Synthesis and Integration of an Fe(II) Coordination Compound into Green Resin Matrices for Multifunctional Dielectric, Piezoelectric, Energy Harvesting, and Storage Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of [Fe(bpy)3]SO4
2.2. Fabrication of Filaments
3. Results and Discussion
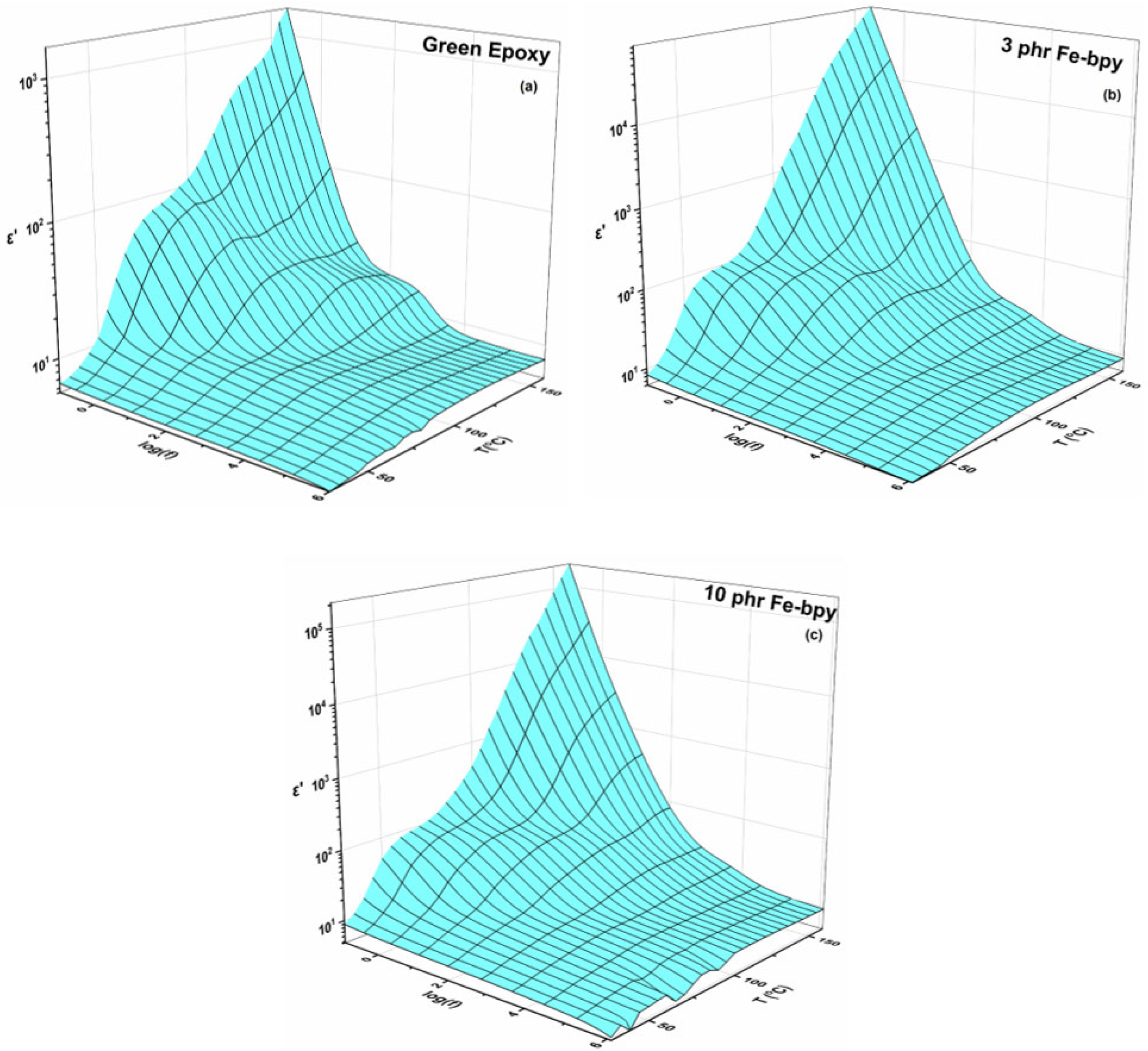
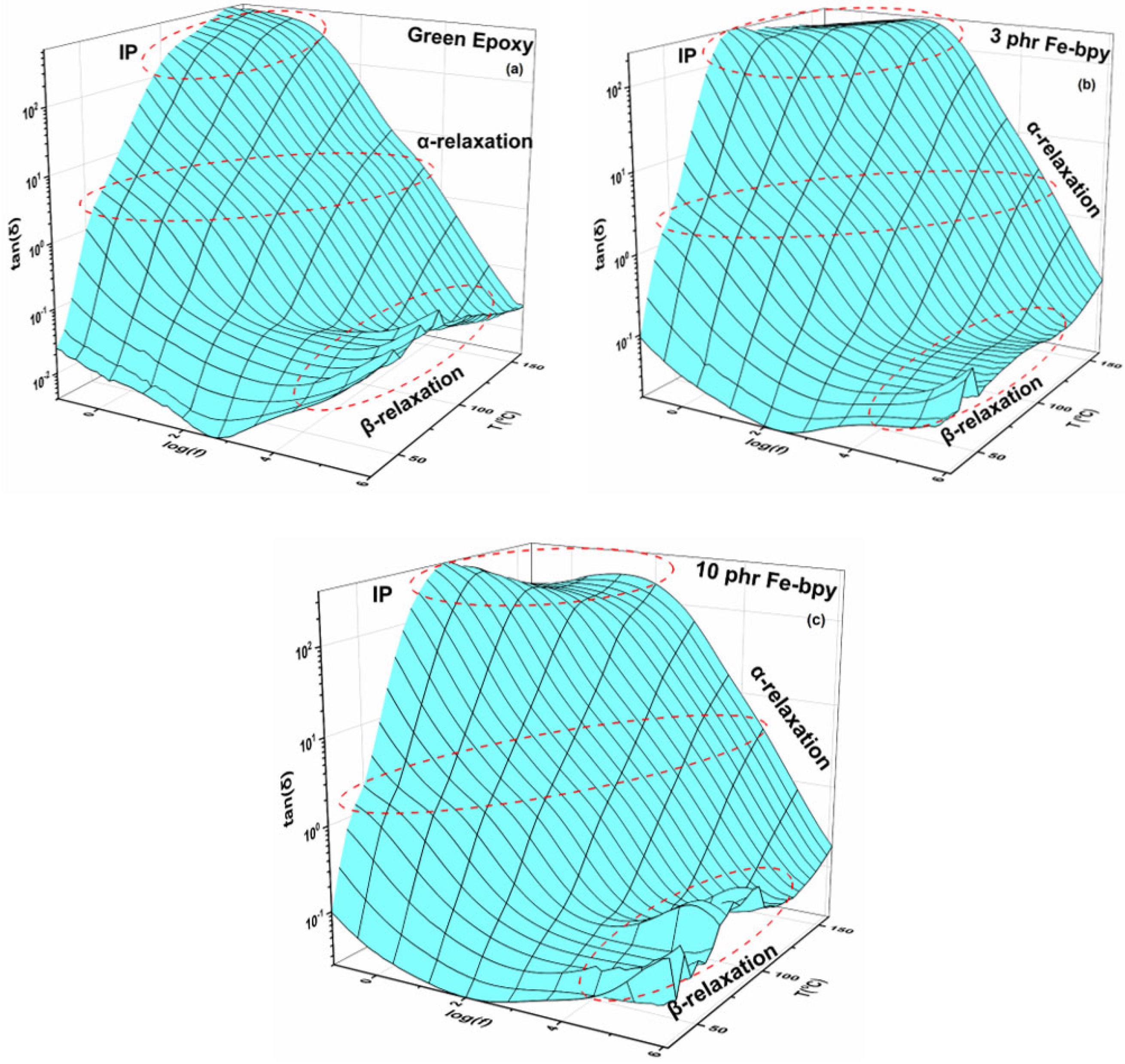
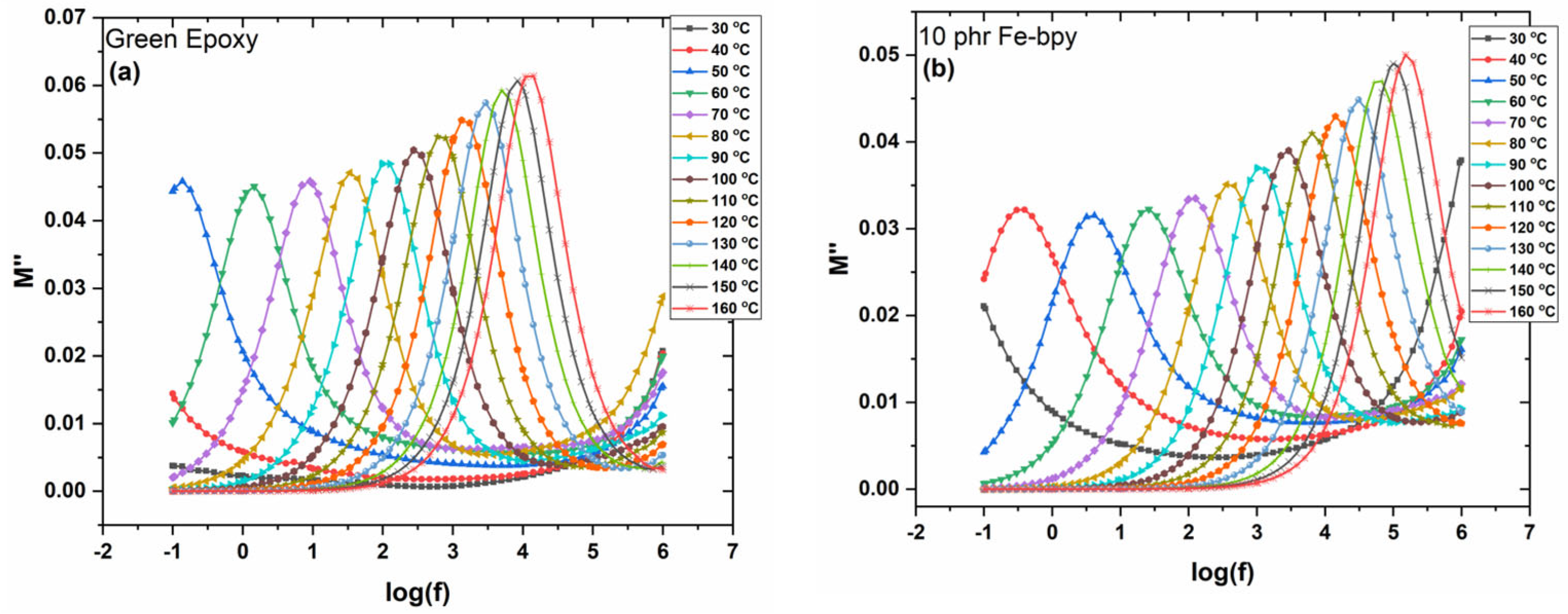
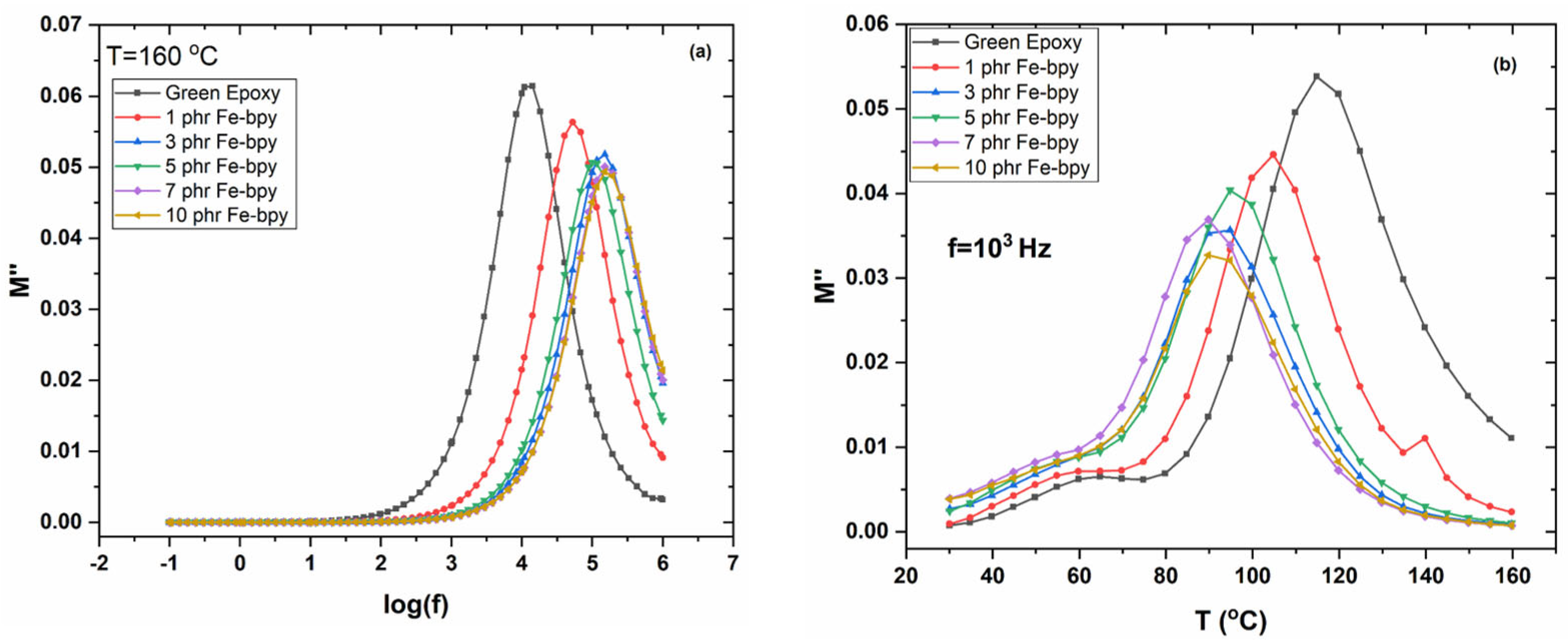
3.1. Dielectric Properties
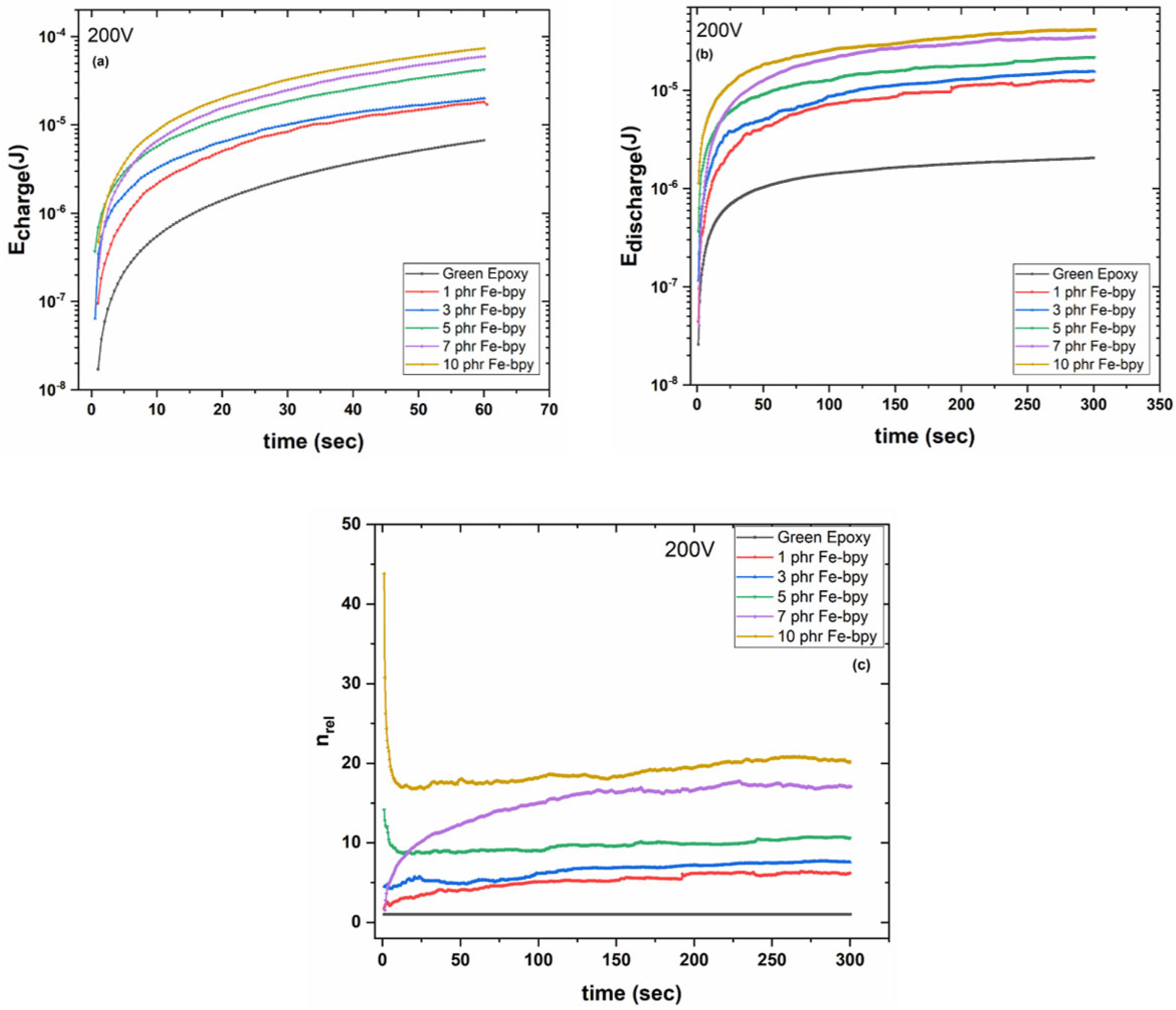
3.2. Energy Storage and Harvesting Properties
3.3. Piezoelectricity Properties
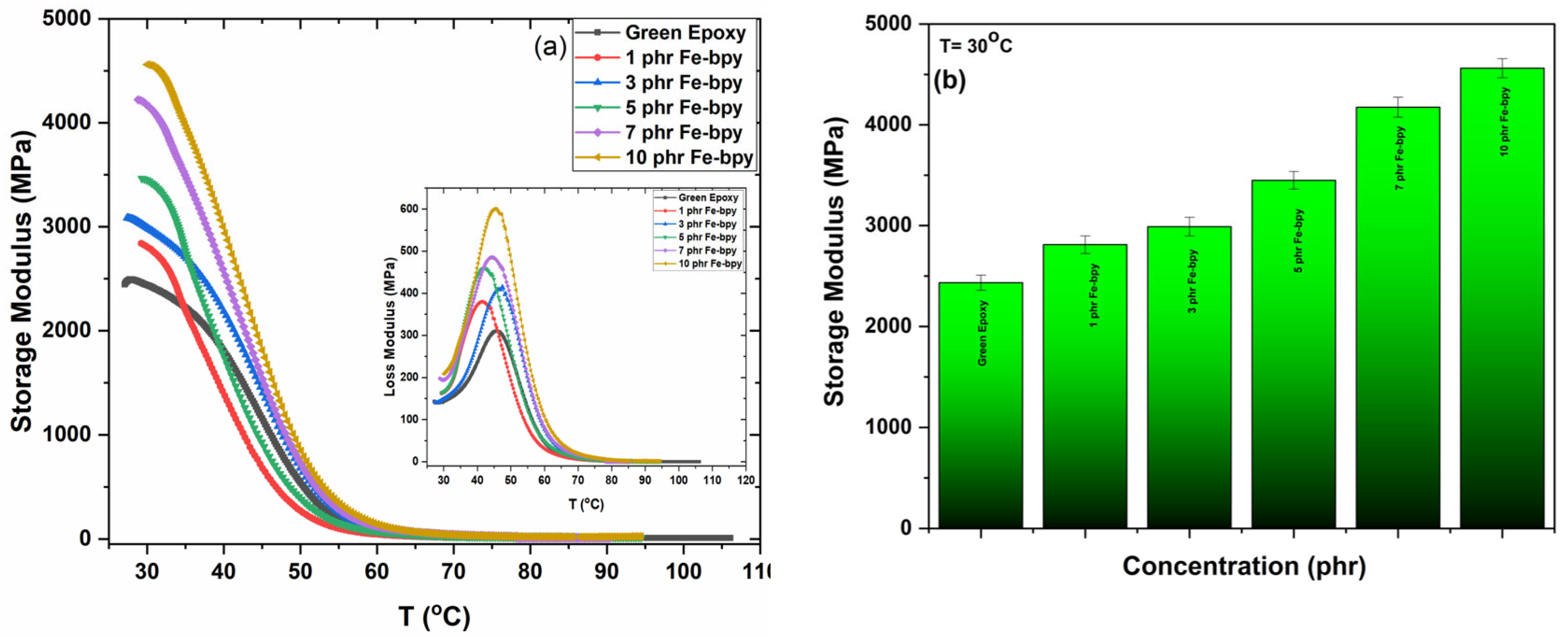
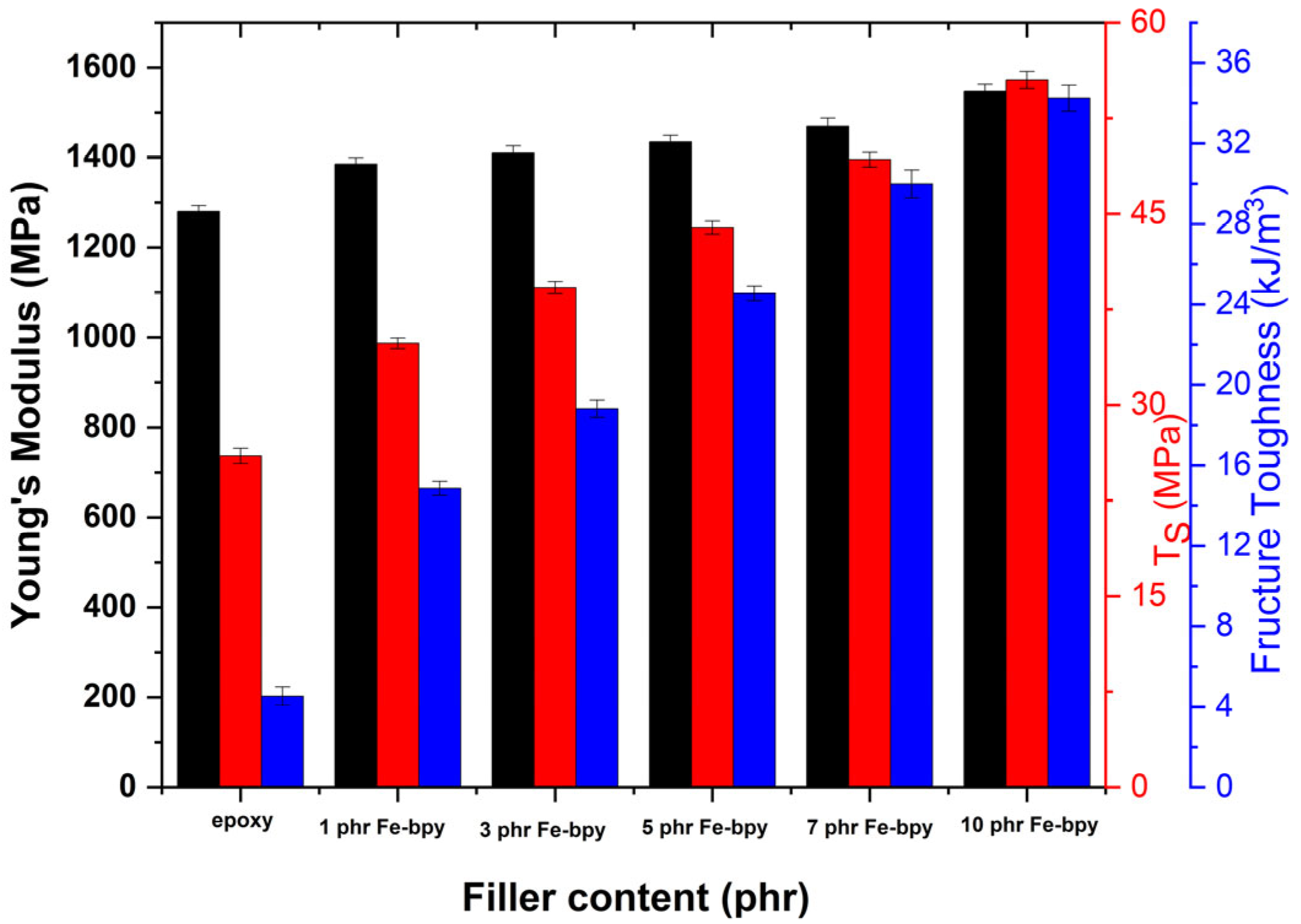
3.4. Thermomechanical Properties
3.5. Comparative Analysis of the Dielectric Properties and Energy Storage Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Psarras, G.C. Polymer nanocomposites and transport (in their) properties. Express Polym. Lett. 2023, 17, 563. [Google Scholar] [CrossRef]
- Azeez, A.A.; Rhee, K.Y.; Park, S.J.; Hui, D. Epoxy clay nanocomposites—Processing, properties and applications: A review. Compos. Part B Eng. 2013, 45, 308–320. [Google Scholar] [CrossRef]
- Joshi, M.; Adak, B.; Butola, B.S. Polyurethane nanocomposite based gas barrier films, membranes and coatings: A review on synthesis, characterization and potential applications. Prog. Mater. Sci. 2018, 97, 230–282. [Google Scholar] [CrossRef]
- Kumar, V.; Alam, M.N. Multifunctional Polymer Composite Materials. Polymers 2025, 17, 1636. [Google Scholar] [CrossRef] [PubMed]
- Quaresimin, M.; Bertani, R.; Zappalorto, M.; Pontefisso, A.; Simionato, F.; Bartolozzi, A. Multifunctional polymer nanocomposites with enhanced mechanical and anti-microbial properties. Compos. Part B Eng. 2015, 80, 108–115. [Google Scholar] [CrossRef]
- Wu, Z.; Ding, X.; Chen, X.; Chen, J.; Chang, X.; Liu, Z.; Song, L.; Huang, J.; Zhu, Y. Recent progress of polymer-based piezoelectric nanogenerators. Adv. Compos. Hybrid Mater. 2025, 8, 225. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, Y.; Men, R.; Lei, Z.; Song, J.; Li, Y.; Guo, M. High-energy-density polymer dielectrics via compositional and structural tailoring for electrical energy storage. iScience 2022, 25, 104837. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Yang, Z.; Mu, W.; Liu, T.; Liu, X.; Wang, Q. Ultra-Broadband Perfect Absorbers Based on Biomimetic Metamaterials with Dual Coupling Gradient Resonators. Adv. Mater. 2025, 37, 2416314. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, S.; Li, C.; Yang, N.; He, Y.; Duan, K.; Zhang, J. Frequency insensitive electromagnetic absorption core-shell sandwich structure with excellent electromagnetic damage tolerance. Compos. Part B Eng. 2025, 289, 111946. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Wang, B.; He, Q.; Cao, J.; Zhu, Y.; Su, K.; Yan, H.; Sun, P.; Li, R.; et al. Ultra-Bandwidth Microwave Absorption and Low Angle Sensitivity in Dual-Network Aerogels with Dual-Scale Pores. Small 2025, 21, 2412744. [Google Scholar] [CrossRef]
- Manika, G.C.; Psarras, G.C. Energy storage and harvesting in BaTiO3/epoxy nanodielectrics. High Volt. 2016, 1, 151–157. [Google Scholar] [CrossRef]
- Sanida, A.; Stavropoulos, S.G.; Speliotis, T.; Psarras, G.C. Investigating the Effect of Zn Ferrite Nanoparticles on the Thermomechanical, Dielectric and Magnetic Properties of Polymer Nanocomposites. Materials 2019, 12, 3015. [Google Scholar] [CrossRef]
- Qiu, J.; Gu, Q.; Sha, Y.; Huang, Y.; Zhang, M.; Luo, Z. Preparation and application of dielectric polymers with high permittivity and low energy loss: A mini review. J. Appl. Polym. Sci. 2022, 139, 52367. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Liu, Y.; Li, L.; Liu, Y.; Wang, Q. Dielectric polymers for high-temperature capacitive energy storage. Chem. Soc. Rev. 2021, 50, 6369–6400. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Xi, R.; Zhou, C.; He, G.; Yang, F.; Xu, L.; Li, H. Polymer Capacitor Films with Nanoscale Coatings for Dielectric Energy Storage: A Review. Coatings 2024, 14, 1193. [Google Scholar] [CrossRef]
- Aherrao, D.S.; Singh, C.; Srivastava, A.K. Review of ferrite-based microwave-absorbing materials: Origin, synthesis, morphological effects, dielectric/magnetic properties, composites, absorption mechanisms, and optimization. J. Appl. Phys. 2022, 132, 240701. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Zhang, Q.M.; Tan, D.Q. Advanced dielectric polymers for energy storage. Energy Storage Mater. 2022, 44, 29–47. [Google Scholar] [CrossRef]
- Kostishin, V.G.; Isaev, I.M.; Salogub, D.V. Radio-Absorbing Magnetic Polymer Composites Based on Spinel Ferrites: A Review. Polymers 2024, 16, 1003. [Google Scholar] [CrossRef]
- Wang, Q.; Che, J.; Wu, W.; Hu, Z.; Liu, X.; Ren, T.; Chen, Y.; Zhang, J. Contributing Factors of Dielectric Properties for Polymer Matrix Composites. Polymers 2023, 15, 590. [Google Scholar] [CrossRef]
- Carey, M.C.; Adelman, S.L.; McCusker, J.K. Insights into the excited state dynamics of Fe(ii) polypyridyl complexes from variable-temperature ultrafast spectroscopy. Chem. Sci. 2019, 10, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.; Holubowitch, N.E. Isopropyl alcohol and copper hexacyanoferrate boost performance of the iron tris-bipyridine catholyte for near-neutral pH aqueous redox flow batteries. Int. J. Energy Res. 2022, 46, 5864–5875. [Google Scholar] [CrossRef]
- Holubowitch, N.E.; Nguyen, G. Dimerization of Fe(III)(bpy)3 in Aqueous Solutions. Inorg. Chem. 2022, 61, 9541–9556. [Google Scholar] [CrossRef]
- Holubowitch, N.E.; Jabbar, A. Spectroelectrochemistry of next-generation redox flow battery electrolytes: A survey of active species from four representative classes. Microchem. J. 2022, 182, 107920. [Google Scholar] [CrossRef]
- Patsidis, A.C.; Souliotis, M. End-Of-Use Fly Ash as an Effective Reinforcing Filler in Green Polymer Composites. Polymers 2023, 15, 3418. [Google Scholar] [CrossRef]
- Gioti, S.; Sanida, A.; Mathioudakis, G.N.; Patsidis, A.C.; Speliotis, T.; Psarras, G.C. Multitasking Performance of Fe3O4/BaTiO3/Epoxy Resin Hybrid Nanocomposites. Materials 2022, 15, 1784. [Google Scholar] [CrossRef] [PubMed]
- Manika, G.C.; Andrikopoulos, K.S.; Psarras, G.C. On the Ferroelectric to Paraelectric Structural Transition of BaTiO3 Micro-/Nanoparticles and Their Epoxy Nanocomposites. Molecules 2020, 25, 2686. [Google Scholar] [CrossRef]
- ASTM D150-18; Standard Test Methods for AC Loss Characteristics and Permittivity (Dielectric Constant) of Solid Electrical Insulation. American Society for Testing and Materials (ASTM International): West Conshohocken, PA, USA, 2022.
- ASTM D570-22; Standard Test Method for Water Absorption of Plastics. American Society for Testing and Materials (ASTM International): West Conshohocken, PA, USA, 2022.
- ASTM D257-14(2021)e1; Standard Test Methods for DC Resistance or Conductance of Insulating Materials. American Society for Testing and Materials (ASTM International): West Conshohocken, PA, USA, 2021.
- Manika, G.C.; Psarras, G.C. Barium titanate/epoxy resin composite nanodielectrics as compact capacitive energy storing systems. Express Polym. Lett. 2019, 13, 749–758. [Google Scholar] [CrossRef]
- Psarras, G.C. Hopping conductivity in polymer matrix–metal particles composites. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1545–1553. [Google Scholar] [CrossRef]
- Andrew, K.J. Dielectric relaxation in solids. J. Phys. D Appl. Phys. 1999, 32, R57. [Google Scholar] [CrossRef]
- Dyre, J.C.; Maass, P.; Roling, B.; Sidebottom, D.L. Fundamental questions relating to ion conduction in disordered solids. Rep. Prog. Phys. 2009, 72, 046501. [Google Scholar] [CrossRef]
- Gatos, K.G.; Martínez Alcázar, J.G.; Psarras, G.C.; Thomann, R.; Karger-Kocsis, J. Polyurethane latex/water dispersible boehmite alumina nanocomposites: Thermal, mechanical and dielectrical properties. Compos. Sci. Technol. 2007, 67, 157–167. [Google Scholar] [CrossRef]
- Tsangaris, G.M.; Psarras, G.C.; Kouloumbi, N. Electric modulus and interfacial polarization in composite polymeric systems. J. Mater. Sci. 1998, 33, 2027–2037. [Google Scholar] [CrossRef]
- Grohens, Y.; Hamon, L.; Reiter, G.; Soldera, A.; Holl, Y. Some relevant parameters affecting the glass transition of supported ultra-thin polymer films. Eur. Phys. J. E 2002, 8, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.; Gorbatschow, W.; Hauwede, J.; Kremer, F. Molecular dynamics in thin films of isotactic poly(methyl methacrylate). Eur. Phys. J. E 2002, 8, 145–154. [Google Scholar] [CrossRef]
- Mathioudakis, G.N.; Patsidis, A.C.; Psarras, G.C. Dynamic electrical thermal analysis on zinc oxide/epoxy resin nanodielectrics. J. Therm. Anal. Calorim. 2014, 116, 27–33. [Google Scholar] [CrossRef]
- Psarras, G.C. Fundamentals of Dielectric Theories. In Dielectric Polymer Materials for High-Density Energy Storage; Dang, Z.-M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 11–57. [Google Scholar]
- Patsidis, A.C.; Koufakis, E.I.; Mathioudakis, G.N.; Vryonis, O.; Psarras, G.C. Development, Dielectric Response, and Functionality of ZnTiO3/BaTiO3/Epoxy Resin Hybrid Nanocomposites. J. Compos. Sci. 2024, 8, 225. [Google Scholar] [CrossRef]
- Dang, Z.-M.; Yuan, J.-K.; Zha, J.-W.; Zhou, T.; Li, S.-T.; Hu, G.-H. Fundamentals, processes and applications of high-permittivity polymer–matrix composites. Prog. Mater. Sci. 2012, 57, 660–723. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, H.; Yin, C.; Jung, Y.H.; Min, S.; Zhang, Y.; Zhang, C.; Chen, Q.; Lee, K.J.; Chi, Q. Recent progress in polymer dielectric energy storage: From film fabrication and modification to capacitor performance and application. Prog. Mater. Sci. 2023, 140, 101207. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-Active Polymers for Energy Storage Nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef]
- Chen, M.; Chen, R.; Zhitomirsky, I.; He, G.; Shi, K. Redox-active molecules for aqueous electrolytes of energy storage devices: A review on fundamental aspects, current progress, and prospects. Mater. Sci. Eng. R Rep. 2024, 161, 100865. [Google Scholar] [CrossRef]
- Sundar, U.; Lao, Z.; Cook-Chennault, K. Investigation of Piezoelectricity and Resistivity of Surface Modified Barium Titanate Nanocomposites. Polymers 2019, 11, 2123. [Google Scholar] [CrossRef]
- Stuber, V.L.; Mahon, T.R.; van der Zwaag, S.; Groen, P. The effect of the intrinsic electrical matrix conductivity on the piezoelectric charge constant of piezoelectric composites. Mater. Res. Express 2020, 7, 015703. [Google Scholar] [CrossRef]
- Mystiridou, E.; Patsidis, A.C.; Bouropoulos, N. Development and Characterization of 3D Printed Multifunctional Bioscaffolds Based on PLA/PCL/HAp/BaTiO3 Composites. Appl. Sci. 2021, 11, 4253. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Naffakh, M.; Gómez, M.A.; Marco, C.; Ellis, G.; Martínez, M.T.; Ansón, A.; González-Domínguez, J.M.; Martínez-Rubi, Y.; Simard, B. Development and characterization of PEEK/carbon nanotube composites. Carbon 2009, 47, 3079–3090. [Google Scholar] [CrossRef]
- You, L.; Liu, B.; Hua, H.; Jiang, H.; Yin, C.; Wen, F. Energy Storage Performance of Polymer-Based Dielectric Composites with Two-Dimensional Fillers. Nanomaterials 2023, 13, 2842. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Hou, C.; Shen, Z.; Sun, H.; Zhang, G.; Luo, Z.; Dai, Z.; Wang, C.; Chen, X.; Li, L.; et al. Negatively Charged Nanosheets Significantly Enhance the Energy-Storage Capability of Polymer-Based Nanocomposites. Adv. Mater. 2020, 32, 1907227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sheng, Q.; Ye, L.; Long, S.; Zhou, B.; Wen, F.; Yang, J.; Wang, G.; Bai, W. Two-dimensional SrTiO3 platelets induced the improvement of energy storage performance in polymer composite films at low electric fields. Ceram. Int. 2022, 48, 7145–7152. [Google Scholar] [CrossRef]
- Chen, J.; Shen, Z.; Kang, Q.; Qian, X.; Li, S.; Jiang, P.; Huang, X. Chemical adsorption on 2D dielectric nanosheets for matrix free nanocomposites with ultrahigh electrical energy storage. Sci. Bull. 2022, 67, 609–618. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, B.; Zhai, J.; Yao, L.; Yang, K.; Shen, B. NaNbO3 two-dimensional platelets induced highly energy storage density in trilayered architecture composites. Nano Energy 2017, 40, 587–595. [Google Scholar] [CrossRef]
- Li, M.; Yao, M.; Su, Z.; Gao, W.; Yao, X. Improved breakdown strength and energy density of Al2O3/nano-ZrO2 composite films via enhanced interfacial repairing behavior. Ceram. Int. 2018, 44, 21428–21436. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, Y.; Dong, J.; Xu, X.; Yuan, Q.; Niu, Y.; Wang, Q.; Wang, H. Significantly improved breakdown strength and energy density of tri-layered polymer nanocomposites with optimized graphene oxide. Compos. Sci. Technol. 2020, 186, 107912. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, X.; Xu, C.; Han, B.; Xu, L. Enhanced dielectric property and energy density in poly(vinylidene fluoride-chlorotrifluoroethylene) nanocomposite incorporated with graphene functionalized with hyperbranched polyethylene-graft-poly(trifluoroethyl methacrylate) copolymer. J. Mater. Chem. C 2018, 6, 11144–11155. [Google Scholar] [CrossRef]
- Panda, S.; Pasha, S.K.K. Amplified Dielectric Properties of PVDF–HFP/SrTiO3 Nanocomposites for a Flexible Film Capacitor. Langmuir 2023, 39, 13345–13358. [Google Scholar] [CrossRef]
- Wang, L.; Gao, F.; Xu, J.; Zhang, K.; Kong, J.; Reece, M.; Yan, H. Enhanced dielectric tunability and energy storage properties of plate-like Ba0.6Sr0.4TiO3/poly(vinylidene fluoride) composites through texture arrangement. Compos. Sci. Technol. 2018, 158, 112–120. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Y.; Sun, C.; Zhan, S.; Yuan, Q.; Yang, H. Energy storage performance in polymer dielectrics by introducing 2D SrBi4Ti4O15 nanosheets. Ceram. Int. 2020, 46, 15270–15275. [Google Scholar] [CrossRef]
- Pan, Z.; Ding, Q.; Yao, L.; Huang, S.; Xing, S.; Liu, J.; Chen, J.; Zhai, J. Simultaneously enhanced discharge energy density and efficiency in nanocomposite film capacitors utilizing two-dimensional NaNbO3@Al2O3 platelets. Nanoscale 2019, 11, 10546–10554. [Google Scholar] [CrossRef]
- Wen, R.; Guo, J.; Zhao, C.; Liu, Y. Nanocomposite Capacitors with Significantly Enhanced Energy Density and Breakdown Strength Utilizing a Small Loading of Monolayer Titania. Adv. Mater. Interfaces 2018, 5, 1701088. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Feng, Y.; Chen, D.; Li, Y.; Yue, D.; Huang, B.; Yin, J. Constructing bidirectional-matched interface between polymer and 2D nanosheets for enhancing energy storage performance of the composites. Energy Storage Mater. 2023, 54, 605–614. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Tan, S.; Peng, B.; Qiao, B.; Zhang, Z.; Huang, X.; Sui, H. Improving Energy Storage Density and Efficiency of Polymer Dielectrics by Adding Trace Biomimetic Lysozyme-Modified Boron Nitride. ACS Appl. Energy Mater. 2020, 3, 7952–7963. [Google Scholar] [CrossRef]
- Wen, F.; Zhu, C.; Li, L.; Zhou, B.; Zhang, L.; Han, C.; Li, W.; Yue, Z.; Wu, W.; Wang, G.; et al. Enhanced energy storage performance of polymer nanocomposites using hybrid 2D ZnO@MoS2 semiconductive nano-fillers. Chem. Eng. J. 2022, 430, 132676. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, X.; Xie, J.; Liu, Y.; Sun, S. Influence of organic Na+-MMT on the dielectric and energy storage properties of maleic anhydride-functionalized polypropylene nanocomposites. J. Polym. Res. 2022, 29, 182. [Google Scholar] [CrossRef]
- Feng, M.; Feng, Y.; Zhang, T.; Li, J.; Chen, Q.; Chi, Q.; Lei, Q. Recent Advances in Multilayer-Structure Dielectrics for Energy Storage Application. Adv. Sci. 2021, 8, 2102221. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Zhang, S.; Ren, K. 2D MoS2 Nanosheet-Based Polyimide Nanocomposite with High Energy Density for High Temperature Capacitor Applications. Macromol. Mater. Eng. 2021, 306, 2100079. [Google Scholar] [CrossRef]
- Jiang, H.; Ye, H.; Xu, L. One-pot synthesis of hexafluorobutyl acrylate hyperbranched copolymer for graphene/poly(vinylidenefluoride-trifluoroethylene- chlorofluoroethylene) dielectric composite. Nanotechnology 2022, 33, 215703. [Google Scholar] [CrossRef]
- Yu, S.; Ding, C.; Liu, Y.; Liu, Y.; Zhang, Y.; Luo, H.; Zhang, D.; Chen, S. Enhanced breakdown strength and energy density over a broad temperature range in polyimide dielectrics using oxidized MXenes filler. J. Power Sources 2022, 535, 231415. [Google Scholar] [CrossRef]
- Liu, F.; Li, Q.; Li, Z.; Liu, Y.; Dong, L.; Xiong, C.; Wang, Q. Poly(methyl methacrylate)/boron nitride nanocomposites with enhanced energy density as high temperature dielectrics. Compos. Sci. Technol. 2017, 142, 139–144. [Google Scholar] [CrossRef]



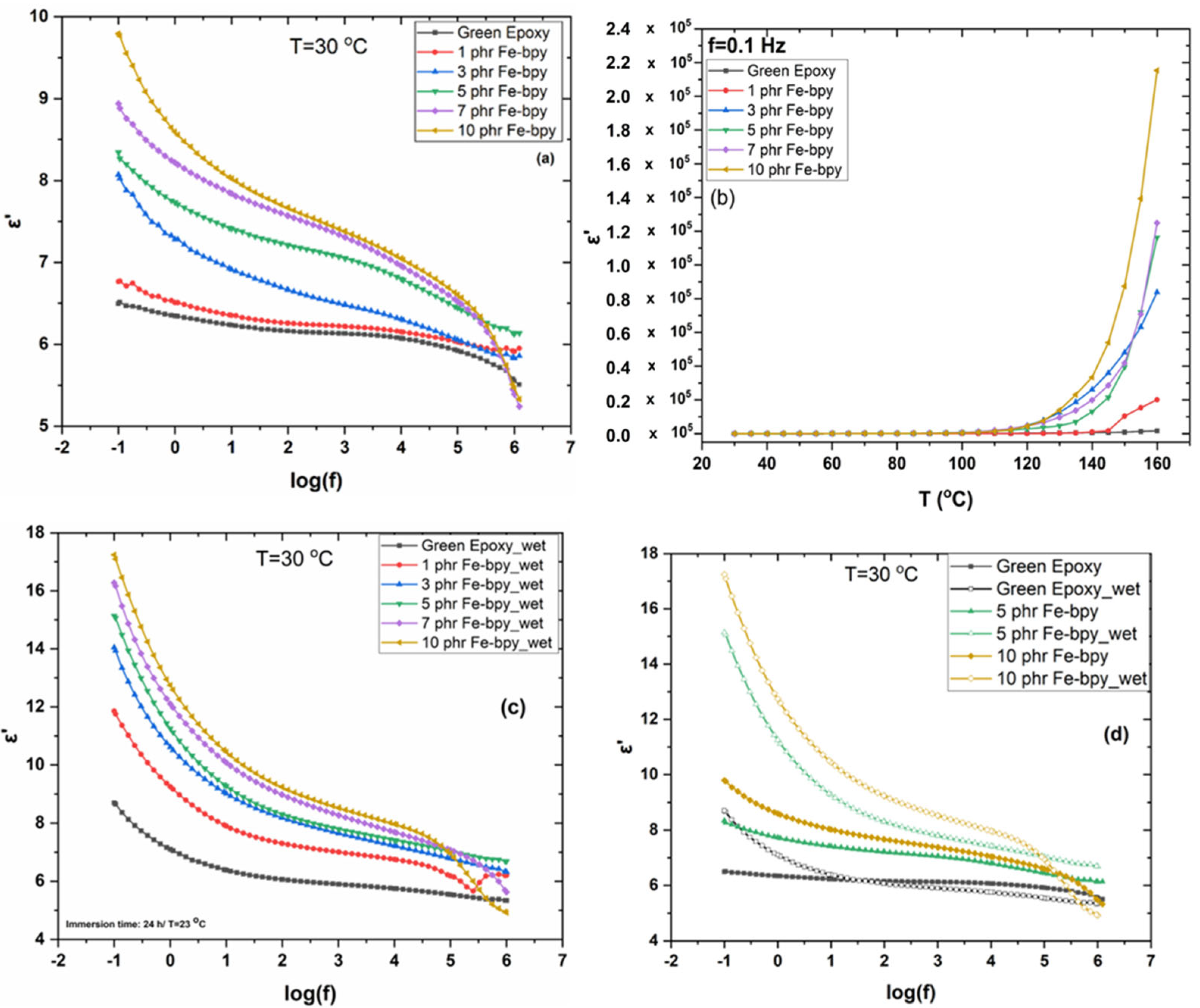

| Sample | mafter immersion − minitial (g) | Absorption (%) | mfinal (g) After Re-Drying | ε′rel Τ = 30 °C, f = 10−1 Hz |
|---|---|---|---|---|
| Neat epoxy | 0.0089 | 0.460 | 1.9310 | 0.339 |
| 1 phr Fe-bpy | 0.0081 | 0.398 | 2.0332 | 0.753 |
| 3 phr Fe-bpy | 0.0095 | 0.484 | 1.9608 | 0.740 |
| 5 phr Fe-bpy | 0.0106 | 0.554 | 1.9113 | 0.815 |
| 7 phr Fe-bpy | 0.0107 | 0.559 | 1.9120 | 0.738 |
| 10 phr Fe-bpy | 0.0109 | 0.599 | 1.8166 | 0.760 |
| Filler | Polymer | Content | ε’ | tanδ | η (%) | Ref. |
|---|---|---|---|---|---|---|
| [Fe(bpy)3]SO4 | Green Epoxy Resin | 3–10 wt.% | 40.7 (0.1 Hz)/7.8 (1 kHz, 30 °C) | 0.05 | 44 | This Study |
| BaTiO3 | P(VDF-TrFE-CTFE) | 15% | 90.2 | 0.1 | 74.2 | [51] |
| BaTiO3 | PVDF | 0.3% | 11.9 | <0.04 | 55 | [50] |
| SrTiO3 | PVDF | 1 wt.% | 10.66 | - | 57.2 | [57] |
| NaNbO3 | P(VDF-HFP) | 3% | 12 | <0.05 | 70.1 | [58] |
| K0.5Na0.5NbO3 | PVDF | 3% | 12 | <0.05 | 80.2 | [59] |
| Na0.5Bi4.5Ti4O15 | PVDF | 1 wt.% | 16 | 0.1 | 52.3 | [60] |
| Ca2Nb3O10 | PVDF | 2.1 wt.% | 10.5 | - | 61.2 | [61] |
| Ca2Nb3O10 | P(VDF-HFP) | 0.1% | - | - | - | [62] |
| Sr2Nb2O7 | PVDF | 5 wt.% | 11 | <0.05 | 71 | [63] |
| ZrO2 | PVDF | 1 wt.% | 10 | <0.04 | 67.4 | [54] |
| Ti0.87O2 | PVDF | 1 wt.% | 12 | <0.03 | 60 | [52] |
| TiO2β | PMMA/P(VDFHFP) | 5 wt.% | 10 | ~0.04 | 63 | [53] |
| MMT | PVDF | 0.2 wt.% | 28 | 0.032 | >60 | [64] |
| Na+/MMT | PVDF | - | 15 | <0.02 | 81 | [65] |
| MoS2 | PVDF | 0.4% | 11.3 | 0.07 | ~72 | [55] |
| Bi2Te3 | PVDF | 10 vol.% | 140 | 0.05 | - | [66] |
| MoS2 | Chitin | 5 wt.% | ~9.8 | ~0.025 | >80 | [67] |
| MoS2 | g-PMMA/PI | 3 wt.% | 4.2 | 0.015 | 61.7 | [68] |
| MoS2 | P(VDF-CTFE-DB) | 2 wt.% | 12.9 | 0.047 | 83 | [69] |
| Graphene | P(VDF-TrFE-CFE) | 0.1 wt.% | ~15 | ~0.04 | 78.1 | [56] |
| Graphene | P(VDF-CTFE) | 0.8 vol.% | 24.8 | 0.06 | 62 | [55] |
| GO | P(VDF-HFP) | 2 wt.% | ~11 | ~0.1 | 77 | [55] |
| BNNS | P(VDF-TrFE-CFE) | 12 wt.% | 38 | 0.03 | 78 | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patsidis, A.C.; Papageorgiou, I.T.; Lada, Z.G. Synthesis and Integration of an Fe(II) Coordination Compound into Green Resin Matrices for Multifunctional Dielectric, Piezoelectric, Energy Harvesting, and Storage Applications. Polymers 2025, 17, 2509. https://doi.org/10.3390/polym17182509
Patsidis AC, Papageorgiou IT, Lada ZG. Synthesis and Integration of an Fe(II) Coordination Compound into Green Resin Matrices for Multifunctional Dielectric, Piezoelectric, Energy Harvesting, and Storage Applications. Polymers. 2025; 17(18):2509. https://doi.org/10.3390/polym17182509
Chicago/Turabian StylePatsidis, Anastasios C., Ioanna Th. Papageorgiou, and Zoi G. Lada. 2025. "Synthesis and Integration of an Fe(II) Coordination Compound into Green Resin Matrices for Multifunctional Dielectric, Piezoelectric, Energy Harvesting, and Storage Applications" Polymers 17, no. 18: 2509. https://doi.org/10.3390/polym17182509
APA StylePatsidis, A. C., Papageorgiou, I. T., & Lada, Z. G. (2025). Synthesis and Integration of an Fe(II) Coordination Compound into Green Resin Matrices for Multifunctional Dielectric, Piezoelectric, Energy Harvesting, and Storage Applications. Polymers, 17(18), 2509. https://doi.org/10.3390/polym17182509









