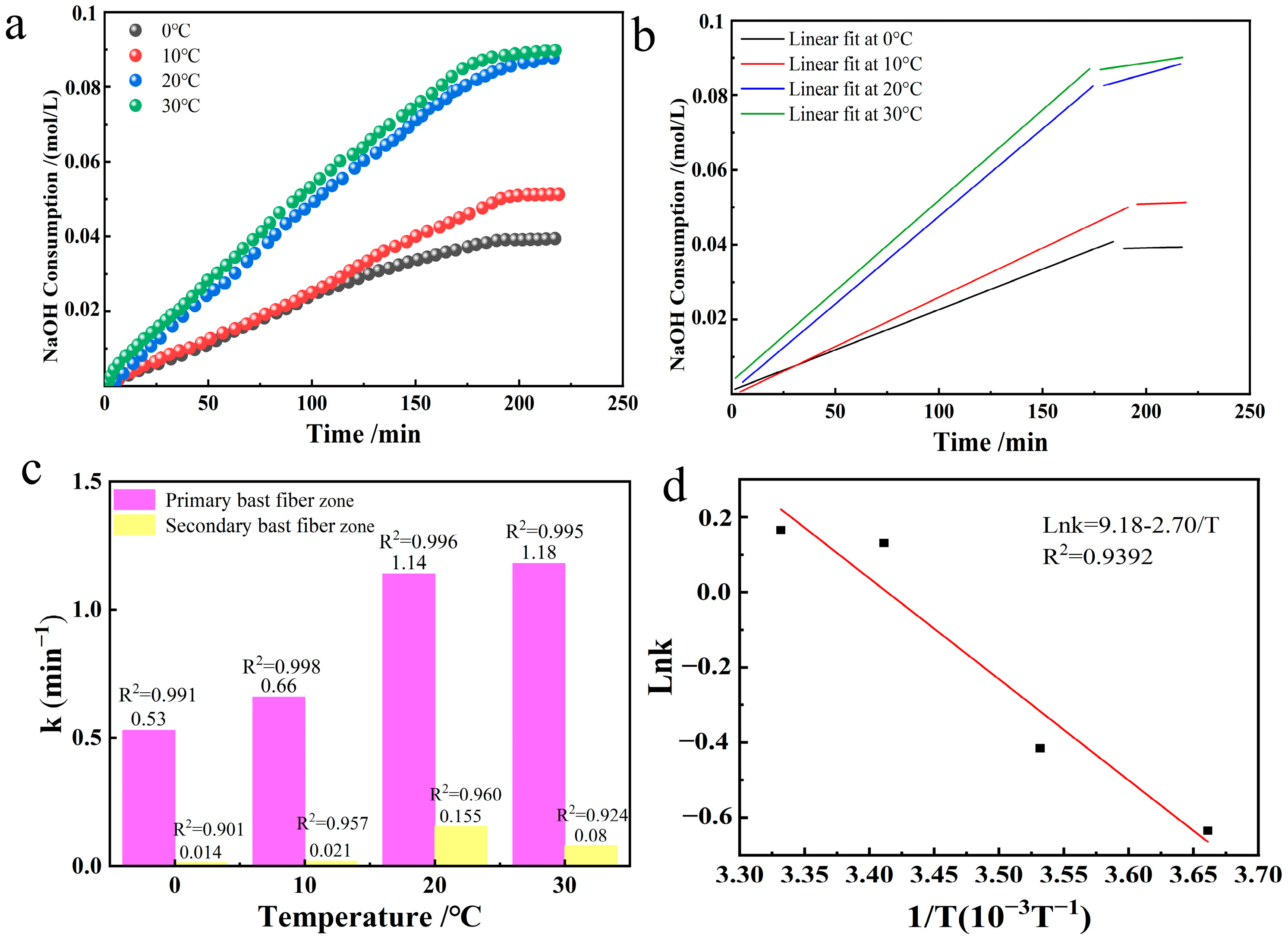
3.5. Effects of Temperature Changes
The effects produced during the selective oxidation of industrial hemp staple fibers were observed by varying the reaction temperature in the TEMPO/NaBr/NaClO system. We took 0.213 mmol/L of TEMPO, 3.24 mmol/L of NaBr, 109.26 mmol/L of NaClO, and 10 g of industrial hemp staple fiber dissolved in 750 mL of deionized water, adjusted the solution pH = 10, and compared the changes in the rate constants of the reaction at the temperatures of 0 °C, 10 °C, 20 °C, and 30 °C. The NaOH consumption in the reaction of oxidized industrial hemp staple fibers at different temperatures and its linear fitting graphs were plotted as shown in
Figure 7a,b.
According to the
Figure 7c data, the oxidation reaction in the primary bast fibers exhibits a high coefficient of determination, indicating that the reaction in this region complies with the first-order kinetic equation at different temperatures. The selective oxidation reaction rate constants increased significantly with increasing temperature, and the two were positively correlated. Although the time required for the reaction was similar for the four temperature conditions, the difference in reaction rates resulted in different final outputs of glucuronic acid. Specifically, at higher temperatures, molecular motion is intensified, the collision frequency is elevated, and more cellulose molecular chains are involved in the oxidation reaction [
24], thereby increasing the selective oxidation efficiency, resulting in more intense oxidation of industrial hemp staple fibers. In particular, the reaction rate constant at 30 °C was 2.2 times higher than that at 0 °C, a finding that highlights the critical role of temperature in modulating oxidation reactions.
According to the Arrhenius Equation:
taking logarithmic values yields
. The Arrhenius equation was plotted and fitted using the data for temperature and reaction rate constants from
Table 2. It is shown in
Figure 7c. From
Figure 7c, there is a good linear relationship between the different temperatures and the reaction rate constants. The slope after linear regression was −2.70, from which the apparent activation energy was calculated as 22.45 kJ/mol.
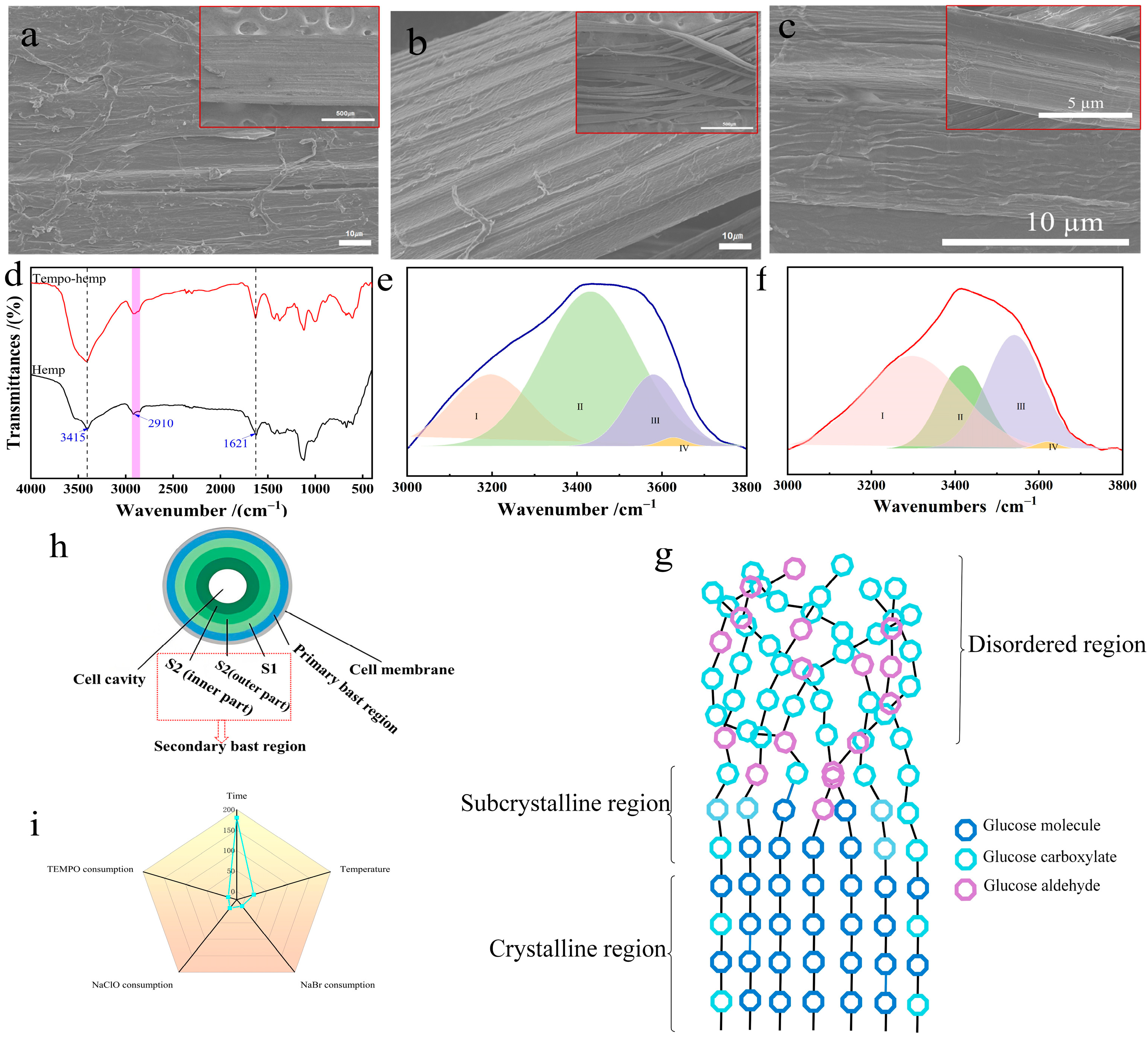
3.7. Chemical Structure and Hydrogen Bonding Analysis
Figure 8d shows the infrared spectra of industrial hemp staple fibers before and after oxidation by the TEMPO system. The industrial hemp staple fibers before and after oxidation of the TEMPO system had broad peaks at 3415 cm
−1 and narrow peaks at 2910 cm
−1, which were attributed to O-H and C-H in cellulose [
27], respectively. Additionally, 1621 cm
−1 corresponds to the C=O tensile vibration of carboxylic acid [
28]. The absorption peak of oxidized industrial hemp staple fiber is larger than that of untreated industrial hemp staple fiber. The results indicate that the carboxylic acid group forms during the oxidation reaction, with its content increasing. It was proved that the experimental method of measuring the consumption of NaOH (mL/min) to indirectly monitor the production of glucuronic acid (mol/L) and thus the oxidation reaction rate was feasible.
Cellulose, the main component of industrial hemp staple fiber, is a polyhydroxy compound with a large number of hydrogen bonds [
29,
30]. The spectral bands of the hydrogen bonding peaks in the range of 3000–3800 cm
−1 were fitted using the Gaussian fitting method [
29,
31], as shown in
Figure 8e,f. IV (cyclic polymers) and I (OH–OH) belong to intramolecular hydrogen bonding, III (OH–ether O) belongs to intermolecular hydrogen bonding, and II (-OH) belongs to the free hydroxyl group [
31]. The fit in the figure shows that as the percentage of intramolecular hydrogen bonding decreases (24.02% to 21.21%), the percentage of intermolecular hydrogen bonding increases (75.98% to 78.79%). This is because the oxygen atom brought by the carboxyl group strengthens the hydrogen bond cooperation network between molecules [
32]. The results showed that the selective oxidation of the oxidation kinetics of the TEMPO system acted mainly on the surface of primary and secondary bast fiber microfibers.
3.9. Microfiber Oxidation Model
For the study of the oxidation process, we should consider not only the spatial structure of each layer of microfibrils, but also the existence of crystalline and amorphous regions within a single microfibril (
Figure 8h). In the process of establishing the oxidation kinetic equation (
Section 3.1), it was found that the reaction rate of the TEMPO/NaBr/NaClO system in oxidizing the primary bast zone of industrial hemp staple fiber was 6.5 times higher than that of the secondary bast zone. We believe the reasons for this phenomenon include the following: (1) the close adhesion between the secondary bast region and the wood pith caused the reaction in the interface region to continue slowly; (2) it was caused by the different arrangement of microfibrils in the secondary bast region [
18]; and (3) the crystal structure of cellulose on a smaller scale, namely the amorphous region and crystalline region [
33,
34]. The first two points have been explained in the reaction rate analysis of oxidation kinetics, so we focus on the third point. The disordered region of cellulose accounts for about 1% to 3% of cellulose [
33,
34]. The disordered arrangement of molecular chains and the loose structure lead to faster reaction speed, and at least one C
6 aldehyde group is formed on each cellulose chain as an intermediate structure [
24]. With the increase in oxidation time, the macromolecular structure of cellulose becomes more relaxed [
13,
35]. By studying the crystal structure of cellulose after TEMPO oxidation, it was found that selective oxidation occurred not only in the amorphous region but also on the surface of the crystalline region [
21,
36], which means that both the primary and secondary phloem regions have crystalline regions involved in the reaction. Zhang Qiang et al. investigated the thermal degradation behavior and thermal degradation kinetics of cotton fibers using the TG-DTG technique under the protection of high-purity nitrogen [
37]. They found that the degradation of cotton fibers is a process that occurs excessively from the non-crystalline region to the crystalline region. The kinetic mechanism of the main degradation stage is diffusion-controlled, and the degradation rate is mainly affected by the temperature and decomposition rate. To express more clearly the oxidation of the crystalline, subcrystalline, and disordered zones within the microfibrils during the oxidation of industrial hemp staple fibers mediated by the TEMPO/NaBr/NaClO system to describe the distribution of atomic groups in the internal structure of the cellulose, an ideal model was developed as shown in
Figure 8g, without taking into account the non-selective oxidative depolymerization behavior of NaClO. Based on the widely accepted literature regarding the microstructure of cellulose microfibrils and the established mechanism of TEMPO-mediated oxidation initiating preferentially in disordered regions [
11,
38,
39], combined with the kinetic results of this study (which reveal a rapid phase corresponding to oxidation in accessible regions and a slow phase reflecting oxidation in recalcitrant regions), we propose a schematic model (
Figure 8g) to illustrate the hypothesized progression of TEMPO-mediated oxidation within the microstructure of industrial hemp bast fibers. This model posits that oxidation advances sequentially from amorphous regions and crystal surfaces toward the interior of the crystals, consistent with the observed biphasic kinetic behavior. It is important to note that this model is a conceptual schematic, intended to visually integrate structural knowledge with kinetic behavior, rather than being derived from computational simulations or representing an atomistically precise structural model.
3.10. Limitations of the Kinetic Model
The pseudo-first-order kinetic model employed in this study successfully captures the overall trends of hemp fiber oxidation within the TEMPO system. However, its applicability rests on several key assumptions: a large excess of oxidants (NaClO/NaBr) is required to maintain a steady concentration of active species, and the chemical reaction itself is the rate-determining step. It is crucial to note that this is a practical simplified model. The rate constant it reports is an “apparent” value, integrating both the chemical reaction and the diffusion process of reactants within the fiber structure. Consequently, the model is more adept at comparing the effects of different parameters under the specific experimental conditions of this study than at precisely predicting all complex scenarios. Deviations from the model are expected, particularly during the later stages of oxidation when targeting structurally dense secondary fibers, where increased diffusion resistance becomes significant. The underlying mechanisms for certain complex nonlinear phenomena observed (such as reaction inhibition at high TEMPO concentrations) are further explained by supplementary concepts like our proposed ‘variable-speed competition mechanism,’ which fall outside the scope of the fundamental kinetic model itself.
While the pseudo-first-order model provides excellent fits (high R2 values) for the primary reaction phase and allows for effective parameter comparison, its limitations must be acknowledged. The model simplifies the complex heterogeneous reality. Diffusional limitations likely become more pronounced in the later stages of oxidation (especially for the secondary bast fibers) and under conditions of high reagent loading, where reagent penetration into the dense fiber microstructure may become rate-influencing.