Characterization and Performance Evaluation of Cotton Fabrics Functionalized via In Situ Green Synthesis of Silver Nanoparticles Using Solanum tuberosum Peel Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Green Synthesis of AgNPs
2.2.2. In Situ Green Synthesis of Silver Nanoparticles onto Cotton Fabric
2.2.3. Durability of the Treated Fabrics to Washing
2.2.4. Qualitative Efficacy Testing of the Treated Fabrics After Bacteria
2.2.5. Characterization of the Physical and Chemical Properties of the Treated Samples
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Green Synthesis of Silver Nanoparticles
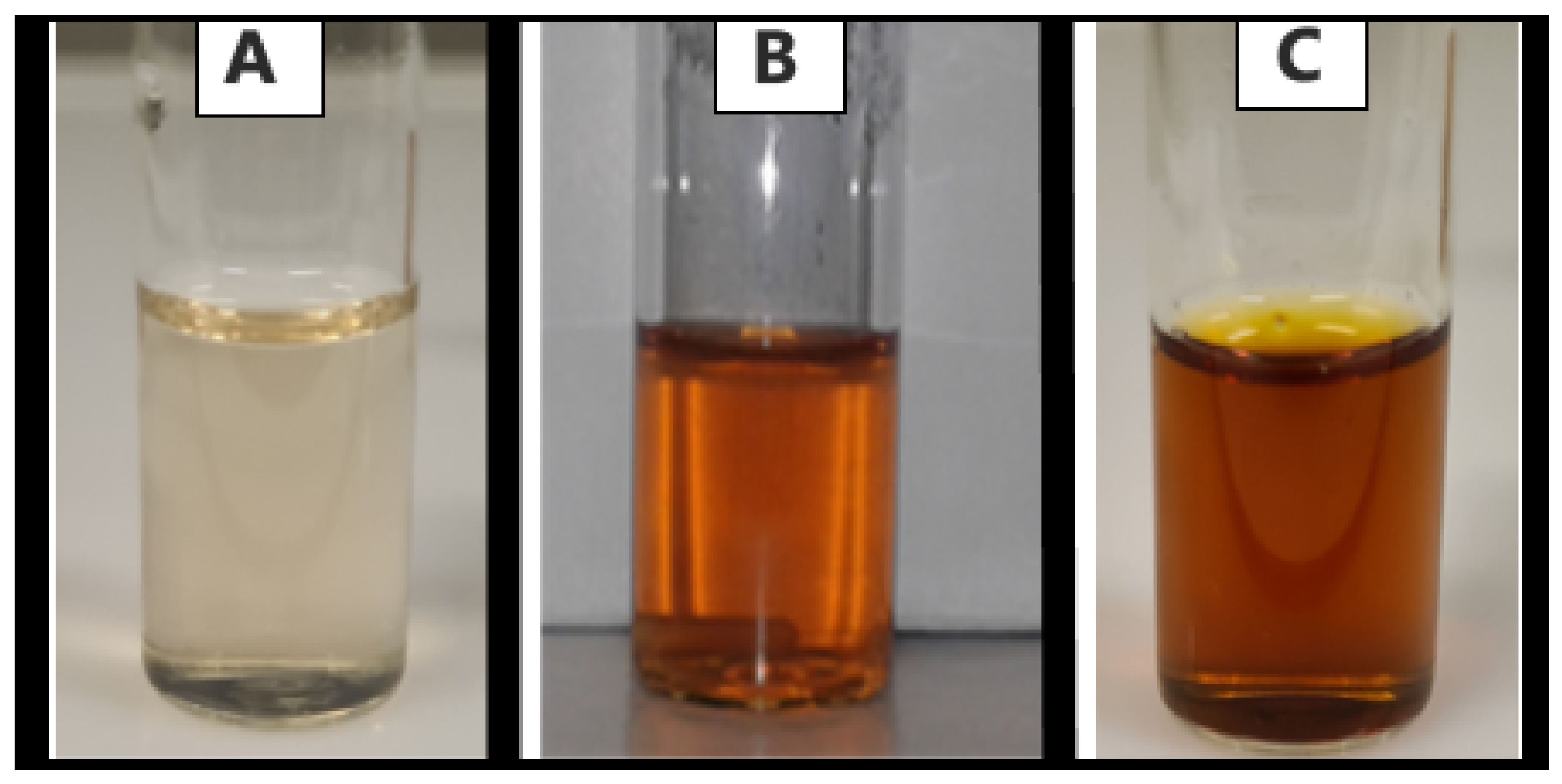
3.1.1. Visual Assessment of Green-Synthesized Nanoparticles
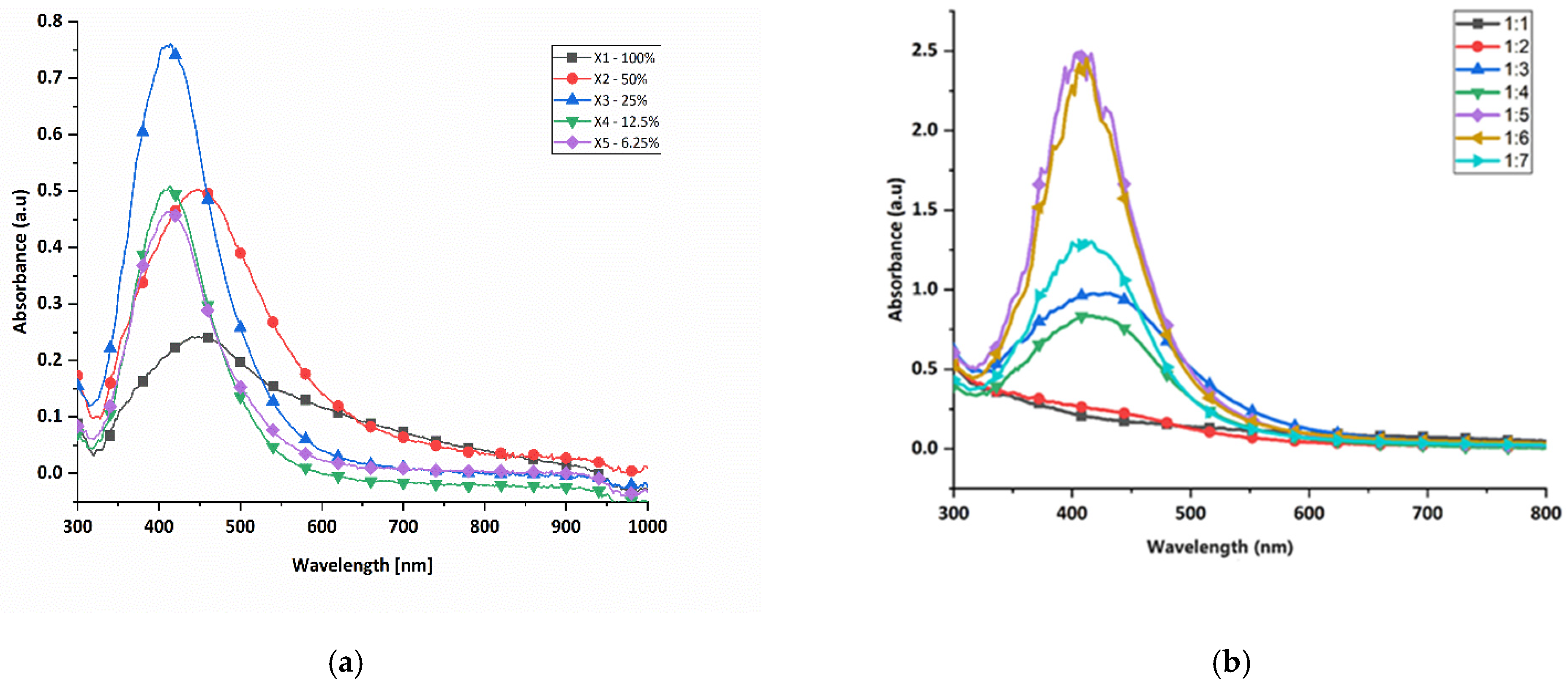
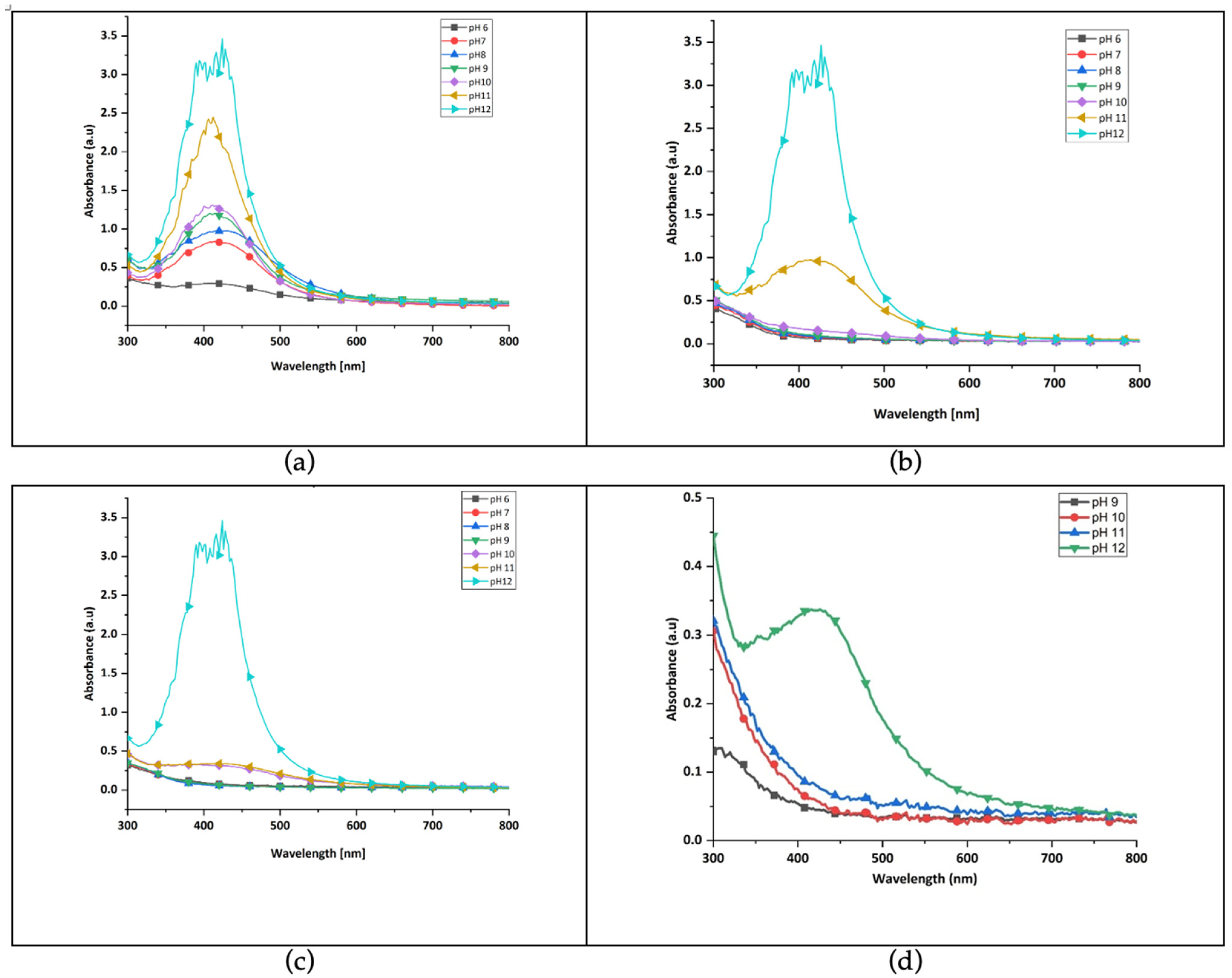
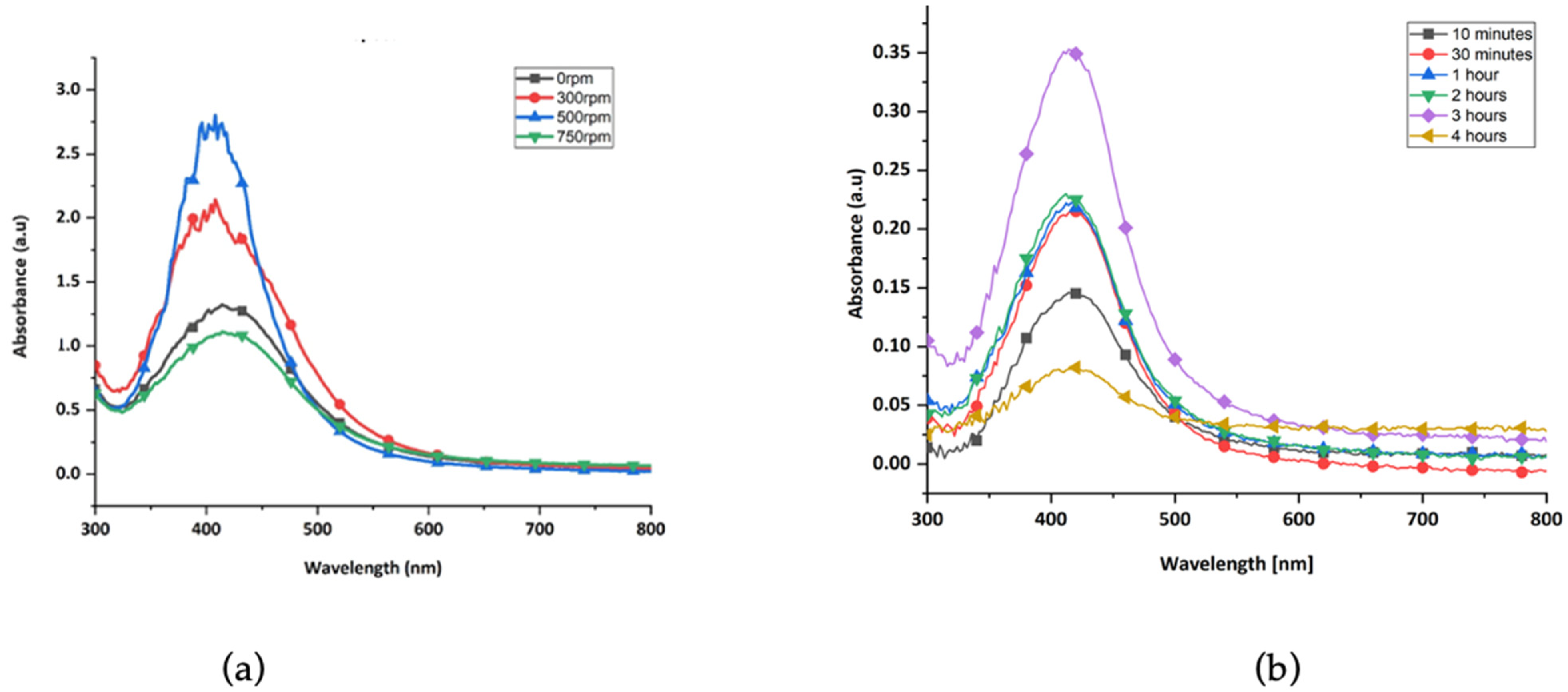
3.1.2. Determination of Suitable Green-Synthesis Parameters
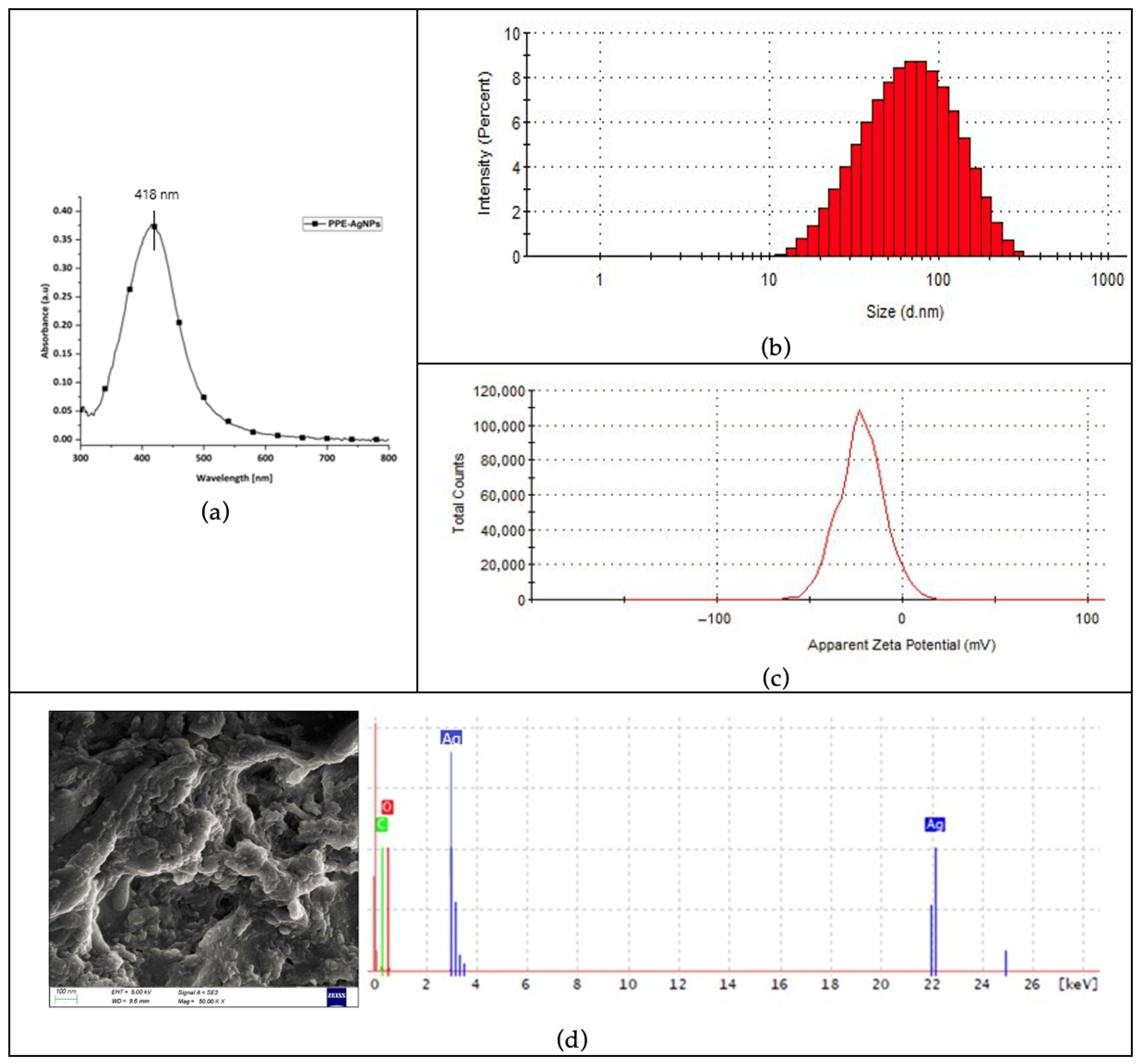
3.1.3. Characterization of Nanoparticles Synthesized Using Optimum Values
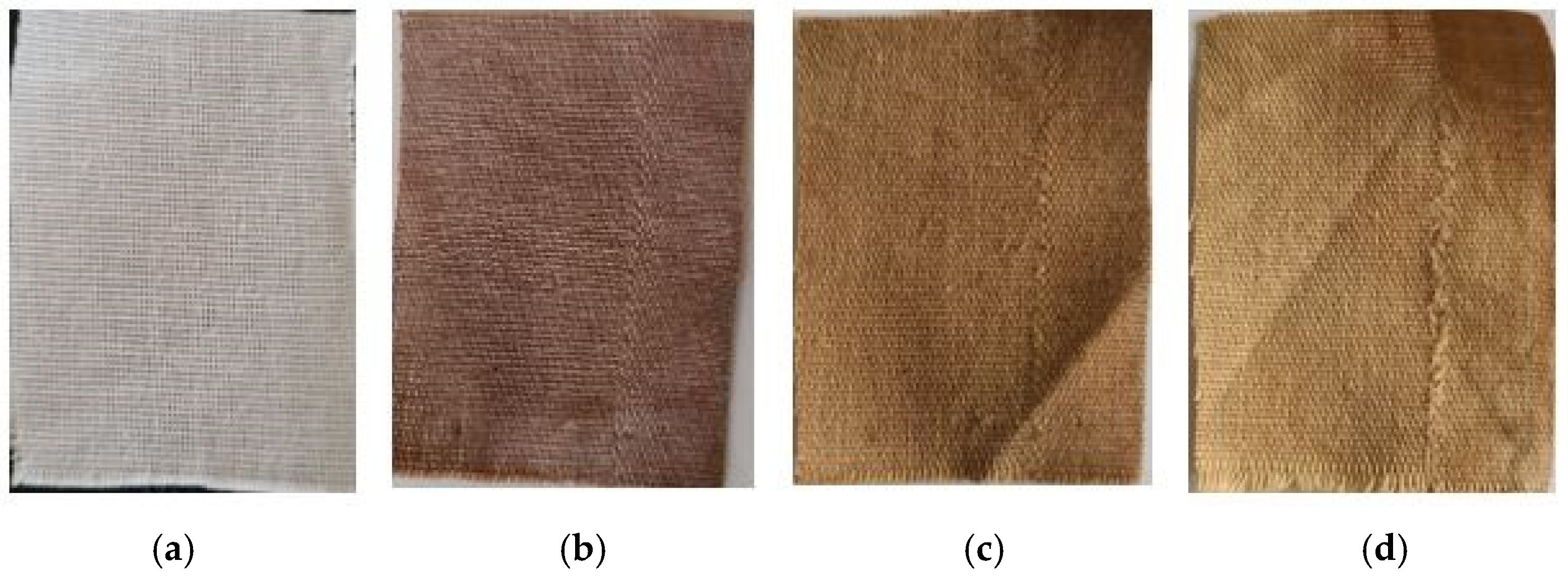
3.2. Visible Effects of the Silver Nanoparticles on Cotton Fabric
3.3. Antibacterial Efficacy Testing of the Treated Fabric Samples
3.4. Characteristics of the Fabric Treated with Silver Nanoparticles
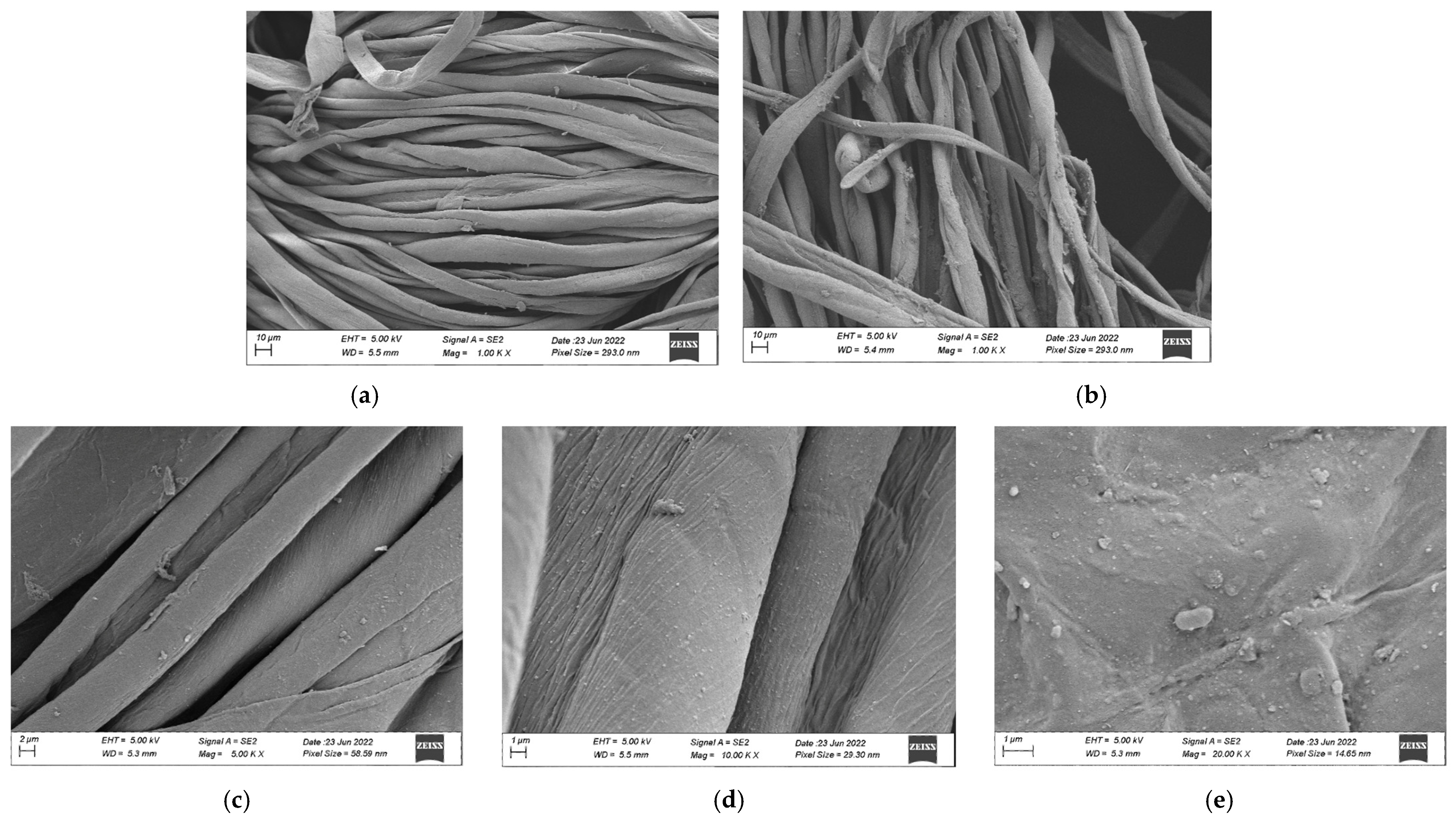
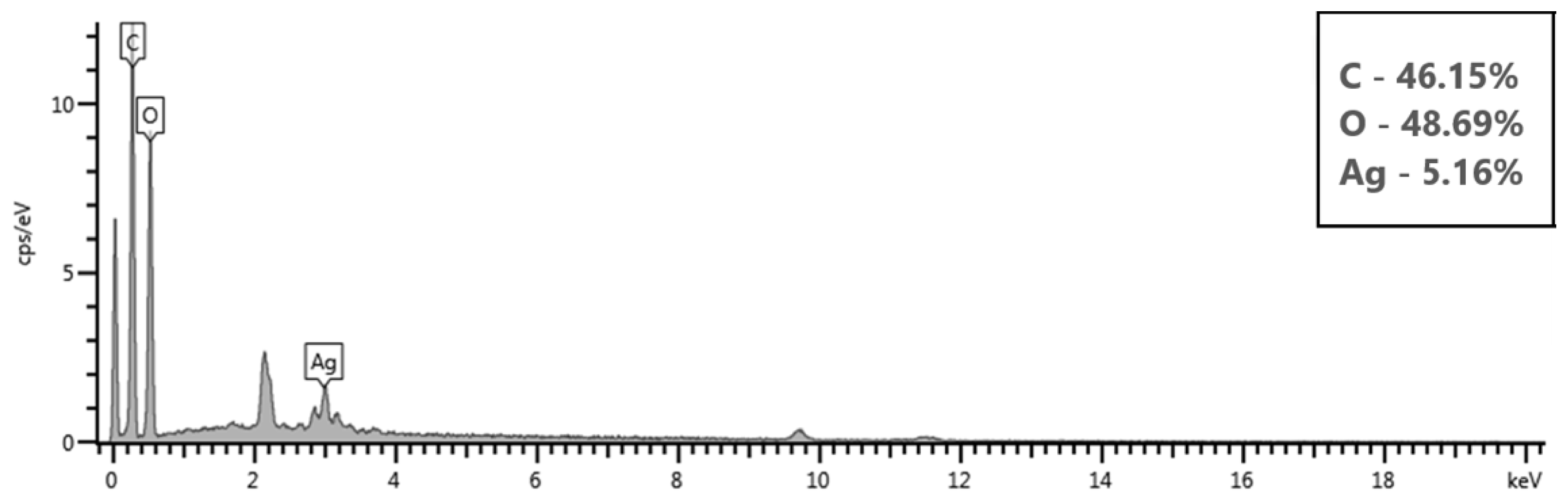
3.4.1. Morphology and Elemental Analysis
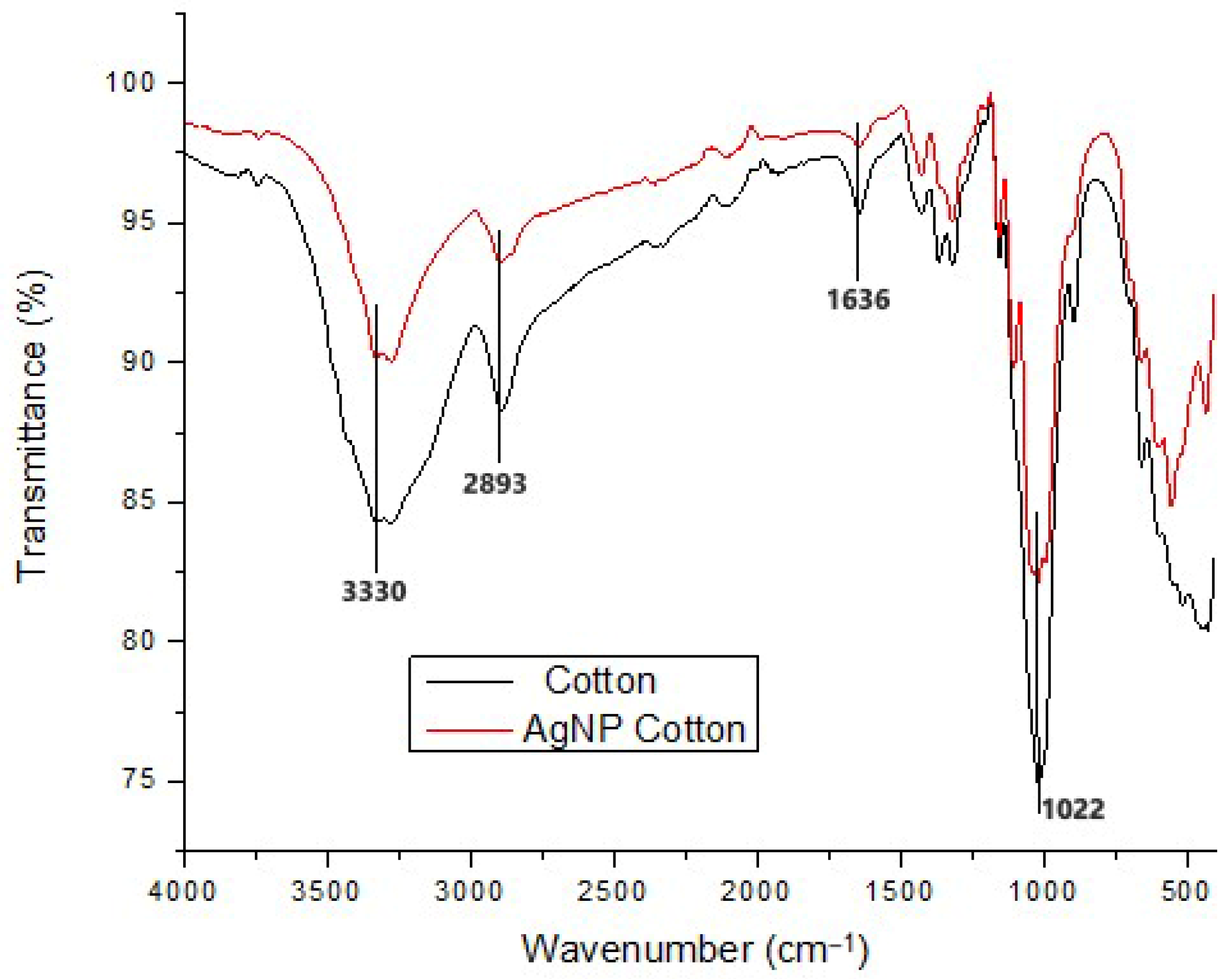
3.4.2. Functional Group Analysis
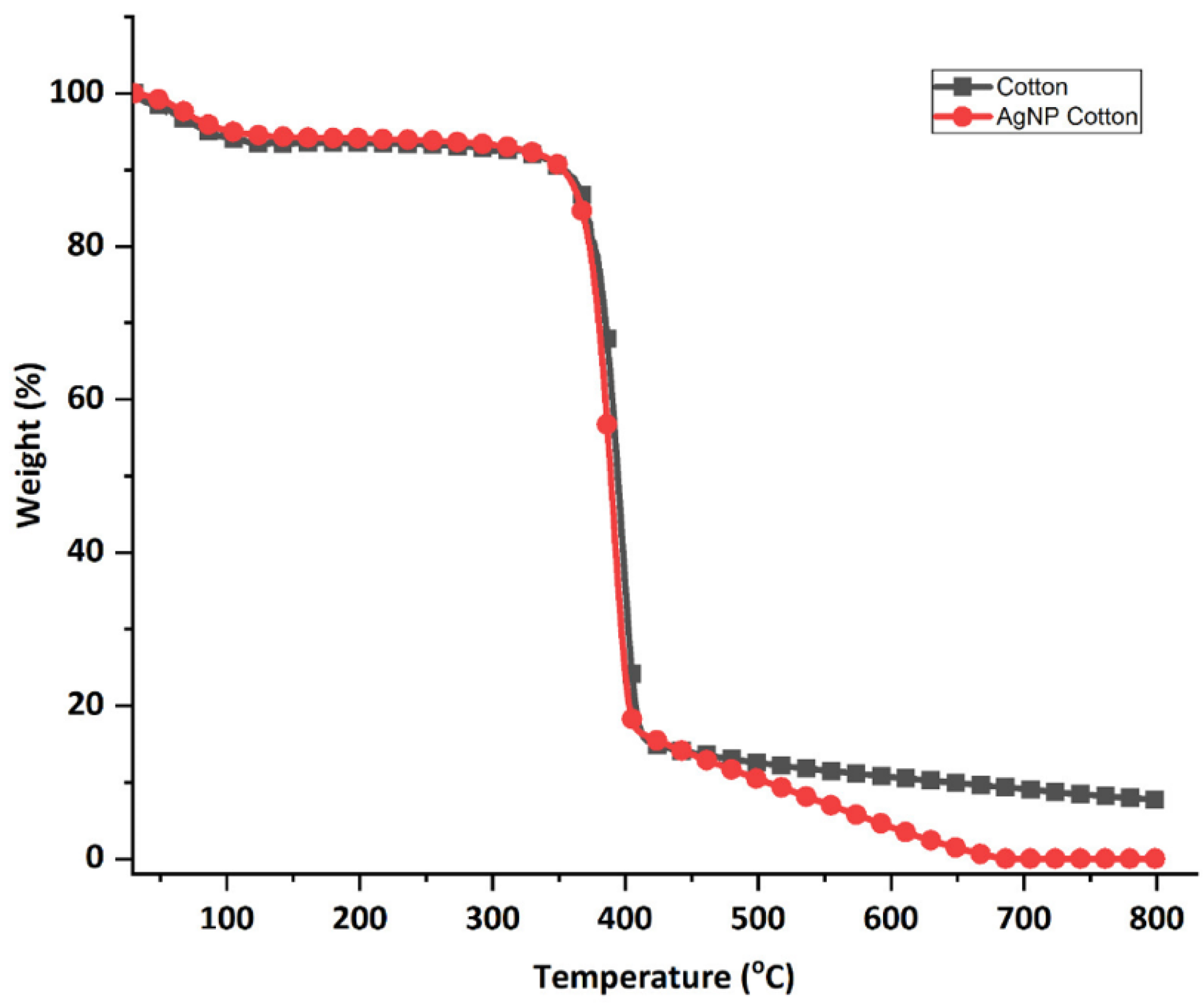
3.4.3. Thermogravimetric Analysis
3.4.4. Air Permeability
3.4.5. Tensile Strength
4. Conclusions and Recommendations
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boryo, D.E.A. The effect of microbes on textile material: A review on the way-out so far. Int. J. Eng. Sci. 2013, 2, 9–13. [Google Scholar]
- Sanders, D.; Grunden, A.; Dunn, R.R. A review of clothing microbiology: The history of clothing and the role of microbes in textiles. Biol. Lett. 2021, 17, 20200700. [Google Scholar] [CrossRef]
- Suliman, Z.A.; Mecha, A.C.; Mwasiagi, J.I. Characterization and Disinfection Performance of α-Fe2O3-TiO2 Based Polyester Membranes for Water Treatment. Univers. J. Green. Chem. 2024, 2, 336–346. [Google Scholar] [CrossRef]
- Khairy, T.; Amin, D.H.; Salama, H.M.; Elkholy, I.M.A.; Elnakib, M.; Gebreel, H.M.; Sayed, H.A.E. Antibacterial activity of green synthesized copper oxide nanoparticles against multidrug-resistant bacteria. Sci. Rep. 2024, 14, 25020. [Google Scholar] [CrossRef]
- Mpofu, N.S.; Igadwa, J.I.; Nganyi, E.O.; Kamhala, E. Antimicrobial and antiviral properties of metal nanoparticles and their potential use in textiles: A review. Ann. Univ. Oradea Fascicle Text Leatherwork 2021, 22, 69–74. [Google Scholar]
- Afraz, N.; Uddin, F.; Syed, U.; Mahmood, A. Antimicrobial Finishes for Textiles. Fash. Technol. Text. Eng. 2019, 4, 87–94. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.M.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial effect of silver nanoparticles synthesized using Murraya koenigii (L.) against multidrug-resistant pathogens. Bioinorg. Chem. Appl. 2019, 2019, 4649506. [Google Scholar] [CrossRef]
- Holubnycha, V.; Husak, Y.; Korniienko, V.; Bolshanina, S.; Tveresovska, O.; Myronov, P.; Holubnycha, M.; Butsyk, A.; Borén, T.; Banasiuk, R.; et al. Antimicrobial Activity of Two Different Types of Silver Nanoparticles against Wide Range of Pathogenic Bacteria. Nanomaterials 2024, 14, 137. [Google Scholar] [CrossRef]
- Ahmed, T.; Ogulata, R.T.; Sezgin Bozok, S. Silver Nanoparticles against SARS-CoV-2 and Its Potential Application in Medical Protective Clothing—A Review. J. Text. Inst. 2022, 113, 2825–2838. [Google Scholar] [CrossRef]
- Zhao, X.; Li, M.; Zhao, J.; Wang, X. Intelligent Sportswear Design: Innovative Applications Based on Conjugated Nanomaterials. Sci. Eng. Compos. Mater. 2024, 31, 20240032. [Google Scholar] [CrossRef]
- Ibrahim, N.A. Nanomaterials for Antibacterial Textiles; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Bisi, M.K.; El-Shafei, A. Green synthesis of silver nanoparticles and their application to cotton fabrics. Int. J. Biol. Macromol. 2015, 72, 1384–1390. [Google Scholar] [CrossRef]
- Xu, J.; Yıldıztekin, M.; Han, D.; Keskin, C.; Baran, A.; Baran, M.F.; Eftekhari, A.; Ava, C.A.; Kandemir, S.İ.; Cebe, D.B.; et al. Biosynthesis, characterization, and investigation of antimicrobial and cytotoxic activities of silver nanoparticles using Solanum tuberosum peel aqueous extract. Heliyon 2023, 9, e19061. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, T.I.; El Aty, A.A.A. In-situ green myco-synthesis of silver nanoparticles onto cotton fabrics for broad spectrum antimicrobial activity. Int. J. Biol. Macromol. 2018, 118, 2121–2130. [Google Scholar] [CrossRef]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 368, 58–63. [Google Scholar] [CrossRef]
- Ahamed, M.; Majeed Khan, M.A.; Siddiqui, M.K.J.; Alsalhi, M.S.; Alrokayan, S.A. Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2011, 43, 1266–1271. [Google Scholar] [CrossRef]
- Almadiy, A.A.; Nenaah, G.E. Ecofriendly Synthesis of Silver Nanoparticles Using Potato Steroidal Alkaloids and Their Activity Against Phytopathogenic Fungi. Braz. Arch. Biol. Technol. 2018, 61, e18180013. [Google Scholar] [CrossRef]
- Wolela, A.D. Antibacterial Finishing of Cotton Textiles with Extract of Citrus Fruit Peels. Fash. Technol. Text. Eng. 2020, 6, 555676. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Kathe, A.A.; Varadarajan, P.V.; Nachane, R.P.; Balasubramanya, R.H. Functional finishing of cotton fabrics using silver nanoparticles. J. Nanosci. Nanotechnol. 2007, 7, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Huang, V.; Quiambao, Q.; Noritake, S.; Liu, J.; Kwon, O.; Chintalapati, S.; Young, J.; Levin, C.E.; Tam, C.; et al. Potato Peels and Their Bioactive Glycoalkaloids and Phenolic Compounds Inhibit the Growth of Pathogenic Trichomonads. J. Agric. Food Chem. 2018, 66, 7942–7947. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Chaidez-Quiroz, C.; López-Cuevas, O.; Ruiz-Cruz, S.; López-Mata, M.A.; Del-Toro-sánchez, C.L.; Marquez-Rios, E.; Ornelas-Paz, J.D.J. Phenolic compounds of potato peel extracts: Their antioxidant activity and protection against human enteric viruses. J. Microbiol. Biotechnol. 2017, 27, 234–241. [Google Scholar] [CrossRef]
- Mpofu, N.S.; Mwasiagi, J.I.; Mecha, C.A.; Nganyi, E.O. Evaluation of solanum tuberosum potato peel waste for use as an eco-friendly antibacterial finish for cotton fabrics. Res. J. Text. Appar. 2025, 29, 203–217. [Google Scholar] [CrossRef]
- Buazar, F.; Bavi, M.; Kroushawi, F.; Halvani, M.; Khaledi-Nasab, A.; Hossieni, S.A. Potato Extract as Reducing Agent and Stabiliser in a Facile Green One-Step Synthesis of ZnO Nanoparticles. J. Exp. Nanosci. 2016, 11, 175–184. [Google Scholar] [CrossRef]
- Mahdieh, Z.M.; Shekarriz, S.; Taromi, F.A. The Effect of Silver Concentration on Ag-TiO2 Nanoparticles Coated Polyester/Cellulose Fabric by In-situ and Ex-situ Photo-reduction Method—A Comparative Study. Fibers Polym. 2021, 22, 87–96. [Google Scholar] [CrossRef]
- Čuk, N.; Šala, M.; Gorjanc, M. Development of antibacterial and UV protective cotton fabrics using plant food waste and alien invasive plant extracts as reducing agents for the in-situ synthesis of silver nanoparticles. Cellulose 2021, 28, 3215–3233. [Google Scholar] [CrossRef]
- Harifi, T.; Montazer, M. A review on textile sonoprocessing: A special focus on sonosynthesis of nanomaterials on textile substrates. Ultrason. Sonochem. 2015, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Cai, Y.; Chen, X.; Ke, Y. Silver-based nanocomposite for fabricating high performance value-added cotton. Cellulose 2022, 29, 723–750. [Google Scholar] [CrossRef]
- Jacob, P.J.S. Cotton based cellulose nanocomposites: Synthesis and application. In Cotton; Intechopen: London, UK, 2022; pp. 1–20. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.H. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. J. Nanomater. 2014, 2014, 417305. [Google Scholar] [CrossRef]
- Seifipour, R.; Nozari, M.; Pishkar, L. Green Synthesis of Silver Nanoparticles using Tragopogon Collinus Leaf Extract and Study of Their Antibacterial Effects. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2926–2936. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Choi, I. Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci. Rep. 2016, 6, 20414. [Google Scholar] [CrossRef]
- Kredy, M.H. The effect of pH, temperature on the green synthesis and biochemical activities of silver nanoparticles from Lawsonia inermis extract. J. Pharm. Sci. Res. 2018, 10, 2022–2026. [Google Scholar]
- Aladpoosh, R.; Montazer, M.; Samadi, N. In situ green synthesis of silver nanoparticles on cotton fabric using Seidlitzia rosmarinus ashes. Cellulose 2014, 21, 3755–3766. [Google Scholar] [CrossRef]
- Liu, H.; Lee, Y.Y.; Norsten, T.B.; Chong, K. In situ formation of anti-bacterial silver nanoparticles on cotton textiles. J. Ind. Text. 2014, 44, 198–210. [Google Scholar] [CrossRef]
- ISO 105-C10: 2006; Textiles—Tests for Colour Fastness—Part c10: Colour Fastness to Washing with Soap or Soap and Soda. International Organisation for Standardisation (ISO): Geneva, Switzerland, 2006.
- Jain, A.; Kongkham, B.; Puttaswamy, H.; Butola, B.S.; Malik, H.K.; Malik, A. Development of Wash-Durable Antimicrobial Cotton Fabrics by In Situ Green Synthesis of Silver Nanoparticles and Investigation of Their Antimicrobial Efficacy against Drug-Resistant Bacteria. Antibiotics 2022, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- ISO 9237; Textiles—Determination of the Permeability of Fabrics to Air. International Organisation for Standardisation (ISO): Geneva, Switzerland, 1995.
- ISO 13934: 2013; Textiles—Tensile properties of fabrics—Part 1: Determination of maximum force and elongation at maximum force using the strip method. International Organisation for Standardisation (ISO): Geneva, Switzerland, 2013.
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- De Leersnyder, I.; Rijckaert, H.; De Gelder, L.; Van Driessche, I.; Vermeir, P. High variability in silver particle characteristics, silver concentrations, and production batches of commercially available products indicates the need for a more rigorous approach. Nanomaterials 2020, 10, 1394. [Google Scholar] [CrossRef]
- Djuhana, D.; Putra, M.H.; Imawan, C.; Fauzia, V.; Harmoko, A.; Handayani, W.; Ardani, H. Numerical study of the plasmonic resonance sensitivity silver nanoparticles coated polyvinyl alcohol (PVA) using Bohren-Huffman-Mie (BHMie) approximation. AIP Conf. Proc. 2016, 1729, 020023. [Google Scholar] [CrossRef]
- Verma, A.; Mehata, M.S. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Radiat. Res. Appl. Sci. 2016, 9, 109–115. [Google Scholar] [CrossRef]
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The antimicrobial and anti-inflammatory effects of silver nanoparticles synthesised from cotyledon orbiculata aqueous extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef]
- Kaur, R.; Avti, P.; Kumar, V.; Kumar, R. Effect of various synthesis parameters on the stability of size controlled green synthesis of silver nanoparticles. Nano Express 2021, 2, 020005. [Google Scholar] [CrossRef]
- Melkamu, W.W.; Bitew, L.T. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) J.F. Gmel plant leaf extract and their antibacterial and anti-oxidant activities. Heliyon 2021, 7, e08459. [Google Scholar] [CrossRef] [PubMed]
- Azarbani, F.; Shiravand, S. Green synthesis of silver nanoparticles by Ferulago macrocarpa flowers extract and their antibacterial, antifungal and toxic effects. Green Chem. Lett. Rev. 2020, 13, 41–49. [Google Scholar] [CrossRef]
- Mahiuddin, M.; Saha, P.; Ochiai, B. Green synthesis and catalytic activity of silver nanoparticles based on piper chaba stem extracts. Nanomaterials 2020, 10, 1777. [Google Scholar] [CrossRef]
- Rao, B.; Tang, R.C. Green synthesis of silver nanoparticles with antibacterial activities using aqueous Eriobotrya japonica leaf extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 015014. [Google Scholar] [CrossRef]
- Jang, M.H.; Lee, S.; Hwang, Y.S. Characterization of silver nanoparticles under environmentally relevant conditions using asymmetrical flow field-flow fractionation (AF4). PLoS ONE 2015, 10, e0143149. [Google Scholar] [CrossRef]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest. Control. 2018, 28, 28. [Google Scholar] [CrossRef]
- Jalab, J.; Abdelwahed, W.; Kitaz, A.; Al-Kayali, R. Green synthesis of silver nanoparticles using aqueous extract of Acacia cyanophylla and its antibacterial activity. Heliyon 2021, 7, e08033. [Google Scholar] [CrossRef]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Cevik, O. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crop. Prod. 2013, 46, 132–137. [Google Scholar] [CrossRef]
- Balamurugan, M.; Saravanan, S.; Soga, T. Coating of green-synthesized silver nanoparticles on cotton fabric. J. Coat. Technol. Res. 2017, 14, 735–745. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, J.; Lu, Z.; Tian, Q.; Shao, J. In Situ Synthesis of Silver Nanoparticles on Flame-Retardant Cotton Textiles Treated with Biological Phytic Acid and Antibacterial Activity. Materials 2022, 15, 2537. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Pervez, N.; Taher, M.A.; Mohiuddin, K.; Liu, H.H. Multifunctional organic cotton fabric based on silver nanoparticles green synthesized from sodium alginate. Text. Res. J. 2020, 90, 1224–1236. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E.; Etemadifar, A. Fabricating multifunctional silver nanoparticles-coated cotton fabric. Arab. J. Chem. 2017, 10, S2355–S2362. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Fulazzaky, M.A.; Salmiati, S.; Roestamy, M. Sticky silver nanoparticles and surface coatings of different textile fabrics stabilised by Muntingia calabura leaf extract. SN Appl. Sci. 2020, 2, 733. [Google Scholar] [CrossRef]
- Novoa, C.C.; Tortella, G.; Seabra, A.B.; Diez, M.C.; Rubilar, O. Cotton Textile with Antimicrobial Activity and Enhanced Durability Produced by L -Cysteine-Capped Silver nanoparticles. Processes 2022, 10, 958. [Google Scholar] [CrossRef]
- Tania, I.S.; Ali, M.; Bhuiyan, R.H. Experimental Study on Dyeing Performance and Antibacterial Activity of Silver Nanoparticle-Immobilized Cotton Woven Fabric. Autex Res. J. 2020, 21, 45–51. [Google Scholar] [CrossRef]
- Salama, A.; Abouzeid, R.E.; Owda, M.E.; Cruz-Maya, I.; Guarino, V. Cellulose–silver composites materials: Preparation and applications. Biomolecules 2021, 11, 1684. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Navaneethaiyer, U.; Mohan, R.; Lee, J.; Kim, S.J. Graphene oxide nanostructures modified multifunctional cotton fabrics. Appl. Nanosci. 2012, 2, 119–126. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M.; Hassan, M.S. Characterization and antimicrobial properties of cotton fabric loaded with green synthesized silver nanoparticles. Carbohydr. Polym. 2016, 151, 841–850. [Google Scholar] [CrossRef]
- Jagadeshvaran, P.L.; Bose, S. Nano silver-deposited cotton textile core with carbon nanostructure-filled shell for suppression of electromagnetic radiation via absorption-reflection-absorption. Mater. Chem. Phys. 2023, 293, 126897. [Google Scholar] [CrossRef]
- Nam, S.; Baek, I.S.; Hillyer, M.B.; He, Z.; Barnaby, J.Y.; Condon, B.D.; Kim, M.S. Thermosensitive textiles made from silver nanoparticle-filled brown cotton fibers. Nanoscale Adv. 2022, 4, 3725–3736. [Google Scholar] [CrossRef]
- Ogulata, R.T. Air Permeability of Woven Fabrics. J. Text. Appar. Technol. Manag. 2006, 5, 473–480. [Google Scholar] [CrossRef]
- Onal, L.; Yildirim, M. Comfort properties of functional three-dimensional knitted spacer fabrics for home-textile applications. Text. Res. J. 2012, 82, 1751–1764. [Google Scholar] [CrossRef]
- Ali, A.; Nguyen, N.H.A.; Baheti, V.; Ashraf, M.; Militky, J.; Mansoor, T.; Noman, M.T.; Ahmad, S. Electrical conductivity and physiological comfort of silver coated cotton fabrics. J. Text. Inst. 2018, 109, 620–628. [Google Scholar] [CrossRef]
- Noman, M.T.; Petru, M.; Amor, N.; Louda, P. Thermophysiological comfort of zinc oxide nanoparticles coated woven fabrics. Sci. Rep. 2020, 10, 21080. [Google Scholar] [CrossRef]
- Gollapudi, V.R.; Mallavarapu, U.; Seetha, J.; Akepogu, P.; Amara, V.R.; Natarajan, H.; Anumakonda, V. In situ generation of silver and silver oxide nanoparticles on cotton fabrics using Tinospora cordifolia as bio reductant. SN Appl. Sci. 2020, 2, 508. [Google Scholar] [CrossRef]
- Xu, Q.B.; Xie, L.J.; Diao, H.; Li, F.; Zhang, Y.Y.; Fu, F.Y.; Liu, X.D. Antibacterial cotton fabric with enhanced durability prepared using silver nanoparticles and carboxymethyl chitosan. Carbohydr. Polym. 2017, 177, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Elmogahzy, Y.; Farag, R. Tensile properties of cotton fibers: Importance, research, and limitations. In Handbook of Properties of Textile and Technical Fibres; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Chapter 7; pp. 223–273. [Google Scholar] [CrossRef]


| Fabric Samples | Zone of Inhibition (mm) Mean ± SD | |
|---|---|---|
| E. coli | S. aureus | |
| Untreated fabric (negative control) | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Positive control | 40.00 ± 0.02 | 30.00 ± 0.04 |
| PPE-treated fabric before washing | 12.00 ± 0.01 | 8.00 ± 0.02 |
| PPE-treated fabric after 20 washes | 5.00 ± 0.00 | 3.50 ± 0.03 |
| AgNP-treated fabric before washing | 23.00 ± 0.05 | 16.00 ± 0.03 |
| AgNP-treated fabric after 20 washes | 13.00 ± 0.02 | 9.00 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpofu, N.S.; Mwasiagi, J.I.; Mecha, C.A.; Nganyi, E.O. Characterization and Performance Evaluation of Cotton Fabrics Functionalized via In Situ Green Synthesis of Silver Nanoparticles Using Solanum tuberosum Peel Extract. Polymers 2025, 17, 2598. https://doi.org/10.3390/polym17192598
Mpofu NS, Mwasiagi JI, Mecha CA, Nganyi EO. Characterization and Performance Evaluation of Cotton Fabrics Functionalized via In Situ Green Synthesis of Silver Nanoparticles Using Solanum tuberosum Peel Extract. Polymers. 2025; 17(19):2598. https://doi.org/10.3390/polym17192598
Chicago/Turabian StyleMpofu, Nonsikelelo Sheron, Josphat Igadwa Mwasiagi, Cleophas Achisa Mecha, and Eric Oyondi Nganyi. 2025. "Characterization and Performance Evaluation of Cotton Fabrics Functionalized via In Situ Green Synthesis of Silver Nanoparticles Using Solanum tuberosum Peel Extract" Polymers 17, no. 19: 2598. https://doi.org/10.3390/polym17192598
APA StyleMpofu, N. S., Mwasiagi, J. I., Mecha, C. A., & Nganyi, E. O. (2025). Characterization and Performance Evaluation of Cotton Fabrics Functionalized via In Situ Green Synthesis of Silver Nanoparticles Using Solanum tuberosum Peel Extract. Polymers, 17(19), 2598. https://doi.org/10.3390/polym17192598







