Performance Evolution of CFRP Strip Anodes in Concrete: An Integrated Electrochemical and Mechanical Study
Abstract
1. Introduction
2. Principle of ICCP with CFRP Strip as Anode
3. Test Program
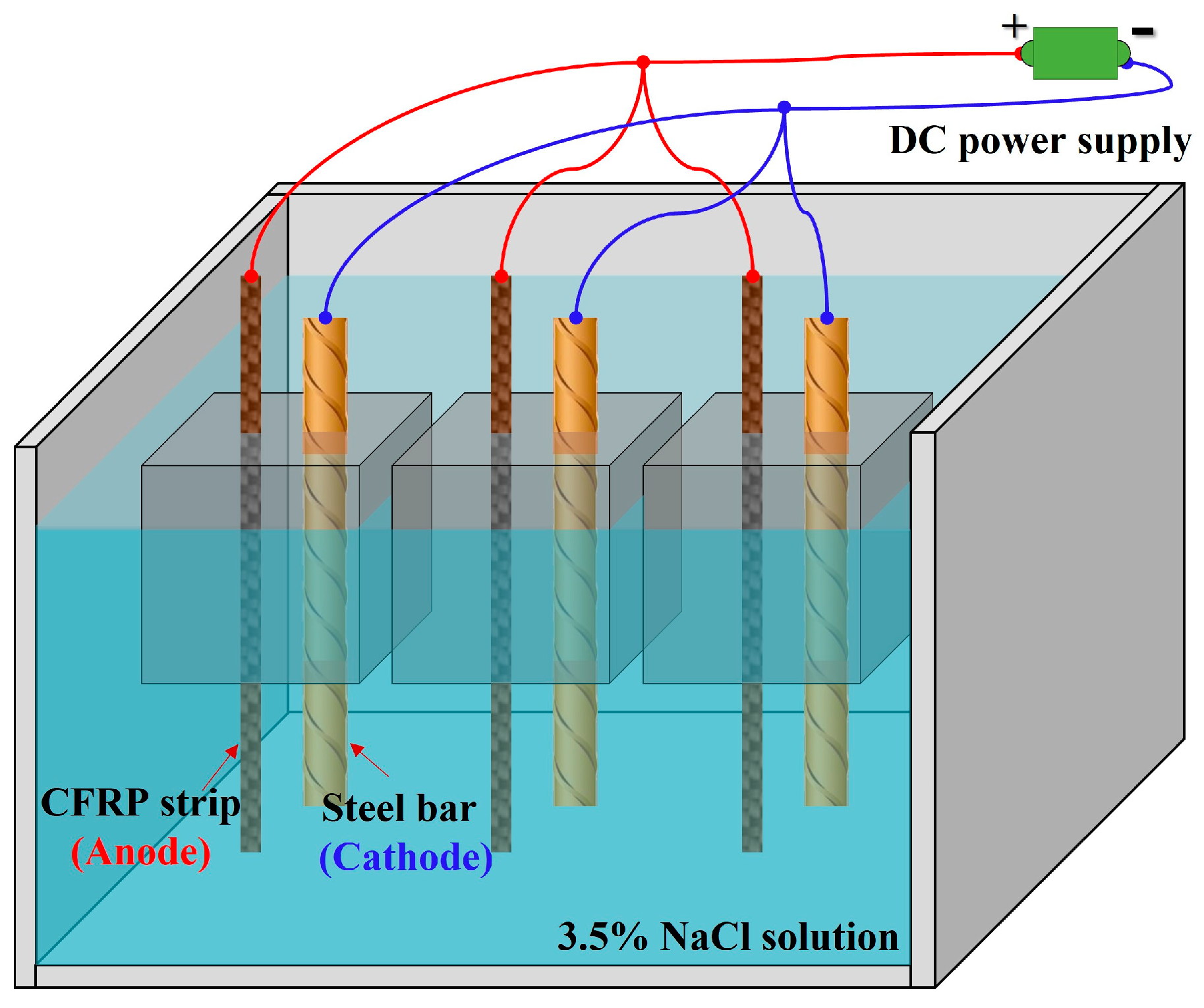
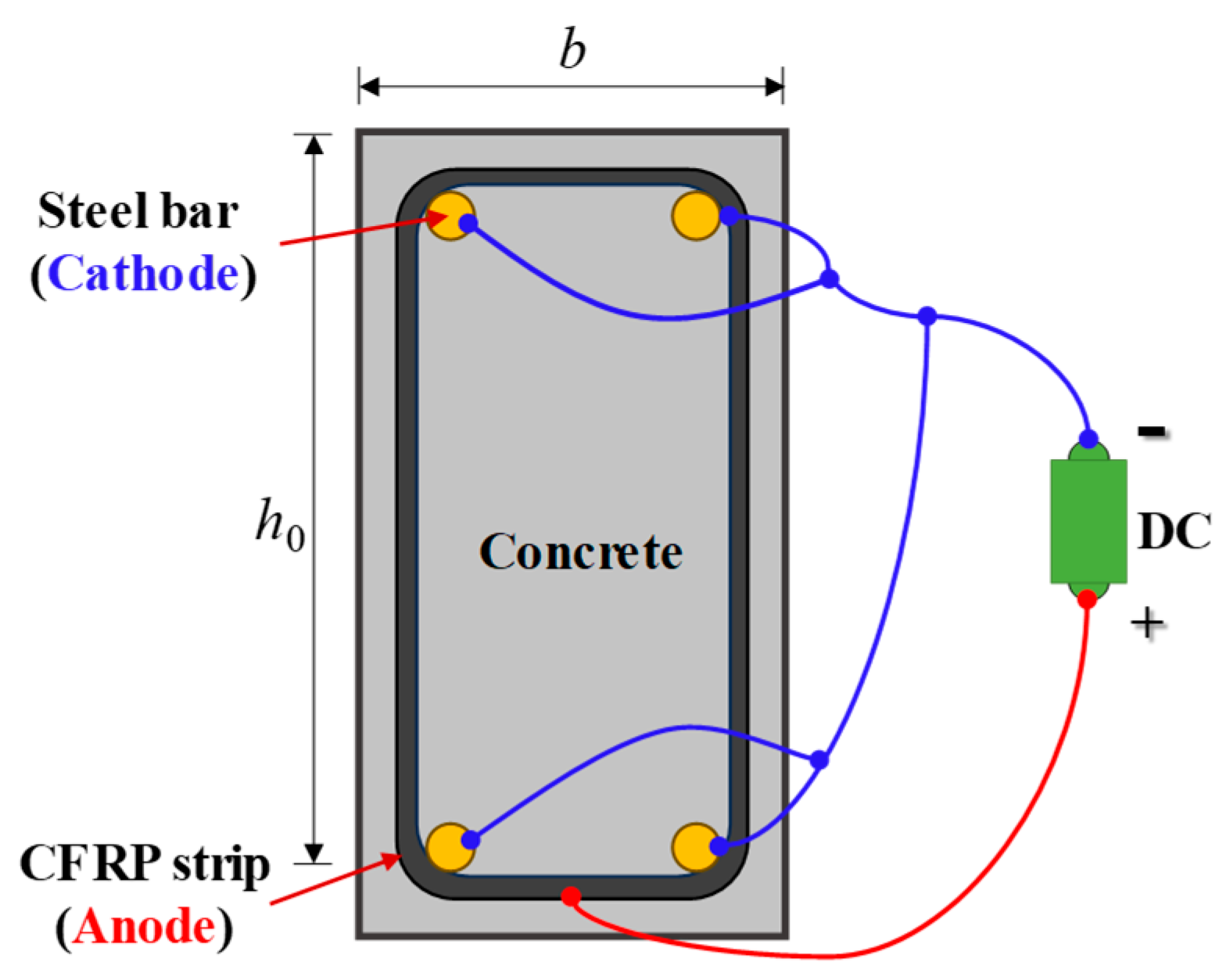
3.1. Specimen Preparation
3.2. Material Properties
3.3. Accelerated Anodic Polarization Test
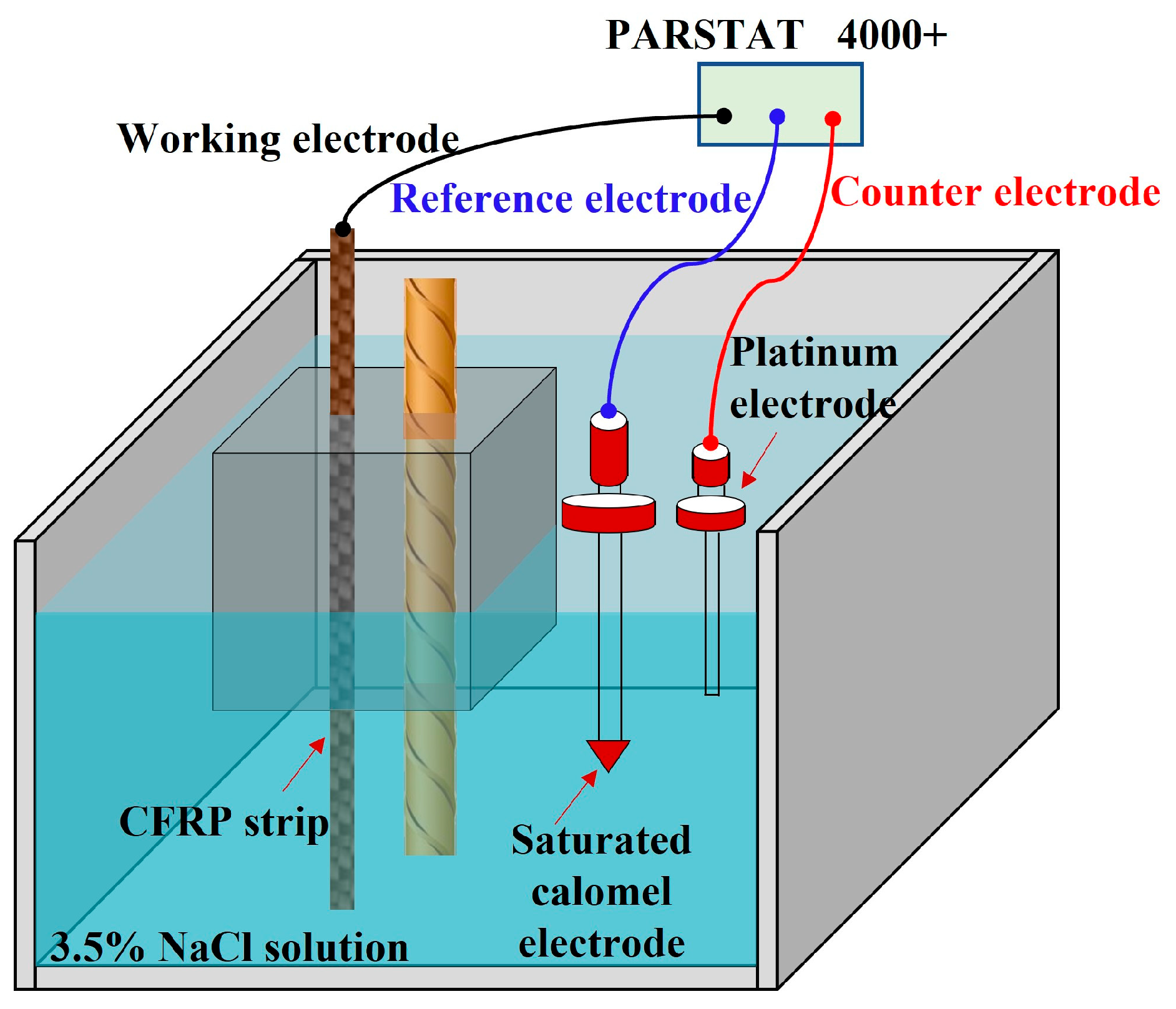
3.4. Electrochemical Impedance Spectroscopy
3.5. Uniaxial Tensile Test
3.6. Acidification Test
4. Results and Discussion
4.1. Experimental Phenomena
4.1.1. Anodic Polarization Phenomenon
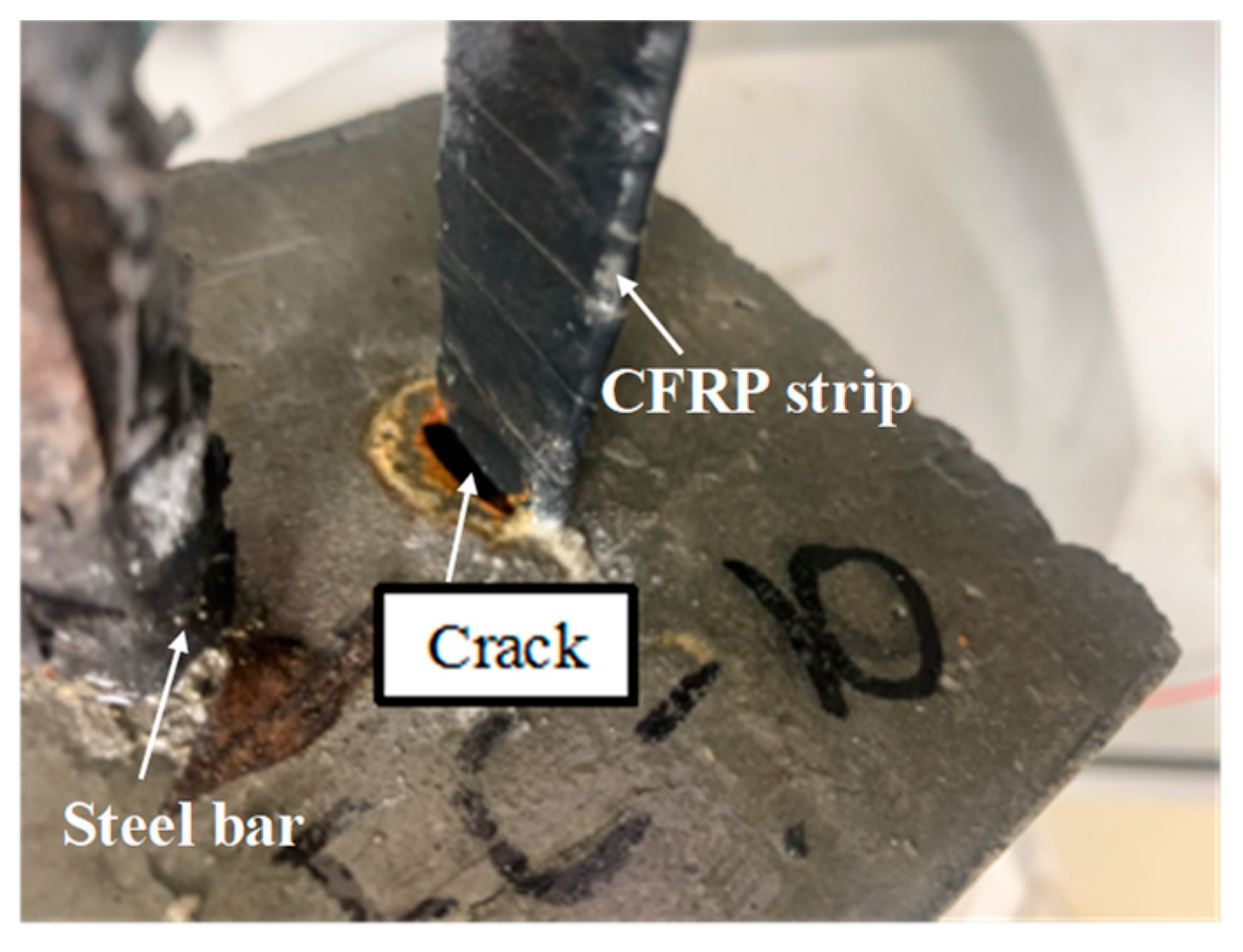
4.1.2. Failure Mode
4.2. Feeding Voltage
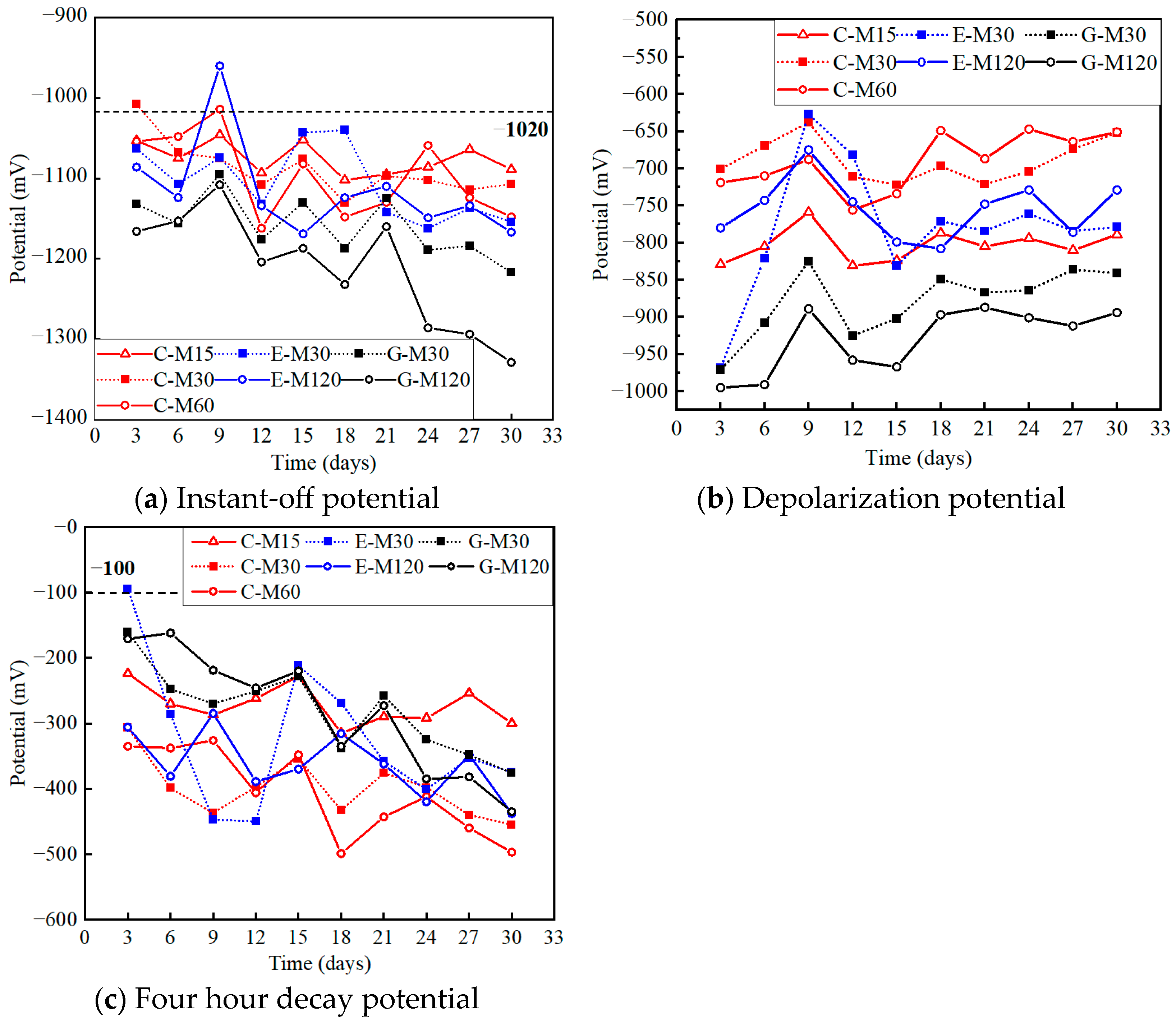
4.3. Electrochemical Potentials
4.3.1. Instant-Off Potential
4.3.2. Depolarization Potential
4.3.3. Four Hour Decay Potential
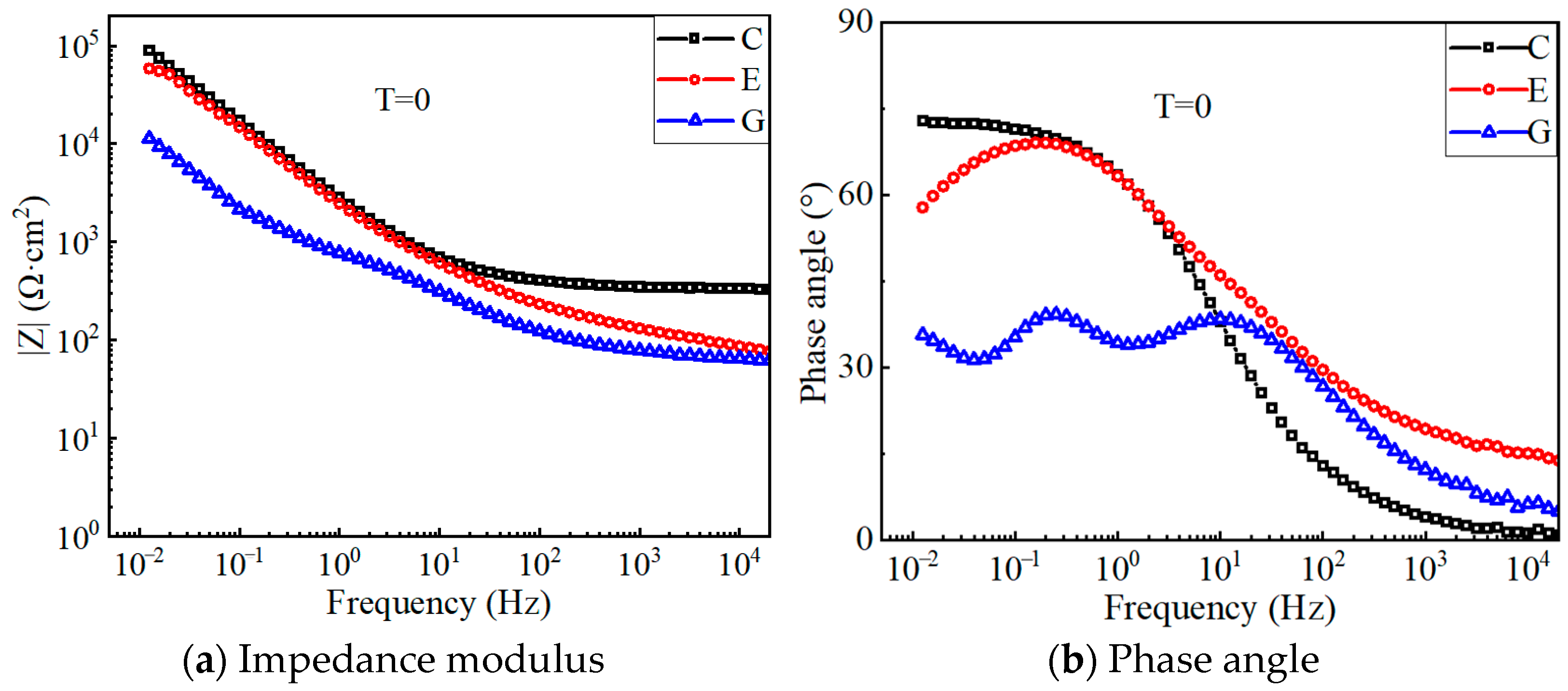
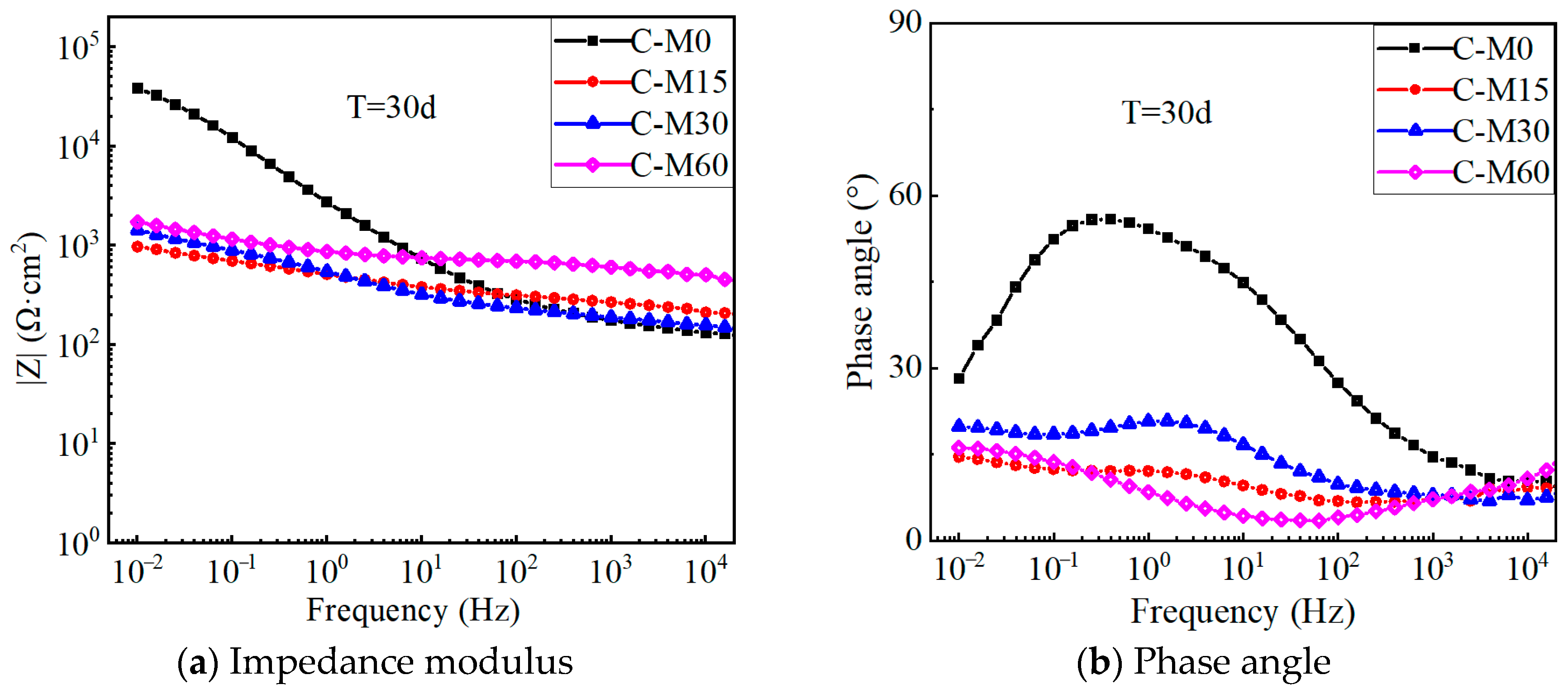
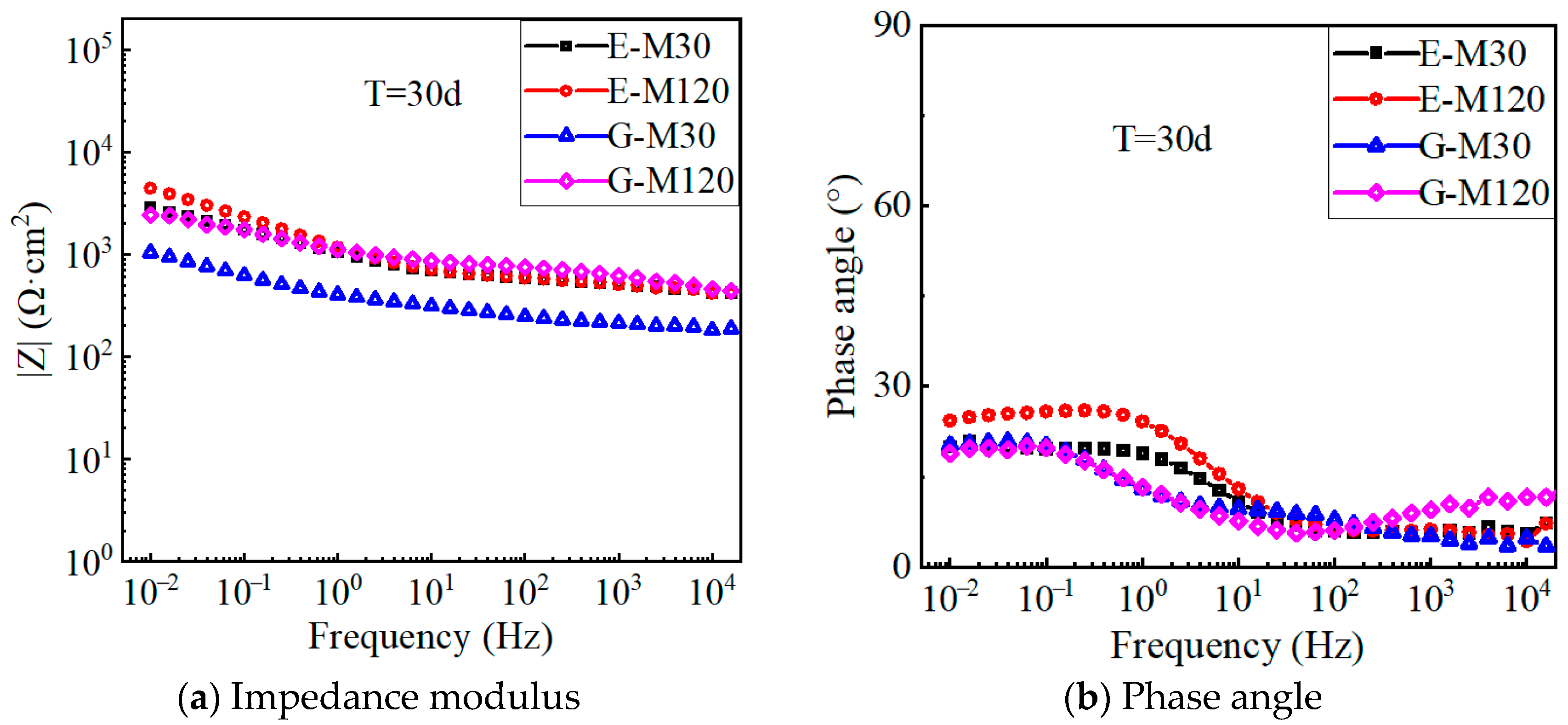
4.4. EIS Results
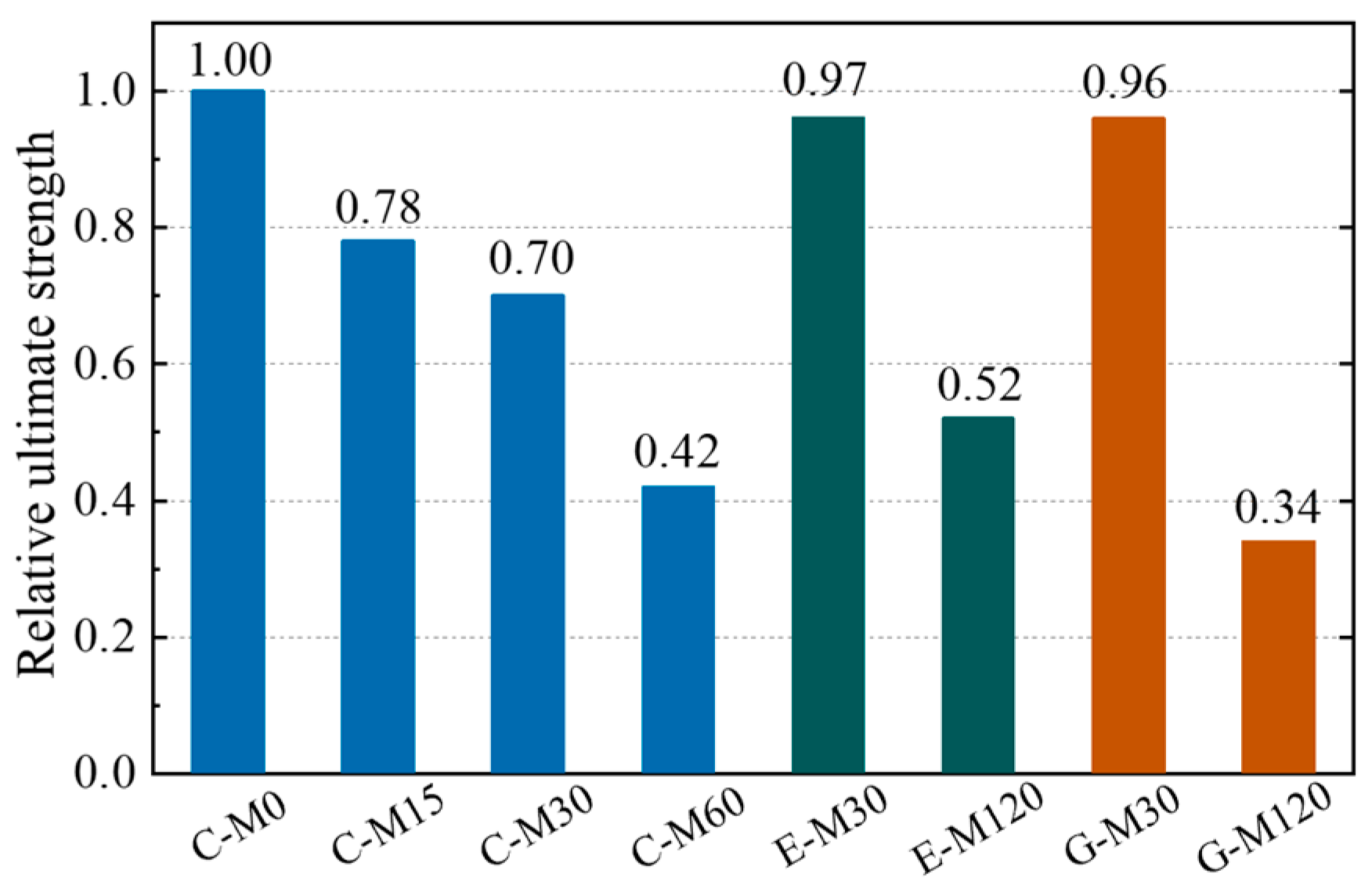
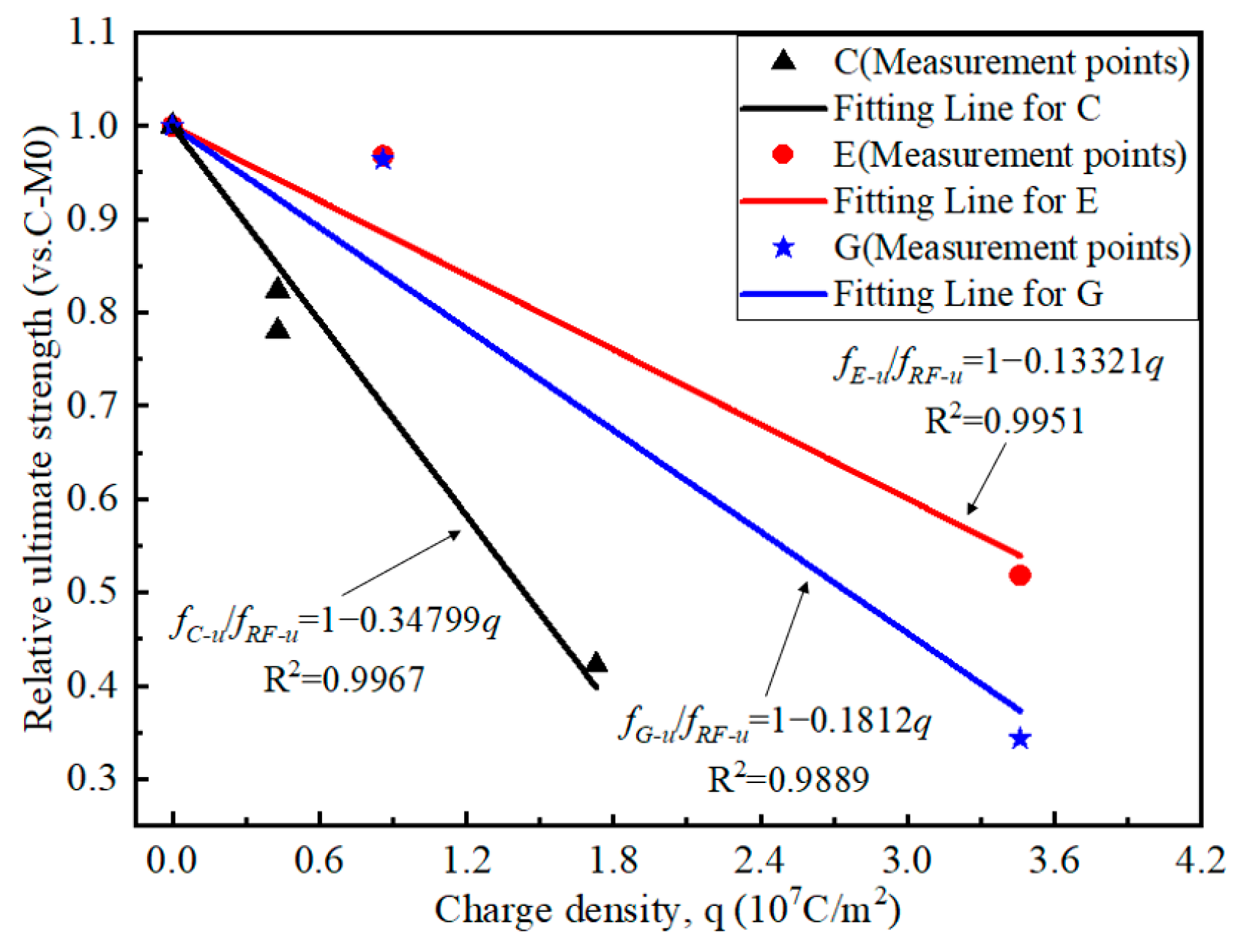
4.5. Uniaxial Tensile Test Results
4.6. Acidification Analysis of CFRP–Concrete Interface
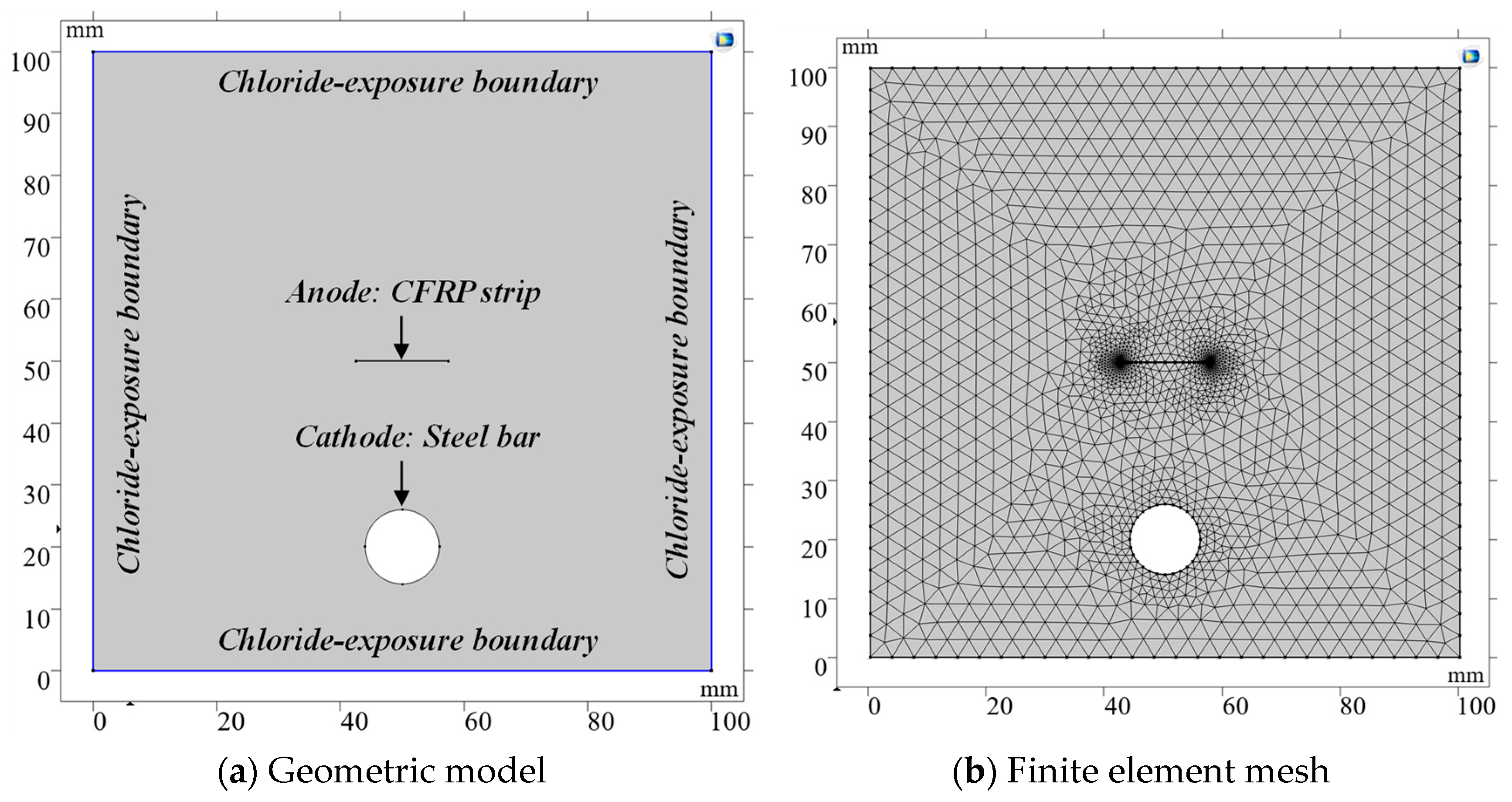
5. Numerical Analysis
5.1. Computational Model
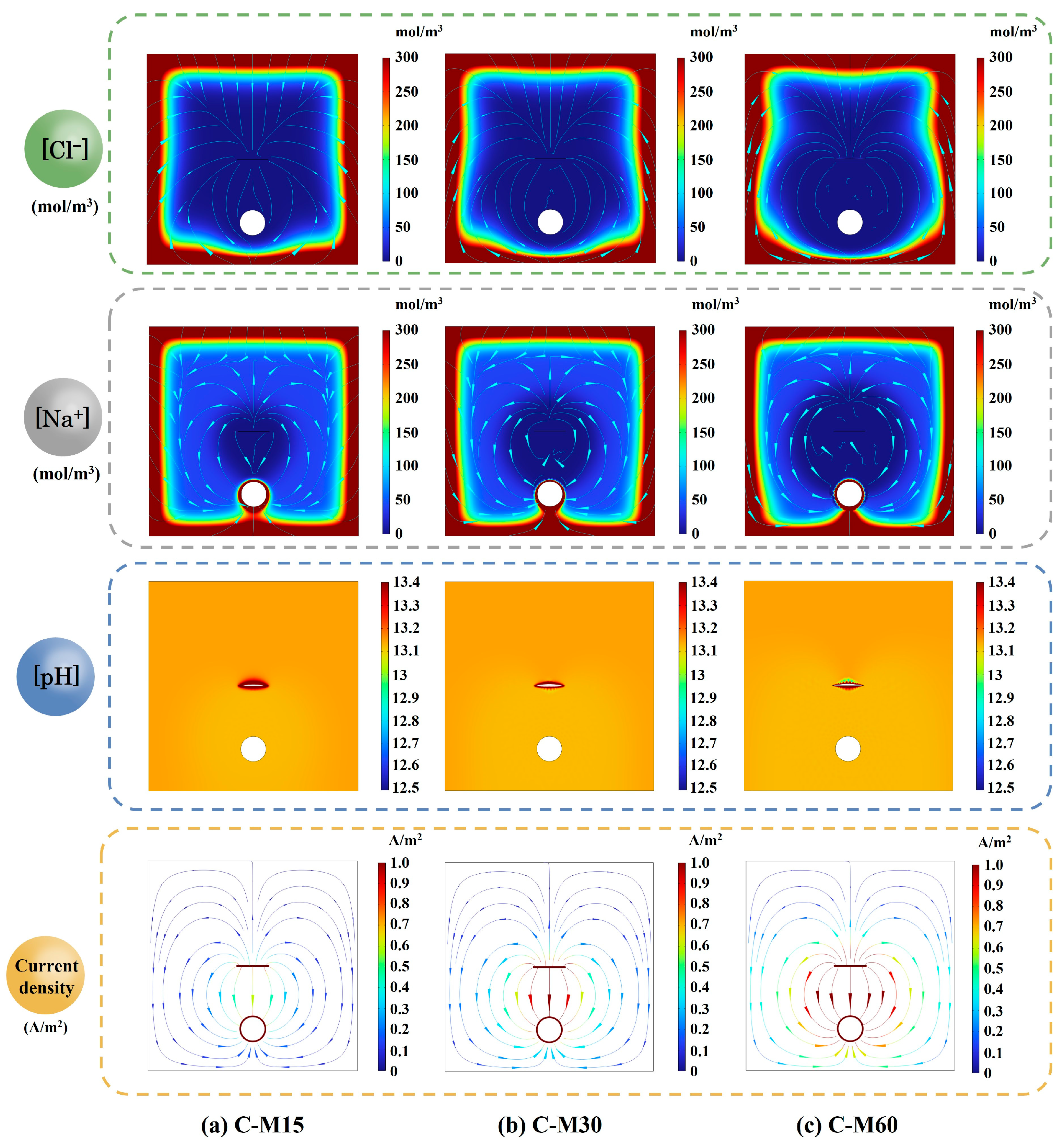
5.2. Effect of Current Density
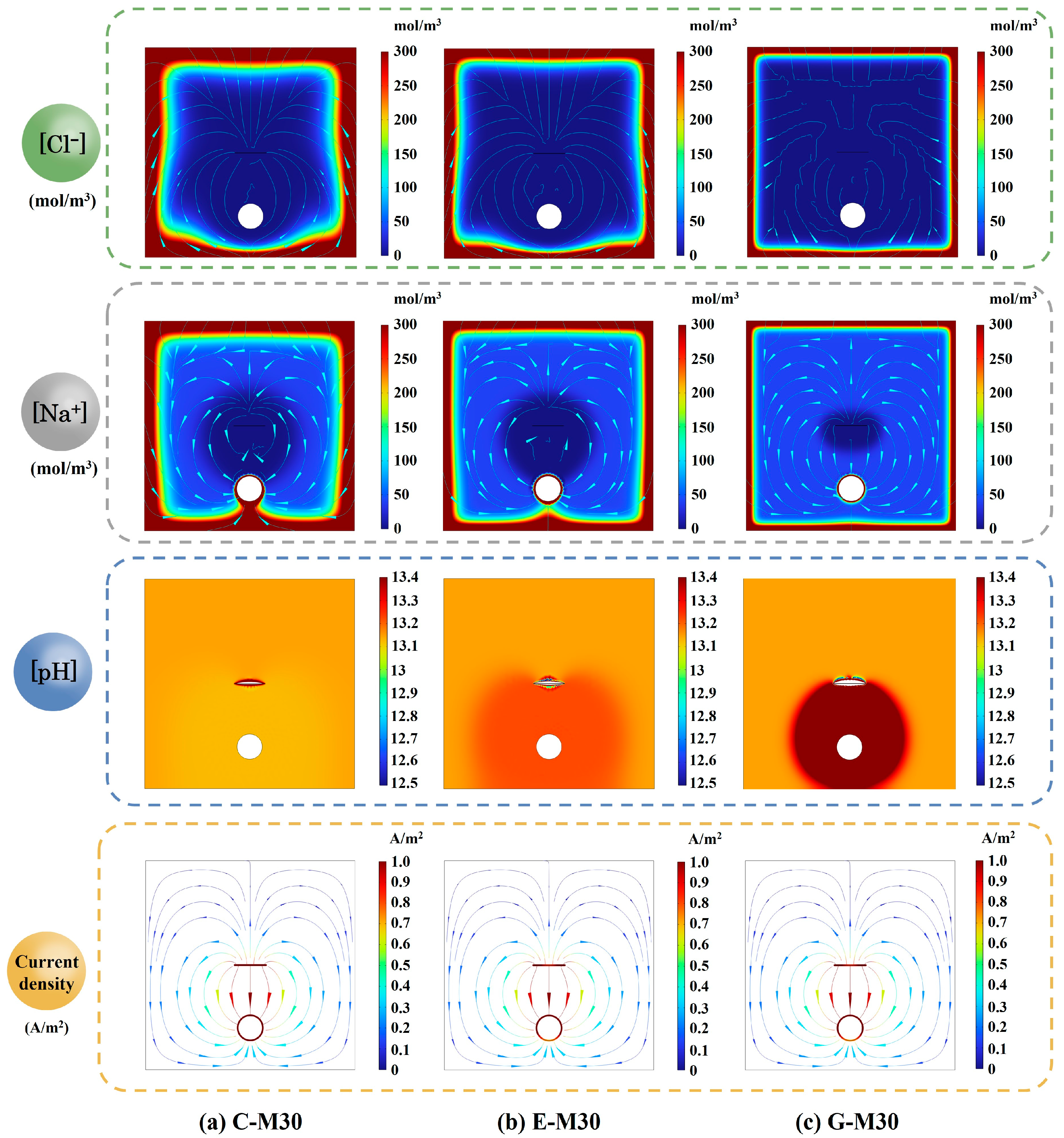
5.3. Effect of Concrete Type
6. Life Assessment of the Proposed ICCP System
6.1. Strength Prediction
6.2. Service Life Assessment
6.2.1. Prediction Model
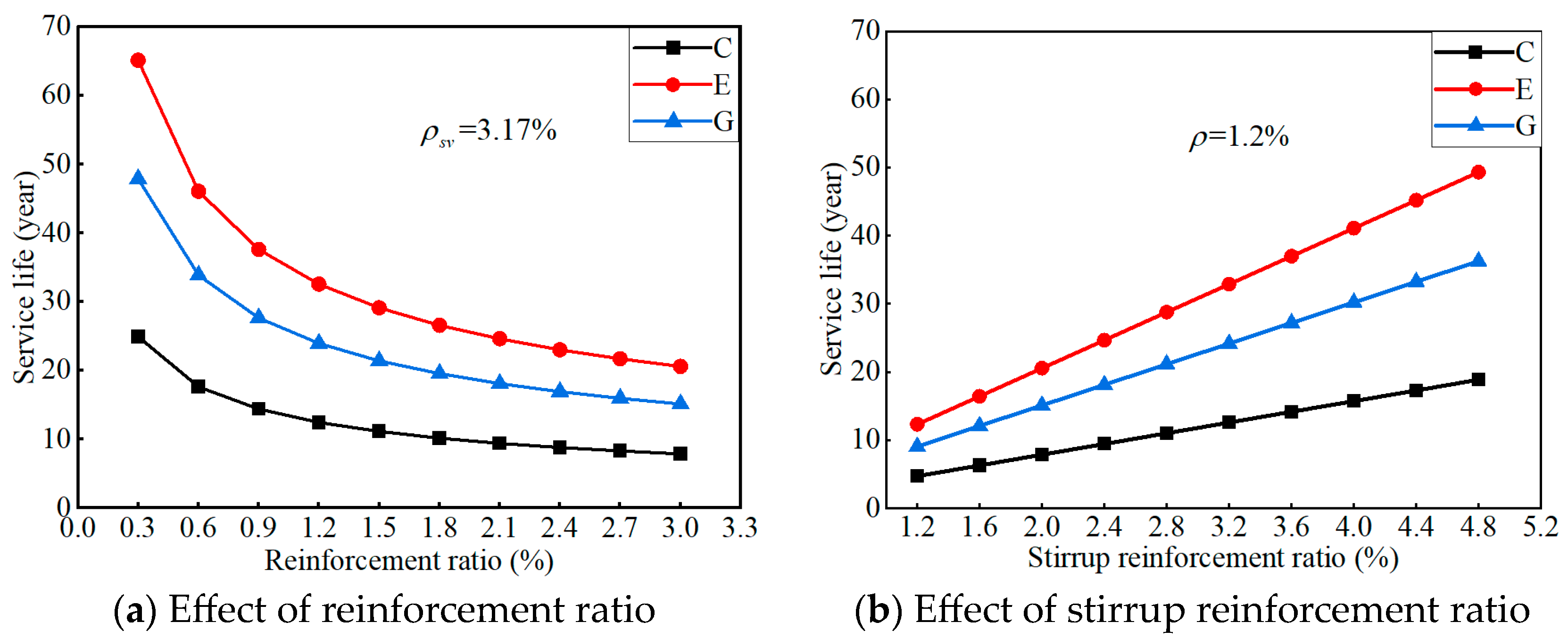
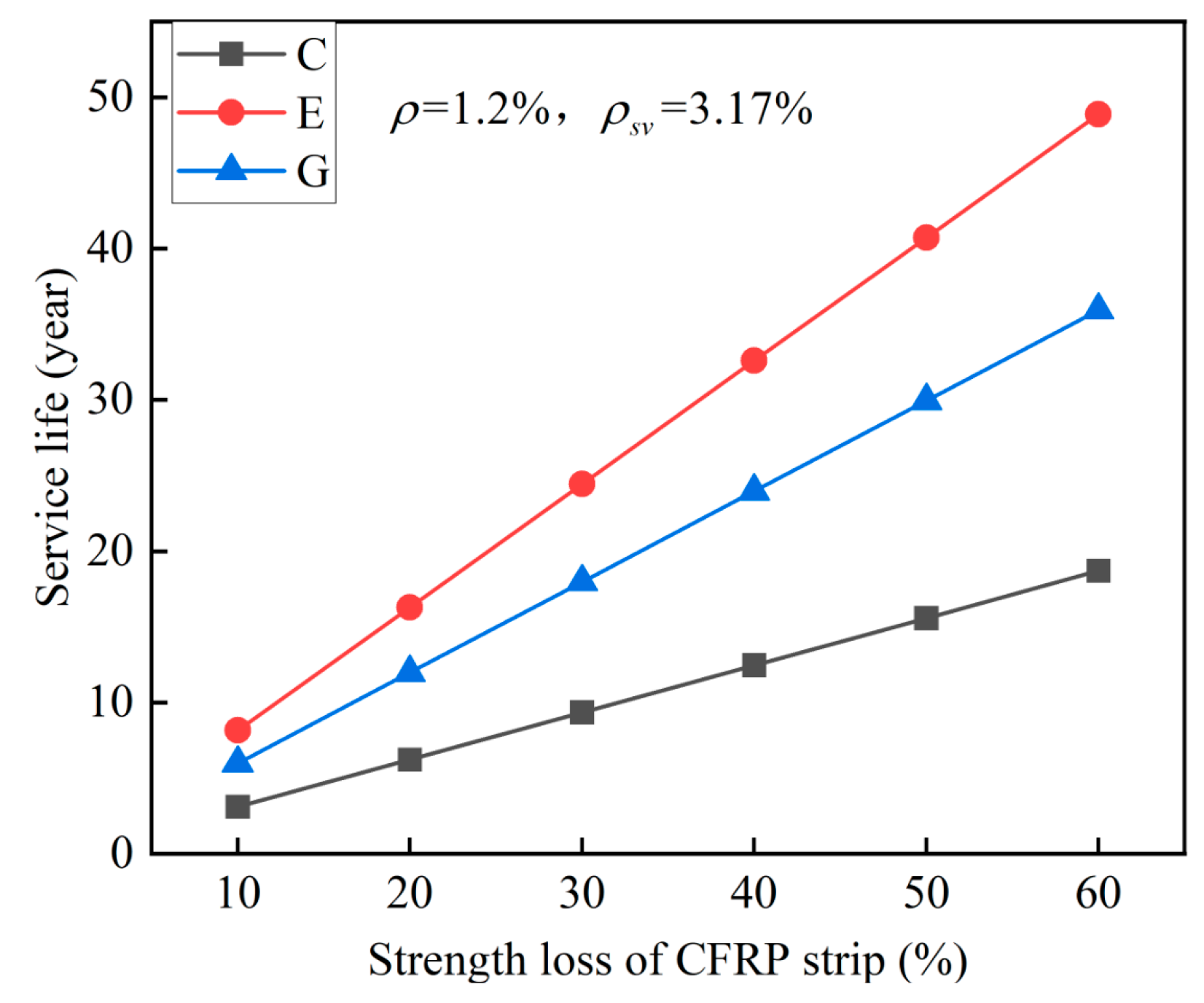
6.2.2. Parameter Analysis
7. Conclusions
- (1)
- As anodes in ICCP systems, CFRP strips exhibit excellent conductivity and relatively low output resistance within concrete environments. Notably, ECC concrete exhibits higher feeding voltages due to its superior impermeability and lower electrolyte content. Furthermore, the significant ICCP effectiveness was verified by the electrochemical potential.
- (2)
- EIS results indicate that enlarged anode current densities enhance electron exchange at the CFRP/concrete interface, leading to a gradual increase in the low-frequency impedance modulus. The impedance modulus of geopolymer concrete is lower than that of ECC concrete for the same current density.
- (3)
- Due to the deterioration of CFRP strips during the accelerated anodic polarization, the strength of the CFRP strips decreases gradually as the current density increases. For the specimens with a current density enlarged by 30 times, the strength of the CFRP strip in geopolymer and ECC concrete is about 38% higher than that of ordinary concrete. Moreover, the deterioration rate of the CFRP strip in ECC concrete is lower than that of geopolymer concrete.
- (4)
- The predicted service life of the proposed ICCP system decreases as the reinforcement ratio increases, while it increases as the stirrup ratio increases. The proposed ICCP system has the longest service life in ECC concrete, followed by geopolymer concrete and OPC concrete: Compared with OPC concrete, the predicted service life for the specific case increased by 92% and 161% for geopolymer concrete and ECC concrete, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.H.; Xu, R.H.; Liu, K.; Sun, S.H. Research progress on durability of marine concrete under the combined action of Cl- erosion, carbonation, and dry-wet cycles. Rev. Adv. Mater. Sci. 2022, 61, 622–637. [Google Scholar] [CrossRef]
- Rodrigues, R.; Gaboreau, S.; Gance, J.; Ignatiadis, I.; Betelu, S. Reinforced concrete structures: A review of corrosion mechanisms and advances in electrical methods for corrosion monitoring. Constr. Build. Mater. 2021, 269, 121240. [Google Scholar] [CrossRef]
- Zhang, W.P.; Chen, J.P.; Yu, Q.Q.; Gu, X.L. Corrosion evolution of steel bars in RC structures based on Markov chain modeling. Struct. Saf. 2021, 88, 102037. [Google Scholar] [CrossRef]
- Li, Q.; Lan, J.T.; Shen, L.; Yang, J.P.; Chen, C.; Jiang, Z.L.; Wang, C. A state-of-the-art review on monitoring technology and characterization of reinforcement corrosion in concrete. Case Stud. Constr. Mater. 2025, 22, e04780. [Google Scholar] [CrossRef]
- Moreno-Herrera, J.; Vega-Juarez, N.; Varela-Rivera, J.; Fernandez-Baqueiro, L.; Castro-Borges, P. The Effect of Steel Reinforcement Diameter on the Behavior of Concrete Beams with Corrosion. Buildings 2025, 15, 266. [Google Scholar] [CrossRef]
- Polder, R.B.; Peelen, W.H.A.; Courage, W.M.G. Non-traditional assessment and maintenance methods for aging concrete structures—Technical and non-technical issues. Mater. Corros. Werkst. Korros. 2012, 63, 1147–1153. [Google Scholar] [CrossRef]
- Huang, X.X.; Zhou, Y.W.; Zheng, X.B.; Xing, F.; Sui, L.L.; Hu, B. Bond performance between corroded steel bars and concrete in cathodic protection system with CFRP as anode. Compos. Struct. 2023, 309, 116739. [Google Scholar] [CrossRef]
- Pedeferri, P. Cathodic protection and cathodic prevention. Constr. Build. Mater. 1996, 10, 391–402. [Google Scholar] [CrossRef]
- Anwar, M.S.; Sujitha, B.; Vedalakshmi, R. Light-weight cementitious conductive anode for impressed current cathodic protection of steel reinforced concrete application. Constr. Build. Mater. 2014, 71, 167–180. [Google Scholar] [CrossRef]
- Lambert, P.; Nguyen, C.V.; Mangat, P.S.; O’Flaherty, F.J.; Jones, G. Dual function carbon fibre fabric strengthening and impressed current cathodic protection (ICCP) anode for reinforced concrete structures. Mater. Struct. 2015, 48, 2157–2167. [Google Scholar] [CrossRef]
- Zhu, J.H.; Wei, L.L.; Moahmoud, H.; Redaelli, E.; Xing, F.; Bertolini, L. Investigation on CFRP as dual-functional material in chloride-contaminated solutions. Constr. Build. Mater. 2017, 151, 127–137. [Google Scholar] [CrossRef]
- Kara, I.F.; Ashour, A.F. Flexural performance of FRP reinforced concrete beams. Compos. Struct. 2012, 94, 1616–1625. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Chen, X.; Wang, X.H.; Sui, L.L.; Huang, X.X.; Guo, M.H.; Hu, B. Seismic performance of large rupture strain FRP retrofitted RC columns with corroded steel reinforcement. Eng. Struct. 2020, 216, 110744. [Google Scholar] [CrossRef]
- Lee-Orantes, F.T.-A.A.; Martínez-Madrid, M.; López-Cajún, C. Cathodic protection in reinforced concrete elements, using carbon fibers base composites. ECS Trans. 2007, 3, 93–98. [Google Scholar] [CrossRef]
- Van Nguyen, C.L.; Lambert, P.; Mangat, P.; O’Flaherty, F.; Jones, G. The performance of carbon fibre composites as iccp anodes for reinforced concrete structures. ISRN Corros. 2012, 2012, 814923. [Google Scholar] [CrossRef]
- Sun, H.F.; Wei, L.L.; Zhu, M.C.; Han, N.X.; Zhu, J.H.; Xing, F. Corrosion behavior of carbon fiber reinforced polymer anode in simulated impressed current cathodic protection system with 3% NaCl solution. Constr. Build. Mater. 2016, 112, 538–546. [Google Scholar] [CrossRef]
- Zhu, J.H.; Wei, L.L.; Guo, G.P.; Zhu, A.Z. Mechanical and Electrochemical Performance of Carbon Fiber Reinforced Polymer in Oxygen Evolution Environment. Polymers 2016, 8, 393. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Zheng, X.B.; Xing, F.; Sui, L.L.; Zheng, Y.W.; Huang, X.X. Investigation on the electrochemical and mechanical performance of CFRP and steel-fiber composite bar used for impressed current cathodic protection anode. Constr. Build. Mater. 2020, 255, 119377. [Google Scholar] [CrossRef]
- Zhang, E.Q.; Abbas, Z.; Tang, L.P. Predicting degradation of the anode-concrete interface for impressed current cathodic protection in concrete. Constr. Build. Mater. 2018, 185, 57–68. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Hu, J.Y.; Li, S.; Tang, W.S. Active and passive protection of steel reinforcement in concrete column using carbon fibre reinforced polymer against corrosion. Electrochim. Acta 2018, 278, 124–136. [Google Scholar] [CrossRef]
- Gadve, S.; Mukherjee, A.; Malhotra, S.N. Corrosion Protection of Fiber-Reinforced Polymer-Wrapped Reinforced Concrete. ACI Mater. J. 2010, 107, 349–356. [Google Scholar]
- Gadve, S.; Mukherjee, A.; Malhotra, S.N. Active Protection of Fiber-Reinforced Polymer-Wrapped Reinforced Concrete Structures Against Corrosion. Corrosion 2011, 67, 025002-025001–025002-025011. [Google Scholar] [CrossRef]
- Zhu, J.H.; Su, M.N.; Huang, J.Y.; Ueda, T.; Xing, F. The ICCP-SS technique for retrofitting reinforced concrete compressive members subjected to corrosion. Constr. Build. Mater. 2018, 167, 669–679. [Google Scholar] [CrossRef]
- Su, M.N.; Zeng, C.Q.; Li, W.Q.; Zhu, J.H.; Lin, W.H.; Ueda, T.; Xing, F. Flexural performance of corroded continuous RC beams rehabilitated by ICCP-SS. Compos. Struct. 2020, 232, 111556. [Google Scholar] [CrossRef]
- Feng, R.; Wang, J.; Zhu, J.H.; Dong, Z.J. Fatigue behavior of corroded reinforced concrete continuous beams with multi-intervention system. Eng. Struct. 2021, 231, 111748. [Google Scholar] [CrossRef]
- Wei, L.L.; Zhu, J.H.; Ueda, T.; Matsumoto, K. Performance of FRCM composites and FRCM-strengthened RC beams subjected to anodic polarization and cyclic loading. Eng. Struct. 2022, 250, 113475. [Google Scholar] [CrossRef]
- Zhu, M.C.; Zhu, J.H.; Ueda, T.; Matsumoto, K. Degradation behavior of multifunctional carbon fabric-reinforced cementitious matrix composites under anodic polarization. Constr. Build. Mater. 2022, 341, 127751. [Google Scholar] [CrossRef]
- Fatemi, H.; Hadigheh, S.A.; Tao, Y.Y.; Adam, G. Development of a novel and specialised cementitious matrix overlay for anode embedment in impressed current cathodic protection (ICCP) systems for reinforced concrete bridges. Case Stud. Constr. Mater. 2024, 20, e02908. [Google Scholar] [CrossRef]
- GB/T.50081(2019); Standard for Test Methods of Concrete Physical and Mechanical Properties. C.A.B. Press: Beijing, China, 2019.
- GB/T.228(2010); Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. S.A.O. China: Beijing, China, 2010.
- GB/T.30022(2013); Fiber-Reinforced Plastic Composites—Determination of Compressive Properties in the In-Plane Direction. S.A.O. China: Beijing, China, 2013.
- Bahekar, P.V.; Gadve, S.S. Impressed current cathodic protection of rebar in concrete using Carbon FRP laminate. Constr. Build. Mater. 2017, 156, 242–251. [Google Scholar] [CrossRef]
- Huang, X.X.; Zhou, Y.W.; Zheng, X.B.; Lan, Y.B.; Xing, F.; Sui, L.L.; Hu, B.A.; Li, S. Behavior and Modeling of Hybrid CFRP/Steel Reinforced Concrete Beams with Cathodic Protection in a Marine Environment. J. Compos. Constr. 2022, 26, 4022079. [Google Scholar] [CrossRef]
- NACE.SP0290(2000); Standard Practice: Detection, Repair, and Mitigation of Cracking of Refinery Equipment in Wet H2S Environments. NACE International: Houston, TX, USA,, 2000.
- Cavigliasso, G.E.; Esplandiu, M.J.; Macagno, V.A. Influence of the forming electrolyte on the electrical properties of tantalum and niobium oxide films: An EIS comparative study. J. Appl. Electrochem. 1998, 28, 1213–1219. [Google Scholar] [CrossRef]
- Bertolini, L.; Bolzoni, F.; Pastore, T.; Pedeferri, P. Effectiveness a conductive cementitious mortar anode for cathodic protection of steel in concrete. Cem. Concr. Res. 2004, 34, 681–694. [Google Scholar] [CrossRef]
- BS EN.12954(2019); Cathodic Protection of Buried or Immersed Metallic Structures. General Principles and Application for Pipelines. British Standards Institution (BSI): London, UK, 2019.
- ASTM C876-09; Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concret. ASTM International: West Conshohocken, PA, USA, 2009.
- EN.12696(2000); Cathodic protection of steel in concrete. Brussels: European Committee for Standardization. ECF Standardization: Brussels, Belgium, 2000.
- Li, T. Research on the Mechanism and Numerical Simulation of Non-uniform Corrosion of Reinforcement in Chloride Environment Concrete. Master’s Thesis, Zhejiang University, Hangzhou, China, 2020. [Google Scholar]
- Muehlenkamp, E.B.; Koretsky, M.D.; Westall, J.C. Effect of moisture on the spatial uniformity of cathodic protection of steel in reinforced concrete. Corrosion 2005, 61, 519–533. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In U.S. Geological Survey Techniques and Methods; U.S. Geological Survey: Reston, VA, USA, 2013; Volume 6, p. 497. [Google Scholar]
- Tennakoon, C.; Shayan, A.; Sanjayan, J.G.; Xu, A.M. Chloride ingress and steel corrosion in geopolymer concrete based on long term tests. Mater. Des. 2017, 116, 287–299. [Google Scholar] [CrossRef]
- Vanysek, P. Ionic Conductivity and Diffusion at Infinite Dilution; CRC press: Boca Raton, FL, USA, 2000; pp. 76–78. [Google Scholar]
- Wessel, F.A. Modeling of Galvanic Corrosion-in Presence of External Direct and Alternating Currents. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2022. [Google Scholar]
- Wu, H.; Zhou; Leung, C.K. Experimental study on chloride-induced corrosion of soil nail with engineered cementitious composites (ECC) grout. Infrastructures 2021, 6, 161. [Google Scholar] [CrossRef]
- Zhong, B. Research on Performance Evaluation Methods and Failure Modes of Carbon Fiber Composite Anodes for ICCP-SS. Master’s Thesis, Shenzhen University, Shenzhen, China, 2023. [Google Scholar]
- Cheng, X. Research on the Transport Mechanism and Reaction Behavior of Multiple Ions in Concrete under Electric Field Effects. Master’s Thesis, Zhejiang University, Shenzhen, China, 2022. [Google Scholar]
- Guo, B.B.; Qiao, G.F.; Li, Z.M.; Li, D.S.; Dai, J.H.; Wang, Y. Impressed current cathodic protection of chloride-contaminated RC structures with cracking: A numerical study. J. Build. Eng. 2021, 44, 102943. [Google Scholar] [CrossRef]
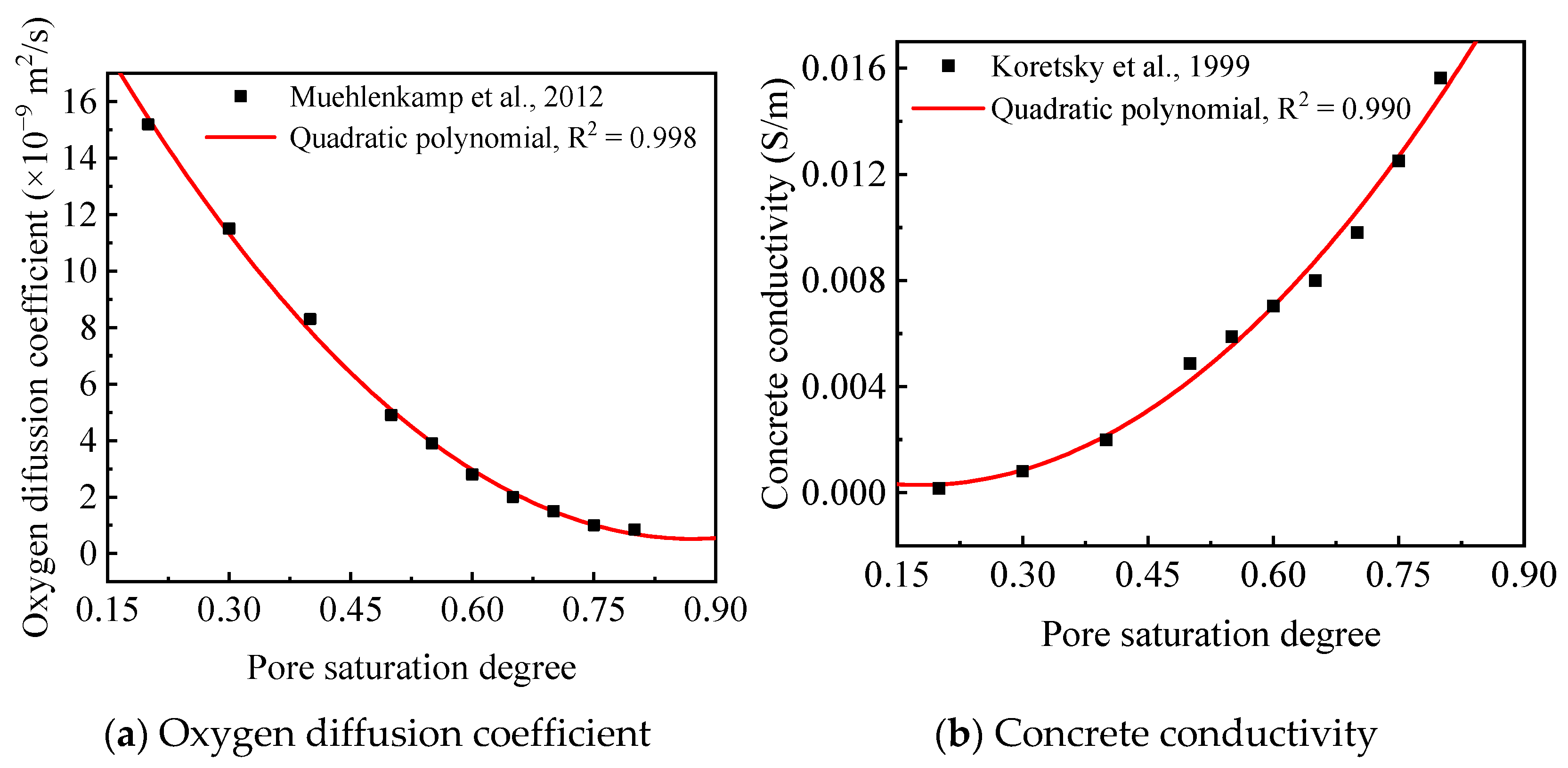
- Koretsky, M.D.; Abooameri, F.; Westall, J.C. Effect of concrete pore saturation on cathodic protection of steel-reinforced concrete bridges. Corrosion 1999, 55, 52–64. [Google Scholar] [CrossRef]
- Yang, Q.B. Inner relative humidity and degree of saturation in high-performance concrete stored in water or salt solution for 2 years. Cem. Concr. Res. 1999, 29, 45–53. [Google Scholar] [CrossRef]
- Tian, L.Y.; He, D.P.; Zhao, J.N.; Wang, H.G. Durability of geopolymers and geopolymer concretes: A review. Rev. Adv. Mater. Sci. 2021, 60, 1–14. [Google Scholar] [CrossRef]
- García, J.; Almeraya, F.; Barrios, C.; Gaona, C.; Núñez, R.; López, I.; Rodríguez, M.; Martínez-Villafañe, A.; Bastidas, J.M. Effect of cathodic protection on steel-concrete bond strength using ion migration measurements. Cem. Concr. Compos. 2012, 34, 242–247. [Google Scholar] [CrossRef]
- Guo, W.H.; Hu, J.; Ma, Y.W.; Huang, H.L.; Yin, S.H.; Wei, J.X.; Yu, Q.J. The application of novel lightweight functional aggregates on the mitigation of acidification damage in the external anode mortar during cathodic protection for reinforced concrete. Corros. Sci. 2020, 165, 108366. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.Y.; Ma, Y.W.; Wei, J.X.; Yu, Q.J. Identification on acidification damage of external anode system induced by impressed current cathodic protection for reinforced concrete. Math. Biosci. Eng. 2019, 16, 7510–7525. [Google Scholar] [CrossRef]
- NACE.TM0294(2007); Evaluation of Pipeline and Pressure Vessel Steels for Resistance to Hydrogen-Induced Cracking. NACE International: Houston, TX, USA, 2007.
- Chess, P.M.; Broomfield, J.P. Cathodic Protection of Steel in Concrete and Masonry; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–320. [Google Scholar]










| (a) Traditional OPC Concrete | |||||||
| Mixing Ratio | Compressive Strength | ||||||
| Cement | Sand | Aggregate | Water | ||||
| 489 | 764 | 934 | 215 | 55.72 MPa | |||
| (b) ECC Concrete | |||||||
| Mixing Ratio | Compressive Strength | ||||||
| Cement | Mineral Powder | Silica Fume | Cenosphere | Water | PE Fiber | HRWRA | |
| 700 | 188 | 230 | 216 | 230 | 20 | 45 | 61.28 MPa |
| (c) Geopolymer Concrete | |||||||
| Mixing Ratio | Compressive Strength | ||||||
| Aggregate | Sand | Fly Ash | Mineral Powder | NaOH | Sodium Silicate | Water | |
| 1120 | 530 | 369 | 92 | 14 | 110 | 105 | 43.5 MPa |
| Reinforcement | Diameter (mm) | Yield Strength (MPa) | Ultimate Strength (MPa) | Modulus (GPa) |
|---|---|---|---|---|
| Steel bar | 12 | 416.54 | 578.75 | 201.51 |
| CFRP strip | - | - | 2491.92 | 227.02 |
| Specimen | Current (mA) | Current Density (mA/m2) | Electric Flux Density (107 C/m2) | Duration (Day) |
|---|---|---|---|---|
| C-M0-a,b,c | 0 | 0 | 0 | - |
| C-M15-a,b,c | 2 | 1620 | 0.43 | 30 |
| C-M30-a,b,c | 4 | 3240 | 0.86 | 30 |
| C-M60-a,b,c | 8 | 6480 | 1.73 | 30 |
| E-M30-a,b,c | 4 | 3240 | 0.86 | 30 |
| E-M120-a,b,c | 16 | 12,960 | 3.46 | 30 |
| G-M30-a,b,c | 4 | 3240 | 0.86 | 30 |
| G-M120-a,b,c | 16 | 12,960 | 3.46 | 30 |
| Specimen | Instant-Off Potential | Depolarization Potential | Decay Potential | |||
|---|---|---|---|---|---|---|
| 3 d | 30 d | 3 d | 30 d | 3 d | 30 d | |
| C-M15 | −1053 | −1089 | −829 | −789 | −224 | −300 |
| C-M30 | −1008 | −1107 | −701 | −652 | −307 | −455 |
| C-M60 | −1054 | −1148 | −719 | −651 | −335 | −497 |
| E-M30 | −1063 | −1154 | −968 | −779 | −95 | −375 |
| E-M120 | −1086 | −1167 | −780 | −729 | −306 | −438 |
| G-M30 | −1132 | −1217 | −971 | −841 | −161 | −376 |
| G-M120 | −1166 | −1329 | −995 | −894 | −171 | −435 |
| Parameters | OH | Cl | Fe2+ | Na+ | Ca2+ | O2 | Ref. |
|---|---|---|---|---|---|---|---|
| Initial concentration cini,i (mol/m3) | 140 | 0 | 0 | 40 | 50 | 0.156 | [40] |
| Boundary concentration c0,i (mol/m3) | 0 | 614 | 0 | 614 | 0 | 0.268 | |
| Diffusion coefficient Di in free water (×10−9 m2/s) | 5.27 | 2.03 | 0.719 | 1.33 | 0.793 | - | [42,44] |
| Diffusion coefficient Di in OPC concrete (×10−11 m2/s) | 3.12 | 1.20 | 0.425 | 0.786 | 0.469 | 69 | [40] |
| Diffusion coefficient Di in ECC concrete (×10−11 m2/s) | 1.56 | 0.60 | 0.213 | 0.393 | 0.234 | 149 | [46] |
| Diffusion coefficient Di in geopolymer concrete (×10−11 m2/s) | 0.618 | 0.238 | 0.084 | 0.156 | 0.093 | 54 | [43] |
| Reaction @Electrode | Equilibrium Potential Eeq (SCE) | Tafel Slope (V/dec) | Exchange Current Density (A/m2) | Ref. |
|---|---|---|---|---|
| Fe → Fe2+ @Steel | −0.68 | 0.410 | 7.10 × 10−5 | [41] |
| ORR @Steel | 0.16 | −0.180 | 7.70 × 10−7 | [41] |
| HER @Steel | −0.24 | −0.150 | 1.10 × 10−2 | [41] |
| OER @CFRP | 0.16 | 0.184 | 2.00 × 10−4 | [47] |
| CER @CFRP | 1.12 | 0.098 | 1.40 × 10−2 | [45] |
| HCOR @CFRP | 1.25 | 0.180 | 6.00 × 10−6 | [48] |
| Specimen ID | Experimental Results | Numerical Results | ||
|---|---|---|---|---|
| Rebar Potential (mVSCE) | Feeding Voltage (V) | Rebar Potential (mVSCE) | Feeding Voltage (V) | |
| C-M15 | −1046–−1102 | 2.80–8.30 | −1087.6 | 2.98 |
| C-M30 | −1008–−1130 | 3.05–7.61 | −1134.3 | 4.11 |
| C-M60 | −1014–−1162 | 3.37–11.20 | −1182.9 | 6.25 |
| E-M30 | −1063–−1162 | 3.05–7.61 | −1136.9 | 5.32 |
| G-M30 | −1095–−1217 | 5.48–10.7 | −1184.2 | 3.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Jia, Y.; Zhou, Y.; Xue, C.; Hu, B.; He, Y.; Huang, X. Performance Evolution of CFRP Strip Anodes in Concrete: An Integrated Electrochemical and Mechanical Study. Polymers 2025, 17, 2494. https://doi.org/10.3390/polym17182494
Wu X, Jia Y, Zhou Y, Xue C, Hu B, He Y, Huang X. Performance Evolution of CFRP Strip Anodes in Concrete: An Integrated Electrochemical and Mechanical Study. Polymers. 2025; 17(18):2494. https://doi.org/10.3390/polym17182494
Chicago/Turabian StyleWu, Xuan, Yichen Jia, Yingwu Zhou, Chengcheng Xue, Biao Hu, Yinghou He, and Xiaoxu Huang. 2025. "Performance Evolution of CFRP Strip Anodes in Concrete: An Integrated Electrochemical and Mechanical Study" Polymers 17, no. 18: 2494. https://doi.org/10.3390/polym17182494
APA StyleWu, X., Jia, Y., Zhou, Y., Xue, C., Hu, B., He, Y., & Huang, X. (2025). Performance Evolution of CFRP Strip Anodes in Concrete: An Integrated Electrochemical and Mechanical Study. Polymers, 17(18), 2494. https://doi.org/10.3390/polym17182494