Physical Characterization of Multiwire Polystyrene Produced by Electrospinning Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Analysis Techniques
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabu, T.; Ajitha, A.R.; Cintil, J.-C.; Bejoy, T. (Eds.) Handbook of Biopolymers; Springer Nature: Singapore, 2023. [Google Scholar] [CrossRef]
- Sabu, T.; Kuruvilla, J.; Malhotra, S.K.; Goda, K.; Sreekala, M.S. Polymer Composites; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- Seah, M.Q.; Chua, S.F.; Ang, W.L.; Lau, W.J.; Mansourizadeh, A.; Thamaraiselvan, C. Advancements in polymeric membranes for challenging water filtration environments: A comprehensive review. J. Environ. Chem. Eng. 2024, 12, 112628. [Google Scholar] [CrossRef]
- Cutroneo, M.; Hnatowicz, V.; Mackova, A.; Malinsky, P.; Miksova, R.; Ceccio, G.; Maly, J.; Smejkal, J.; Štofik, M.; Havranek, V. Ion Lithography of Single Ions Irradiation for Spatially, Regular Arrays of Pores in Membranes of Polyethylene Terephthalate. Nanomaterials 2022, 12, 3927. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Ravi, J.; Othman, M.H.D.; Matsuura, T.; Bilad, M.R.; El-badawy, T.H.; Aziz, F.; Ismail, A.F.; Rahman, M.A. Polymeric membranes for desalination using membrane distillation: A review. Desalination 2020, 490, 114530. [Google Scholar] [CrossRef]
- Gopal, R.; Kaur, S.; Ma, Z.; Chan, C.; Ramakrishna, S.; Matsuura, T. Electrospun nanofibrous filtration membrane. J. Membr. Sci. 2006, 281, 581–586. [Google Scholar] [CrossRef]
- Khil, M.S.; Cha, D.; Kim, H.; Kim, I.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 675–679. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, X.; Tian, Q.; Lin, Y.; Li, P.; Tao, Y.; Wang, Z.; Ma, J.; Xu, H.; Liu, Y. Hierarchical porous biomass-derived carbon with rich nitrogen doping for high-performance microwave absorption and tensile strain sensing. Carbon 2024, 224, 119083. [Google Scholar] [CrossRef]
- Cooley, J.F. Apparatus for Electrically Dispersing Fluids. U.S. Patent US692631A, 4 February 1902. [Google Scholar]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, L.; de Antonellis, S.; Tomaino, G.; Freni, A.; Malara, A.; Frontera, P.; Fotia, A. Electrospun hybrid microfibers for desiccant cooling/air dehumidification. Appl. Therm. Eng. 2025, 258, 124524. [Google Scholar] [CrossRef]
- Fotia, A.; Frontera, P.; Bonaccorsi, L.; Sciacca, B.; Malara, A. Encapsulation of Disodium-EDTA in Electrospun Polymeric Fibers for the Detection of Heavy Metals. Adv. Sustain. Syst. 2024, 8, 2300463. [Google Scholar] [CrossRef]
- Fotia, A.; Malara, A.; Paone, E.; Bonaccorsi, L.; Frontera, P.; Serrano, G.; Caneschi, A. Self standing mats of blended polyaniline produced by electrospinning. Nanomaterials 2021, 11, 1269. [Google Scholar] [CrossRef]
- Wannatong, L.; Sirivat, A.; Supaphol, P. Effects of solvents on electrospun polymeric fibers: Preliminary study on polystyrene. Polym. Int. 2004, 53, 1851–1859. [Google Scholar] [CrossRef]
- Jarusuwannapoom, T.; Hongrojjanawiwat, W.; Jitjaicham, S.; Wannatong, L.; Nithitanakul, M.; Pattamaprom, C.; Koombhongse, P.; Rangkupan, R.; Supaphol, P. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur. Polym. J. 2005, 41, 409–421. [Google Scholar] [CrossRef]
- RAbdulhussain; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Maul, J.; Frushour, B.G.; Kontoff, J.R.; Eichenauer, H.; Ott, K.H.; Schade, C. Polystyrene and Styrene Copolymers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar] [CrossRef]
- Torrisi, L.; Cutroneo, M.; Havranek, V.; Silipigni, L.; Fazio, B.; Fazio, M.; Di Marco, G.; Stassi, A.; Torrisi, A. Self-supporting graphene oxide films preparation and characterization methods. Vacuum 2019, 160, 1–11. [Google Scholar] [CrossRef]
- Pierini, F.; Lanzi, M.; Nakielski, P.; Pawłowska, S.; Zembrzycki, K.; Kowalewski, T.A. Electrospun poly(3-hexylthiophene)/poly(ethylene oxide)/graphene oxide composite nanofibers: Effects of graphene oxide reduction. Polym. Adv. Technol. 2016, 27, 1465–1475. [Google Scholar] [CrossRef]
- Cutroneo, M.; Havranek, V.; Mackova, A.; Malinsky, P.; Torrisi, L.; Lorincik, J.; Luxa, J.; Szokolova, K.; Sofer, Z.; Stammers, J. Localized deoxygenation of graphene oxide foil by ion microbeam writing. Vacuum 2019, 163, 10–14. [Google Scholar] [CrossRef]
- Torrisi, L.; Silipigni, L.; Manno, D.; Serra, A.; Nassisi, V.; Cutroneo, M.; Torrisi, A. Investigations on graphene oxide for ion beam dosimetry applications. Vacuum 2020, 178, 109451. [Google Scholar] [CrossRef]
- Manno, D.; Serra, A.; Buccolieri, A.; Calcagnile, L.; Cutroneo, M.; Torrisi, A.; Silipigni, L.; Torrisi, L. Structural and spectroscopic investigations on graphene oxide foils irradiated by ion beams for dosimetry application. Vacuum 2021, 188, 110185. [Google Scholar] [CrossRef]
- Torrisi, L.; Silipigni, L.; Cutroneo, M.; Proverbio, E.; Torrisi, A. Linear Energy Transfer (LET) dependence of graphene oxide dosimeter for different ionizing radiations. Vacuum 2022, 203, 111240. [Google Scholar] [CrossRef]
- Er, S.; Demirkol, D.O. Graphene oxide incorporated polystyrene electrospun nanofibers for immunosensing of CD36, as a marker of diabetic plasma. Bioelectrochemistry 2022, 145, 108083. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, L.; Yu, H.; Zhang, Y.; Dong, F. Graphene oxide/polystyrene composite nanofibers on quartz crystal microbalance electrode for the ammonia detection. RSC Advances 2015, 5, 40620–40627. [Google Scholar] [CrossRef]
- Cutroneo, M.; Havranek, V.; Mackova, A.; Malinsky, P.; Silipigni, L.; Slepicka, P.; Fajstavr, D.; Torrisi, L. Synthesis of Porous Polydimethylsiloxane Gold Nanoparticles Composites by a Single Step Laser Ablation Process. Int. J. Mol. Sci. 2022, 22, 12155. [Google Scholar] [CrossRef]
- Vivekchand, S.R.C.; Kam, K.C.; Gundiah, G.; Govindaraj, A.; Cheetham, A.K.; Rao, C.N.R. Electrical properties of inorganic nanowire-polymer composites. J. Mater. Chem. 2005, 15, 4922–4927. [Google Scholar] [CrossRef]
- Silipigni, L.; Cutroneo, M.; Salvato, G.; Torrisi, A.; Torrisi, L. Thermally treated GO foil dielectric properties under low and medium vacuum conditions. Radiat. Eff. Defects Solids 2025, 180, 4–15. [Google Scholar] [CrossRef]
- Yabagi, J.A.; Kimpa, M.I.; Muhammad, M.N.; Rashid, S.B.; Zaidi, E.; Agam, M.A. The effect of gamma irradiation on chemical, morphology and optical properties of polystyrene nanosphere at various exposure time. IOP Conf. Ser. Mater. Sci. Eng. 2018, 298, 012004. [Google Scholar] [CrossRef]
- Eyssa, H.M.; Osman, M.; Kandil, S.A.; Abdelrahman, M.M. Effect of ion and electron beam irradiation on surface morphology and optical properties of PVA. Nucl. Sci. Tech. 2016, 26, 1–6. [Google Scholar]
- Song, X.; Dai, Z.; Xiao, X.; Li, W.; Zheng, X.; Shang, X.; Zhang, X.; Cai, G.; Wu, W.; Meng, F.; et al. Formation of Carbonized Polystyrene Sphere/hemisphere Shell Arrays by Ion Beam Irradiation and Subsequent Annealing or Chloroform Treatment. Sci. Rep. 2015, 5, 17529. [Google Scholar] [CrossRef] [PubMed]
- Infrared Spectra of Some Common Functional Groups. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Wade)_Complete_and_Semesters_I_and_II/Map%3A_Organic_Chemistry_(Wade)/11%3A_Infrared_Spectroscopy_and_Mass_Spectrometry/11.05%3A_Infrared_Spectra_of_Some_Common_Functional_Groups (accessed on 17 September 2025).
- Yüce, M.Y.; Demirel, L. The effect of nanoparticles on the surface hydrophobicity of polystyrene. Eur. Phys. J. B 2008, 64, 493–497. [Google Scholar] [CrossRef]
- Huan, S.; Liu, G.; Han, G.; Cheng, W.; Fu, Z.; Wu, Q.; Wang, Q. Effect of Experimental Parameters on Morphological, Mechanical and Hydrophobic Properties of Electrospun Polystyrene Fibers. Materials 2015, 8, 2718–2734. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, X.; Lin, Y.; Wang, Z.; Tao, Y.; Xu, H.; Liu, Y. In-situ growth of MoO2/MoS2 microspheres on reduced graphene oxide with enhanced dielectric polarization and impedance matching for boosting electromagnetic wave absorption. Carbon 2025, 238, 120298. [Google Scholar] [CrossRef]
- Torrisi, L.; Cutroneo, M.; Silipigni, L.; Fazio, M.; Torrisi, A. Effects of the Laser Irradiation on Graphene Oxide Foils in Vacuum and Air. Phys. Solid State 2019, 61, 1327–1331. [Google Scholar] [CrossRef]
- Torrisi, L.; Silipigni, L.; Cutroneo, M.; Torrisi, A. Mass spectrometry of graphene oxide thermal reduction in vacuum. Rad. Eff. Defects Solids 2023, 178, 28–39. [Google Scholar] [CrossRef]
- Ishii, Y. Geometrical and electrostatic densities in a highly sparse as-electrospun polystyrene microfiber Mat. Mater. Lett. 2023, 20, 100221. [Google Scholar] [CrossRef]
- Jensen, M.G.; O’Shaughnessy, P.T.; Shaffer, M.; Yu, S.; Choi, Y.Y.; Christiansen, M.; Stanier, C.O.; Hartley, M.; Huddle, J.; Johnson, J.; et al. Simple fabrication of an electrospun polystyrene microfiberfilter that meets N95 filtering facepiece respirator filtration and breathability standards. J. Appl. Polym. Sci. 2023, 140, e53406. [Google Scholar] [CrossRef]
- Torrisi, L.; Cutroneo, M.; Torrisi, A.; Silipigni, L. Nitrogen diffusion in graphene oxide and reduced graphene oxide foils. Vacuum 2021, 194, 110632. [Google Scholar] [CrossRef]
- AlMaadeed, M.A.A.; Ponnamma, D.; Carignano, M.A. Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-816808-0. [Google Scholar]
- Zuppolini, S.; Borriello, A.; Pellegrino, M.; Venditto, V.; Ambrosio, L.; Nicolais, L. Potential contact and intraocular lenses based on hydrophilic/hydrophobic sulfonated syndiotactic polystyrene membranes. J. King Saud Univ.-Sci. 2017, 29, 487–493. [Google Scholar] [CrossRef]
- Mohammed, M.; Jawad, A.J.M.; Mohammed, A.M.; Oleiwi, J.K.; Adam, T.; Osman, A.F.; Dahham, O.S.; Betar, B.O.; Gopinath, S.C.B.; Jaafar, M. Challenges and advancement in water absorption of natural fiber-reinforced polymer Composites. Polym. Test. 2023, 124, 108083. [Google Scholar] [CrossRef]
- Esteves, R.J.A.; Gornick, V.; Alqurwani, D.S.; Koenig-Lovejoy, J.; Abdelrazeq, H.; Khraisheh, M.; Forzano, A.V.; Gad-El-Hak, M.; Tafreshi, H.V.; McLeskey, J.T. Activated carbon-doped polystyrene fibers for direct contact membrane desalination. Emergent Mater. 2020, 3, 807–814. [Google Scholar] [CrossRef]
- Kovtun, A.; Bianchi, A.; Zambianchi, M.; Bettini, C.; Corticelli, F.; Ruani, G.; Bocchi, L.; Stante, F.; Gazzano, M.; Marforio, T.D.; et al. Core-shell graphene oxide-polymer hollow fibers as water filters with enhanced performance and selectivity. Faraday Discuss. 2021, 227, 274–290. [Google Scholar] [CrossRef] [PubMed]
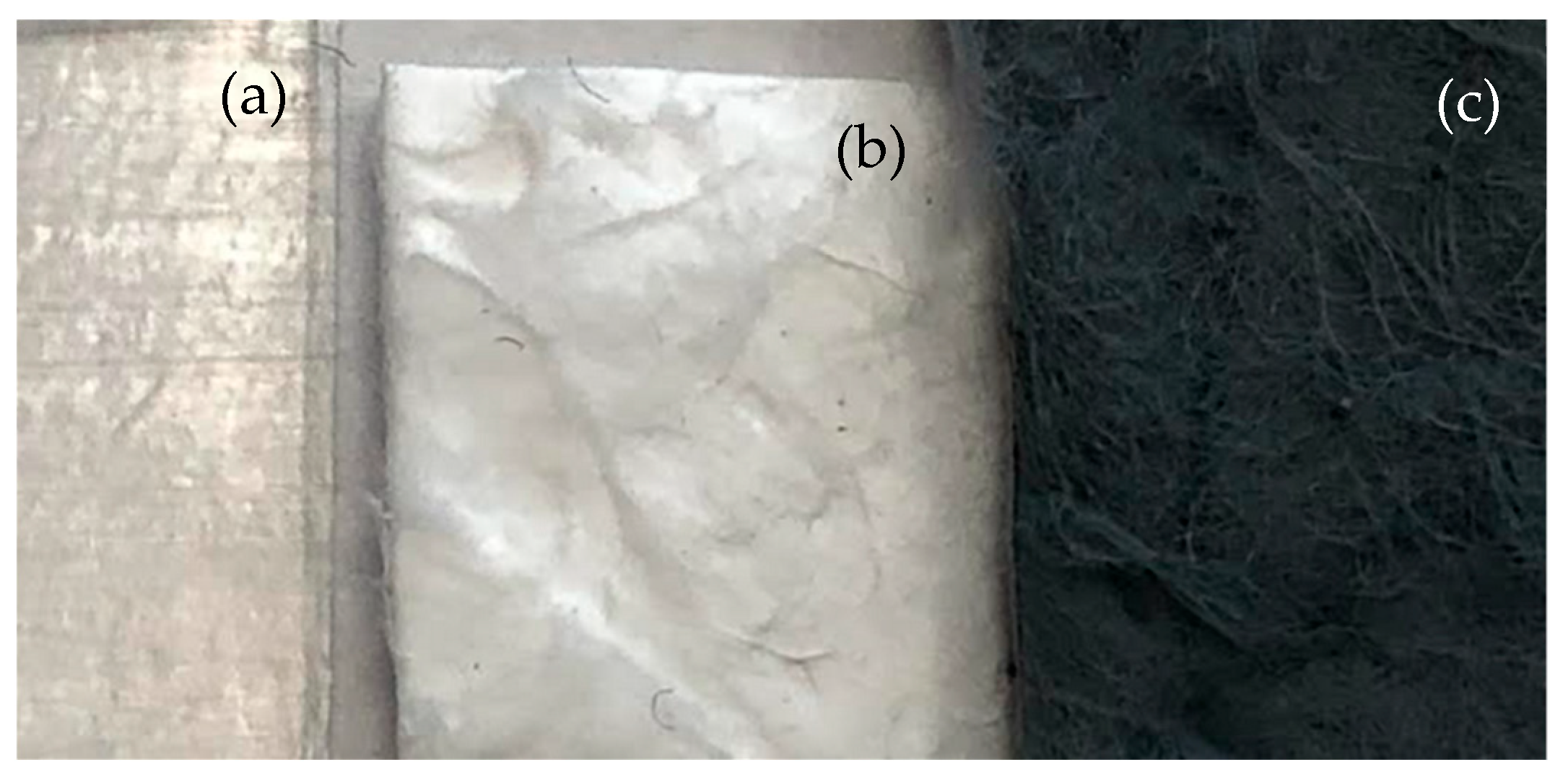

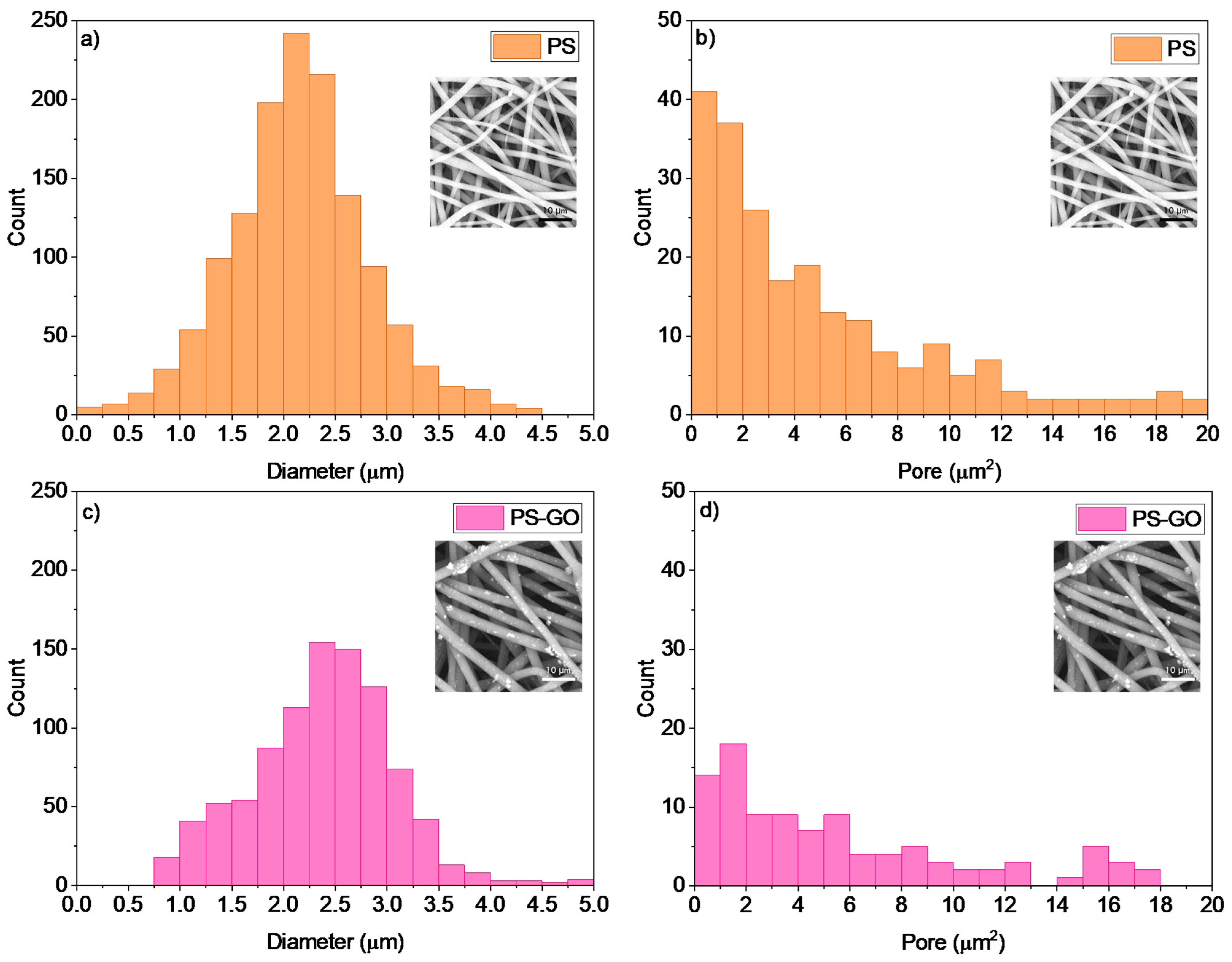
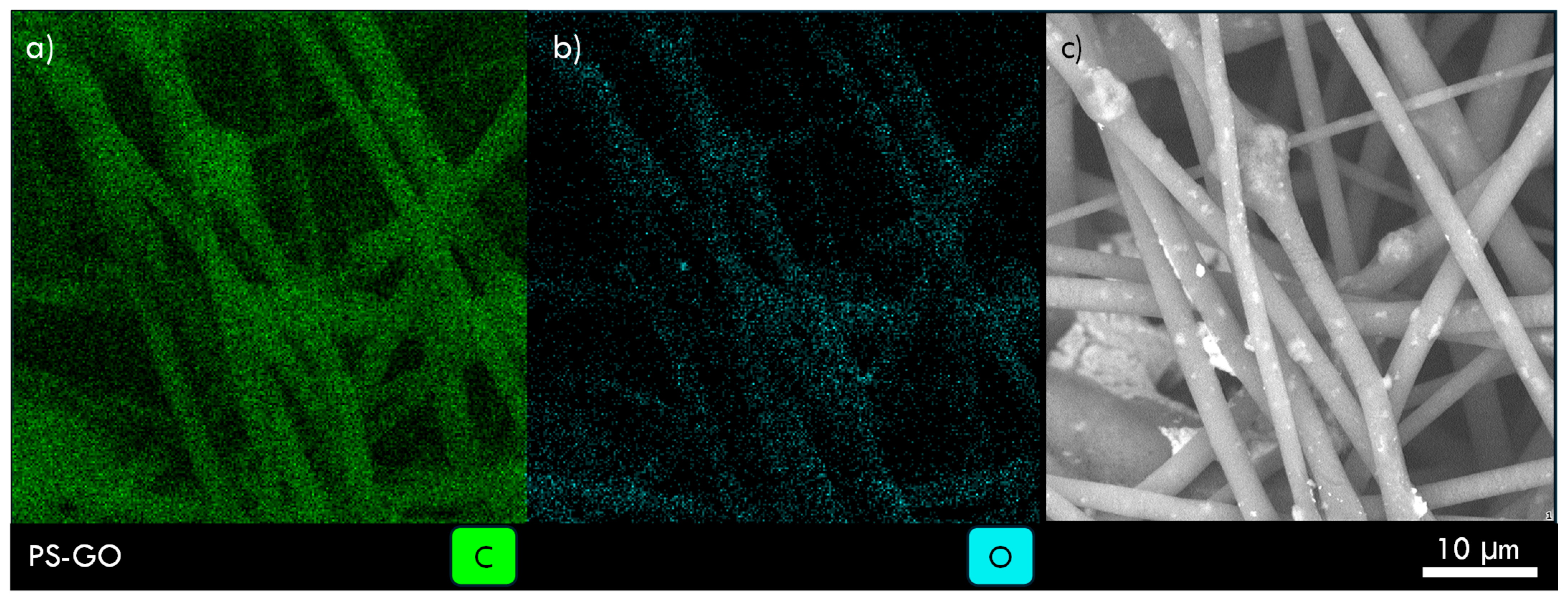




| Sample Code | Weight Ratio Polymer/Solvent | Weight Ratio Additive/Polymer | Voltage (kV) | Flow Rate (µL/min) | Needle to Collector Distance (cm) |
|---|---|---|---|---|---|
| PS | 21.9/78.1 | - | 12.5 | 15.0 | 15.0 |
| PS-GO | 21.9/78.1 | 10.0/90.0 | 12.0 | 15.0 | 15.0 |
| Property | PS Bulk | Multiwire PS | Multiwire PS + GO 10 wt% |
|---|---|---|---|
| Density (g/cm3) | 0.98–1.05 | 0.020 | 0.022 |
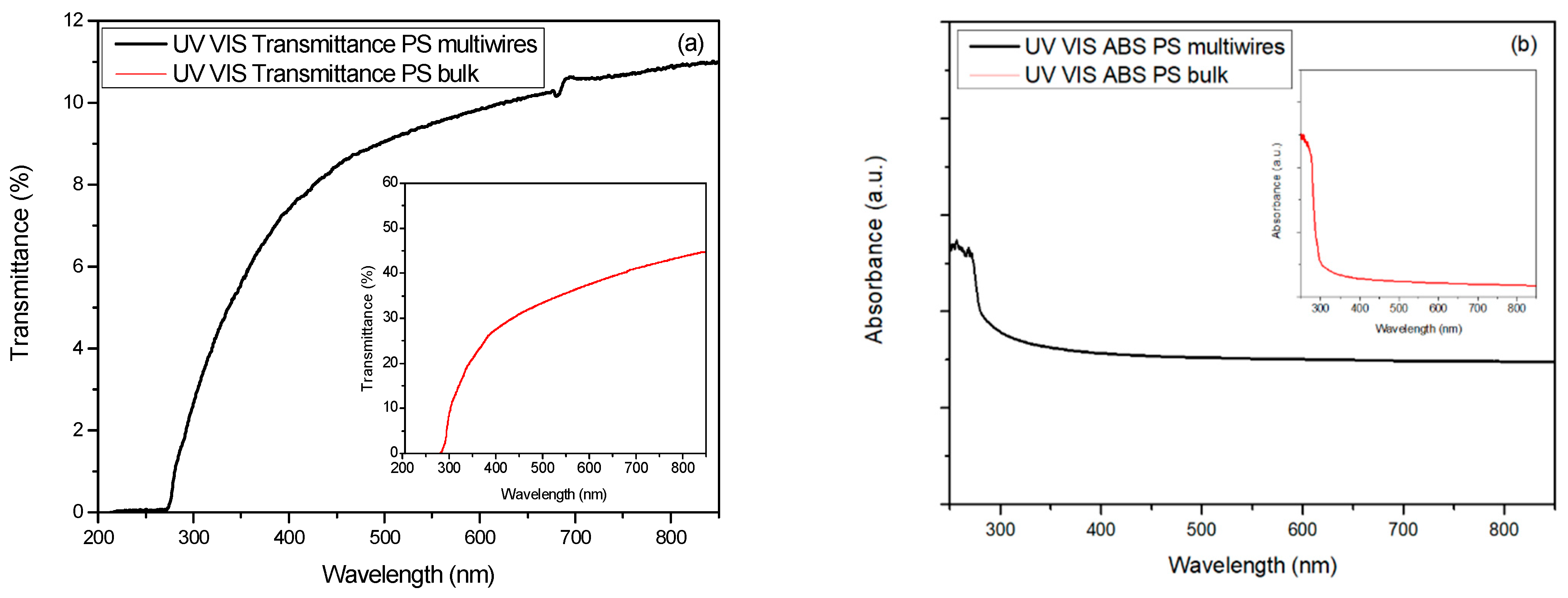
| Transmittance (550 nm) | 35% (30 μm) | 9.5% (250 μm) | 4% (250 μm) |
| Contact angle (300 K, degrees) | 92 | 125 | 144 |
| Relative dielectric permittivity (100 KHz) | 2.05 | 1.05 | 1.06 |
| Water absorption (wt%) | 0 | 1900 | 1500 |
| Air diffusion coefficient (×108 cm2/s) | 6 | 0.05 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrisi, L.; Silipigni, L.; Torrisi, A.; Cutroneo, M.; Malara, A.; Fotia, A.; Nunnari, C.; Frontera, P. Physical Characterization of Multiwire Polystyrene Produced by Electrospinning Technique. Polymers 2025, 17, 2587. https://doi.org/10.3390/polym17192587
Torrisi L, Silipigni L, Torrisi A, Cutroneo M, Malara A, Fotia A, Nunnari C, Frontera P. Physical Characterization of Multiwire Polystyrene Produced by Electrospinning Technique. Polymers. 2025; 17(19):2587. https://doi.org/10.3390/polym17192587
Chicago/Turabian StyleTorrisi, Lorenzo, Letteria Silipigni, Alfio Torrisi, Mariapompea Cutroneo, Angela Malara, Antonio Fotia, Chiara Nunnari, and Patrizia Frontera. 2025. "Physical Characterization of Multiwire Polystyrene Produced by Electrospinning Technique" Polymers 17, no. 19: 2587. https://doi.org/10.3390/polym17192587
APA StyleTorrisi, L., Silipigni, L., Torrisi, A., Cutroneo, M., Malara, A., Fotia, A., Nunnari, C., & Frontera, P. (2025). Physical Characterization of Multiwire Polystyrene Produced by Electrospinning Technique. Polymers, 17(19), 2587. https://doi.org/10.3390/polym17192587








