Study on the Regeneration-Cycle Mechanism of Cu-BTC@MWS Composites Following Mercury Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
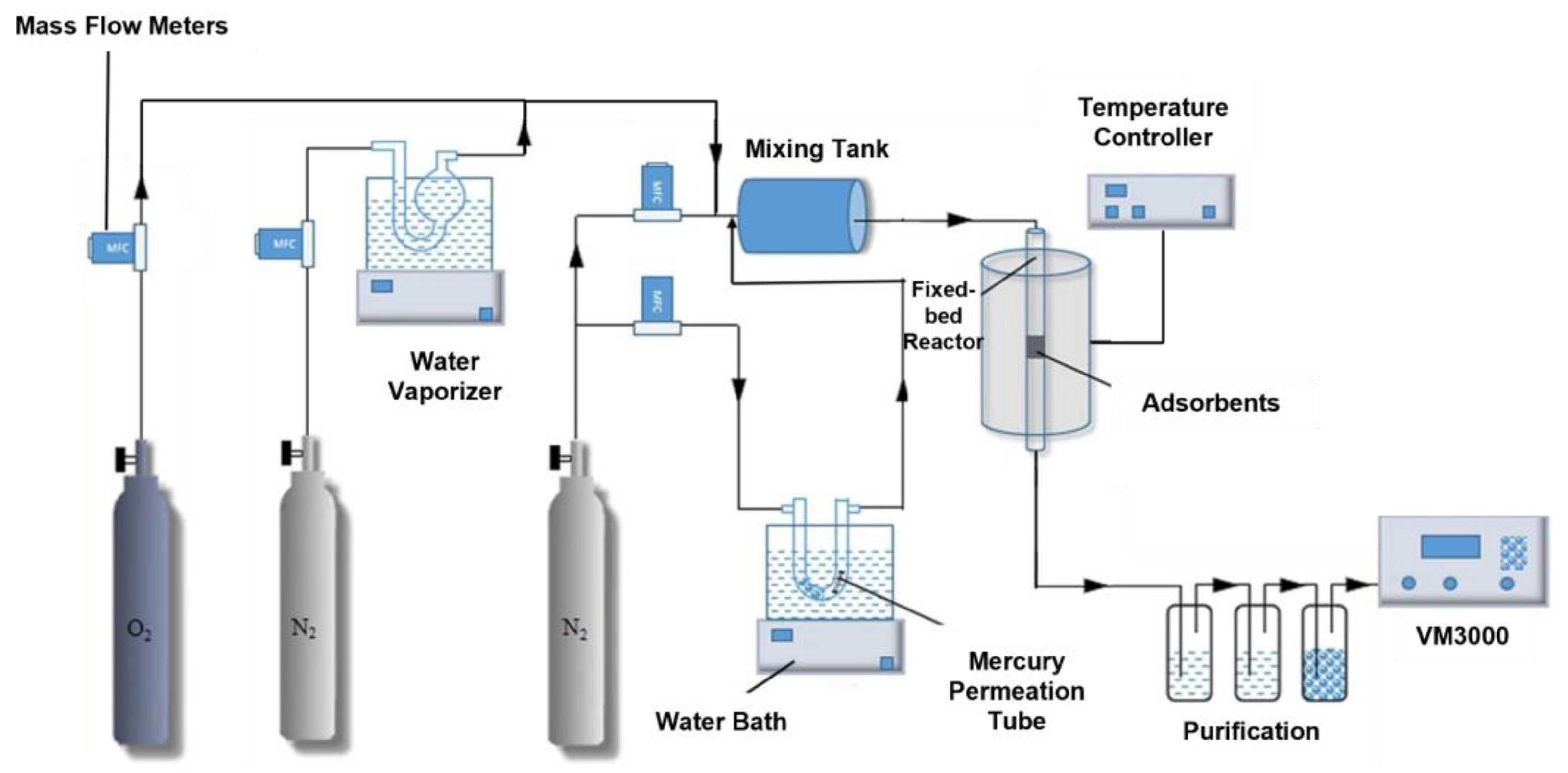
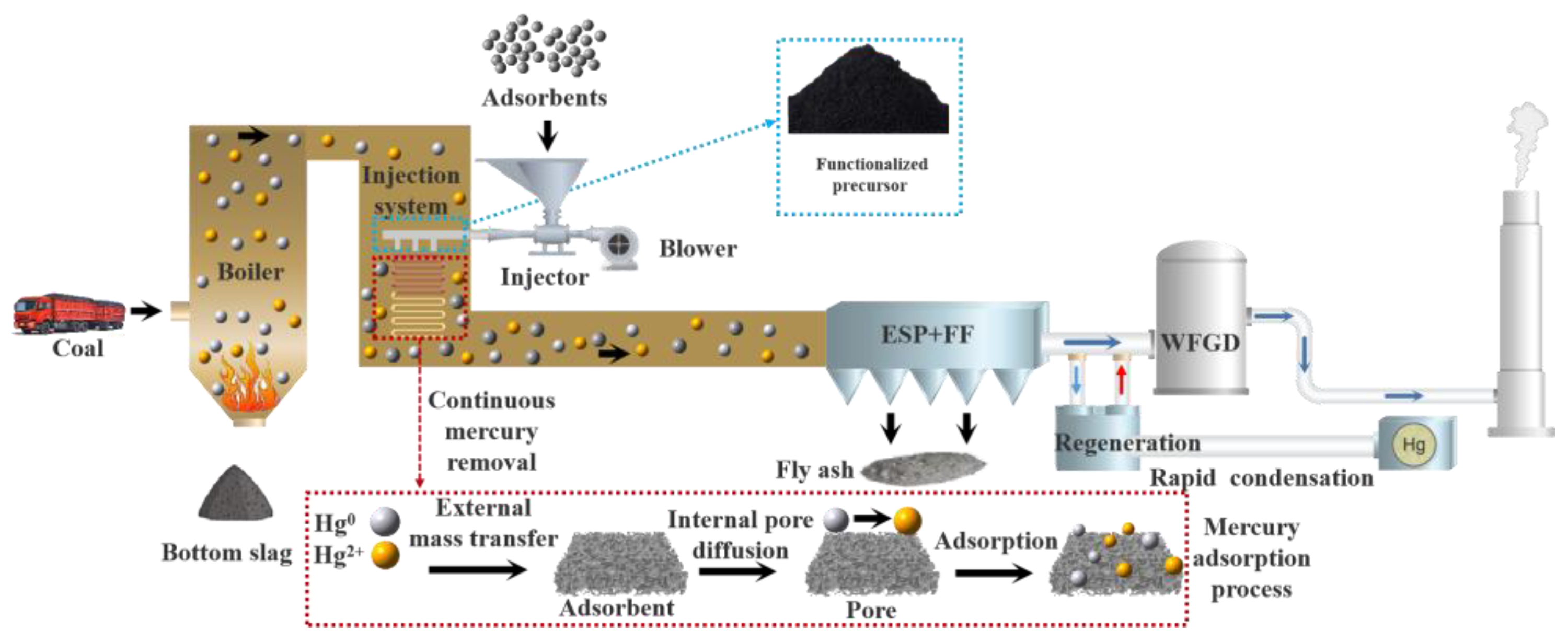
2.3. Hg0 Fixed-Bed Adsorption/Regeneration Experiment System
2.4. Model Construction and Simulation Calculation Method
3. Results
3.1. Adsorption and Regeneration Cycle Characteristics
3.2. Microscopic Characterization
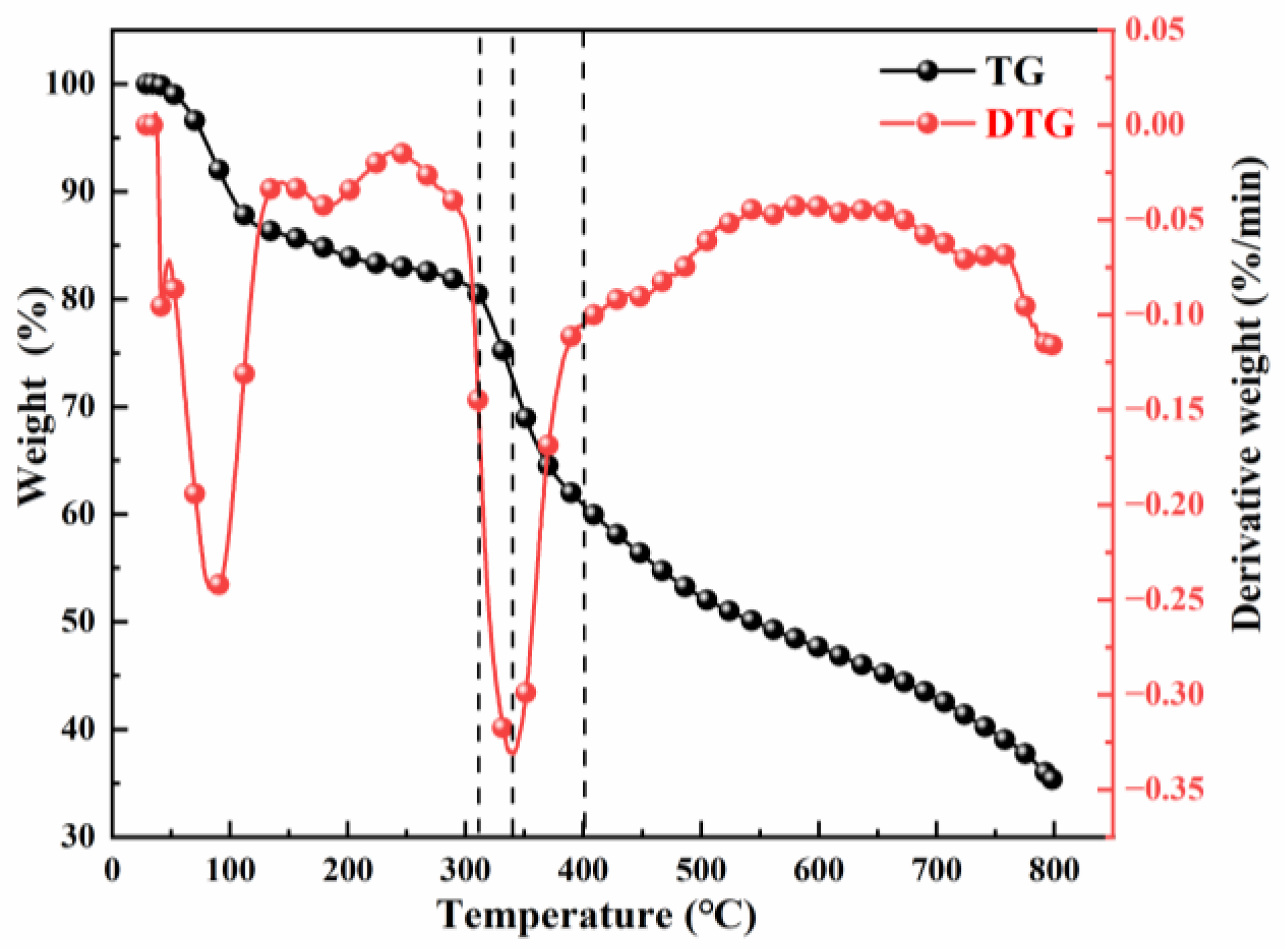
3.2.1. Weight Loss Characteristics
3.2.2. Ultimate Analysis
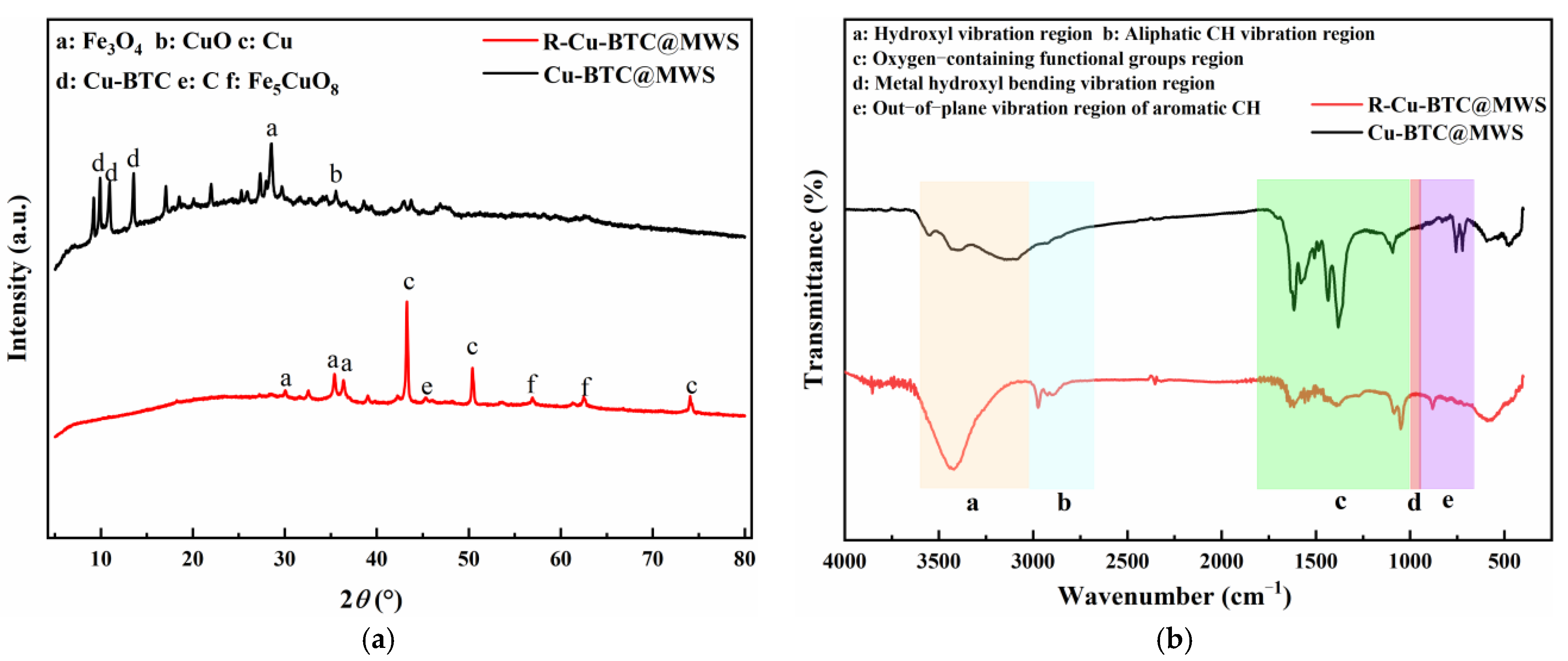
3.2.3. Crystal Phase Structure and Surface Chemical Characteristics
3.2.4. Microscopic Morphology
3.2.5. Carbon Chain Structure Composition
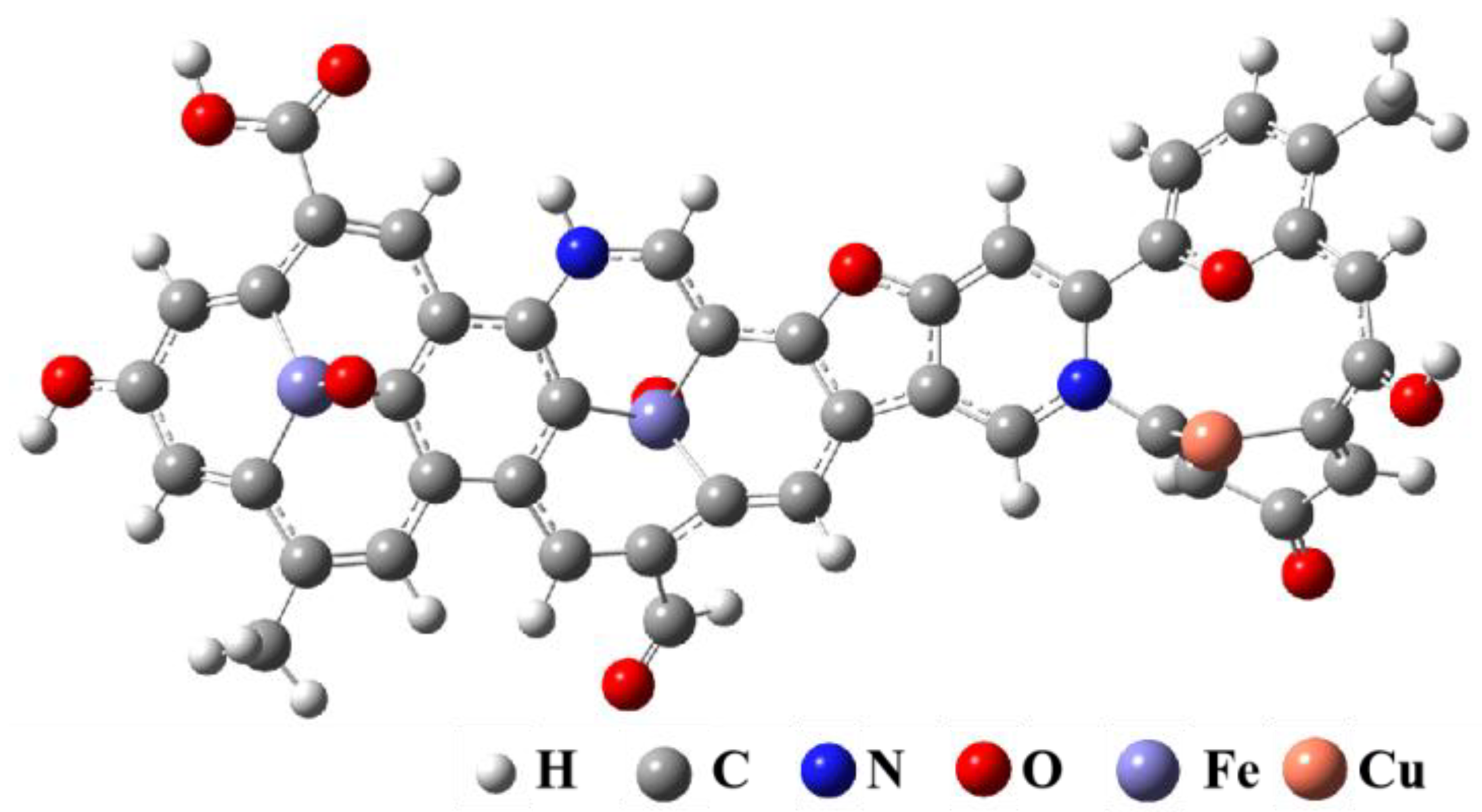
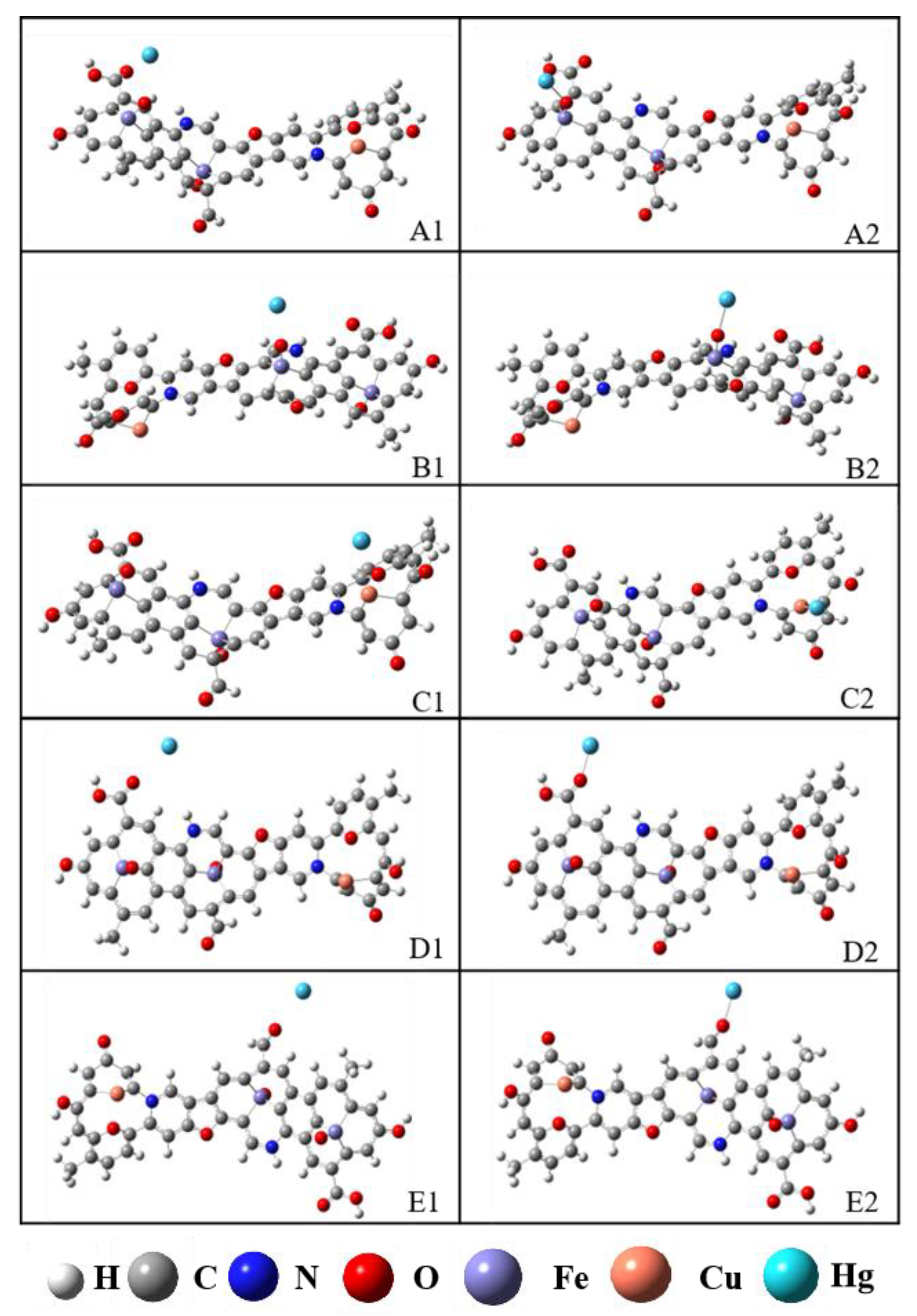
3.3. Molecular Modeling of Regenerated Samples
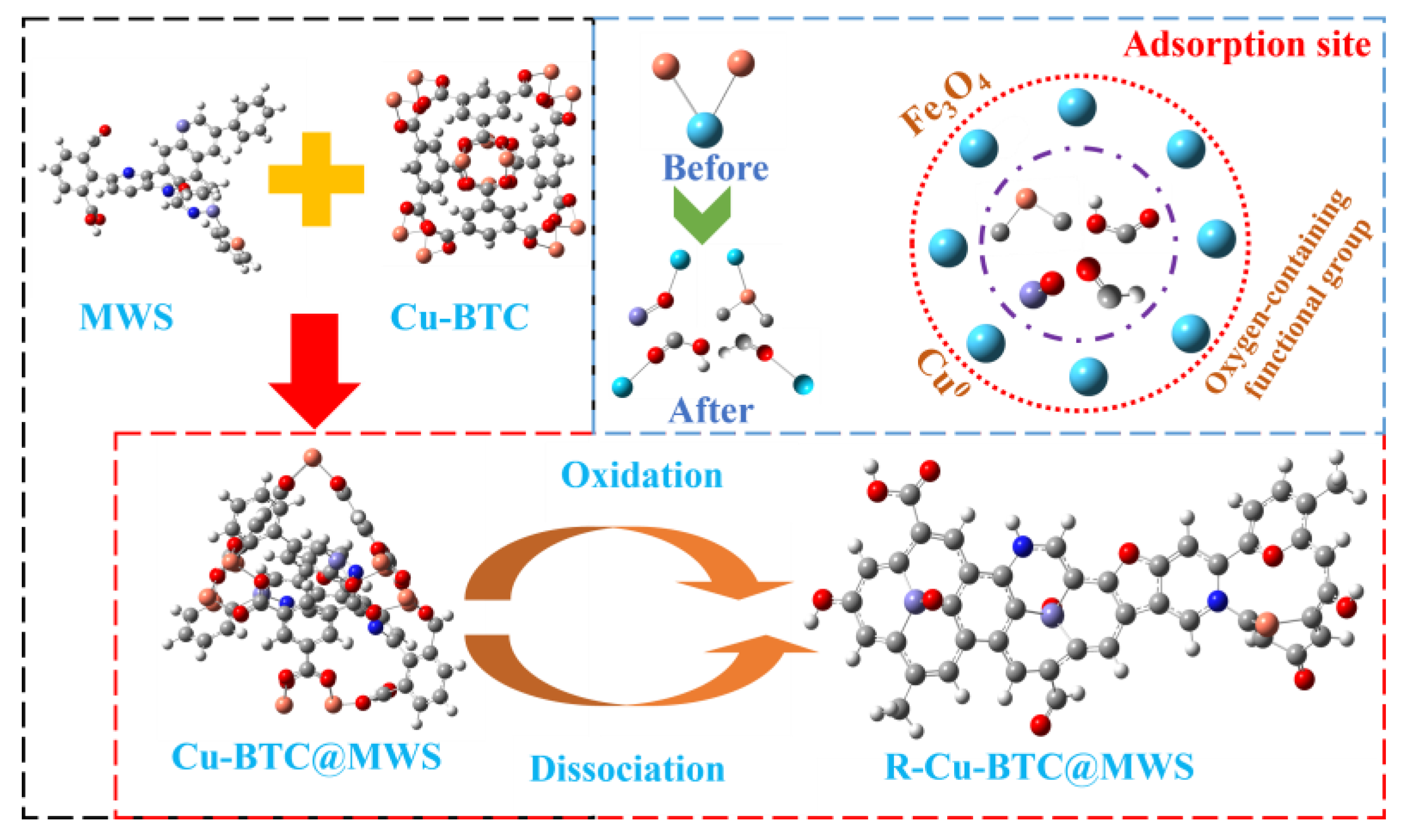
3.4. Adsorption and Regeneration Mechanism
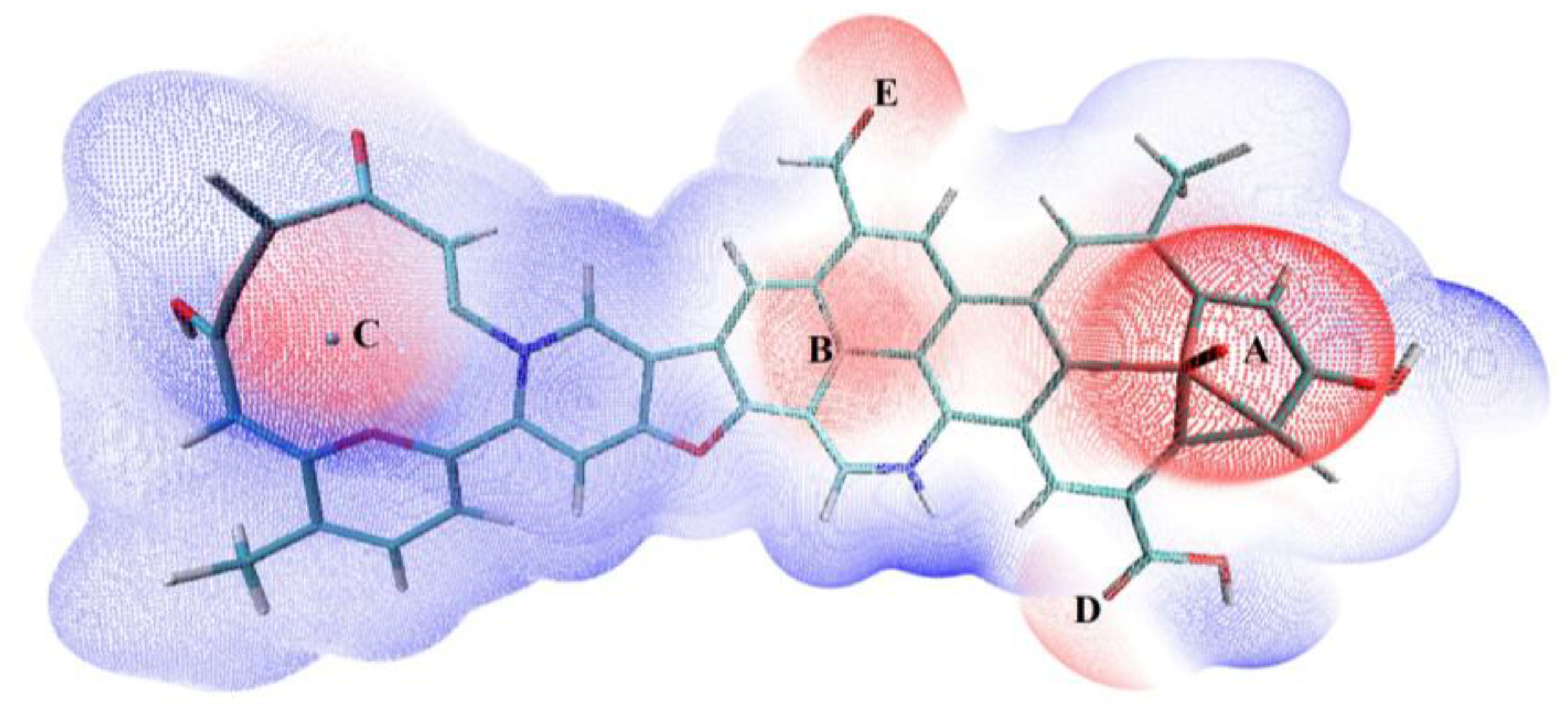
3.4.1. Determination of Adsorption Sites
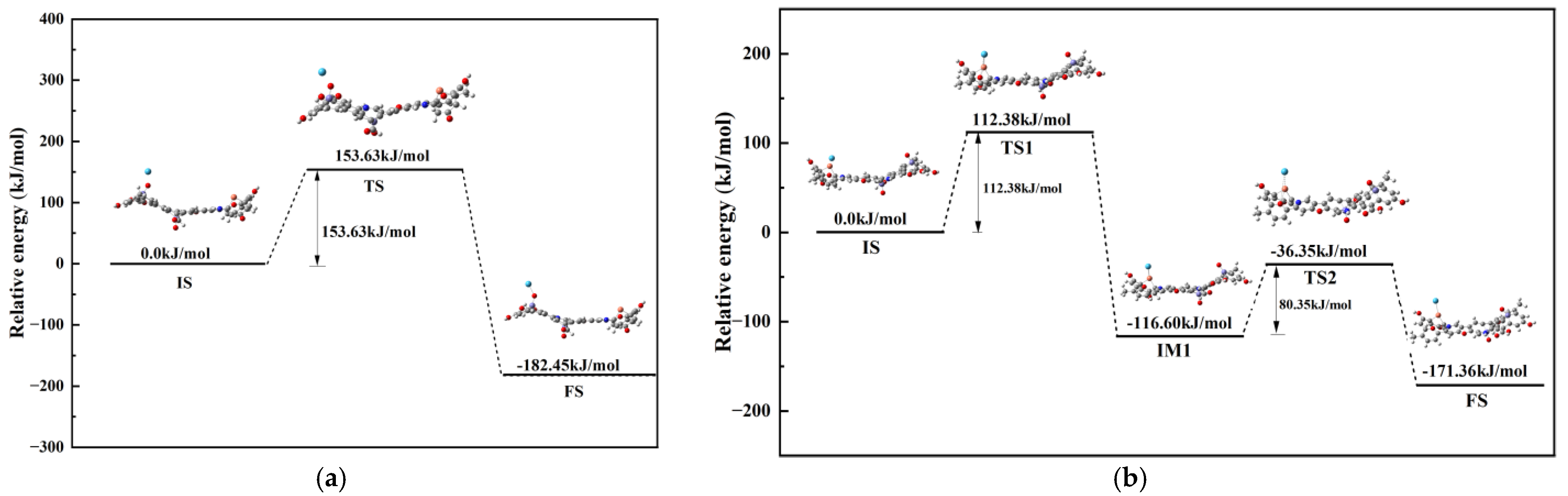
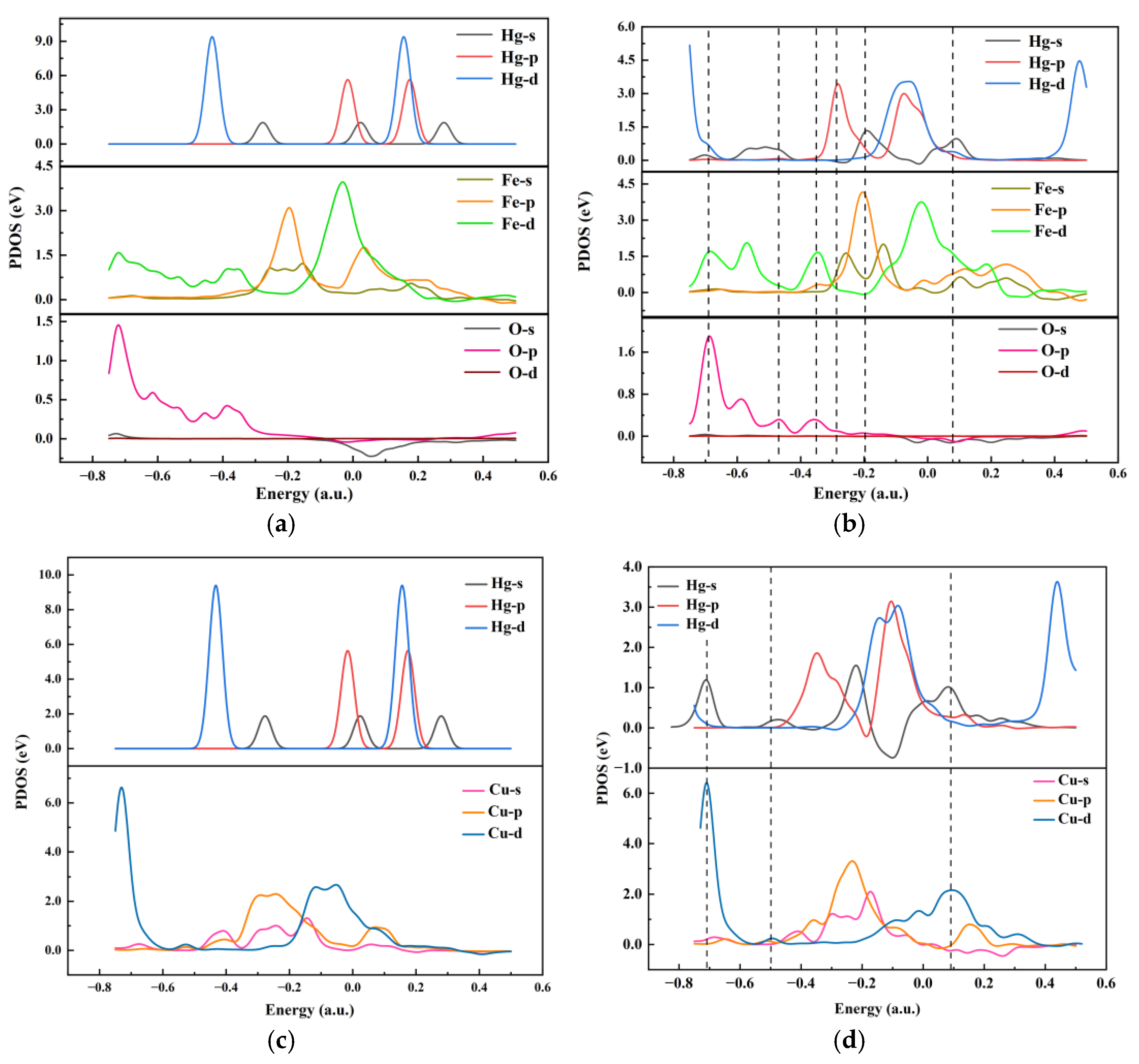
3.4.2. Reaction Mechanisms
4. Regeneration Mechanism
5. Industrial Application
6. Conclusions
- Temperature and O2 volume significantly affect the regeneration process. For R-Cu-BTC@MWS, the ideal regeneration conditions were 350 °C and an atmosphere of 5% O2 + 95% N2. Under these conditions, the maximum regeneration efficiency reached 92% of the fresh sample’s adsorbed capacity. The regeneration cycle efficiency showed a gradual decline as the number of cycles increased.
- Under optimal regeneration conditions, the overall structure of the Cu-BTC-based modified biochar composite adsorbent samples collapsed and was accompanied by the formation of the derived carbon material R-Cu-BTC@MWS. The molecular structure of the regenerated samples included two anthracene-benzenes, two pyridinium nitriles, and one furan.
- During the adsorption of Hg0 on the surface of R-Cu-BTC@MWS, Fe3O4, as the main adsorption site, was directly involved in the redox reaction of Hg0 to realize the mercury removal, and the corresponding adsorption energy value of the adsorption configuration was the largest; the Cu0 needed to rely on the O2− that escaped to the surface of R-Cu-BTC@MWS and the lattice oxygen and oxygen vacancies in the oxygen-stored solid solution to carry out the redox of Hg0 and itself and finally complete the removal process.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, A.; Shen, B.; Kang, D.; Yang, J.; Luo, J. Emission Control Strategies of Hazardous Trace Elements from Coal-Fired Power Plants in China. J. Environ. Sci. 2020, 93, 66–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, X.; Zhang, S.; Xiao, R.; Xu, G.; Wang, X.; Zhang, J. Promotion Effect of Cu Doping on Mercury Removal Properties of Magnetic MnFe2O4 Materials with High Adsorption Capacity. J. Energy Inst. 2024, 113, 101561. [Google Scholar] [CrossRef]
- Zhao, S.; Mei, J.; Xu, H.; Liu, W.; Qu, Z.; Cui, Y.; Yan, N. Research of Mercury Removal from Sintering Flue Gas of Iron and Steel by the Open Metal Site of Mil-101(Cr). J. Hazard. Mater. 2018, 351, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, S.X.; Wang, L.; Hao, J.M. Atmospheric Mercury Concentration and Chemical Speciation at a Rural Site in Beijing, China: Implications of Mercury Emission Sources. Atmos. Chem. Phys. 2013, 13, 10505. [Google Scholar] [CrossRef]
- Dou, Z.; Wang, Y.; Liu, Y.; Zhao, Y.; Huang, R. Enhanced Adsorption of Gaseous Mercury on Activated Carbon by a Novel Clean Modification Method. Sep. Purif. Technol. 2023, 308, 122885. [Google Scholar] [CrossRef]
- Xiang, Z.; Peng, X.; Cheng, X.; Li, X.; Cao, D. CNT@Cu3(BTC)2 and Metal–Organic Frameworks for Separation of CO2/CH4 Mixture. J. Phys. Chem. C 2011, 115, 19864–19871. [Google Scholar] [CrossRef]
- Liu, Y.; Ghimire, P.; Jaroniec, M. Copper Benzene-1,3,5-Tricarboxylate (Cu-BTC) Metal-Organic Framework (MOF) and Porous Carbon Composites as Efficient Carbon Dioxide Adsorbents. J. Colloid Interface Sci. 2019, 535, 122–132. [Google Scholar] [CrossRef]
- Wen, C.; Liu, T.; Wang, D.; Wang, Y.; Chen, H.; Luo, G.; Zhou, Z.; Li, C.; Xu, M. Biochar as the Effective Adsorbent to Combustion Gaseous Pollutants: Preparation, Activation, Functionalization and the Adsorption Mechanisms. Prog. Energy Combust. Sci. 2023, 99, 101098. [Google Scholar] [CrossRef]
- Liu, L.; Sun, X.; Nie, C.; Chen, M.; Wang, Y.; Zhang, Q.; Xu, Y. Salts-Activated Synthesis of Cu/Co Co-Doped Biochar for Efficient Removal of Elemental Mercury from Coal Combustion Flue Gas. Chem. Eng. J. 2024, 499, 156174. [Google Scholar] [CrossRef]
- Yi, L.; Xie, J.; Li, C.; Shan, J.; Liu, Y.; Lv, J.; Li, M.; Gao, L. LaOx Modified MnOx Loaded Biomass Activated Carbon and Its Enhanced Performance for Simultaneous Abatement of NO and Hg0. Environ. Sci. Pollut. Res. 2022, 29, 2258–2275. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, Y.; Zhang, B.; Luo, G.; Sun, P.; Zou, R.; Yao, H. Elemental Mercury Adsorption and Regeneration Performance of Sorbents FeMnOx Enhanced via Non-Thermal Plasma. Chem. Eng. J. 2017, 309, 503–512. [Google Scholar] [CrossRef]
- Boehme, R.; Zimmer, K. Laser-Induced Writing of Submicron Surface Relief Gratings in Fused Silica on the Fly. J. Laser Micro/Nanoeng. 2007, 2, 178–182. [Google Scholar] [CrossRef][Green Version]
- Siyu, G.; Bingguo, L.; Peng, L.; Yifan, N.; Guangxiong, J.; Wang, C.; Enhua, D.; Libo, Z.; Shenghui, G. Microwave Technology for Safe and Efficient Discard Mercury Catalysts Treatment: Dielectric Properties, Processes, Kinetics, and Mechanism Study. Chem. Eng. Sci. 2025, 306, 121240. [Google Scholar] [CrossRef]
- Miguel, G.S.; Lambert, S.D.; Graham, N.J.D. The Regeneration of Field-Spent Granular-Activated Carbons. Water Res. 2001, 35, 2740–2748. [Google Scholar] [CrossRef]
- Zhou, Q.; Tao, X.; Di, G.; Shang, Y.; Lu, P.; Xu, G.; Liu, M.; Zheng, Y.; Dong, L. Elemental Mercury Capture from Flue Gas by Magnetic Recyclable Fe6Mn1-xCexOy Sorbent. Part 1. Performance Evaluation and Regeneration. Fuel 2021, 304, 120723. [Google Scholar] [CrossRef]
- Jia, L.; Cheng, P.; Yu, Y.; Chen, S.; Wang, C.; He, L.; Nie, H.; Wang, J.; Zhang, J.; Fan, B.; et al. Regeneration Mechanism of a Novel High-Performance Biochar Mercury Adsorbent Directionally Modified by Multimetal Multilayer Loading. J. Environ. Manag. 2023, 326, 116790. [Google Scholar] [CrossRef]
- Wang, C.; Jia, L.; Jin, Y.; Qin, S. Study on Regeneration Mechanism of Composite Adsorbent by Mg-MOF-74-Based Modified Biochar. Sci. Total Environ. 2024, 946, 173944. [Google Scholar] [CrossRef]
- Hao, S.; Qiyuan, S.; Yuling, L.; Peng, G. Halogen (Cl, Br, I) Regulation Cu-BTC for NH3-SCR: NO and Hg0 Removal and Mechanism Analysis. Microporous Mesoporous Mater. 2024, 365, 112914. [Google Scholar] [CrossRef]
- Qin, S.; Fan, H.; Jia, L.; Jin, Y.; Li, Z.; Fan, B. Molecular Structure Analysis and Mercury Adsorption Mechanism of Iron-Based Modified Biochar. Energy Fuels 2022, 36, 3184–3200. [Google Scholar] [CrossRef]
- Qin, S.; Wang, C.; Jia, L.; Fan, B.; Wang, Y.; Qiao, X.; Guo, B.; Jin, Y. Study on Mercury Adsorption Mechanism of Iron-Modified Biomass Char in Coal-Fired Flue Gas Based on Density Functional Theory. Fuel Process. Technol. 2023, 247, 107801. [Google Scholar] [CrossRef]
- Quan, Z.; Huang, W.; Liao, Y.; Liu, W.; Xu, H.; Yan, N.; Qu, Z. Study on the Regenerable Sulfur-Resistant Sorbent for Mercury Removal from Nonferrous Metal Smelting Flue Gas. Fuel 2019, 241, 451–458. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, J.; Zhou, Q.; Xu, X.; Xie, C. Elemental Mercury Removal from Coal Gas by CeMnTi Sorbents and Their Regeneration Performance. J. Zhejiang Univ.-Sci. A 2021, 22, 222–234. [Google Scholar] [CrossRef]
- Gómez-Giménez, C.; Izquierdo, M.T.; de las Obras-Loscertales, M.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Mercury Capture by a Structured Au/C Regenerable Sorbent under Oxycoal Combustion Representative and Real Conditions. Fuel 2017, 207, 821–829. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Wang, Z.; Long, Y.; Ding, J. Interface Reaction Activity of Recyclable and Regenerable Cu-Mn Spinel-Type Sorbent for Hg0 Capture from Flue Gas. Chem. Eng. J. 2019, 372, 697–707. [Google Scholar] [CrossRef]
- Yang, K.; Wang, H.; Zeng, Q.; Ran, H.; Wu, J.; Wu, J.; Liu, D. Insight into the Mercury Removal Mechanism of Copper Salt-Modified Biomass Coke in an Oxyfuel Combustion Atmosphere. Energy Fuels 2023, 37, 8431–8443. [Google Scholar] [CrossRef]
- Wang, C.; Jia, L.; Qin, S.; He, L.; Wu, Y.; Liu, Q.; Jin, Y. Study on the Decarbonization Mechanism of Composite Adsorbent by Mg-MOF-74-Based Modified Biochar. Fuel 2024, 357, 129959. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Ding, J.; Yu, Y.; Zhang, J. Mercury/Oxygen Reaction Mechanism over CuFe2O4 Catalyst. J. Hazard. Mater. 2022, 424, 127556. [Google Scholar] [CrossRef]
- Lim, D.-H.; Choi, S.; Park, J.; Senthamaraikannan, T.G.; Min, Y.; Lee, S.-S. Fundamental Mechanisms of Mercury Removal by FeCl3- and CuCl2-Impregnated Activated Carbons: Experimental and First-Principles Study. Energy Fuels 2020, 34, 16401–16410. [Google Scholar] [CrossRef]
- Luo, J.; Niu, Q.; Jin, M.; Cao, Y.; Ye, L.; Du, R. Study on the Effects of Oxygen-Containing Functional Groups on Hg0 Adsorption in Simulated Flue Gas by XAFS and XPS Analysis. J. Hazard. Mater. 2019, 376, 21–28. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, C.; Wu, H.; Liu, J.; Yang, H. Molecular Study of Heterogeneous Mercury Conversion Mechanism over Cu-MOFs: Oxidation Pathway and Effect of Halogen. Fuel 2021, 290, 120030. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Zhang, B.; Zhao, Y.; Chen, X.; Shen, F. Experimental and Theoretical Studies of Mercury Oxidation over CeO2−WO3/TiO2 Catalysts in Coal-Fired Flue Gas. Chem. Eng. J. 2017, 317, 758–765. [Google Scholar] [CrossRef]




| Samples | Ultimate Analysis/% | Atomic Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| C | H | O | Fe | Cu | N | H/C | O/C | |
| Cu-BTC@MWS | 37.3 | 0.9 | 21.7 | 1.1 | 4.4 | 34.6 | 0.02 | 0.58 |
| R-Cu-BTC@MWS | 57.6 | 2.7 | 17.4 | 12.3 | 6.9 | 3.1 | 0.05 | 0.30 |
| Adsorption Configurations | Adsorption Energy (kJ/mol) | Key Length (Å) |
|---|---|---|
| A | −182.45 | 2.18 |
| B | −171.36 | 2.20 |
| C | −156.83 | 2.58 |
| D | −98.71 | 2.32 |
| E | −122.08 | 2.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Yu, Y. Study on the Regeneration-Cycle Mechanism of Cu-BTC@MWS Composites Following Mercury Adsorption. Polymers 2025, 17, 2474. https://doi.org/10.3390/polym17182474
Wang F, Yu Y. Study on the Regeneration-Cycle Mechanism of Cu-BTC@MWS Composites Following Mercury Adsorption. Polymers. 2025; 17(18):2474. https://doi.org/10.3390/polym17182474
Chicago/Turabian StyleWang, Feng, and Yue Yu. 2025. "Study on the Regeneration-Cycle Mechanism of Cu-BTC@MWS Composites Following Mercury Adsorption" Polymers 17, no. 18: 2474. https://doi.org/10.3390/polym17182474
APA StyleWang, F., & Yu, Y. (2025). Study on the Regeneration-Cycle Mechanism of Cu-BTC@MWS Composites Following Mercury Adsorption. Polymers, 17(18), 2474. https://doi.org/10.3390/polym17182474






