Synthesis and Studies of PAM-Ag-g/WS2/Ti3C2Tx Hydrogel and Its Possible Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Materials Characterization
2.3. Synthesis
2.4. Mechanical Testing of PAM-Ag-hg/WS2/Ti3C2Tx Hydrogel
2.5. Sensor Characteristic of PAM-Ag-g/WS2/Ti3C2Tx Hydrogel
2.6. First-Principles Modeling
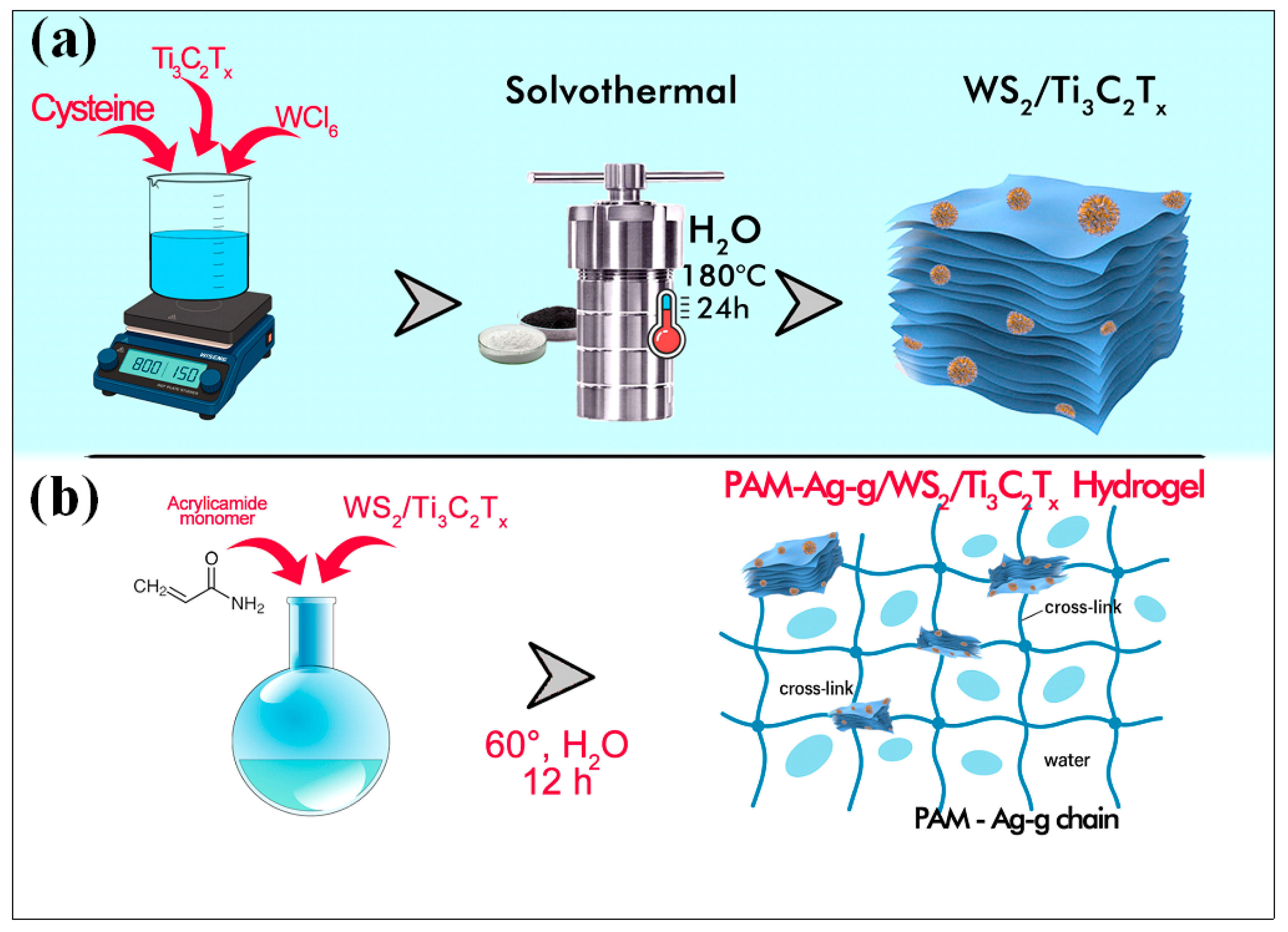
3. Results and Discussion
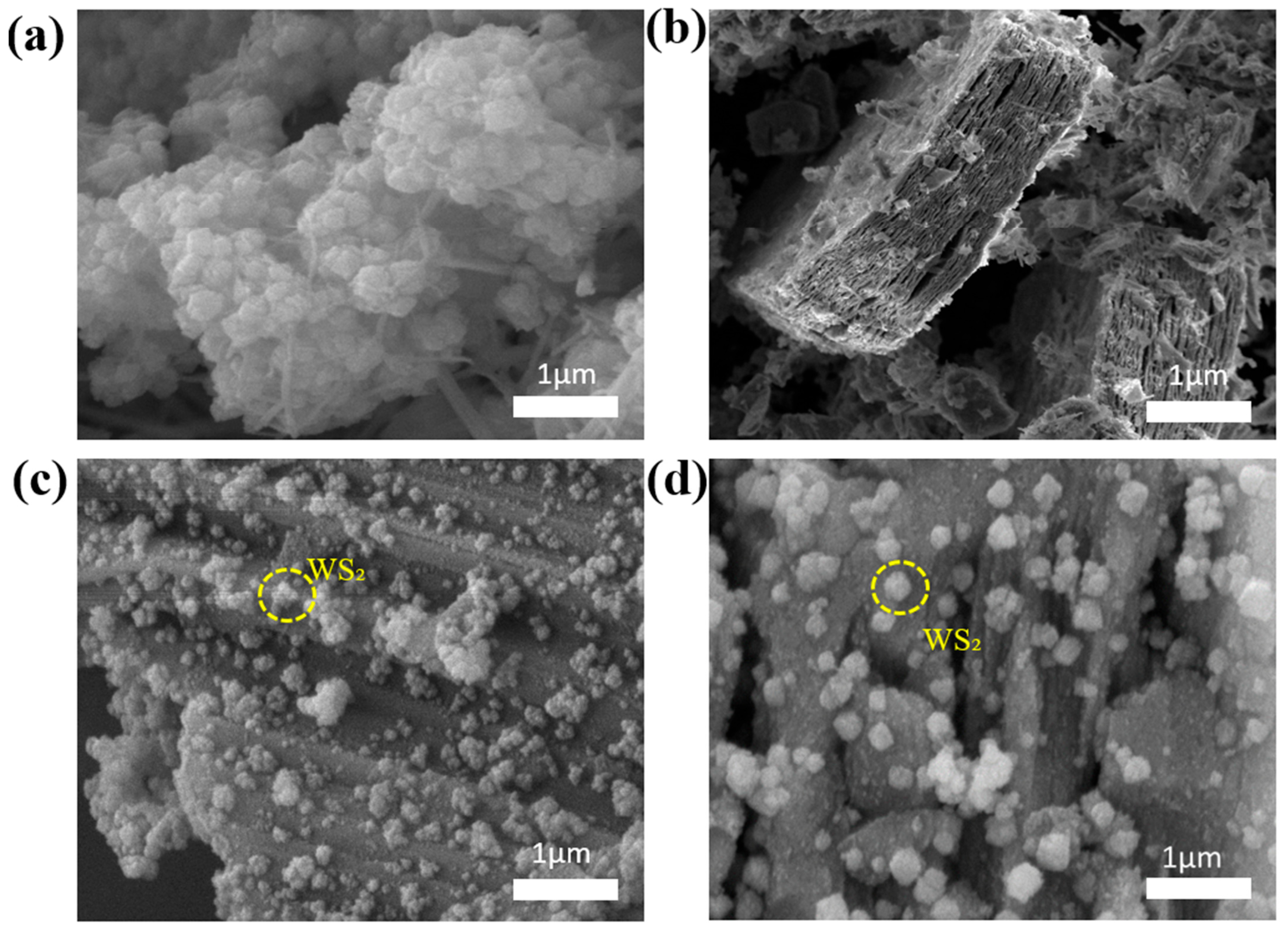
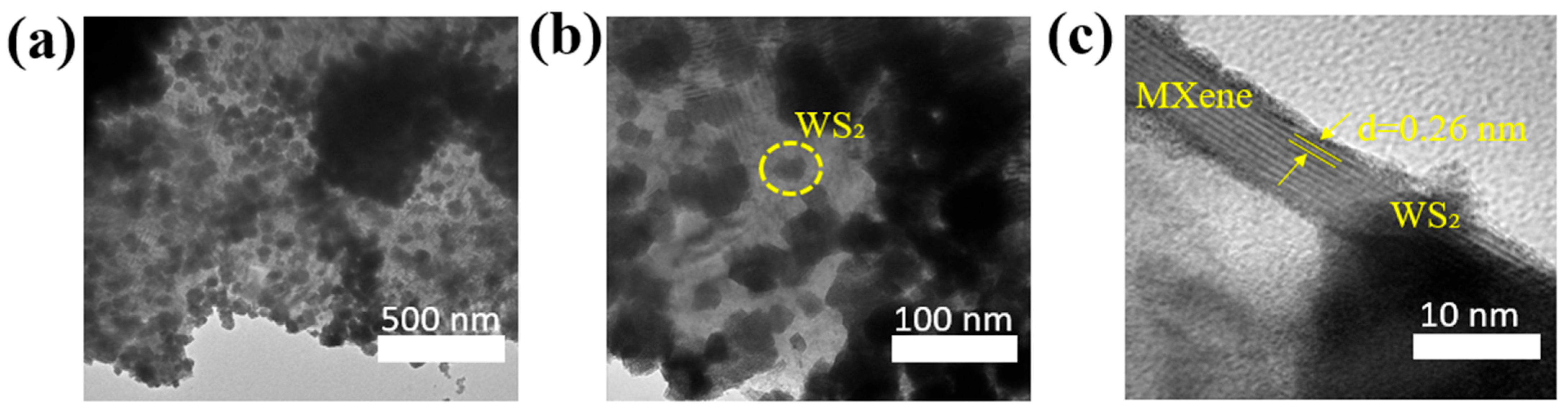
3.1. Chemical Characteristics of WS2/Ti3C2Tx
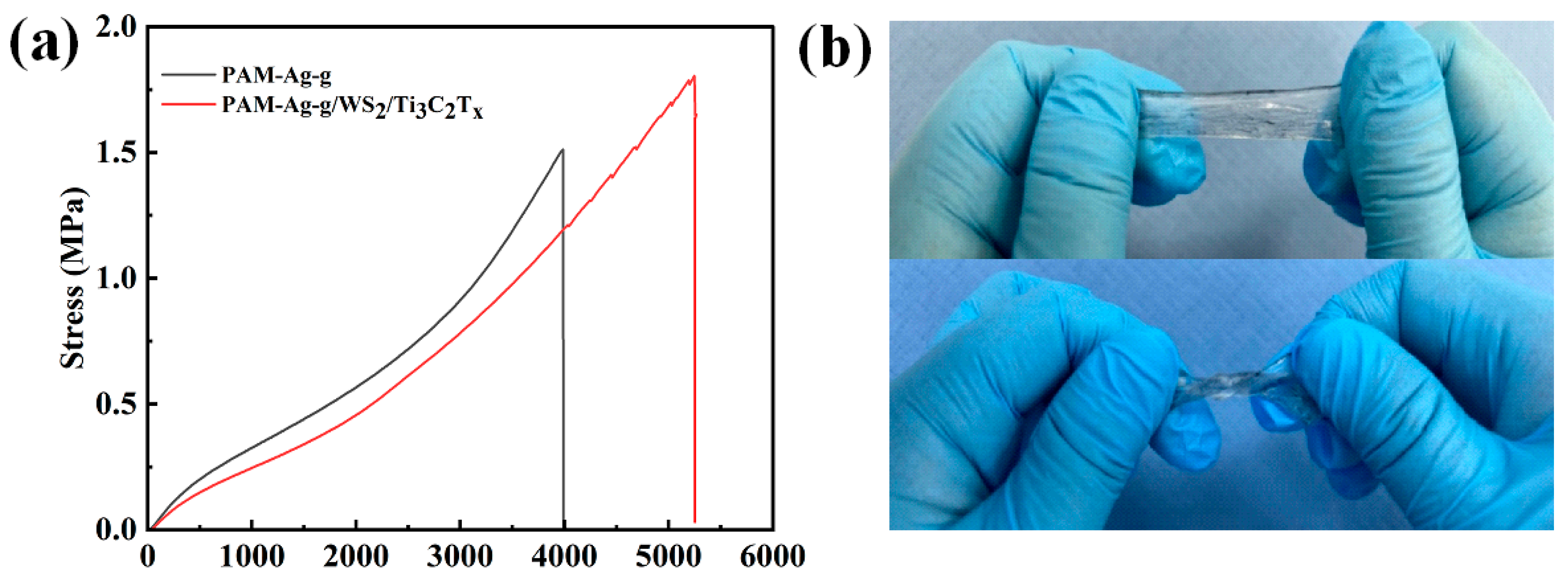
3.2. Mechanical Properties of PAM-Ag-g/WS2/Ti3C2Tx Hydrogel
3.3. Theoretical Simulations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, J.; Zhou, X.; Gong, H.; Lin, Z.; Xiang, H.; Liu, X.; Chen, X.; Li, H.; Liu, T.; Liu, S. Covalently Functionalized MoS2 Initiated Gelation of Hydrogels for Flexible Strain Sensing. ACS Appl. Mater. Interfaces 2023, 15, 36636–36646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and Optoelectronics of Two-Dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.N.; Luscombe, J.H.; Bate, R.T. Heterostructures and Quantum Devices; Elsevier: London, UK, 1994; Volume 24, ISBN 9780122341243. [Google Scholar]
- Feng, S.; Wang, X.; Wang, M.; Bai, C.; Cao, S.; Kong, D. Crumpled MXene Electrodes for Ultrastretchable and High-Area-Capacitance Supercapacitors. Nano Lett. 2021, 21, 7561–7568. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Shuck, C.E.; Maleski, K.; Alshareef, H.N.; Zhou, J.; Gogotsi, Y.; Huang, L. An Aqueous 2.1 V Pseudocapacitor with MXene and V–MnO2 Electrodes. Nano Res. 2022, 15, 535–541. [Google Scholar] [CrossRef]
- Zhu, M.; Du, X.; Liu, S.; Li, J.; Wang, Z.; Ono, T. A Review of Strain Sensors Based on Two-Dimensional Molybdenum Disulfide. J. Mater. Chem. C 2021, 9, 9083–9101. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wang, S.; Liu, X.; Chen, Y.; Qi, P.; Liu, X. Molybdenum Disulfide Enhanced Polyacrylamide-Acrylic Acid-Fe3+ Ionic Conductive Hydrogel with High Mechanical Properties and Anti-Fatigue Abilities as Strain Sensors. Colloids Surf. A 2021, 610, 125692. [Google Scholar] [CrossRef]
- Zhang, J.; Su, E.; Li, C.; Xu, S.; Tang, W.; Cao, L.N.Y.; Li, D.; Wang, Z.L. Enhancing Artifact Protection in Smart Transportation Monitoring Systems via a Porous Structural Triboelectric Nanogenerator. Electronics 2023, 12, 3031. [Google Scholar] [CrossRef]
- Rasool, F.; Pirzada, B.M.; Talib, S.H.; Alkhidir, T.; Anjum, D.H.; Mohamed, S.; Qurashi, A. In Situ Growth of Interfacially Nanoengineered 2D-2D WS2/Ti3C2Tx MXene for the Enhanced Performance of Hydrogen Evolution Reactions. ACS Appl. Mater. Interfaces 2024, 16, 14229–14242. [Google Scholar] [CrossRef]
- Han, F.; Chen, S.; Wang, F.; Liu, M.; Li, J.; Liu, H.; Yang, Y.; Zhang, H.; Liu, D.; He, R.; et al. High-Conductivity, Self-Healing, and Adhesive Ionic Hydrogels for Health Monitoring and Human-Machine Interactions Under Extreme Cold Conditions. Adv. Sci. 2025, 12, 2412726. [Google Scholar] [CrossRef]
- Lei, H.; Zhao, J.; Ma, X.; Li, H.; Fan, D. Antibacterial Dual Network Hydrogels for Sensing and Human Health Monitoring. Adv. Healthcare Mater. 2021, 10, 2101089. [Google Scholar] [CrossRef]
- Hou, W.; Sheng, N.; Zhang, X.; Luan, Z.; Qi, P.; Lin, M.; Tan, Y.; Xia, Y.; Li, Y.; Su, K. Design of Injectable Agar/NaCl/Polyacrylamide Ionic Hydrogels for High Performance Strain Sensors. Carbohydr. Polym. 2019, 211, 322–328. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Liu, Z.; Tang, Z.; Yang, Q.; Zhao, Y.; Du, S.; Chen, Q.; Zhi, C. A Highly Elastic and Reversibly Stretchable All-Polymer Supercapacitor. Angew. Chem. Int. Ed. 2019, 58, 15707–15711. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. Quantum Espresso: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Casarin, M.; Forrer, D.; Pavone, M.; Sambi, M.; Vittadini, A. Role and effective treatment of dispersive forces in materials: Polyethylene and graphite crystals as test cases. J. Comput. Chem. 2009, 30, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, Y.; Wang, K.; Lv, C.; Wei, W.; Wang, L.; Han, W. Highly Stable Cross-Linked Cationic Polyacrylamide/Ti3C2Tx MXene Nanocomposites for Flexible Ammonia-Recognition Devices. Adv. Mater. Technol. 2020, 5, 200048. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Li, L.; Wang, Y.; Wang, J.; Xiong, J.; Yu, J. MXene/Polymer Nanocomposites: Preparation, Properties, and Applications. Polym. Rev. 2020, 61, 80–115. [Google Scholar] [CrossRef]
- Wahba, M.I.; Hassan, M.E. Agar-Carrageenan Hydrogel Blend as a Carrier for the Covalent Immobilization of β-D-Galactosidase. Macromol. Res. 2017, 25, 913–923. [Google Scholar] [CrossRef]
- Vattikuti, V.S.V.; Chan, B. Effect of CTAB Surfactant on Textural, Structural, and Photocatalytic Properties of Mesoporous WS2. Sci. Adv. Mater. 2015, 7, 2639–2645. [Google Scholar] [CrossRef]
- Yang, J.; Voiry, D.; Ahn, S.J.; Kang, D.; Kim, A.Y.; Chhowalla, M.; Shin, H.S. Two-dimensional hybrid nanosheets of tungsten disulfide and reduced graphene oxide as catalysts for enhanced hydrogen evolution. Angew. Chem. Int. Ed. 2013, 125, 13996–13999. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, Y.; Wang, P.; Wang, Y.; Wang, Q.; Yang, Z.; Tang, C.; Xiang, S.; Luo, S.; Huang, S.; et al. Facile synthesis of a sandwiched Ti3C2Tx MXene/nZVI/fungal hypha nanofiber hybrid membrane for enhanced removal of Be(II) from Be(NH2)2 complexing solutions. Chem. Eng. J. 2021, 421, 129682. [Google Scholar] [CrossRef]
- Miah, M.Y.; Halder, S.; Saikat, S.P.; Dewanjee, S.; Ashaduzzaman, M.; Bhowmik, S. Microwave-assisted one-step synthesis of polyacrylamide/NiO nanocomposite for biomedical applications. RSC Adv. 2025, 15, 18971–18985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, A.; Qiao, S.; Zhang, Q.; Huang, C.; Lei, D.; Shi, X.; He, G.; Zhang, F. Mott-Schottky MXene@WS2 Heterostructure: Structural and Thermodynamic Insights and Application in Ultra Stable Lithium−Sulfur Batteries. ChemSusChem 2023, 16, e202300507. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, C.; Deng, Y.; Huang, Z.; Zhou, G.; Lv, W.; He, Y.B.; Wan, Y.; Kang, F.; Yang, Q.H. Optimized Catalytic WS2–WO3 Heterostructure Design for Accelerated Polysulfide Conversion in Lithium–Sulfur Batteries. Adv. Energy Mater. 2020, 10, 2000091. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, L.; Li, D.; Cao, J.; Han, W. Carbon-Reinforced Nb2CTx MXene/MoS2 Nanosheets as a Superior Rate and High-Capacity Anode for Sodium-Ion Batteries. ACS Nano 2021, 15, 7439–7450. [Google Scholar] [CrossRef]
- Wang, X.; Luo, D.; Wang, J.; Sun, Z.; Cui, G.; Chen, Y.; Wang, T.; Zheng, L.; Zhao, Y.; Shui, L.; et al. Recent Advances in Layered Transition Metal Dichalcogenides for Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 2371–2378. [Google Scholar] [CrossRef]
- Biswas, M.C.; Chakraborty, S.; Bhattacharjee, A.; Mohammed, Z. 4D Printing of Shape Memory Materials for Textiles: Mechanism, Mathematical Modeling, and Challenges. Adv. Funct. Mater. 2021, 31, 2100257. [Google Scholar] [CrossRef]
- Leong, W.X.R.; Al-Dhahebi, A.M.; Ahmad, M.R.; Saheed, M.S.M. Ti3C2Tx MXene-Polymeric Strain Sensor with Huge Gauge Factor for Body Movement Detection. Micromachines 2022, 13, 1302. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Chuchvaga, N.A.; Rakhimzhanov, M.K.; Shongalova, A.; Serikkanov, A.S.; Osipov, V.Y. The Effect of the Metal Impurities on the Stability, Chemical, and Sensing Properties of MoSe2. Surfaces 2025, 8, 56. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arinova, A.; Boukhvalov, D.W.; Umirzakov, A.; Bondar, E.; Shongalova, A.; Mustafa, L.; Kemelbekova, A.; Dmitriyeva, E. Synthesis and Studies of PAM-Ag-g/WS2/Ti3C2Tx Hydrogel and Its Possible Applications. Polymers 2025, 17, 2588. https://doi.org/10.3390/polym17192588
Arinova A, Boukhvalov DW, Umirzakov A, Bondar E, Shongalova A, Mustafa L, Kemelbekova A, Dmitriyeva E. Synthesis and Studies of PAM-Ag-g/WS2/Ti3C2Tx Hydrogel and Its Possible Applications. Polymers. 2025; 17(19):2588. https://doi.org/10.3390/polym17192588
Chicago/Turabian StyleArinova, Anar, Danil W. Boukhvalov, Arman Umirzakov, Ekaterina Bondar, Aigul Shongalova, Laura Mustafa, Ainagul Kemelbekova, and Elena Dmitriyeva. 2025. "Synthesis and Studies of PAM-Ag-g/WS2/Ti3C2Tx Hydrogel and Its Possible Applications" Polymers 17, no. 19: 2588. https://doi.org/10.3390/polym17192588
APA StyleArinova, A., Boukhvalov, D. W., Umirzakov, A., Bondar, E., Shongalova, A., Mustafa, L., Kemelbekova, A., & Dmitriyeva, E. (2025). Synthesis and Studies of PAM-Ag-g/WS2/Ti3C2Tx Hydrogel and Its Possible Applications. Polymers, 17(19), 2588. https://doi.org/10.3390/polym17192588






