Theoretical Study on the Grafting Reaction of Benzophenone Compounds to Polyethylene in the UV Radiation Cross-Linking Process
Abstract
1. Introduction
2. Computation Methods
3. Results and Discussion
3.1. Stationary Point Geometries
3.2. Frontier MOs
3.3. Energetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Orton, H. History of underground power cables. IEEE Electr. Insul. Mag. 2013, 29, 52–57. [Google Scholar] [CrossRef]
- Teyssedre, G.; Laurent, C. Advances in high-field insulating polymeric materials over the past 50 years. IEEE Electr. Insul. Mag. 2013, 29, 26–36. [Google Scholar] [CrossRef]
- He, J.L.; Dang, B.; Zhou, Y.; Hu, J. Reviews on Research Progress and Key Technology in Extruded Cables for HVDC Transmission. High Volt. Eng. 2015, 41, 1417–1429. [Google Scholar] [CrossRef]
- Montanari, G.C.; Laurent, C.; Teyssedre, G.; Campus, A.; Nilsson, U.H. From LDPE to XLPE: Investigating the change of electrical properties. Part I. Space charge, conduction and lifetime. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 438–446. [Google Scholar] [CrossRef]
- Mazzanti, G.; Montanari, G.C. Electrical aging and life models: The role of space charge. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 876–890. [Google Scholar] [CrossRef]
- Montanari, G.C.; Mazzanti, G.; Palmieri, F.; Motori, A.; Perego, G.; Serra, S. Space-charge trapping and conduction in LDPE, HDPE and XLPE. J. Phys. D Appl. Phys. 2001, 34, 2902–2911. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Zhao, H.; Han, B.Z.; Li, Z.S. Mechanisms on electrical breakdown strength increment of polyethylene by acetophenone and its analogues addition: A theoretical study. J. Mol. Model. 2013, 19, 4477–4485. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.C. Insulation thickness determination of polymeric power cables. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 624–629. [Google Scholar] [CrossRef]
- Bostrom, J.O.; Marsden, E.; Hampton, R.N.; Nilsson, U. Electrical stress enhancement of contaminants in XLPE insulation used for power cables. IEEE Electr. Insul. Mag. 2003, 19, 6–12. [Google Scholar] [CrossRef]
- Englund, V.; Huuva, R.; Gubanski, S.M.; Hjertberg, T. Synthesis and efficiency of voltage stabilizers for XLPE cable insulation. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1455–1461. [Google Scholar] [CrossRef]
- Ashcraft, A.; Eichhorn, R.M.; Shaw, R.G. Laboratory studies of treeing in solid dielectrics and voltage stabilization of polyethylene. In Proceedings of the IEEE International Symposium on Electrical Insulation, Montreal, QC, Canada, 14–16 June 1976. [Google Scholar]
- Zhang, H.; Zhao, H.; Wang, X.; Shang, Y.; Han, B.Z.; Li, Z.S. Theoretical study on the mechanisms of polyethylene electrical breakdown strength increment by the addition of voltage stabilizers. J. Mol. Model. 2014, 20, 2211. [Google Scholar] [CrossRef]
- Fu, Y.W.; Sun, W.F.; Wang, X. UV-Initiated Crosslinking Reaction Mechanism and Electrical Breakdown Performance of Crosslinked Polyethylene. Polymers 2020, 12, 420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shang, Y.; Li, M.X.; Zhao, H.; Wang, X.; Han, B.Z. Theoretical study on the radical reaction mechanism in the cross-linking process of polyethylene. RSC Adv. 2015, 5, 90343–90353. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Li, M.X.; Zhao, H.; Wang, X.; Han, B.Z. Theoretical Study on the Reaction Mechanism in the UV radiation Cross-linking Process of Polyethylene. RSC Adv. 2016, 112, 110831–110839. [Google Scholar] [CrossRef]
- Horne, J.K.; Urmy, S.S.; Barbee, D.H. Increase in breakdown strength of PE film by additives of azocompounds. IEEE Trans. Dielectr. Electr. Insul. 1998, 5, 270–275. [Google Scholar] [CrossRef]
- Yamano, Y. Roles of polycyclic compounds in increasing breakdown strength of LDPE film. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 773–781. [Google Scholar] [CrossRef]
- Jarvid, M.; Johansson, A.; Englund, V.; Gubanski, S.; Andersson, M.R. Electrical tree inhibition by voltage stabilizers. In Proceedings of the Annual Repot Conference on Electrical Insulation and Dielectric Phenomena, Montreal, QC, Canada, 14–17 October 2012. [Google Scholar]
- Jarvid, M.; Johansson, A.; Bjuggren, J.M.; Wutzel, H.; Englund, V.; Gubanski, S.; Müller, C.; Andersson, M.R. Tailored side-chain architecture of Benzil voltage stabilizers for enhanced dielectric strength of cross-linked polyethylene. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1047–1054. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Zhao, H.; Han, B.Z.; Li, Z.S. Study of the effect of valence bond isomerizations on electrical breakdown by adding acetophenone to polyethylene as voltage stabilizers. Comput. Theor. Chem. 2015, 5, 99–104. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Wang, X.; Zhao, H.; Han, B.Z.; Li, Z.S. Mechanisms on electrical breakdown strength increment of polyethylene by aromatic carbonyl compounds addition: A theoretical study. J. Mol. Model. 2013, 19, 5429–5438. [Google Scholar] [CrossRef] [PubMed]
- Jarvid, M.; Johansson, A.; Englund, V.; Lundin, A.; Gubanski, S.; Müller, C.; Andersson, M.R. High electron affinity a guiding criterion for voltage stabilizer design. J. Mater. Chem. A 2015, 3, 7273–7286. [Google Scholar] [CrossRef]
- Rånby, B.; Rabek, J.F. Photooxidation, Photodegradation and Photostabilisation of Polymers; Wiley: New York, NY, USA, 1975. [Google Scholar]
- McKellar, J.F.; Allen, N.S. Photochemistry of Man-Made Polymers; Applied Science Publishers Ltd.: London, UK, 1979. [Google Scholar]
- Hardy, W.B. Developments in Polymerphotochemistry; Applied Science Publishers Ltd.: London, UK, 1982. [Google Scholar]
- Allen, N.S. Photostabilising Action of ortho-Hydroxy Aromatic Compounds: A Critical Review. Polym. Photochem. 1983, 3, 167–187. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, C.C.; Zhao, H.; Zhang, H.; Wang, X.; Han, B.Z. Grafted UV absorber as voltage stabilizer against electrical degradation and breakdown in cross-linked polyethylene for high voltage cable insulation. Polym. Degrad. Stab. 2021, 185, 109498. [Google Scholar] [CrossRef]
- Englund, V.; Huuva, R.; Gubanski, S.M.; Hjertberg, T. High efficiency voltage stabilizers for XLPE cable insulation. Polym. Degrad. Stab. 2009, 94, 823–833. [Google Scholar] [CrossRef]
- Wutzel, H.; Jarvid, M.; Bjuggren, J.M.; Johansson, A.; Englund, V.; Gubanski, S.; Andersson, M.R. Thioxanthone derivatives as stabilizers against electrical breakdown in cross-linked polyethylene for high voltage cable applications. Polym. Degrad. Stab. 2015, 112, 63–69. [Google Scholar] [CrossRef]
- Gao, L.Y.; Tu, D.M.; Qiu, B.; Huang, G.L.; Wang, L.H. Effect of ferrocene derivatives on the dielectric of polyethylene. In Proceedings of the 3rd International Conference on Properties and Applications of Dielectric Materials, Tokyo, Japan, 8–12 July 1991. [Google Scholar]
- Martinotto, L.; Peruzzotti, F.; Brenna, M.D. Cable, in Particular for Transport or Distribution of Electrical Energy and Insulating Composition. U.S. Patent 6,154,696, 1 October 2003. [Google Scholar]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Truong, T.N.; Duncan, W.T.; Bell, R.L. Chemical Applications of Density-Functional Theory; American Chemical Society: Washington, DC, USA, 1996. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. A 1965, 140, 1133. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200. [Google Scholar] [CrossRef]
- Zangwill, A.; Soven, P. Density-functional approach to local-field effects in finite systems: Photoabsorption in the rare gases. Phys. Rev. A 1980, 21, 1561–1572. [Google Scholar] [CrossRef]
- Levine, Z.H.; Soven, P. Time-dependent local-density theorey of dielectric effects in small molecules. Phys. Rev. A 1984, 29, 625–635. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision D.01); Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Wei, Z.J.; Liu, H.Y.; Yu, L.W.; Xiao, S.W.; Hou, Y.X.; Chen, X.R. Delocalized aromatic molecules with matched electron-donating and electron-withdrawing groups enhancing insulating performance of polyethylene blends. J. Appl. Polym. Sci. 2020, 137, e49185. [Google Scholar] [CrossRef]
- Aloïse, S.; Ruckebusch, C.; Blanchet, L.; Réhault, J.; Huvenne, J.P. The benzophenone S1 (n, π*) → T1 (n, π*) states intersystem crossing reinvestigated by ultrafast absorption spectroscopy and multivariate curve resolution. J. Phys. Chem. A 2008, 112, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Favero, L.; Granucci, G.; Persicob, M. Surface hopping investigation of benzophenone excited state dynamics. Phys. Chem. Chem. Phys. 2016, 18, 10499–10506. [Google Scholar] [CrossRef] [PubMed]
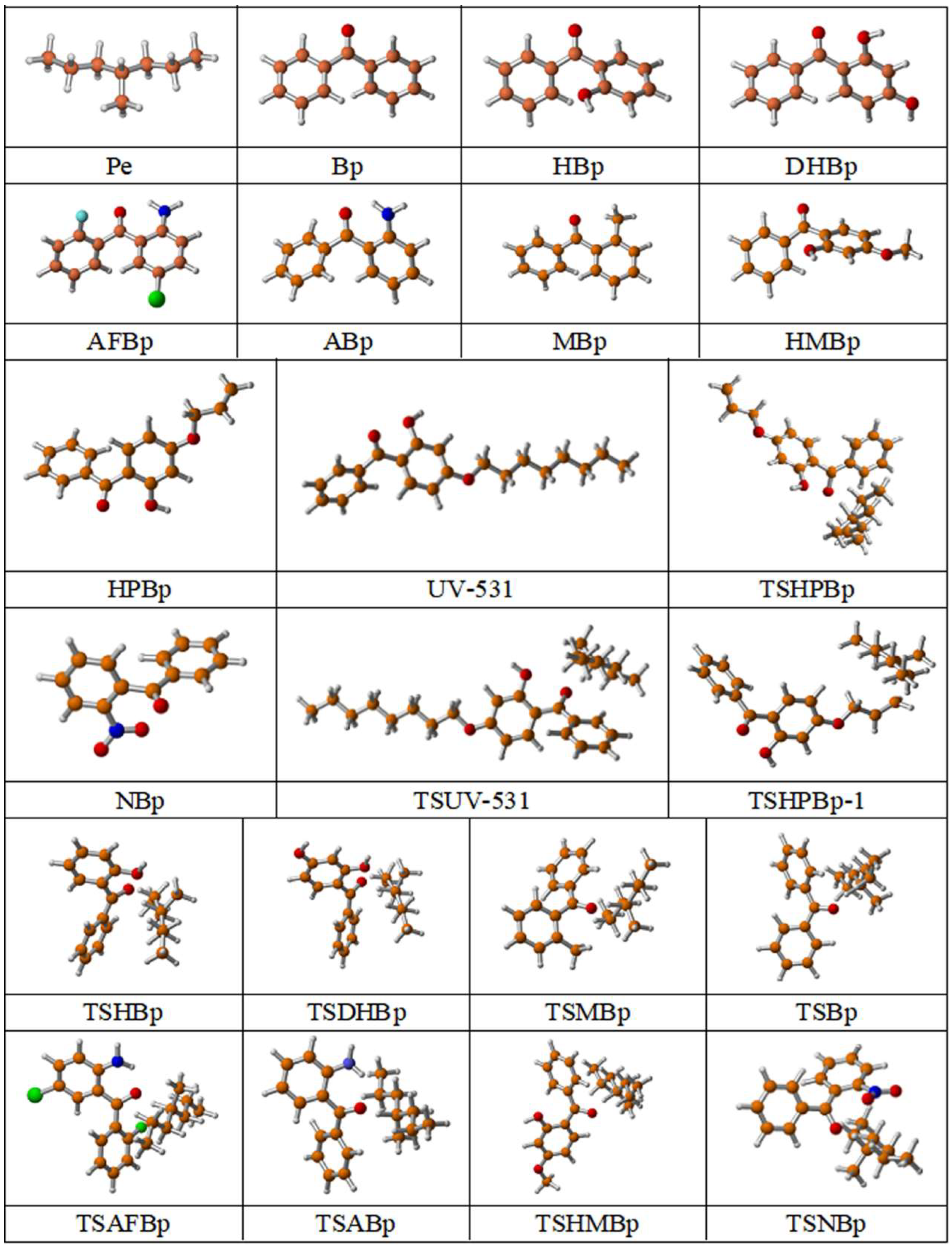
| Molecular Formula | Molecular Name | ab. | Molecular Formula | Molecular Name | ab. |
|---|---|---|---|---|---|
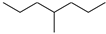 | 4-Methylheptane | Pe |  | 2-Methylbenzophenone | MBp |
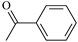 | Acetophenone | Ap |  | 2-Aminobenzophenone | ABp |
 | Benzophenone | Bp |  | 2-Nitrobenzophenone | NBp |
 | 2-Hydroxybenzophenone | HBp | 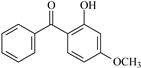 | 2-Hydroxy-4-methoxybenzophenone | HMBp |
 | 2-Amino-2’-fluoro-5-chlorobenzophenone | AFBp |  | 2-Hydroxy-4-(2-propenyloxy)lbenzophenone | HPBp |
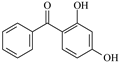 | 2,4-Dihydroxybenzophenone | DHBp | 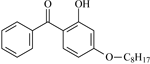 | 2-Hydroxy-4-octyloxybenzophenone | UV-531 |
| Reaction Equation | B3LYP/6-311+G(d,p) | |||||||
|---|---|---|---|---|---|---|---|---|
| ΔG≠ | ΔG | Reactant | b/f | Product | Freq. | |||
| ① | TSBp |  | 0.71 | −0.46 | 1.100 | 1.228/1.395 | 0.964 | 835 i |
| ② | TSHBp |  | 0.72 | −0.60 | 1.100 | 1.224/1.407 | 0.965 | 1019 i |
| ③ | TSAFBp |  | 1.12 | 0.06 | 1.100 | 1.247/1.371 | 0.983 | 1151 i |
| ④ | TSDHBp |  | 0.75 | −0.36 | 1.100 | 1.239/1.375 | 0.965 | 1156 i |
| ⑤ | TSMBp | 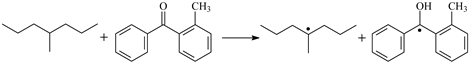 | 0.80 | −0.38 | 1.100 | 1.251/1.362 | 0.965 | 1149 i |
| ⑥ | TSABp |  | 1.05 | −0.08 | 1.100 | 1.262/1.349 | 0.965 | 1331 i |
| ⑦ | TSHMBp |  | 0.79 | −0.36 | 1.100 | 1.251/1.354 | 0.965 | 1181 i |
| ⑧ | TSHPBp | 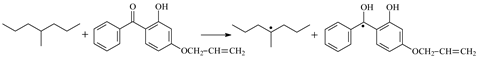 | 0.78 | −0.34 | 1.100 | 1.247/1.358 | 0.963 | 1149 i |
| ⑨ | TSHPBp-1 |  | 0.99 | −0.49 | 1.100 | 1.329/1.420 | 1.092 | 1654 i |
| ⑩ | TSUV-531 |  | 0.80 | −0.34 | 1.100 | 1.252/1.349 | 0.965 | 1216 i |
| ⑪ | TSNBp | 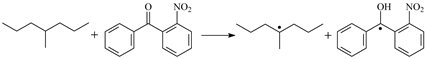 | 0.80 | 0.33 | 1.100 | 1.222/1.442 | 1.018 | 537 i |
| Molecular Formula | ab. | Eg | EA (a) | IP (a) | EA (v) | IP (v) |
|---|---|---|---|---|---|---|
 | Pe | 8.38 | −1.09 | 9.41 | −1.10 | 10.03 |
 | Ap | 5.20 | 0.33 (0.33) | 8.95 (9.1 ± 0.1) | 0.09 | 9.19 |
 | Bp | 4.90 | 0.73 (0.69 ± 0.05) | 8.52 (9.05) | 0.50 | 8.67 |
 | HBp | 4.73 | 0.60 | 8.14 | 0.35 | 8.38 |
 | AFBp | 3.91 | 0.89 | 7.60 | 0.60 | 7.81 |
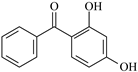 | DHBp | 4.63 | 0.47 | 7.83 | 0.24 | 8.17 |
 | MBp | 4.89 | 0.67 | 8.30 | 0.46 | 8.55 |
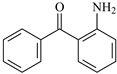 | ABp | 3.98 | 0.67 | 7.51 (8.3 ± 0.1) | 0.37 | 7.70 |
 | HMBp | 4.62 | 0.48 | 7.73 | 0.23 | 8.05 |
 | HPBp | 4.59 | 0.46 | 7.62 | 0.24 | 8.00 |
 | UV-531 | 4.49 | 0.41 | 7.50 | 0.19 | 7.84 |
 | NBp | 4.46 | 1.62 | 8.85 | 1.12 | 9.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Deng, C.; Zhang, H.; Du, X.; Shang, Y.; Wang, X. Theoretical Study on the Grafting Reaction of Benzophenone Compounds to Polyethylene in the UV Radiation Cross-Linking Process. Polymers 2025, 17, 2595. https://doi.org/10.3390/polym17192595
Du Y, Deng C, Zhang H, Du X, Shang Y, Wang X. Theoretical Study on the Grafting Reaction of Benzophenone Compounds to Polyethylene in the UV Radiation Cross-Linking Process. Polymers. 2025; 17(19):2595. https://doi.org/10.3390/polym17192595
Chicago/Turabian StyleDu, Yang, Chi Deng, Hui Zhang, Xia Du, Yan Shang, and Xuan Wang. 2025. "Theoretical Study on the Grafting Reaction of Benzophenone Compounds to Polyethylene in the UV Radiation Cross-Linking Process" Polymers 17, no. 19: 2595. https://doi.org/10.3390/polym17192595
APA StyleDu, Y., Deng, C., Zhang, H., Du, X., Shang, Y., & Wang, X. (2025). Theoretical Study on the Grafting Reaction of Benzophenone Compounds to Polyethylene in the UV Radiation Cross-Linking Process. Polymers, 17(19), 2595. https://doi.org/10.3390/polym17192595







