An Effect of a Matrix Made of Cell Wall Polysaccharides from Apple on the Rheological Properties of Various Food Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Modified Cell Wall Polysaccharide Matrix (MPS) from Apples
2.2. Chemical Composition
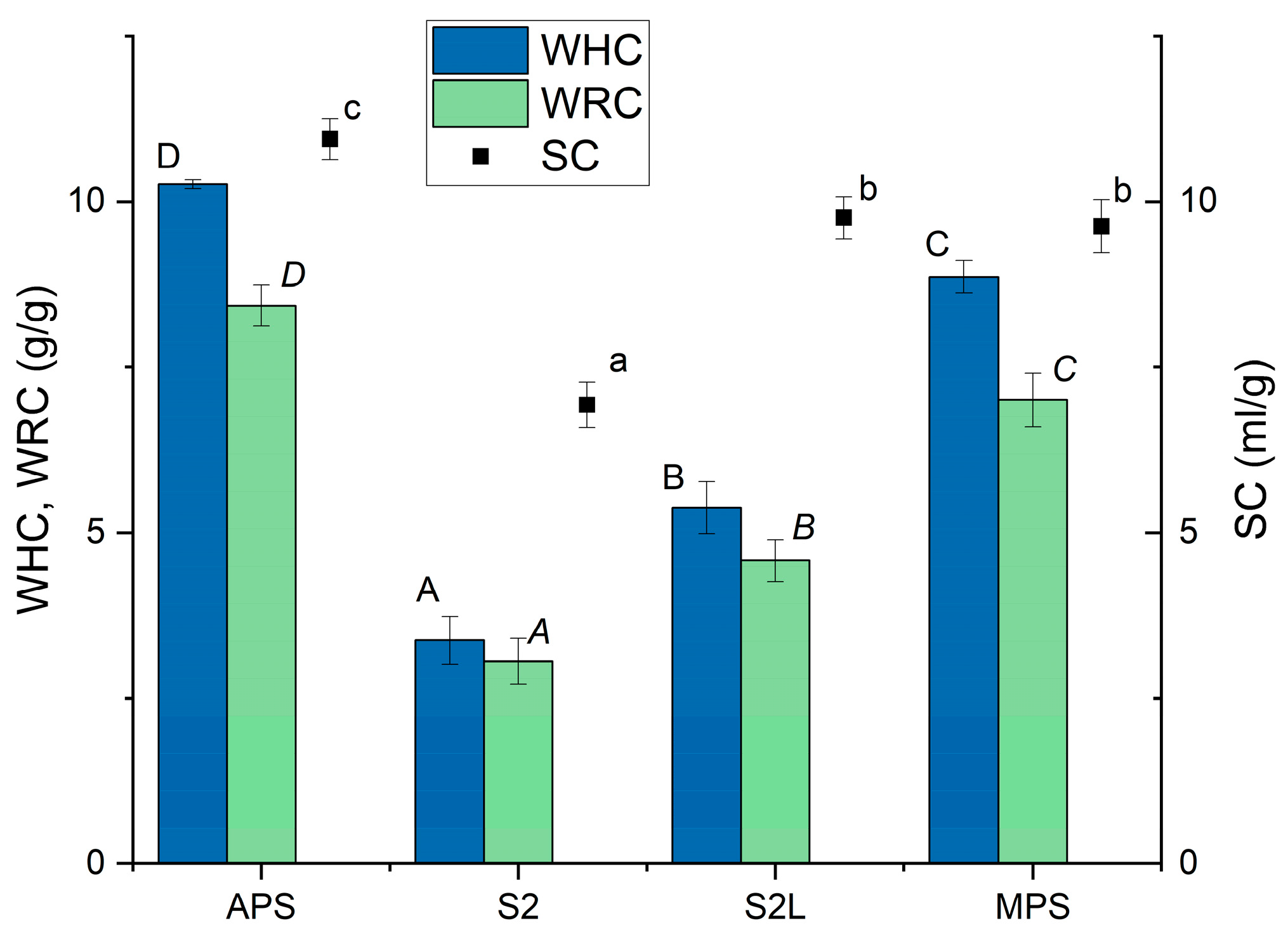
2.3. Water Retention Capacity
2.4. Water Holding Capacity
2.5. Swelling Capacity
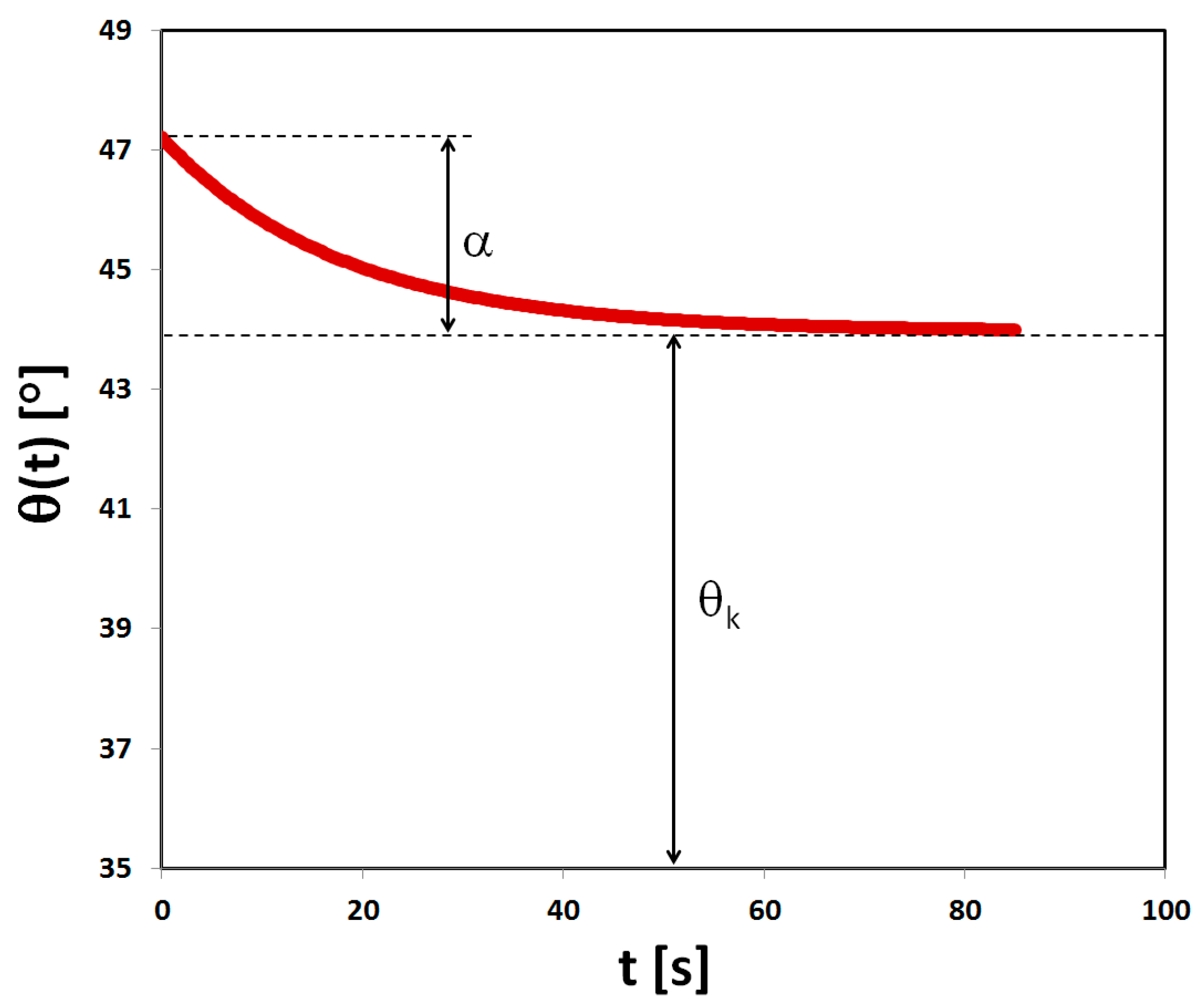
2.6. Measurements of Wetting Angles
- θk—theoretical, limit value of the wetting angle;
- α—value by which the wetting angle changes from the initial value to achieve the limit value;
- β—constant describing the change in the contact angle per unit time;
- t—wetting time.
2.7. Preparation of a Suspension of MPS in Food Products
2.8. Determination of Rheological Properties of MPS in Food Product Suspensions
2.9. Statistical Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Wang, L.; Yu, X.; Wang, X.; Zheng, Y.; Hu, X.; Zhang, P.; Sun, Q.; Wang, Q.; Li, N. Effect of polysaccharide addition on food physical properties: A review. Food Chem. 2024, 431, 137099. [Google Scholar] [CrossRef]
- CODEX STAN 192-1995; General Standard for Food Additives. Codex Alimentarius Commission: Rome, Italy, 1995.
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Himashree, P.; Sengar, A.S.; Sunil, C.K. Food thickening agents: Sources, chemistry, properties and applications—A review. Int. J. Gastron. Food Sci. 2022, 27, 100468. [Google Scholar] [CrossRef]
- Jones, J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr. J. 2014, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Idrovo Encalada, A.M.; Pérez, C.D.; Rossetti, L.; Rojas, A.M.; Fissore, E.N. Carrot pectin enriched fraction as a functional additive: Antioxidant and gelling effects in a model spreadable chia oil-in-water emulsion. Food Hydrocoll. 2020, 108, 106037. [Google Scholar] [CrossRef]
- Liu, T.; Lei, H.; Zhen, X.; Liu, J.; Xie, W.; Tang, Q.; Gou, D.; Zhao, J. Advancements in modifying insoluble dietary fiber: Exploring the microstructure, physicochemical properties, biological activity, and applications in food industry—A review. Food Chem. 2024, 458, 140154. [Google Scholar] [CrossRef]
- Pattarapanawan, M.; Phuengjayaem, S.; Akrimajirachoote, N.; Kotatha, D. Partial enhancement of soluble fiber through pyrodextrinization of the residual starch in cassava pulp: Developing a novel dietary fiber with modified functional and improved prebiotic properties. Food Res. Int. 2025, 217, 116747. [Google Scholar] [CrossRef]
- Calton, A.; Ma, H.; Nordlund, E.; Poutanen, K.; Sozer, N. Instant properties of ingredients used for point of consumption production of high-moisture food structures selectively fortified with protein and dietary fibre. J. Food Eng. 2019, 263, 204–212. [Google Scholar] [CrossRef]
- Swackhamer, C.; Jang, S.; Park, B.-R.; Hamaker, B.R.; Keun Jung, S. Structure-based standardization of prebiotic soluble dietary fibers based on monosaccharide composition, degree of polymerization, and linkage composition. Carbohydr. Polym. 2025, 367, 123949. [Google Scholar] [CrossRef]
- Liu, T.; Zhen, X.; Zheng, N.; Ma, Y.; Zhang, Y.; Lei, H.; Liu, J.; Zhang, R.; Zhao, J. Functional properties and application potential of black soybean meal insoluble dietary fiber remodeled by ultrasound combined with ultrahigh pressure. LWT 2025, 221, 117594. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Zhang, X.; Kazem, A.E.; Iacomini, M.; Hamaker, B.R.; Cordeiro, L.M.C. Microwave treatment enhances human gut microbiota fermentability of isolated insoluble dietary fibers. Food Res. Int. 2021, 143, 110293. [Google Scholar] [CrossRef] [PubMed]
- Opperman, C.; Majzoobi, M.; Farahnaky, A.; Shah, R.; Hao Van, T.T.; Ratanpaul, V.; Blanch, E.W.; Brennan, C.; Eri, R. Beyond soluble and insoluble: A comprehensive framework for classifying dietary fibre’s health effects. Food Res. Int. 2025, 206, 115843. [Google Scholar] [CrossRef]
- Mierczyńska, J.; Cybulska, J.; Sołowiej, B.; Zdunek, A. Effect of Ca2+, Fe2+ and Mg2+ on rheological properties of new food matrix made of modified cell wall polysaccharides from apple. Carbohydr. Polym. 2015, 133, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, J. Method of production of universal food additive for texture stabilization or thickening, especially made of apple pomace, and an additive made according to this method. PL Patent No. 226317, 31 July 2017. [Google Scholar]
- Cybulska, J.; Góźdź, J. Laboratory dryer for drying of agricultural and food materials. PL Patent No. 228048, 28 February 2018. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Robertson, J.A.; Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibault, J.-F. Hydration Properties of Dietary Fibre and Resistant Starch: A European Collaborative Study. LWT−Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Z.; Zhang, Z.; Liu, H.; Liu, G.; Yu, B.; Zhao, H.; Tao, H.; Cui, B. Water-insoluble citrus fiber enhances the textural properties and water-holding capacity of thermally generated kappa-carrageenan gels via skeletal reinforcement. Food Hydrocoll. 2025, 166, 11263. [Google Scholar] [CrossRef]
- Fitzgibbon, A.W.; Fisher, R.B. A Buyer’s Guide to Conic Fitting. In Proceedings of the 5th British Machine Vision Conference, York, UK, 13–16 September 1994. [Google Scholar]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Muñoz-Almagro, N.; Pacheco, M.T.; Antón, M.J.; Dapena, E.; Ruiz, L.; Margolles, A.; Villamiel, M.; Moreno, F.J. Apple pomaces derived from mono-varietal Asturian ciders production are potential source of pectins with appealing functional properties. Carbohydr. Polym. 2021, 264, 117980. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Bouchet, B.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material. Part III: Study on model polysaccharides. BBA Gen. Subj. 2005, 1725, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, J.; Zdunek, A.; Psonka-Antonczyk, K.M.; Stokke, B.T. The relation of apple texture with cell wall nanostructure studied using an atomic force microscope. Carbohydr. Polym. 2013, 92, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, Q.; Jia, R.; Wang, Z.; Fu, J.; Guo, J.; Yang, H.; Zhao, Z. Comparison of cell wall changes of two different types of apple cultivars during fruit development and ripening. J. Integr. Agric. 2023, 22, 2705–2718. [Google Scholar] [CrossRef]
- Kaczmarska, A.; Pieczywek, P.M.; Cybulska, J.; Cieśla, J.; Zdunek, A. Structural and rheological properties of diluted alkali soluble pectin from apple and carrot. Food Chem. 2024, 446, 138869. [Google Scholar] [CrossRef]
- Wang, W.; Hu, J.; Xu, Y.; Li, X.; Bi, J. Insights into the structural alterations of different pectin fractions and their impact on enzymatic browning during apple pulp processing. Food Chem. 2025, 492, 145660. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Demir, A.E.; Armağan, H.S. Valorization of black carrot pomace by using microwave-assisted hydrothermal extraction method: An optimization and comparison research on pectin extraction. Food Hum. 2025, 4, 100637. [Google Scholar] [CrossRef]
- Vaz, A.A.; Bellí, G.; Oms-Oliu, G.; Martín-Belloso, O.; Odriozola-Serrano, I. Exploring the prebiotic potential of unpurified apple dietary fibre concentrate. LWT 2025, 222, 117608. [Google Scholar] [CrossRef]
- De la Peña-Armada, R.; Villanueva-Suárez, M.J.; Molina-García, A.D.; Rupérez, P.; Mateos-Aparicio, I. Novel rich-in-soluble dietary fiber apple ingredient obtained from the synergistic effect of high hydrostatic pressure aided by Celluclast®. LWT 2021, 146, 111421. [Google Scholar] [CrossRef]
- Zhang, M.; Chu, L.; Chen, J.; Qi, F.; Li, X.; Chen, X.; Yu, D.-G. Asymmetric wettability fibrous membranes: Preparation and biologic applications. Compos. B Eng. 2024, 269, 111095. [Google Scholar] [CrossRef]
- Rezvani, Z.; Goli, S.A.H. Fabrication, physicochemical properties and structural characteristics of nanoparticles from carrot pomace and its insoluble dietary fiber. Food Hydrocoll. 2023, 145, 109131. [Google Scholar] [CrossRef]
- Lang, C.V.; Jung, J.; Wang, T.; Zhao, Y. Investigation of mechanisms and approaches for improving hydrophobicity of molded pulp biocomposites produced from apple pomace. Food Bioprod. Process. 2022, 133, 1–15. [Google Scholar] [CrossRef]
- Kowalska, M.; Konopska, J.; Feszterová, M.; Zbikowska, A.; Kowalska, B. Quality Assessment of Natural Juices and Consumer Preferences in the Range of Citrus Fruit Juices. Appl. Sci. 2023, 13, 765. [Google Scholar] [CrossRef]
- Jeevarathinam, G.; Ramniwas, S.; Singh, P.; Rustagi, S.; Asdaq, S.M.B.; Pandiselvam, R. Macromolecular, thermal, and nonthermal technologies for reduction of glycemic index in food-A review. Food Chem. 2024, 445, 138742. [Google Scholar] [CrossRef]
- Sikora, M.; Adamczyk, G.; Krystyjan, M. Thixotropy as a measure of liquid food products. Zywn.- Nauk. Technol. Ja. 2011, 1, 5–14. [Google Scholar]
- Tabilo-Munizaga, G.; Barbosa-Cánovas, G.V. Rheology for the food industry. J. Food Eng. 2005, 67, 147–156. [Google Scholar] [CrossRef]
- Penna, A.L.B.; Sivieri, K.; Oliveira, M.N. Relation between quality and rheological properties of lactic beverages. J. Food Eng. 2001, 49, 7–13. [Google Scholar] [CrossRef]
- Chen, J.; Cui, Y.; Shi, W.; Ma, Y.; Zhang, S. The interaction between wheat starch and pectin with different esterification degree and its influence on the properties of wheat starch-pectin gel. Food Hydrocoll. 2023, 145, 109062. [Google Scholar] [CrossRef]
- Wilbanks, D.J.; Yazdi, S.R.; Lucey, J.A. Effects of varying casein and pectin concentrations on the rheology of high-protein cultured milk beverages stored at ambient temperature. J. Dairy Sci. 2022, 105, 72–82. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R. Re-evaluation of alginic acid and its sodium, potassium, ammonium and calcium salts (E 400-E 404) as food additives. EFSA J. 2017, 15, e05049. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Degen, G.; Engel, K.-H.J.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; et al. Re-evaluation of xanthan gum (E 415) as a food additive in foods for infants below 16 weeks of age and follow-up of its re-evaluation as a food additive for uses in foods for all population groups. EFSA J. 2023, 21, e07951. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aquilina, G.; Castle, L.; Degen, G.; Engel, K.-H.J.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; et al. Re-evaluation of locust bean gum (E 410) as a food additive in foods for infants below 16 weeks of age and follow-up of its re-evaluation as a food additive for uses in foods for all population groups. EFSA J. 2023, 21, e07775. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on the substantiation of health claims related to pectins and reduction of post-prandial glycaemic responses (ID 786), maintenance of normal blood cholesterol concentrations (ID 818) and increase in satiety leading to a reduction in energy intake (ID 4692) pursuant to Article 13(1) of regulation (EC) No. 1924/2006. EFSA J. 2010, 8, 1747. [Google Scholar] [CrossRef]


| Tomato Juice | Apple Juice | Buttermilk | Instant Soup * | Salad Dressing | Instant Kissel * | |
|---|---|---|---|---|---|---|
| Energy (kJ/kcal) | 77/18 | 189/45 | 178/42 | 92/22 | 727/179 | 223/52 |
| Protein (g) | 0.8 | <0.5 | 3.5 | 1.0 | 0.8 | 0.0 |
| Carbohydrate (g) | 2.7 | 11.0 | 4.8 | 3.8 | 8.0 | 13.0 |
| of which sugars (g) | 2.7 | 11.0 | 4.0 | 1.2 | 6.0 | 7.7 |
| of which starch (g) | 0.1 | nd | nd | 0.7 | 1.9 | 5.1 |
| Fat (g) | <0.5 | <0.5 | 1.0 | 0.2 | 16.0 | 0.0 |
| of which saturates (g) | <0.1 | 0.0 | 0.6 | 0.1 | 1.2 | 0.0 |
| Fibre (g) | 0.5 | nd | nd | 0.1 | nd | nd |
| Salt (g) | 0.70 | 0.00 | 0.08 | 0.66 | 2.30 | 0.01 |
| APS | S2 | S2L | MPS | |
|---|---|---|---|---|
| pH | 3.26 ± 0.01 | 3.90 ± 0.01 | 4.21 ± 0.01 | 3.4 ± 0.0 |
| Galacturonic acid content (mg/g) | 694.1 ± 1.0 | 172.5 ± 5.6 | 82.5 ± 3.1 | 389.8 ± 5.6 |
| Dietary fibre fractions (%) | ||||
| Cellulose | 0.0 ± 0.0 | 37.8 ± 0.7 | 48.8 ± 0.7 | 20.8 ± 0.6 |
| Hemicellulose | 1.1 ± 0.1 | 26.1 ± 0.5 | 33.4 ± 0.5 | 10.5 ± 0.7 |
| Pectin * | 98.9 ± 0.1 | 30.8 ± 0.4 | 9.0 ± 0.4 | 65.4 ± 0.3 |
| Lignin | 0.0 ± 0.0 | 5.3 ± 0.4 | 8.8 ± 0.3 | 3.3 ± 0.1 |
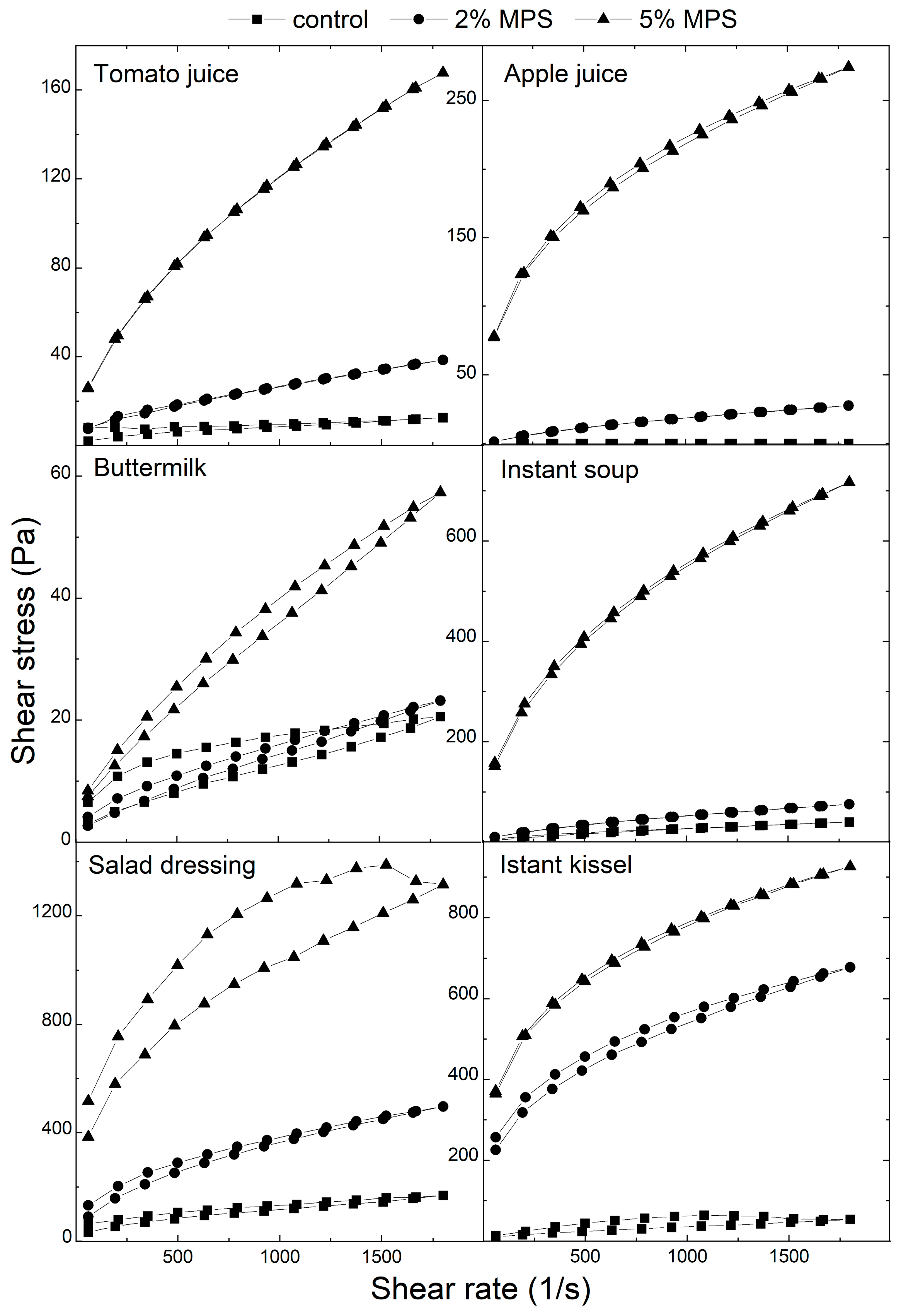
| Tomato Juice | Apple Juice | Buttermilk | Instant Soup | Salad Dressing | Instant Kissel | ||
|---|---|---|---|---|---|---|---|
| Viscosity (Pa·s) | Control | 0.011 a ± 0.001 | 0.001 a ± 0.001 | 0.020 a ± 0.001 | 0.035 a ± 0.009 | 0.089 a ± 0.013 | 0.530 a ± 0.091 |
| 2% MPS | 0.025 b ± 0.004 | 0.020 b ± 0.001 | 0.014 b ± 0.001 | 0.014 b ± 0.001 | 0.327 b ± 0.052 | 0.530 a ± 0.086 | |
| 5% MPS | 0.128 c ± 0.020 | 0.214 c ± 0.016 | 0.038 c ± 0.012 | 0.519 c ± 0.137 | 0.671 c ± 0.041 | 0.718 b ± 0.096 | |
| Thixotropic effect (kPa·s−1) | Control | 0.300 a ± 0.137 | nd | 8.640 a ± 2.973 | 7.726 a ± 1.554 | 43.228 a ± 6.861 | 69.532 a ± 4.526 |
| 2% MPS | 5.742 b ± 0.991 | 3.254 b ± 0.951 | 5.197 b ± 1.322 | 3.542 b ± 0.344 | 92.837 b ± 32.594 | 134.215 b ± 10.333 | |
| 5% MPS | 21.221 c ± 6.666 | 28.055 c ± 5.758 | 7.574 c ± 3.142 | 76.235 c ± 5.211 | 570.483 c ±192.995 | 115.458 c ± 19.591 | |
| Food Product | Sample | Upward Curve | Downward Curve | ||||
|---|---|---|---|---|---|---|---|
| K (Pasn) | n | R2 | K (Pasn) | n | R2 | ||
| Tomato juice | Control | 1.00 ± 0.00 | 0.30 ± 0.01 | 0.99 | 2.76 ± 1.52 | 0.20 ± 0.01 | 0.99 |
| 2% MPS | 1.06 ± 0.01 | 0.47 ± 0.04 | 0.99 | 1.00 ± 0.00 | 0.47 ± 0.03 | 0.99 | |
| 5% MPS | 2.11 ± 0.61 | 0.57 ± 0.06 | 0.99 | 2.077 ± 0.74 | 0.57 ± 0.01 | 0.99 | |
| Apple juice | Control | nd | nd | nd | nd | nd | nd |
| 2% MPS | 1.08 ± 0.06 | 0.45 ± 0.03 | 0.98 | 1.00 ± 0.00 | 0.43 ± 0.01 | 0.98 | |
| 5% MPS | 14.82 ± 5.61 | 0.38 ± 0.02 | 0.96 | 14.86 ± 6.21 | 0.38 ± 0.03 | 0.95 | |
| Buttermilk | Control | 2.64 ± 1.42 | 0.29 ± 0.11 | 0.99 | 1.00 ± 0.01 | 0.37 ± 0.02 | 0.99 |
| 2% MPS | 1.00 ± 0.00 | 0.40 ± 0.02 | 0.99 | 1.005 ± 0.00 | 0.37 ± 0.02 | 0.99 | |
| 5% MPS | 1.02 ± 0.03 | 0.52 ± 0.06 | 0.99 | 1.007 ± 0.00 | 0.51 ± 0.05 | 0.99 | |
| Instant soup | Control | 1.29 ± 0.05 | 0.46 ± 0.05 | 0.99 | 1.002 ± 0.02 | 0.47 ± 0.01 | 0.99 |
| 2% MPS | 1.74 ± 1.25 | 0.39 ± 0.02 | 0.99 | 1.91 ± 1.58 | 0.44 ± 0.09 | 0.99 | |
| 5% MPS | 16.05 ± 2.72 | 0.48 ± 0.02 | 0.99 | 15.73 ± 2.49 | 0.49 ± 0.02 | 0.99 | |
| Salad dressing | Control | 16.41 ± 6.23 | 0.32 ± 0.06 | 0.99 | 3.32 ± 0.85 | 0.53 ± 0.01 | 0.99 |
| 2% MPS | 22.54 ± 10.88 | 0.41 ± 0.07 | 0.99 | 10.21 ± 1.54 | 0.51 ± 0.01 | 0.99 | |
| 5% MPS | 186.97 ± 82.02 | 0.28 ± 0.05 | 0.99 | 83.20 ± 29.87 | 0.36 ± 0.03 | 0.99 | |
| Instant kissel | Control | 6.53 ± 1.12 | 0.58 ± 0.03 | 0.99 | 8.21 ± 0.03 | 0.55 ± 0.01 | 0.99 |
| 2% MPS | 67.16 ± 7.69 | 0.29 ± 0.00 | 0.99 | 47.47 ± 6.21 | 0.34 ± 0.00 | 0.99 | |
| 5% MPS | 107.58 ± 8.05 | 0.27 ± 0.01 | 0.99 | 110.21 ± 9.30 | 0.27 ± 0.01 | 0.99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierczyńska, J.; Pieczywek, P.M.; Cybulska, J. An Effect of a Matrix Made of Cell Wall Polysaccharides from Apple on the Rheological Properties of Various Food Products. Polymers 2025, 17, 2547. https://doi.org/10.3390/polym17182547
Mierczyńska J, Pieczywek PM, Cybulska J. An Effect of a Matrix Made of Cell Wall Polysaccharides from Apple on the Rheological Properties of Various Food Products. Polymers. 2025; 17(18):2547. https://doi.org/10.3390/polym17182547
Chicago/Turabian StyleMierczyńska, Joanna, Piotr Mariusz Pieczywek, and Justyna Cybulska. 2025. "An Effect of a Matrix Made of Cell Wall Polysaccharides from Apple on the Rheological Properties of Various Food Products" Polymers 17, no. 18: 2547. https://doi.org/10.3390/polym17182547
APA StyleMierczyńska, J., Pieczywek, P. M., & Cybulska, J. (2025). An Effect of a Matrix Made of Cell Wall Polysaccharides from Apple on the Rheological Properties of Various Food Products. Polymers, 17(18), 2547. https://doi.org/10.3390/polym17182547








