Bone Regeneration in SLS-Manufactured Resorbable 3D-Scaffolds—An Experimental Pilot Study in Minipigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Scaffold Production
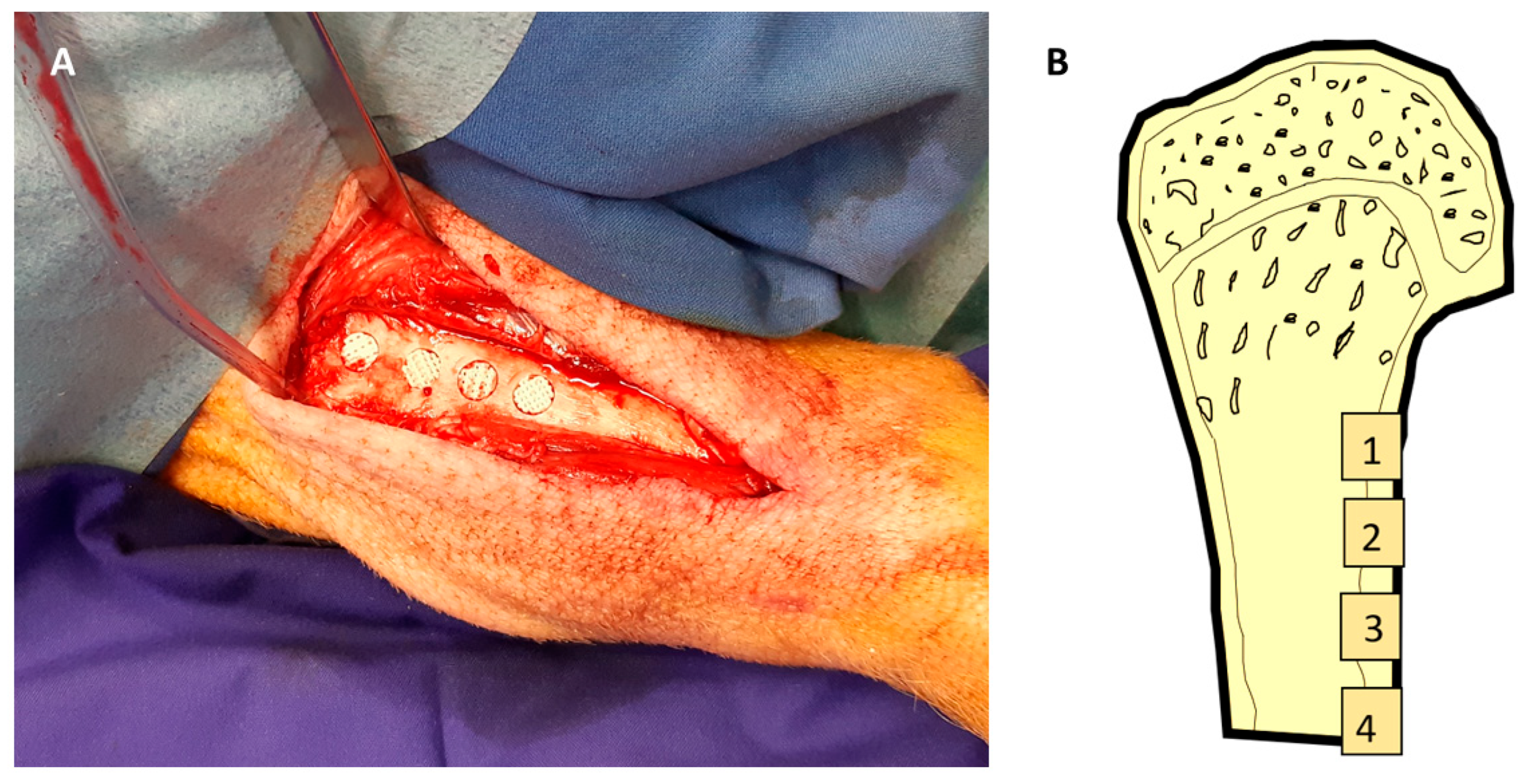
2.3. Surgical Procedures
2.4. Outcome Parameter
- -
- Primary outcome parameters:
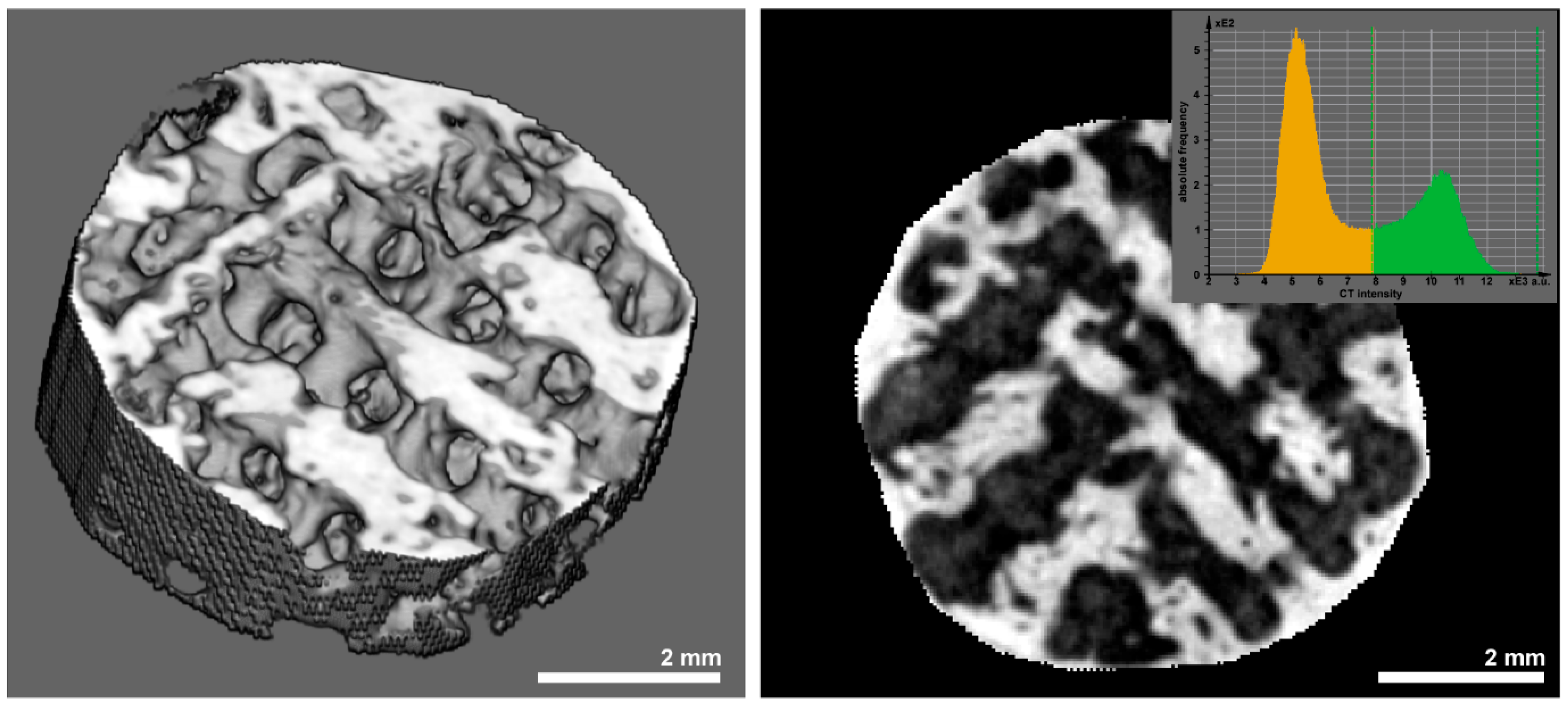
2.5. Micro-CT, Histologic Preparation, and Morphometry
- -
- Surface staining for histomorphometric assessment of bone formation using Alizarine red/Methylene Blue and van Gieson stains.
- -
- Immunohistochemical staining of osteocalcin using peroxidase staining. For immunohistochemical staining, the specimens were mounted on Adhesive Microscope Slides (3800200AE, Leica Biosystems, Wetzlar, Germany) and deplastisized (according to ROWIAK, Hannover, Germany) by incubation in a mixture of xylene and MMA (1:1; Methyl methacrylate, Merck, Darmstadt, Germany) for 24 h, followed by incubation in xylene for 20 h. The specimens were then rehydrated in descending concentrations of ethanol (100%, 95%, 70%, for 5 min each) and washed in deionized water for 5 min. Deplasticized and rehydrated bone tissue sections were incubated in 1× citrate-based Target Retrieval Solution, pH 6.0 (Agilent Dako, Waldbronn, Germany) at 60 °C overnight. Afterwards, the sections were washed for 10 min in deionized water and three times in TBS for 5 min each, following treatment with 1 mL Trypsin Solution and 3 mL Trypsin Buffer (Trypsin Pretreatment Kit, Zytomed Systems, Berlin, Germany) in a humidity chamber at 37 °C for 20 min. The sections were washed for 5 min in deionized water and three times in TBS, 5 min each. To block endogenous peroxidase activity, the specimens were incubated with peroxidase-Blocking Solution (Dako, Waldbronn, Germany) in a humidity chamber at room temperature (RT) for 17 min and rinsed with TBS three times, 5 min each. Next, the samples were incubated in a humidity chamber for 1 h at RT in blocking buffer (10% goat Serum Block in PBS, Histoprime Biozol, Eching, Germany). Immunostaining was performed by incubation with a 1:50 dilution of BGLAP (Osteocalcin) monoclonal antibody (ABN-H00000632-M01, Abnova Biozol, Eching, Germany; humidity chamber, overnight at 4°C) followed by washing three times in TBS for 5 min each and incubation with an HRP-conjugated secondary antibody (Goat anti-Mouse, A10551, Thermo Fisher Scientific, Rockford, IL, USA; 1:250, humidity chamber, 1 h at RT). The antibodies were diluted using Antibody Diluent (Agilent Dako, Waldbrunn, Germany). After the samples were washed three times in TBS, 5 min each, the reactivity was detected using DAB (3,3’-Diaminobenzidine) substrate (Liquid DAB Substrate-Chromogen System, Agilent Dako, Waldbrunn, Germany; 20 min at RT). The reaction was stopped with deionized water, and the sections were rinsed four times in deionized water. Subsequently, specimens were counterstained with Mayer’s hemalaun solution (Merck, Darmstadt, Germany) for 4 s at RT, followed by incubation for 5 min in tap water at RT. The samples were dehydrated in ascending concentrations of ethanol (70% for 2 min, 96% twice for 2 min each, 100% twice for 5 min each) followed by clarification in xylene for 5 min. Finally, the specimens were mounted with Entellan (Merck, Darmstadt, Germany) and dried for 48 h at RT. Sections stained without the primary antibody served as controls.
2.6. Statistics
3. Results
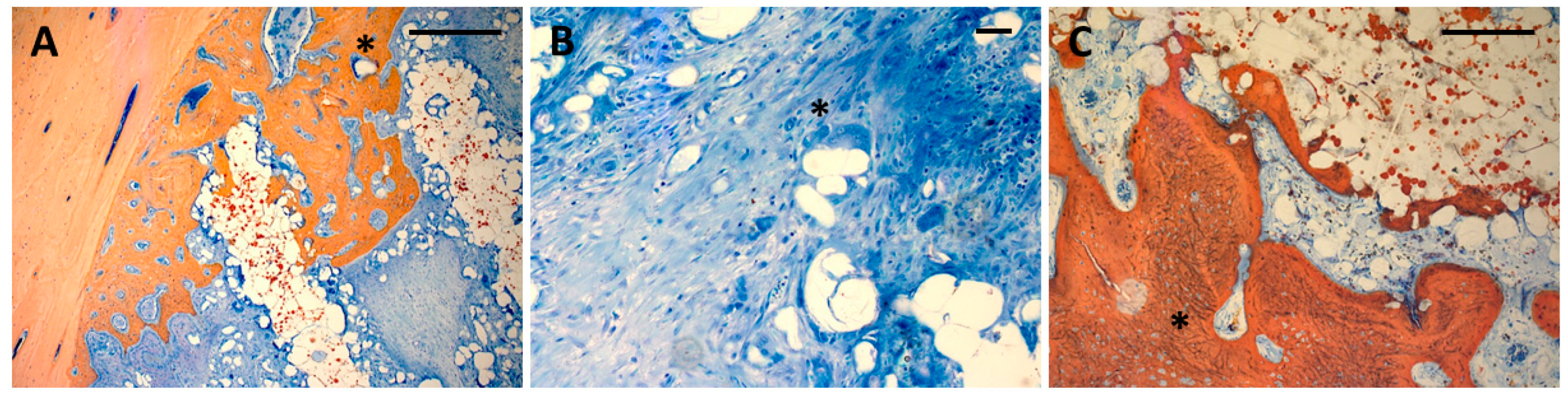
3.1. Histology
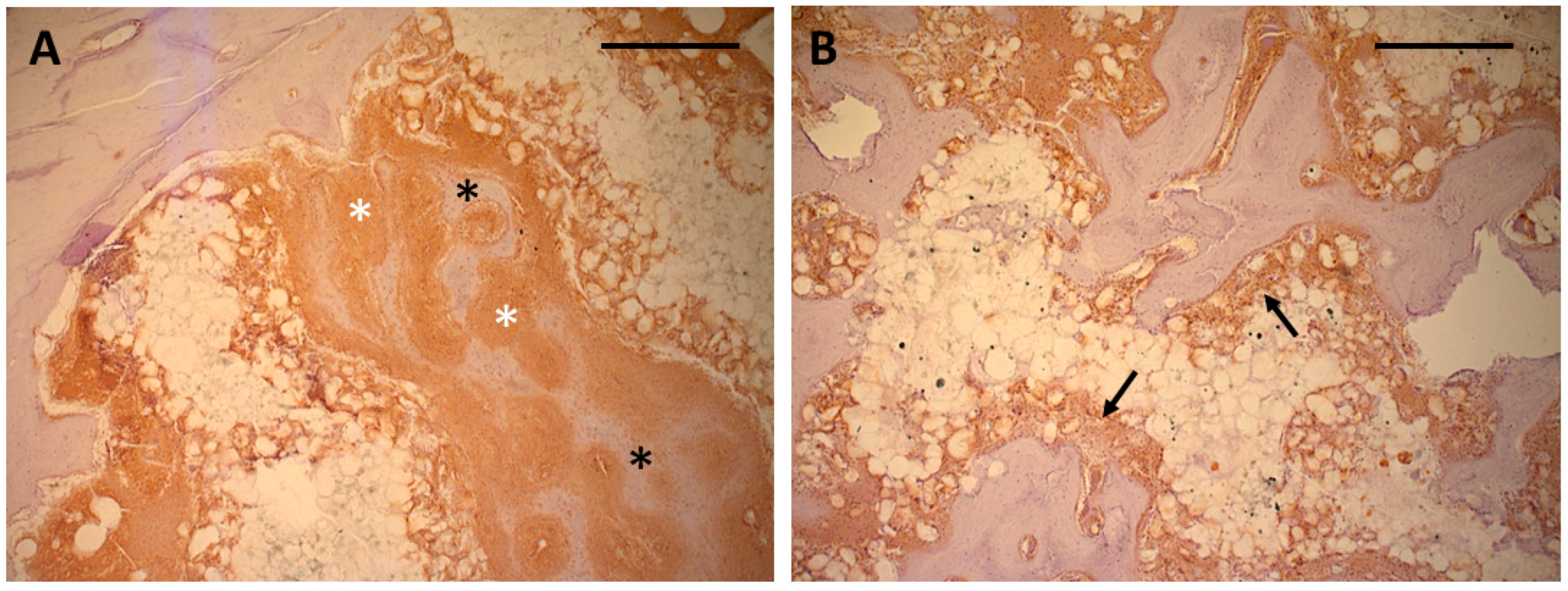
3.2. Immunohistochemistry
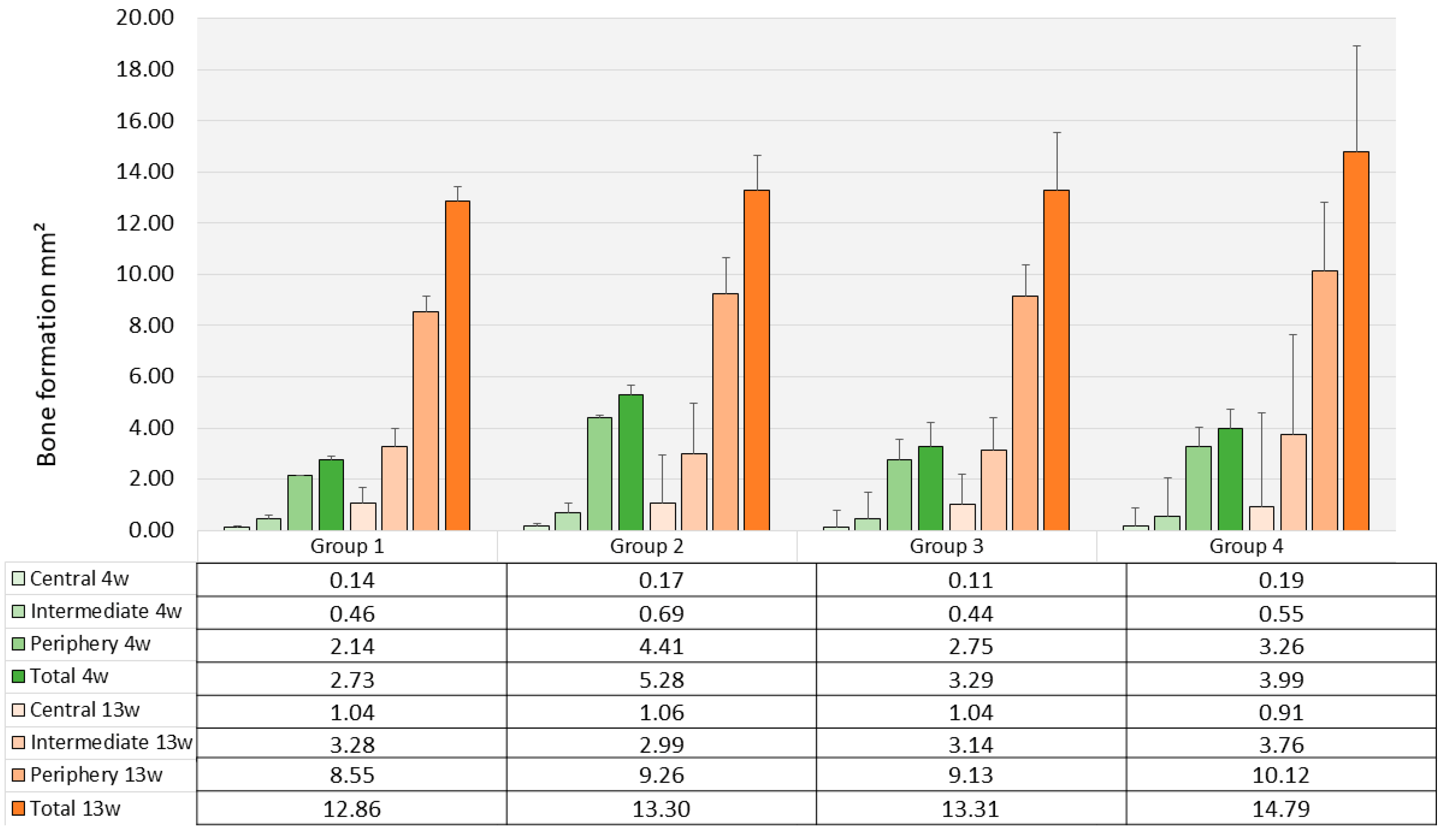
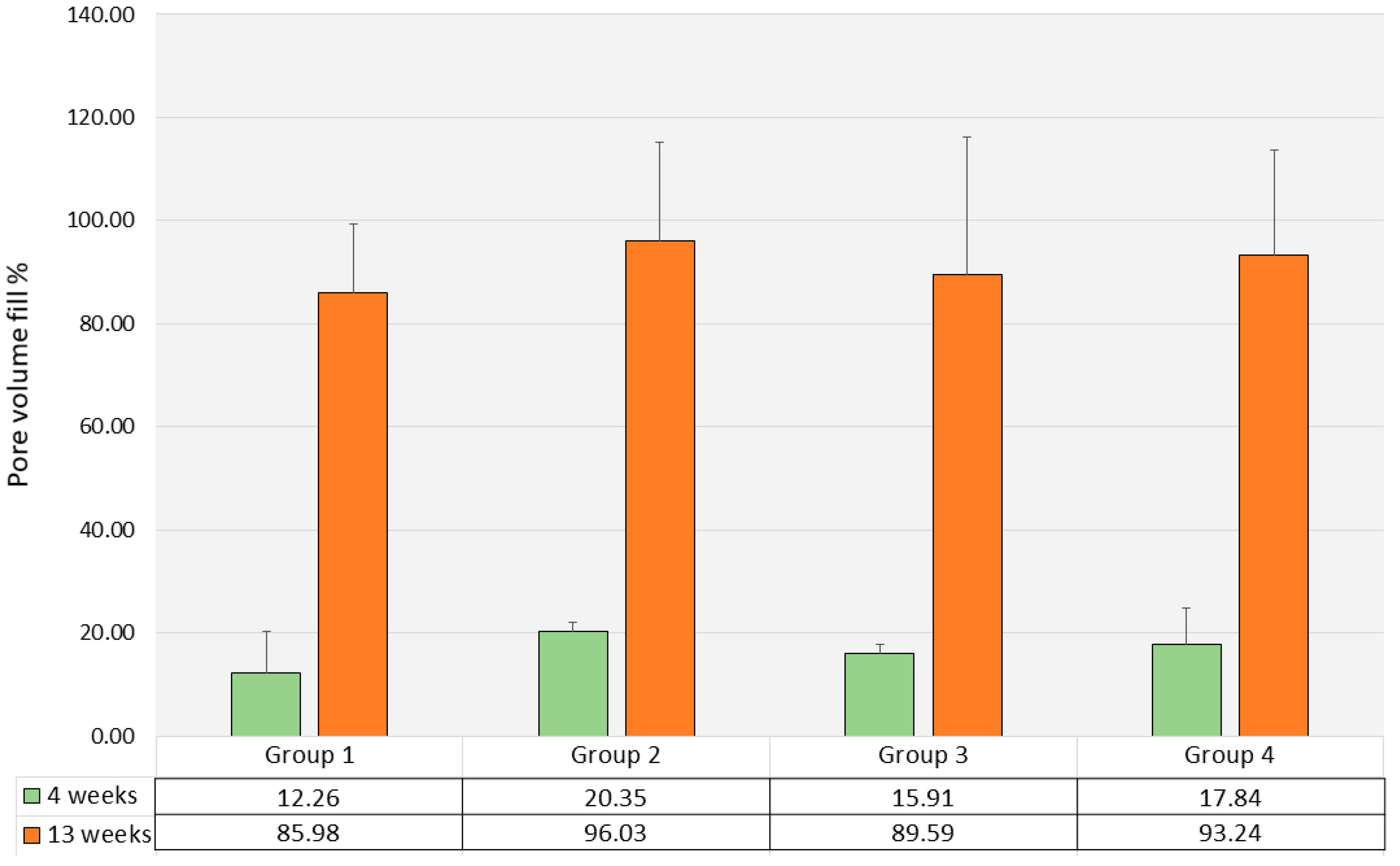
3.3. Histomorphometry and Μct
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindner, M.; Hoeges, S.; Meiners, W.; Wissenbach, K.; Smeets, R.; Telle, R.; Poprawe, R.; Fischer, H. Manufacturing of individual biodegradable bone substitute implants using selective laser melting technique. J. Biomed. Mater. Res. Part A 2011, 97, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Fernández, I.; Haugen, H.J.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz, F. Use of 3D-printed polylactic acid/bioceramic composite scaffolds for bone tissue engineering in preclinical in vivo studies: A systematic review. Acta Biomater. 2023, 168, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Raeisdasteh Hokmabad, V.; Davaran, S.; Ramazani, A.; Salehi, R. Design and fabrication of porous biodegradable scaffolds: A strategy for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, Y.; Zhou, P.; Cheng, X.; Xie, Y.; Liang, C.; Li, C.; Xu, S. Selective laser sintering fabrication of nano-hydroxyapatite/poly-ε-caprolactone scaffolds for bone tissue engineering applications. Int. J. Nanomed. 2013, 8, 4197–4213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Z.; Wu, F.; Gao, H.; Cui, K.; Xian, M.; Zhong, J.; Tian, Y.; Fan, S.; Wu, G. A Dexamethasone-Eluting Porous Scaffold for Bone Regeneration Fabricated by Selective Laser Sintering. ACS Appl. Bio Mater. 2020, 3, 8739–8747. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Peng, Z.; Lv, Q.; Li, L.; Zhang, C.; Pang, L.; Zhang, C.; Li, Y.; Chen, Y.; Tang, X. SLS 3D Printing To Fabricate Poly(vinyl alcohol)/Hydroxyapatite Bioactive Composite Porous Scaffolds and Their Bone Defect Repair Property. ACS Biomater. Sci. Eng. 2023, 9, 6734–6744. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.M.; Zhong, S.-P.; Doherty, P.; Williams, D.F. Mechanisms of polymer degradation in implantable devices: I. Poly(caprolactone). Biomaterials 1993, 14, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.; Hürzeler, M.B.; Schliephake, H. A review of material properties of biodegradable and bioresorbable polymers and devices for GTR and GBR applications. Int. J. Oral Maxillofac. Implants 1996, 11, 667–678. [Google Scholar] [PubMed]
- Pihlajamäki, H.; Böstman, O.; Tynninen, O.; Laitinen, O. Long-term tissue response to bioabsorbable poly-L-lactide and metallic screws: An experimental study. Bone 2006, 39, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, H.K.; Salminen, S.T.; Tynninen, O.; Böstman, O.M.; Laitinen, O. Tissue restoration after implantation of polyglycolide, polydioxanone, polylevolactide, and metallic pins in cortical bone: An experimental study in rabbits. Calcif. Tissue Int. 2010, 87, 90–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Nonhoff, M.; Puetzler, J.; Hasselmann, J.; Fobker, M.; Gosheger, G.; Schulze, M. The Potential for Foreign Body Reaction of Implanted Poly-L-Lactic Acid: A Systematic Review. Polymers 2024, 16, 817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schiller, C.; Rasche, C.; Wehmöller, M.; Beckmann, F.; Eufinger, H.; Epple, M.; Weihe, S. Geometrically structured implants for cranial reconstruction made of biodegradable polyesters and calcium phosphate/calcium carbonate. Biomaterials 2004, 25, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Sukegawa, S.; Nakano, K.; Takabatake, K.; Ono, S.; Nagatsuka, H.; Furuki, Y. Biological Effects of Bioresorbable Materials in Alveolar Ridge Augmentation: Comparison of Early and Slow Resorbing Osteosynthesis Materials. Materials 2021, 14, 3286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akagi, H.; Iwata, M.; Ichinohe, T.; Amimoto, H.; Hayashi, Y.; Kannno, N.; Ochi, H.; Fujita, Y.; Harada, Y.; Tagawa, M.; et al. Hydroxyapatite/poly-L-lactide acid screws have better biocompatibility and femoral burr hole closure than does poly-L-lactide acid alone. J. Biomater. Appl. 2013, 28, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Gayer, C.; Ritter, J.; Bullemer, M.; Grom, S.; Jauer, L.; Meiners, W.; Pfister, A.; Reinauer, F.; Vučak, M.; Wissenbach, K.; et al. Development of a solvent-free polylactide/calcium carbonate composite for selective laser sintering of bone tissue engineering scaffolds. Mater. Sci. Eng. C 2019, 101, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.M.; Krohn, S.; Mauth, C.; Dard, M.; Molenberg, A.; Lange, K.; Perske, C.; Schliephake, H. Mandibular reconstruction using a calcium phosphate/polyethylene glycol hydrogel carrier with BMP-2. J. Clin. Periodontol. 2014, 41, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Mead, R. The Design of Experiments; Cambridge University Press: Cambridge, UK, 1988; p. 620. [Google Scholar]
- Festing, M.F.W. How to Reduce the Number of Animals Used in Research by Improving Experimental Design and Statistics; ANZCCART Fact Sheet T10: Urrbrae, Australia, 2011; pp. 1–11. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012, 20, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; He, X.; Li, Y.; Lou, X. Conditioned Medium Enhances Osteogenic Differentiation of Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2019, 16, 141–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camassari, J.R.; de Sousa, I.T.C.; Cogo-Müller, K.; Puppin-Rontani, R.M. The Self-assembling peptide P11-4 influences viability and osteogenic differentiation of stem cells of the apical papilla (SCAP). J. Dent. 2023, 134, 104551. [Google Scholar] [CrossRef] [PubMed]
- Amiryaghoubi, N.; Fathi, M.; Pesyan, N.N.; Samiei, M.; Barar, J.; Omidi, Y. Bioactive polymeric scaffolds for osteogenic repair and bone regenerative medicine. Med. Res. Rev. 2020, 40, 1833–1870. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Grotheer, V.C.; Nicolini, L.F.; Latz, D.; Pishnamaz, M.; Greven, J.; Taday, R.; Wergen, N.M.; Hildebrand, F.; Windolf, J.; et al. Biomechanical validation of a tibial critical-size defect model in minipigs. Clin. Biomech. 2024, 120, 106336. [Google Scholar] [CrossRef] [PubMed]
- van Oirschot, B.A.; Geven, E.J.; Mikos, A.G.; van den Beucken, J.J.J.P.; Jansen, J.A. A Mini-Pig Mandibular Defect Model for Evaluation of Craniomaxillofacial Bone Regeneration. Tissue Eng. Part C Methods 2022, 28, 193–201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jungbluth, P.; Hakimi, A.; Grassmann, J.; Schneppendahl, J.; Betsch, M.; Kröpil, P.; Thelen, S.; Sager, M.; Herten, M.; Wild, M.; et al. The early phase influence of bone marrow concentrate on metaphyseal bone healing. Injury 2013, 44, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Spies, C.K.; Schnürer, S.; Gotterbarm, T.; Breusch, S.J. Efficacy of Bone Source™ and Cementek™ in comparison with Endobon™ in critical size metaphyseal defects, using a minipig model. J. Appl. Biomater. Biomech. 2010, 8, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Tumedei, M.; Savadori, P.; Del Fabbro, M. Synthetic Blocks for Bone Regeneration: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 4221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eldokmak, M.M.; Essawy, M.M.; Abdelkader, S.; Abolgheit, S. Bioinspired poly-dopamine/nano-hydroxyapatite: An upgrading biocompatible coat for 3D-printed polylactic acid scaffold for bone regeneration. Odontology 2024, 113, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, D.; Chen, X.; Jiang, L. Biomimetic cuttlebone polyvinyl alcohol/carbon nanotubes/hydroxyapatite aerogel scaffolds enhanced bone regeneration. Colloids Surf. B Biointerfaces 2022, 210, 112221. [Google Scholar] [CrossRef] [PubMed]
- Suba Sri, M.; Usha, R. An insightful overview on osteogenic potential of nano hydroxyapatite for bone regeneration. Cell Tissue Bank. 2025, 26, 13. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kauffmann, P.; Wolfer, S.; Gellhaus, T.; Behrens, C.; Dullin, C.; Reinauer, F.; Wolfram, T.; Grom, S.; Vučak, M.; Hauspurg, S.; et al. Bone Regeneration in SLS-Manufactured Resorbable 3D-Scaffolds—An Experimental Pilot Study in Minipigs. Polymers 2025, 17, 2498. https://doi.org/10.3390/polym17182498
Kauffmann P, Wolfer S, Gellhaus T, Behrens C, Dullin C, Reinauer F, Wolfram T, Grom S, Vučak M, Hauspurg S, et al. Bone Regeneration in SLS-Manufactured Resorbable 3D-Scaffolds—An Experimental Pilot Study in Minipigs. Polymers. 2025; 17(18):2498. https://doi.org/10.3390/polym17182498
Chicago/Turabian StyleKauffmann, Philipp, Susanne Wolfer, Tim Gellhaus, Christina Behrens, Christian Dullin, Frank Reinauer, Tobias Wolfram, Stefanie Grom, Marijan Vučak, Sabrina Hauspurg, and et al. 2025. "Bone Regeneration in SLS-Manufactured Resorbable 3D-Scaffolds—An Experimental Pilot Study in Minipigs" Polymers 17, no. 18: 2498. https://doi.org/10.3390/polym17182498
APA StyleKauffmann, P., Wolfer, S., Gellhaus, T., Behrens, C., Dullin, C., Reinauer, F., Wolfram, T., Grom, S., Vučak, M., Hauspurg, S., Rode, C., Wyrwa, R., & Schliephake, H. (2025). Bone Regeneration in SLS-Manufactured Resorbable 3D-Scaffolds—An Experimental Pilot Study in Minipigs. Polymers, 17(18), 2498. https://doi.org/10.3390/polym17182498





