Potential of Landfill Mined Combustible Polymer Composite and Soil-like Fraction for Energy Recovery, Chemical Recycling, and Resource Recovery †
Abstract
1. Introduction
2. Materials and Methods
2.1. Landfill Sites and Sample Preparation
2.2. Evaluation of Energy Recovery Potential
2.3. Evaluation of Chemical Recycling Potential
2.4. Evaluation of Resource Recovery Potential
3. Results and Discussion
3.1. Energy Recovery Potential of Polymer Fraction
3.2. Chemical Recycling Potential of Polymer Fraction
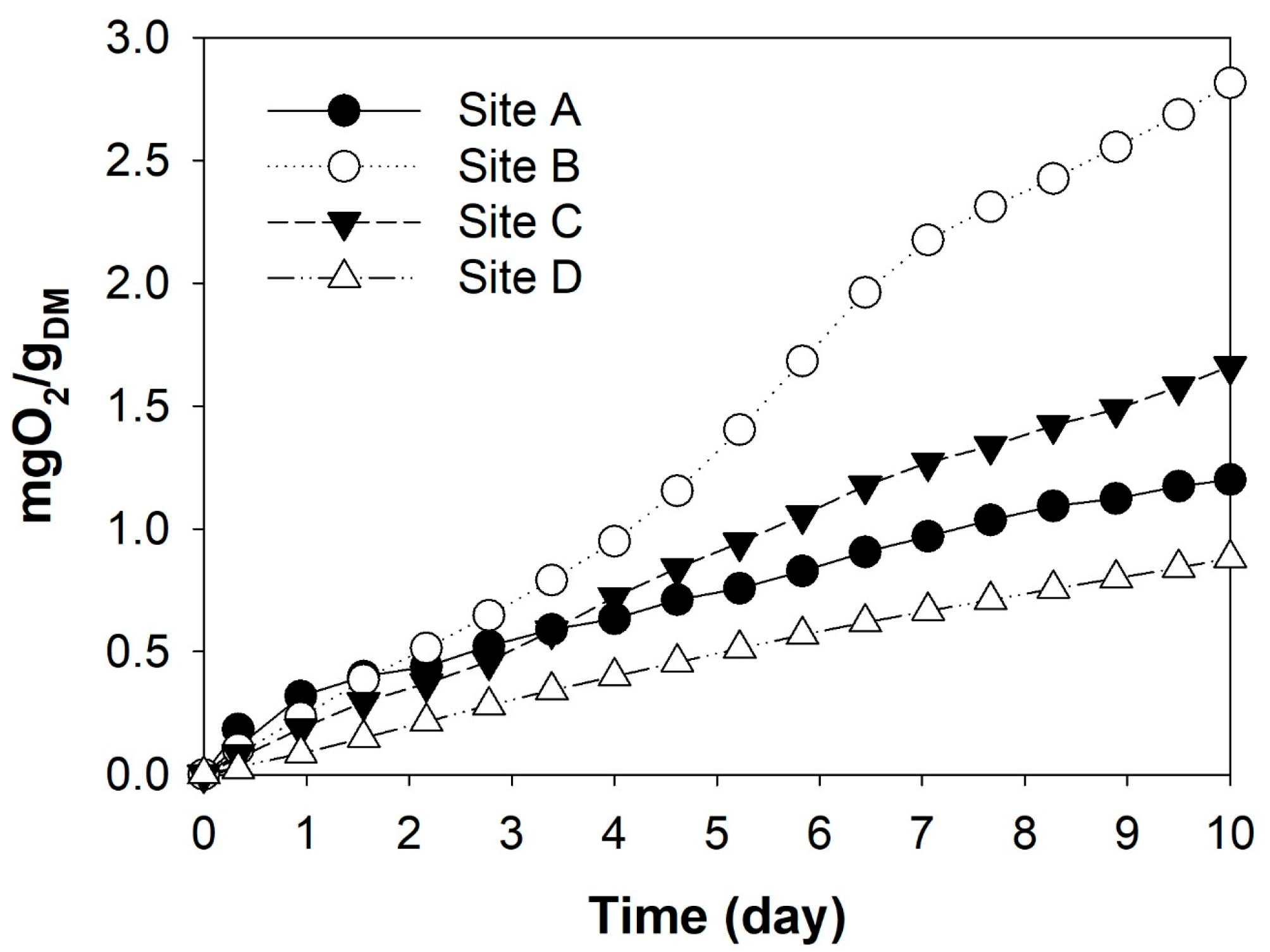
3.3. Resource Recovery Potential of Soil-like Fraction
4. Challenges and Perspectives
5. Conclusions
- CPCs revealed high LHV and acceptable Cl concentrations for energy recovery.
- Styrene-rich pyrolytic oils were produced by CPCs pyrolysis reaction.
- Catalytic pyrolysis of CPCs enhanced the yield of high-value BTEXs and styrene.
- SLFs showed chemical and biological stability for resource recovery.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Goli, V.S.N.S.; Singh, D.N. Valorization of landfill mined plastic waste and soil-like fractions in polymer composites—A comprehensive solution for sustainable landfill mining. J. Clean. Prod. 2023, 420, 138349. [Google Scholar] [CrossRef]
- Jagodzińska, K.; Garcia Lopez, C.G.; Yang, W.; Jönsson, P.G.; Pretz, T.; Raulf, K. Characterisation of excavated landfill waste fractions to evaluate the energy recovery potential using Py-GC/MS and ICP techniques. Resour. Conserv. Recycl. 2021, 168, 105446. [Google Scholar] [CrossRef]
- Jagodzińska, K.; Jönsson, P.G.; Yang, W. Pyrolysis and in-line catalytic decomposition of excavated landfill waste to produce carbon nanotubes and hydrogen over Fe- and Ni-based catalysts—Investigation of the catalyst type and process temperature. Chem. Eng. J. 2022, 446, 136808. [Google Scholar] [CrossRef]
- Yi, S. Resource recovery potentials by landfill mining and reclamation in South Korea. J. Environ. Manag. 2019, 242, 178–185. [Google Scholar] [CrossRef]
- Kwon, Y.; Lee, S.; Bae, J.; Park, S.; Moon, H.; Lee, T.; Kim, K.; Kang, J.; Jeon, T. Evaluation of incinerator performance and policy framework for effective waste management and energy recovery: A case study of South Korea. Sustainability 2024, 16, 448. [Google Scholar] [CrossRef]
- Yang, W.-S.; Lee, Y.-J.; Kang, J.-G.; Shin, S.-K.; Jeon, T.-W. Assessment of quality test methods for solid recovered fuel in South Korea. Waste Manag. 2020, 103, 240–250. [Google Scholar] [CrossRef]
- Jagodzińska, K.; Zaini, I.N.; Svanberg, R.; Yang, W.; Jönsson, P.G. Pyrolysis of excavated waste from landfill mining: Characterisation of the process products. J. Clean. Prod. 2021, 279, 123541. [Google Scholar] [CrossRef]
- Reddy, A.S.; Wanare, R.; Rotte, V.M.; Iyer, K.K.R.; Dave, T.N. Performance of fibre-reinforced landfill mined soil like fraction as an environment friendly fill material. J. Environ. Manag. 2024, 349, 119464. [Google Scholar] [CrossRef]
- Somani, M.; Harbottle, M.; Datta, M.; Ramana, G.V.; Sreekrishnan, T.R. Identification and assessment of appropriate remediation management techniques for the recovery of soil-like material produced in landfill mining. J. Environ. Manag. 2023, 348, 119300. [Google Scholar] [CrossRef]
- Delgado, L.; Catarino, A.S.; Eder, P.; Litten, D.; Luo, Z.; Villanueva, A. End-of-Waste Criteria: Final Report. European Commission (EC). 2009. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC53238 (accessed on 18 August 2025).
- European Council. Council Decision of 19 December 2002 Establishing Criteria and Procedures for the Acceptance of Waste at Landfills Pursuant to Article 16 of and Annex II to Directive 1999/31/EC(2003/33/EC). 2003. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:011:0027:0049:EN:PDF (accessed on 24 December 2024).
- Wulyapash, W.; Phongphiphat, A.; Fellner, J.; Towprayoon, S. Exploring Refuse-Derived Fuel Production from Seafood-Processing Sludge and Landfill-Mined Plastic Waste Co-Pelletization. Recycling 2025, 10, 52. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, J.; Li, J.; Li, H.; Li, F.; Cheng, Z.; Yan, B.; Chen, G. Pyrolysis of combustible fractions in excavated waste: Effect of landfill time on pyrolysis characteristics analyzed by TG-FTIR-MS. J. Anal. Appl. Pyrolysis 2024, 177, 106298. [Google Scholar] [CrossRef]
- Rajput, M.; Li, J.; Yan, B.; Chen, G.; Khan, R.; Sun, Y.; Zhao, J. A comparative study of steam gasification and combustion methods for landfill stale waste (LSW) treatment. J. Environ. Manag. 2025, 380, 124816. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Patil, M.; Dalal, P.H.; Iyer, K.K.R.; Dave, T.N. Sustainable utilization of landfill mined soil like fraction in subbase layer for asphalt road applications. Clean. Mater. 2024, 11, 100218. [Google Scholar] [CrossRef]
- Patil, M.; Dalal, P.H.; Reddy, A.S.; Iyer, K.K.R.; Dave, T.N. Evaluation of the geotechnical characteristics of landfill mined soil like fraction (LMSF) for sustainable fill application. Multiscale Multidiscip. Model. Exp. Des. 2024, 7, 2929–2951. [Google Scholar] [CrossRef]
- De la Torre-Bayo, J.J.D.; Zamorano, M.; Torres-Rojo, J.C.; Rodríguez, M.L.; Martín-Pascual, J. Analyzing the production, quality, and potential uses of solid recovered fuel from screening waste of municipal wastewater treatment plants. Process Saf. Environ. Prot. 2023, 172, 950–970. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.-M.; Jae, J.; Jeon, J.-K.; Jung, S.-C.; Kim, S.C.; Park, Y.-K. Production of aromatic hydrocarbons via catalytic co-pyrolysis of torrefied cellulose and polypropylene. Energy Convers. Manag. 2016, 129, 81–88. [Google Scholar] [CrossRef]
- Woodard & Curran, Inc. Industrial Waste Treatment Handbook, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Hosokai, S.; Matsuoka, K.; Kuramoto, K.; Suzuki, Y. Modification of Dulong’s formula to estimate heating value of gas, liquid and solid fuels. Fuel Process. Technol. 2016, 152, 399–405. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Protocol for the Evaluation of Biodegradable Municipal Waste Sent to Landfill. 2011. Available online: https://www.epa.ie/publications/compliance--enforcement/waste/EPA_Protocol_For-BMW-Final.pdf (accessed on 24 December 2024).
- CEN/TR 15508:2006; Key Properties on Solid Recovered Fuels to Be Used for Establishing a Classification System. European Committee for Standardization: Brussels, Belgium, 2006.
- Garg, A.; Smith, R.; Hill, D.; Simms, N.; Pollard, S. Wastes as co-fuels: The policy framework for solid recovered fuel (SRF) in Europe, with UK implications. Environ. Sci. Technol. 2007, 41, 4868–4874. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Lv, Y.; Cheng, Y.; Wang, Y.; Liu, Y.; Cobb, K.; Chen, P.; Lei, H.; Ruan, R. Pyrolysis technology for plastic waste recycling: A state-of-the-art review. Prog. Energy Combust. Sci. 2022, 93, 101021. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Vainio, E.; Yrjas, P.; Zevenhoven, M.; Brink, A.; Laurén, T.; Hupa, M.; Kajolinna, T.; Vesala, H. The fate of chlorine, sulfur, and potassium during co-combustion of bark, sludge, and solid recovered fuel in an industrial scale BFB boiler. Fuel Process. Technol. 2013, 105, 59–68. [Google Scholar] [CrossRef]
- Vamvuka, D.; Zografos, D.; Alevizos, G. Control methods for mitigating biomass ash-related problems in fluidized beds. Bioresour. Technol. 2008, 99, 3534–3544. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-M.; Huang, S.-W.; Kuan, W.Y. Characteristics of emissions from reclamation of solid-recovered fuel (SRF) in a cogeneration plant. Aerosol Air Qual. Res. 2021, 21, 210112. [Google Scholar] [CrossRef]
- Piaia, E.; Cavali, M.; Nadaleti, W.C.; Matias, M.S.; Russo, M.A.T.; Castilhos Junior, A.B. Production of Solid Recovered Fuel from the Rejected Fraction of Recyclable Materials from Waste Picker Cooperatives: A Case Study in Brazil. Biomass 2023, 3, 238–251. [Google Scholar] [CrossRef]
- Park, J.; Jung, I.; Lee, K.; Kim, M.; Hwang, J.; Choi, W. Case study in Korea of manufacturing SRF for polyurethanes recycling in e-wastes. J. Mater. Cycles Waste Manag. 2018, 20, 1950–1960. [Google Scholar] [CrossRef]
- Predel, M.; Kaminsky, W. Pyrolysis of mixed polyolefins in a fluidised-bed reactor and on a pyro-GC/MS to yield aliphatic waxes. Polym. Degrad. Stab. 2000, 70, 373–385. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Z.; Cecon, V.S.; Curtzwiler, G.; Vorst, K.; Mavrikakis, M.; Huber, G.W. The effects of pyolyolefin structure and source on pyrolysis-derived plastic oil composition. Green Chem. 2024, 26, 11908–11923. [Google Scholar] [CrossRef]
- Shin, T.; Hajima, O.; Chuichi, W. Pyrograms and thermograms of 163 high polymers, and MS data of the major pyrolyzates. In Pyrolysis—GC/MS Data Book of Synthetic Polymers: Pyrograms, Thermograms and MS of Pyrolyzates; Elsevier: Amsterdam, The Netherlands, 2011; pp. 7–335. [Google Scholar] [CrossRef]
- Dogu, O.; Eschenbacher, A.; John Varghese, R.J.; Dobbelaere, M.; D’hooge, D.R.; Van Steenberge, P.H.M.; Van Geem, K.M. Bayesian tuned kinetic Monte Carlo modeling of polystyrene pyrolysis: Unraveling the pathways to its monomer, dimers, and trimers formation. Chem. Eng. J. 2023, 455, 140708. [Google Scholar] [CrossRef]
- Palmay, P.; Haro, C.; Huacho, I.; Barzallo, D.; Bruno, J.C. Production and analysis of the physicochemical properties of the pyrolytic oil obtained from pyrolysis of different thermoplastics and plastic mixtures. Molecules 2022, 27, 3287. [Google Scholar] [CrossRef]
- Das, M. Performance and emission characteristics of biodiesel-diesel blend. Encycl. Renew. Sustain. Mater. 2020, 2, 202–211. [Google Scholar] [CrossRef]
- Park, Y.-K.; Jung, J.; Ryu, S.; Lee, H.W.; Siddiqui, M.Z.; Jae, J.; Watanabe, A.; Kim, Y.-M. Catalytic co-pyrolysis of yellow poplar wood and polyethylene terephthalate over two stage calcium oxide-ZSM-5. Appl. Energy 2019, 250, 1706–1718. [Google Scholar] [CrossRef]
- Sangaré, D.; Moscosa-Santillan, M.; Bostyn, S.; Belandria, V.; De la Cruz Martínez, A.; Van De Steene, L. Multi-step kinetic mechanism coupled with CFD modeling of slow pyrolysis of biomass at different heating rates. Chem. Eng. J. 2024, 479, 147791. [Google Scholar] [CrossRef]
- Siddiqui, M.Z.; Han, T.U.; Park, Y.-K.; Kim, Y.-M.; Kim, S. Catalytic pyrolysis of Tetra Pak over acidic catalysts. Catalysts 2020, 10, 602. [Google Scholar] [CrossRef]
- Wong, S.L.; Armenise, S.; Nyakuma, B.B.; Bogush, A.; Towers, S.; Lee, C.H.; Wong, K.Y.; Lee, T.H.; Rebrov, E.; Muñoz, M. Plastic pyrolysis over HZSM-5 zeolite and fluid catalytic cracking catalyst under ultra-fast heating. J. Anal. Appl. Pyrolysis 2023, 169, 105793. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Gaurh, P.; Pramanik, H. Production of benzene/toluene/ethyl benzene/xylene (BTEX) via multiphase catalytic pyrolysis of hazardous waste polyethylene using low cost fly ash synthesized natural catalyst. Waste Manag. 2018, 77, 114–130. [Google Scholar] [CrossRef]
- Han, T.U.; Kang, J.-G.; Jeon, T.-W. New policy framework for circular economy in South Korea: Achieving both management and chemical recycling of polymeric waste via pyrolysis reaction. Resour. Conserv. Recycl. 2024, 202, 107371. [Google Scholar] [CrossRef]
- Yoo, H.-M.; Kang, J.-H.; Lee, S.-J.; Jang, S.-H.; Yoon, Y.-S.; Kang, Y. Effect analysis on waste recycling by introducing a new policy, “Environmental Assessment of Recycling,” for establishment of the ESG management system in the Republic of Korea. Integr. Environ. Assess. Manag. 2024, 20, 1473–1485. [Google Scholar] [CrossRef]


| Site | Type | Capacity (m3) | Mining Purpose | Location |
|---|---|---|---|---|
| A | Unsanitary | 357,192 | Development of a residential complex | Incheon |
| B | Secure for industrial waste | 2,562,000 | Maintenance and redesign of the landfill site to enlarge landfill capacity | Gunsan |
| C | Sanitary | 2,897,000 | Mokpo | |
| D | Sanitary | 1,508,000 | Gyeong-Ju |
| Site | Proximate Analysis (wt. %) | Ultimate Analysis (wt. %) | Heating Value (MJ/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | Volatiles | Ash | C | H | N | S | Others | ||
| A | 18.73 | 65.18 | 16.09 | 67.02 | 9.20 | 0.25 | 0.28 | 23.25 | 34.28 |
| B | 36.10 | 51.44 | 12.46 | 70.82 | 11.04 | 0.41 | 0.34 | 17.39 | 36.04 |
| C | 20.04 | 62.48 | 17.47 | 64.97 | 9.68 | 0.33 | 0.09 | 24.93 | 32.70 |
| D | 5.72 | 85.25 | 9.03 | 74.88 | 10.00 | 0.31 | 0.14 | 14.67 | 39.30 |
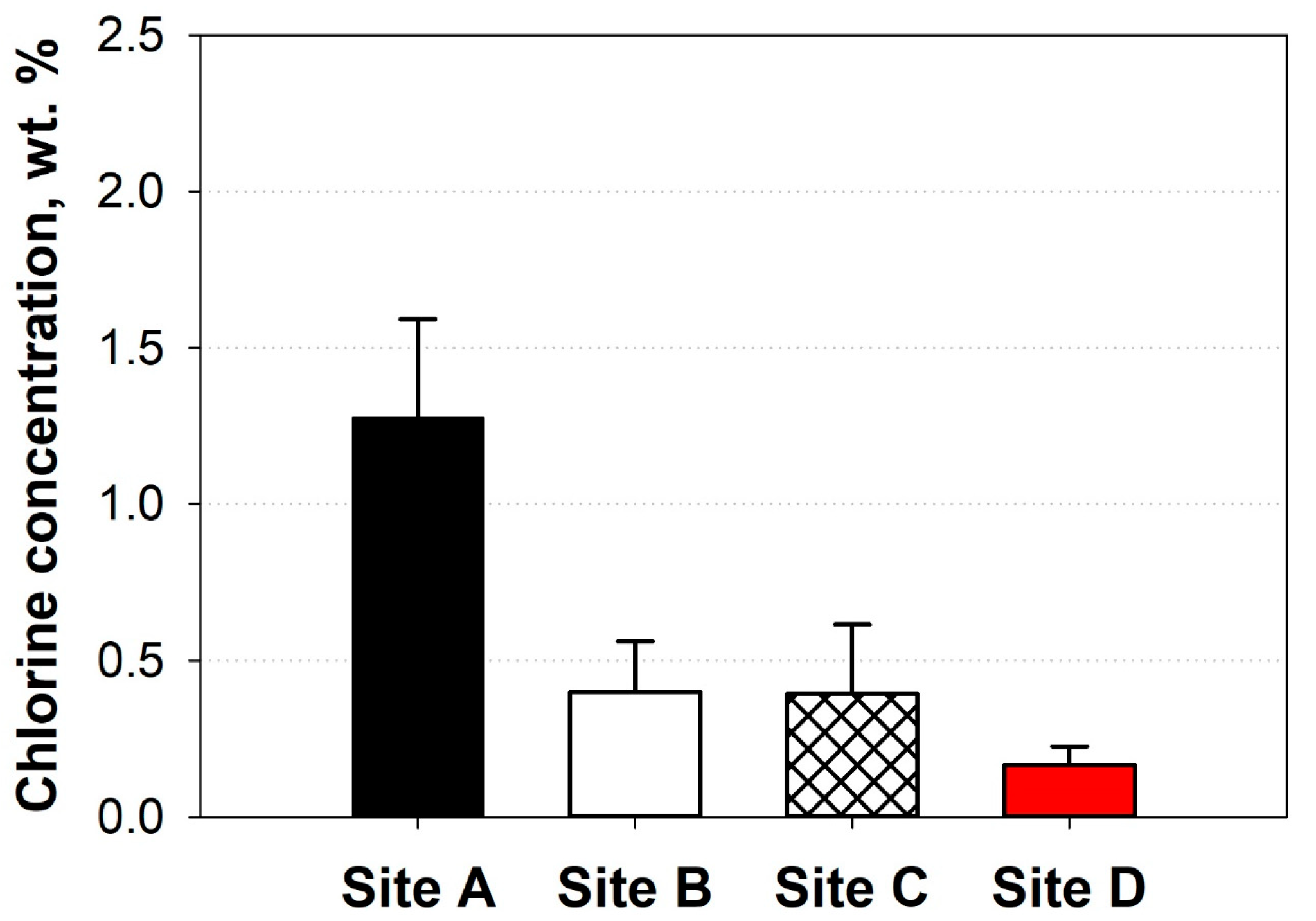
| Point | LHV (MJ/kg) | Water (wt. %) | Ash (wt. %) | Cl Concentration (wt. %) | SRF Classification (Sum of Points) |
|---|---|---|---|---|---|
| 5 | ≥35.0 | ≤5.0 | ≤5.0 | ≤0.5 |
|
| 3 | ≥30.0 | ≤10.0 | ≤10.0 | ≤1.0 | |
| 1 | ≥25.0 | ≤25.0 | ≤20.0 | ≤2.0 | |
| Landfill site | LHV (MJ/kg) | Water (wt. %) | Ash (wt. %) | Cl Concentration (wt. %) | Sum of points (Classification) |
| A | 34.3 | 18.7 | 16.1 | 1.28 | 6 (Class 4) |
| Point | 3 | 1 | 1 | 1 | |
| B | 36.0 | 36.1 | 12.5 | 0.40 | 11 (Class 2) |
| Point | 5 | 0 | 1 | 5 | |
| C | 32.7 | 20.0 | 17.5 | 0.39 | 10 (Class 2) |
| Point | 3 | 1 | 1 | 5 | |
| D | 39.3 | 5.7 | 9.0 | 0.17 | 16 (Class 1) |
| Point | 5 | 3 | 3 | 5 |
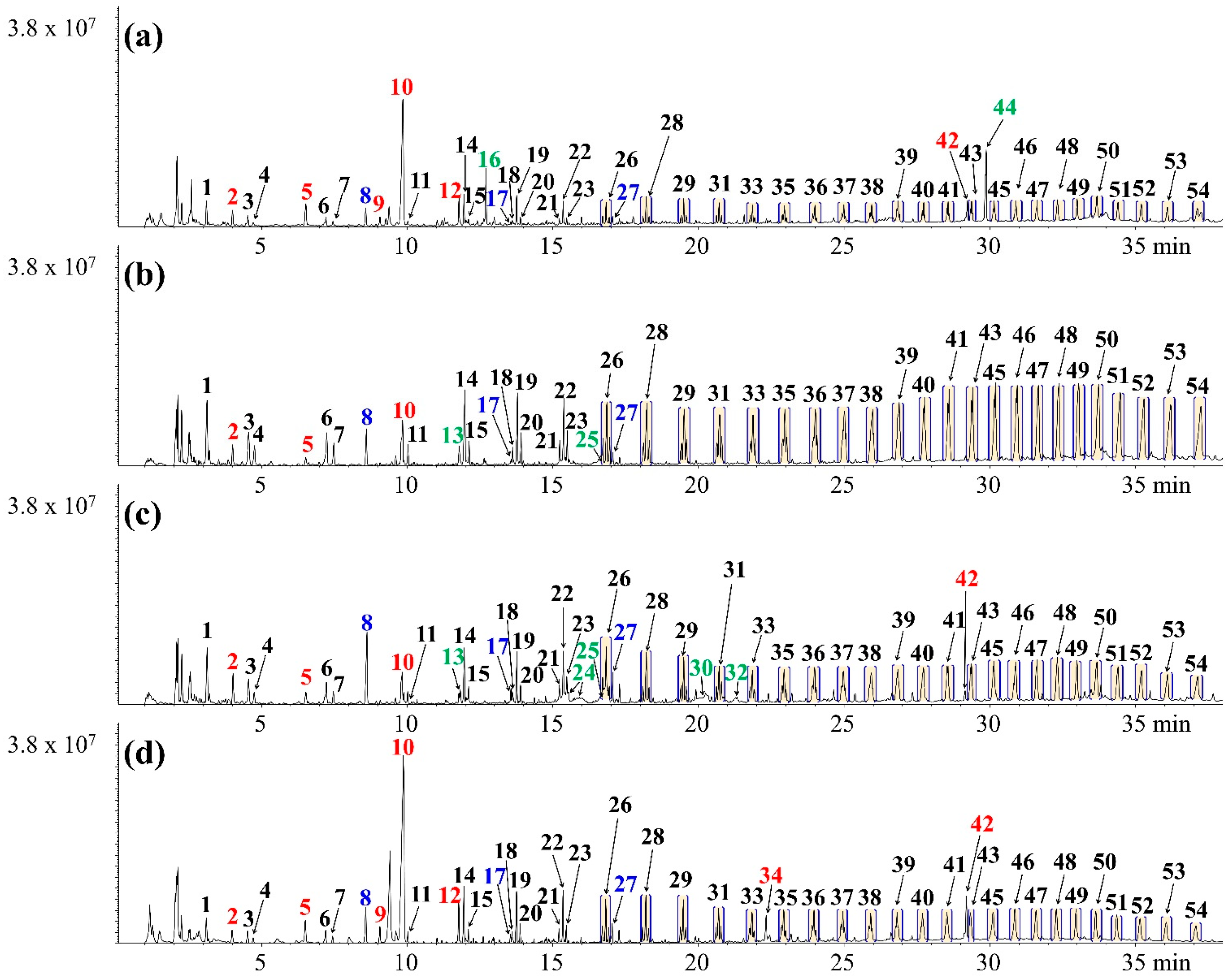
| Peak No. | Compound | MS Peak Area (×10−7) | |||
|---|---|---|---|---|---|
| CPCs-Site A | CPCs-Site B | CPCs-Site C | CPCs-Site D | ||
| 1 | Propylene | 68.3 ± 7.6 | 116.6 ± 15.8 | 71.5 ± 6.6 | 111.8 ± 13.6 |
| 2 | 1-Butene | 55.7 ± 8.0 | 115.1 ± 17.1 | 55.7 ± 8.6 | 88.1 ± 17.8 |
| 3 | 2-Methyl-1-butene | 41.0 ± 7.1 | 71.7 ± 17.5 | 37.3 ± 7.4 | 49.9 ± 7.8 |
| 4 | Benzene | 19.9 ± 2.6 | 17.5 ± 0.7 | 32.6 ± 4.6 | 46.8 ± 4.6 |
| 5 | Toluene | 40.7 ± 0.9 | 32.2 ± 1.1 | 30.9 ± 0.8 | 43.3 ± 2.2 |
| 6 | 2,4-Dimethyl-1-heptene | - | - | 14.0 ± 4.8 | - |
| 7 | Ethylbenzene | 19.5 ± 1.2 | 11.0 ± 0.7 | 14.5 ± 0.3 | 33.7 ± 0.7 |
| 8 | Xylene | 41.7 ± 1.9 | 36.9 ± 1.8 | 31.2 ± 2.5 | 33.7 ± 1.7 |
| 9 | Styrene | 90.6 ± 5.8 | 8.9 ± 0.0 | 14.0 ± 0.3 | 132.3 ± 8.3 |
| 10 | Ethyltoluene | 22.5 ± 0.7 | 17.2 ± 2.8 | 17.9 ± 1.3 | 15.5 ± 1.0 |
| 11 | Cyanobenzene + Methylstyrene | 14.1 ± 1.9 | 17.1 ± 1.9 | 30.7 ± 4.3 | 23.7 ± 0.4 |
| 12 | Trimethylbenzene | 15.5 ± 0.8 | 14.3 ± 0.6 | 14.3 ± 0.7 | 10.4 ± 0.3 |
| 13 | Indene | 11.3 ± 0.5 | - | 6.8 ± 0.2 | 14.8 ± 0.2 |
| 14 | 1-Methylindene | 7.2 ± 0.4 | - | 6.7 ± 0.2 | 7.8 ± 0.6 |
| 15 | 3-Methylindene | 8.2 ± 0.4 | - | 7.9 ± 1.1 | 11.2 ± 0.3 |
| 16 | Methylnaphthalene | 9.6 ± 1.0 | 8.3 ± 1.1 | 19.3 ± 2.3 | 12.1 ± 1.0 |
| 17 | Diphenyl | - | 10.0 ± 0.9 | 7.7 ± 0.4 | - |
| 18 | 1,3-Diphenylpropane | 5.1 ± 0.9 | - | - | 11.1 ± 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Han, T.U. Potential of Landfill Mined Combustible Polymer Composite and Soil-like Fraction for Energy Recovery, Chemical Recycling, and Resource Recovery. Polymers 2025, 17, 2514. https://doi.org/10.3390/polym17182514
Lee S, Han TU. Potential of Landfill Mined Combustible Polymer Composite and Soil-like Fraction for Energy Recovery, Chemical Recycling, and Resource Recovery. Polymers. 2025; 17(18):2514. https://doi.org/10.3390/polym17182514
Chicago/Turabian StyleLee, Suyoung, and Tae Uk Han. 2025. "Potential of Landfill Mined Combustible Polymer Composite and Soil-like Fraction for Energy Recovery, Chemical Recycling, and Resource Recovery" Polymers 17, no. 18: 2514. https://doi.org/10.3390/polym17182514
APA StyleLee, S., & Han, T. U. (2025). Potential of Landfill Mined Combustible Polymer Composite and Soil-like Fraction for Energy Recovery, Chemical Recycling, and Resource Recovery. Polymers, 17(18), 2514. https://doi.org/10.3390/polym17182514






