Nanocomposites from β-Pinene and α-Pinene Copolymer: Synthesis, Characterization, and Antioxidant Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
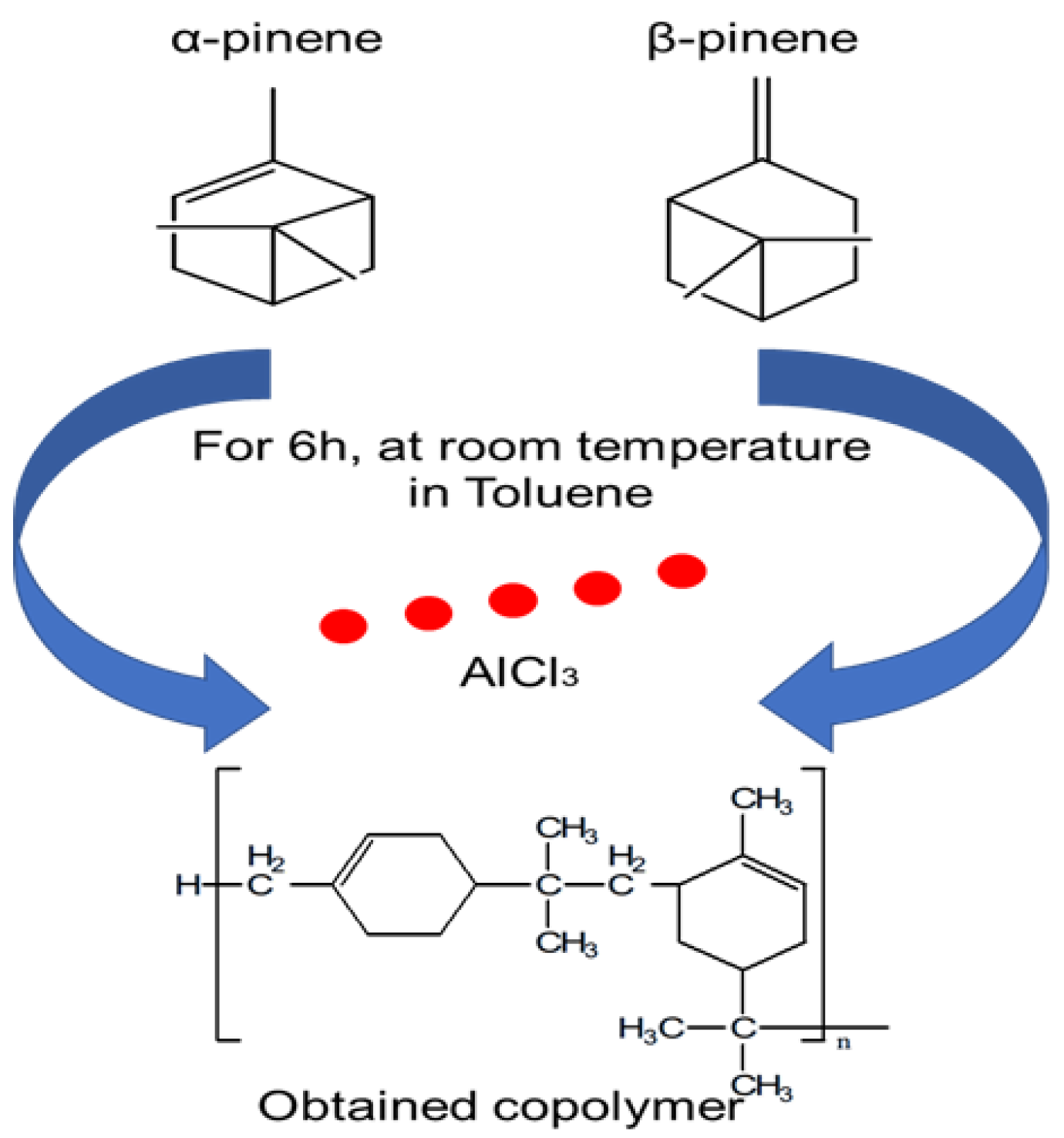
2.2. Copolymerization Procedure
2.3. Elaboration of Nanocomposites Copolymer/Clay (α-co-β-P)/Clay)
2.4. Characterization
3. Results and Discussion
3.1. Characterization of the Obtained Copolymer (α-co-β-P)
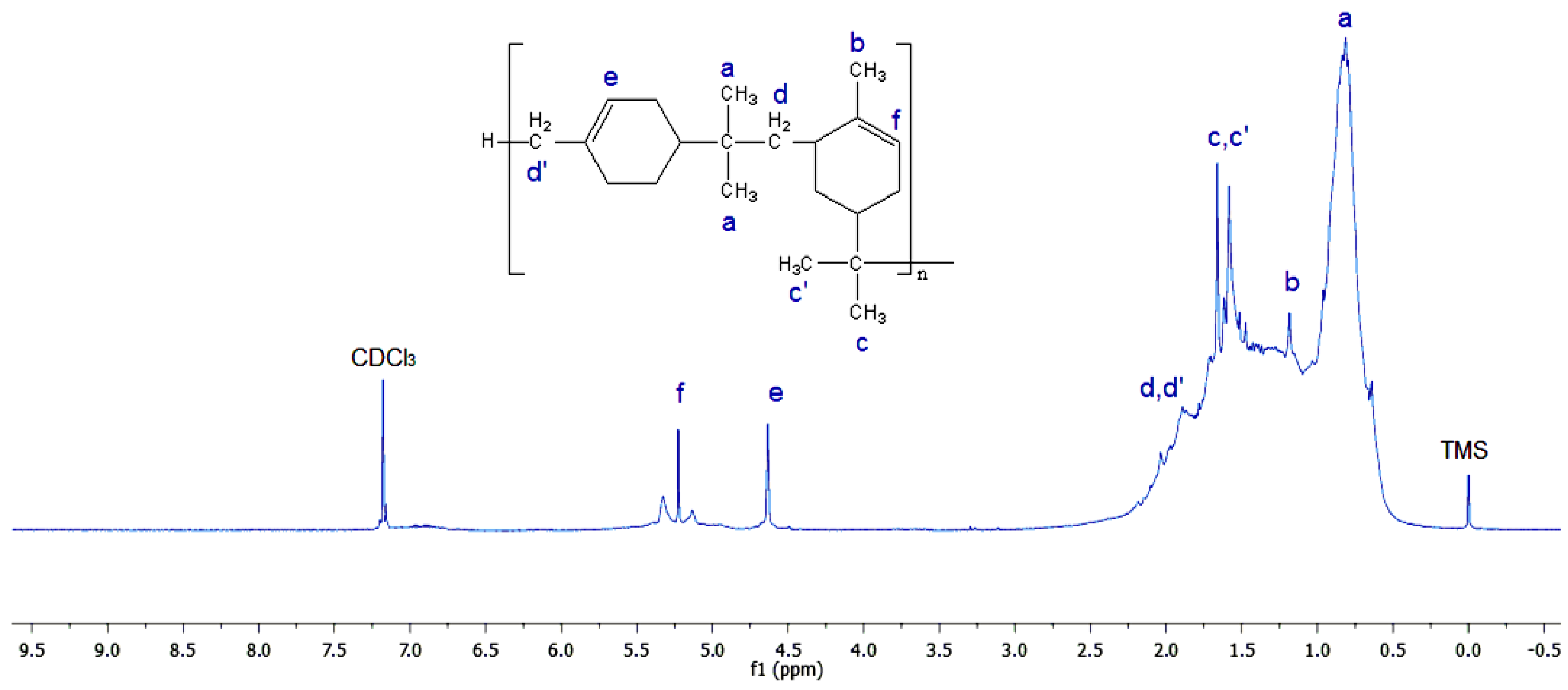
3.1.1. 1H-NMR Measurements
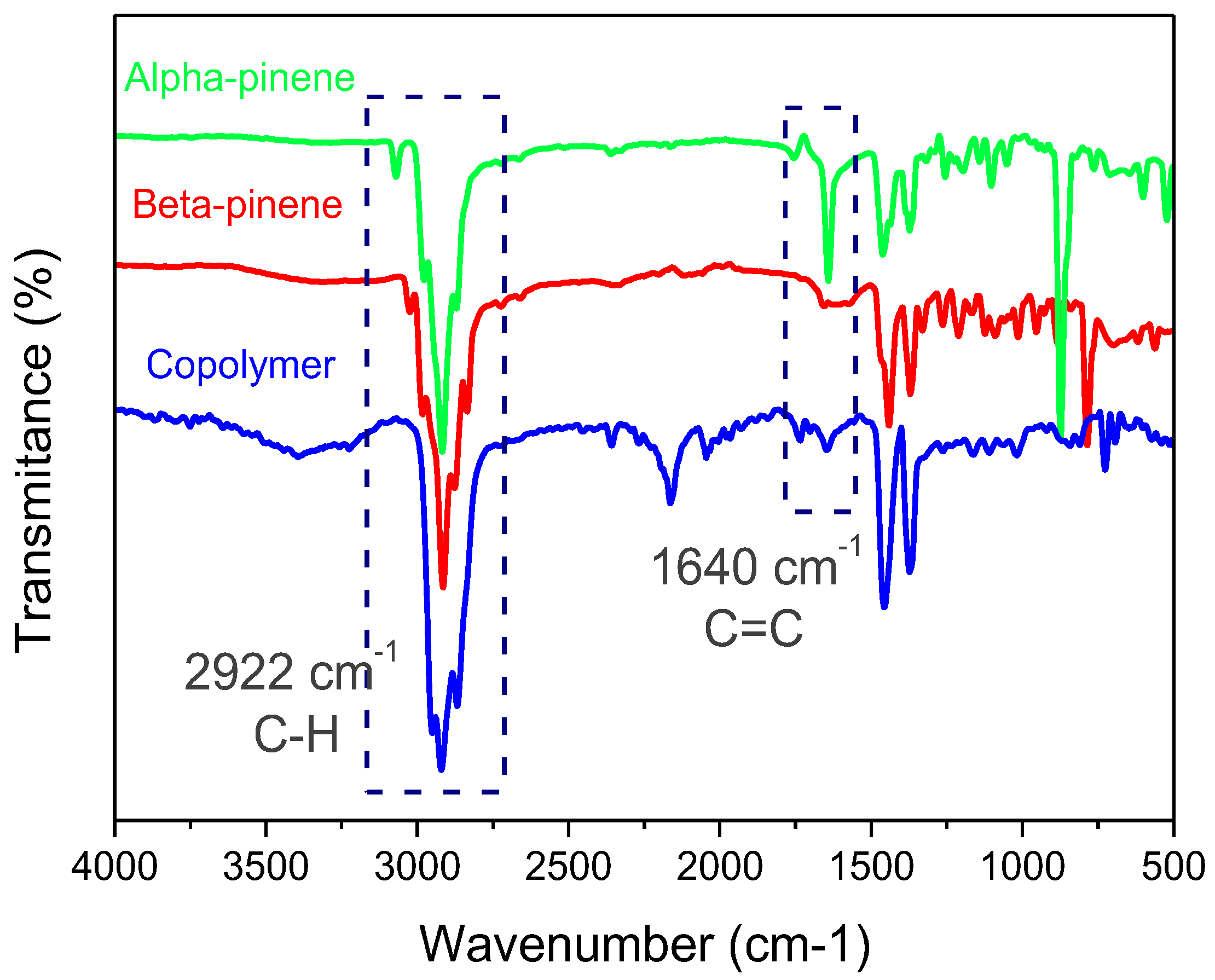
3.1.2. FT-IR Measurements
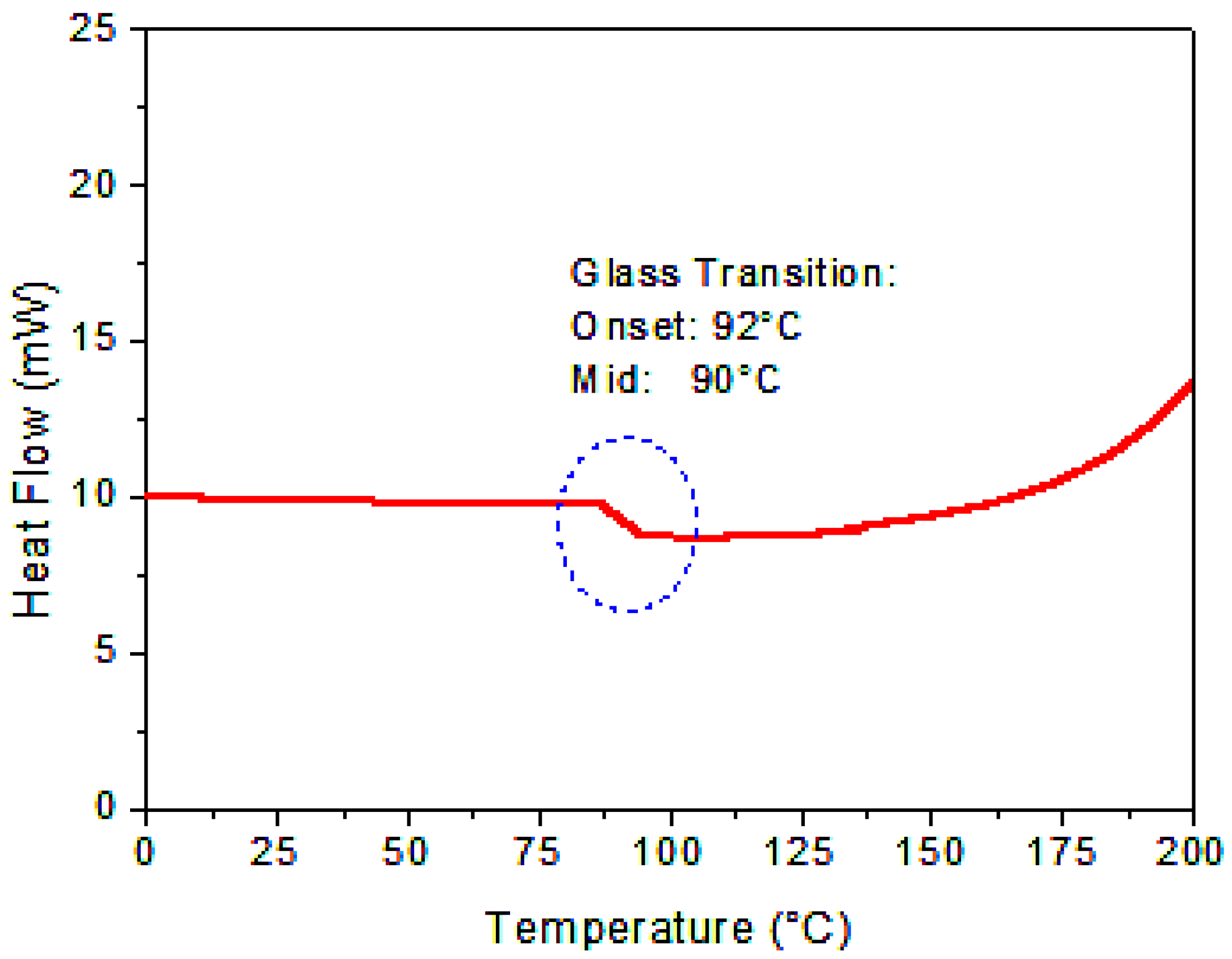
3.1.3. Thermal Study of the Obtained Copolymer with DSC
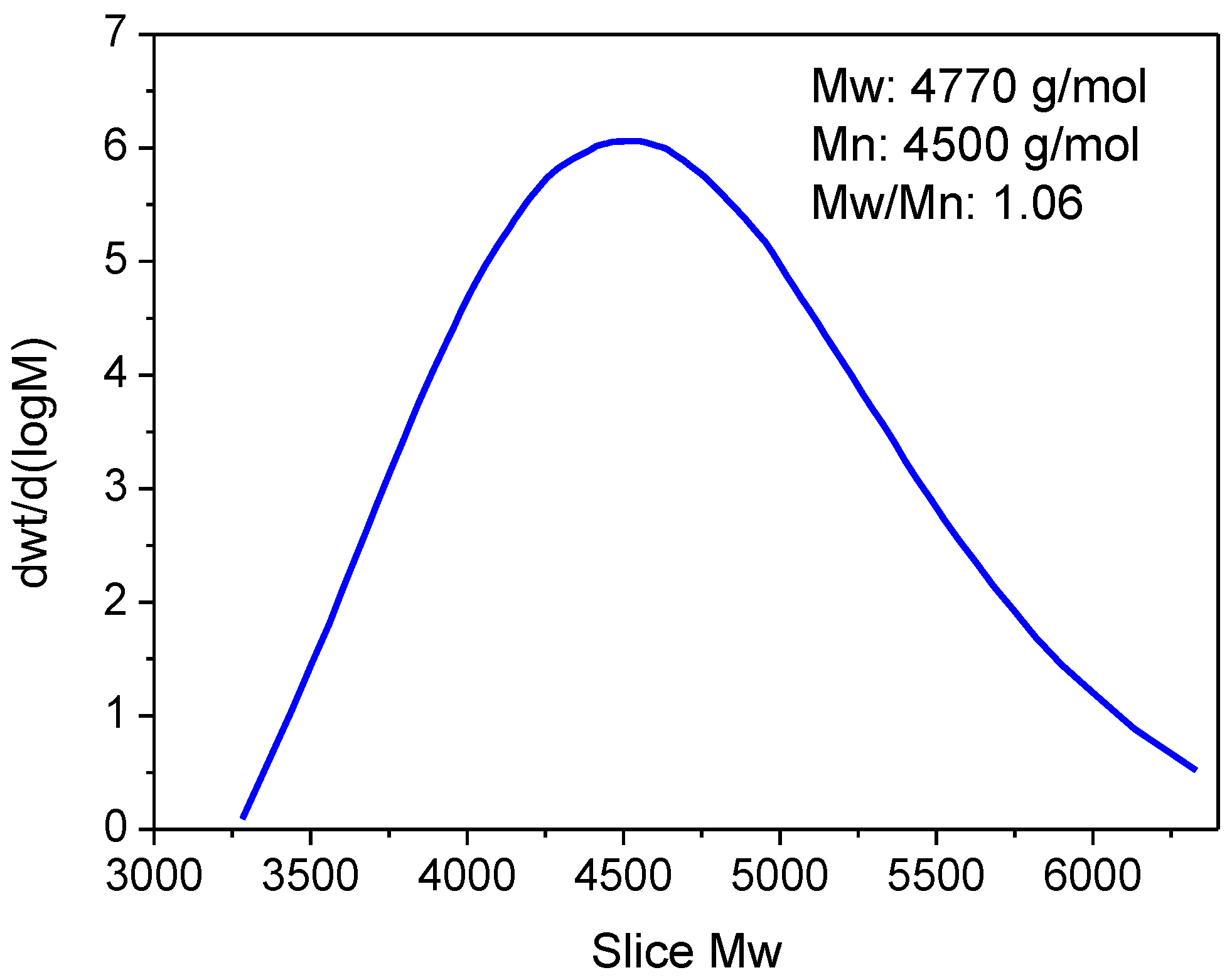
3.1.4. GPC Measurements
3.2. Characterization of the Obtained Nanocomposites (α-co-β-P/Clay)
3.2.1. FT-IR Analysis
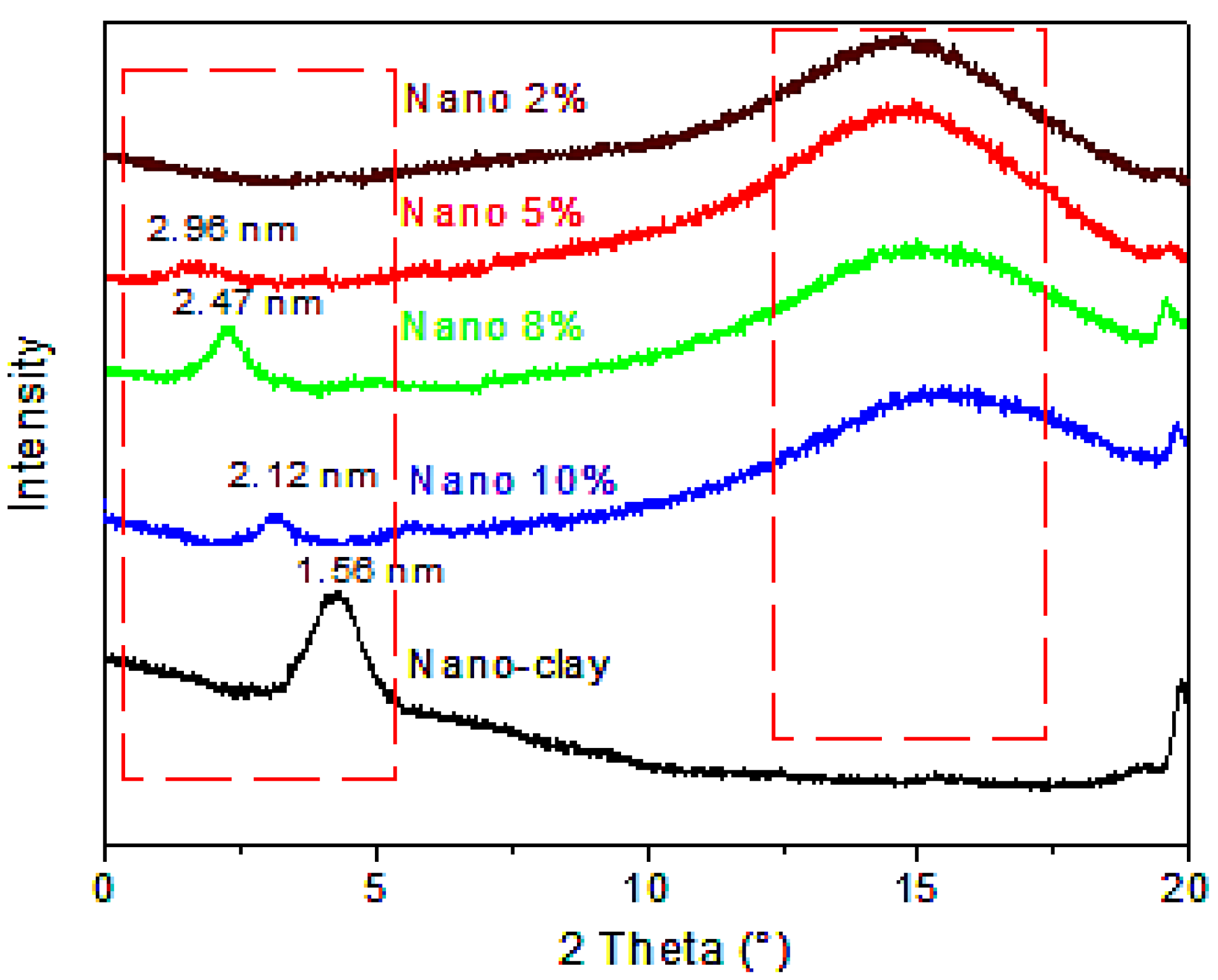
3.2.2. XRD Analysis
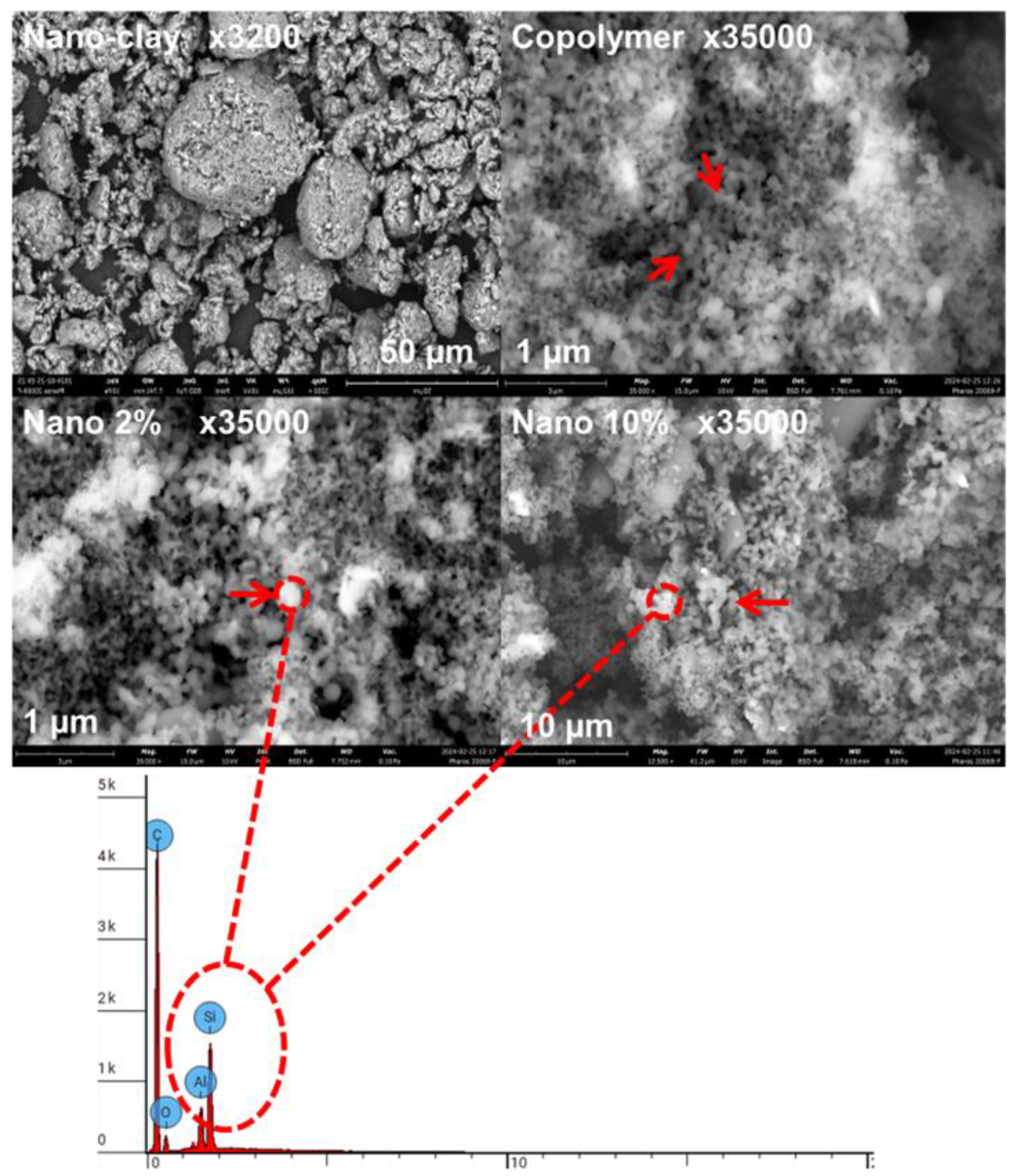
3.2.3. SEM Analysis
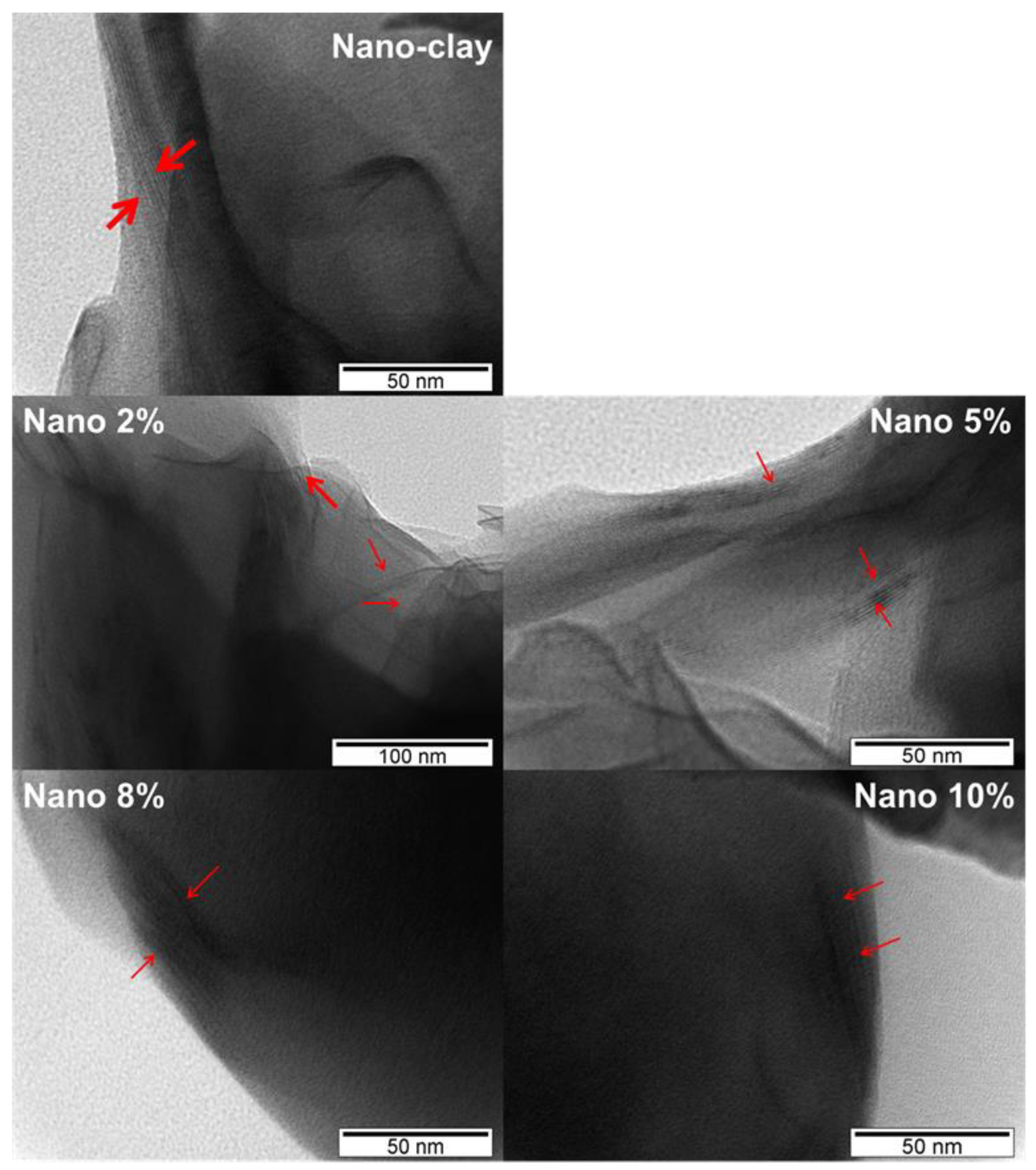
3.2.4. TEM Analysis
3.2.5. TGA
3.3. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-co-β-P | α- and β-pinene based copolymer |
| α-co-β-P/clay | α- and β-pinene copolymer-based nanocomposites |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
References
- Gandini, A.; Lacerda, T.M. Monomers and Macromolecular Materials from Renewable Resources: State of the Art and Perspectives. Molecules 2022, 27, 159. [Google Scholar] [CrossRef] [PubMed]
- Jaillet, F.; Darroman, E.; Ratsimihety, A.; Boutevin, B.; Caillol, S. Synthesis of cardanol oil building blocks for polymer synthesis. Green Mater. 2015, 3, 59–70. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.D.; Sanchez-Ballester, N.M.; Blázquez, M.A. Encapsulated Limonene: A Pleasant Lemon-Like Aroma with Promising Application in the Agri-Food Industry. A Review. Molecules 2020, 25, 2598. [Google Scholar] [CrossRef]
- Satira, A.; Espro, C.; Paone, E.; Calabrò, P.S.; Pagliaro, M.; Ciriminna, R.; Mauriello, F. The Limonene Biorefinery: From Extractive Technologies to Its Catalytic Upgrading into p-Cymene. Catalysts 2021, 11, 387. [Google Scholar] [CrossRef]
- Gaspar, A.S.; Cordeiro, J.B.; Simões, P.A.; Gameiro, D.; Rocha, F.A.; Serra, A.C.; Coelho, J.F.J.; Fonseca, A.C. Poly(β-pinene) as an efficient biobased tackifier for metallocene poly(ethylene) based hotmelt adhesives. Int. J. Adhes. Adhes. 2022, 114, 103111. [Google Scholar] [CrossRef]
- Mosquera, M.E.G.; Jiménez, G.; Tabernero, V.; Vinueza-Vaca, J.; García-Estrada, C.; Kosalková, K.; Sola-Landa, A.; Monje, B.; Acosta, C.; Alonso, R.; et al. Terpenes and Terpenoids: Building Blocks to Produce Biopolymers. Sustain. Chem. 2021, 2, 467–492. [Google Scholar] [CrossRef]
- Scholten, P.B.V.; Moatsou, D.; Detrembleur, C.; Meier, M.A.R. Progress Toward Sustainable Reversible Deactivation Radical Polymerization. Macromol. Rapid Commun. 2020, 41, 2000266. [Google Scholar] [CrossRef]
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. ChemSusChem 2009, 12, 1072–1095. [Google Scholar] [CrossRef]
- Chyau, C.C.; Mau, J.L.; Wu, C.M. Characteristics of the Steam-Distilled Oil and Carbon Dioxide Extract of Zanthoxylumsimulans Fruits. J. Agric. Food Chem. 1996, 44, 1096–1099. [Google Scholar] [CrossRef]
- Yarolimek, M.R.; Coia, B.M.; Bookbinder, H.R.; Kennemur, J.G. Investigating the effect of α-pinene on the ROMP of δ-pinene. Polym. Chem. 2021, 12, 5048–5058. [Google Scholar] [CrossRef]
- Derdar, H.; Belbachir, M.; Harrane, A. A green synthesis of polylimonene using Maghnite-H+, an exchanged montmorillonite clay, as eco-catalyst. Bull. Chem. React. Eng. Catal. 2019, 14, 69–79. [Google Scholar] [CrossRef]
- Derdar, H.; Belbachir, M.; Hennaoui, F.; Akeb, M.; Harrane, A. Green copolymerization of limonene with β-pinene catalyzed by an eco-catalyst Maghnite-H+. Polym. Sci. Ser. B 2018, 60, 555–562. [Google Scholar] [CrossRef]
- Lapuerta, M.; Tobío-Pérez, I.; Ortiz-Alvarez, M.; Donoso, D.; Canoira, L.; Piloto-Rodríguez, R. Heterogeneous Catalytic Conversion of Terpenes into Biofuels: An Open Pathway to Sustainable Fuels. Energies 2023, 16, 2526. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Maréchal, E. Carbocationic Polymerization; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- GunamResul, M.F.M.; Rehman, A.; Saleem, F.; Usman, M.; López Fernández, A.M.; Ezea, V.C.; Harvey, A.P. Recent Advances in Catalytic and Non-Catalytic Epoxidation of Terpenes: A Pathway to Bio-Based Polymers from Waste Biomass. RSC Adv. 2023, 13, 32940–32971. [Google Scholar]
- Tibbetts, J.D.; Bull, S.D. Dimethyl Sulfide Facilitates Acid Catalysed Ring Opening of the Bicyclic Monoterpenes in Crude Sulfate Turpentine to Afford p-Menthadienes in Good Yield. Green Chem. 2021, 23, 597–610. [Google Scholar] [CrossRef]
- Mouren, A.; Avérous, L. Sustainable Cycloaliphatic Polyurethanes: From Synthesis to Applications. Chem. Soc. Rev. 2023, 52, 277–317. [Google Scholar] [CrossRef]
- Nyamwihura, R.J.; Ogungbe, I.V. The pinene scaffold: Its occurrence, chemistry, synthetic utility, and pharmacological importance. RSC Adv. 2022, 12, 11346–11375. [Google Scholar] [CrossRef]
- Griffiths, E.T.; Harries, P.C.; Jeffcoat, R.; Trudgill, P.W. Purification and Properties of α-Pinene Oxide Lyase from Nocardia sp. strain P18. 3. J. Bacteriol. 1987, 169, 4980–4983. [Google Scholar] [CrossRef]
- Fengge, G. Clay/polymer composites: The story. Mater. Today 2004, 7, 50–55. [Google Scholar]
- Avella, M.; Buzarovska, A.; Errico, M.E.; Gentile, G.; Grozdanov, A. Eco-Challenges of Bio-Based Polymer Composites. Materials 2009, 2, 911–925. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef]
- Mykola, S.; Olga, N.; Dmitry, M. The influence of alkylammonium modified clays on the fungal resistance and biodeterioration of epoxy-clay nanocomposites. Int. Biodeterior. Biodegrad. 2016, 110, 136–140. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Pinjari, D.V.; Gogate, P.R.; Sonawane, S.H.; Pandit, A.B. Process intensification of encapsulation of functionalized CaCO3 nanoparticles using ultrasound assisted emulsion polymerization. Chem. Eng. Process. 2011, 50, 1160–1168. [Google Scholar] [CrossRef]
- Derdar, H.; Mitchell, G.R.; Mahendra, V.S.; Beachgoer, M.; Haoue, S.; Cherifi, Z.; Bachari, K.; Harrane, A.; Meghabar, R. Green Nanocomposites from Rosin-Limonene Copolymer and Algerian Clay. Polymers 2020, 12, 1971. [Google Scholar] [CrossRef] [PubMed]
- Derdar, H.; Meghabar, R.; Benachour, M.; Mitchell, G.R.; Bachari, K.; Belbachir, M.; Cherifi, Z.; Baghdadli, M.C.; Harrane, A. Polymer-Clay Nanocomposites: Exfoliation and Intercalation of Organophilic Montmorillonite Nanofillers in Styrene–Limonene Copolymer. Polym. Sci. Ser. A 2021, 63, 568–575. [Google Scholar] [CrossRef]
- Derdar, H.; Mitchell, G.R.; Cherifi, Z.; Belbachir, M.; Benachour, M.; Meghabar, R.; Bachari, K.; Harrane, A. Ultrasound assisted synthesis of polylimonene and organomodified-clay nanocomposites: A structural, morphological and thermal properties. Bull. Chem. React. Eng. Catal. 2020, 15, 798–807. [Google Scholar] [CrossRef]
- Cherifi, Z.; Zaoui, A.; Boukoussa, B.; Derdar, H.; Elabed, Z.O.; Zeggai, F.Z.; Meghabar, R.; Chebout, R.; Bachari, K. Ultrasound-promoted preparation of cellulose acetate/organophilic clay bio-nanocomposites films by solvent casting method. Polym. Bull. 2022, 80, 1831–1843. [Google Scholar] [CrossRef]
- Baghdadli, M.C.; Derdar, H.; Cherifi, Z.; Amine, H.; Meghabar, R. Nanocomposites by in situ polymerization based on styrene-maleic anhydride copolymer and clay. Polym. Bull. 2022, 80, 6869–6883. [Google Scholar] [CrossRef]
- Derdar, H.; Mitchell, G.R.; Mateus, A.; Chaibedraa, S.; Elabed, Z.O.; Mahendra, V.S.; Cherifi, Z.; Bachari, K.; Chebout, R.; Meghabar, R.; et al. Green Copolymers and Nanocomposites from Myrcene and Limonene Using Algerian Nano-Clay as Nano-Reinforcing Filler. Polymers 2022, 14, 5271. [Google Scholar] [CrossRef] [PubMed]
- Akeb, M.; Harrane, A.; Belbachir, M. Polymerization of β-pinene by using natural montmorillonite clay as a green catalyst. Green Mater. 2018, 6, 58–64. [Google Scholar] [CrossRef]
- Ezea, V.C.; Rehman, A.; Patel, M.; Ahmad, S.; Harvey, A.P. Synthesis of cyclic α-pinane carbonate–a potential monomer for bio-based polymers. RSC Adv. 2022, 12, 17454–17465. [Google Scholar] [CrossRef]
- Elabed, Z.O.; Kherroub, D.E.; Derdar, H.; Belbachir, M. Novel Cationic Polymerization of β-Myrcene Using a Proton Exchanged Clay (Maghnite-H+). Polym. Sci. Ser. B. 2021, 63, 480–487. [Google Scholar] [CrossRef]
- Ji, N.; Yu, F.; Yuan, B.; Xie, C.; Yu, S. Polymerization of α-pinene catalyzed by Lewis acidic deep eutectic solvents. New J. Chem. 2024, 48, 4580–4588. [Google Scholar] [CrossRef]
- Kukhta, N.A.; Vasilenko, I.V.; Kostjuk, S.V. Room temperature cationic polymerization of β-pinene using modified AlCl3 catalyst: Toward sustainable plastics from renewable biomass resources. Green Chem. 2011, 13, 2362–2364. [Google Scholar] [CrossRef]
- Thomsett, M.R.; Storr, T.E.; Monaghan, O.R.; Stockman, R.A.; Howdle, S.M. Progress in the Synthesis of Sustainable Polymers from Terpenes and Terpenoids. Green Mater. 2016, 4, 115–134. [Google Scholar] [CrossRef]
- Basturk, S.B. Effect of methyl orange as the modifier on the mechanical, thermal, and thermo-mechanical properties of clay/epoxy composites. J. Appl. Polym. Sci. 2022, 139, 51638. [Google Scholar] [CrossRef]
- Kherroub, D.E.; Boulaouche, T. Maghnite: Novel Inorganic Reinforcement for Single-Step Synthesis of PDMS Nanocomposites with Improved Thermal, Mechanical, and Textural Properties. Res. Chem. Intermed. 2020, 46, 5199–5217. [Google Scholar] [CrossRef]
- Cherifi, Z.; Boukoussa, B.; Zaoui, A.; Belbachir, M.; Meghabar, R. Structural, morphological and thermal properties of nanocomposites poly(GMA)/clay prepared by ultrasound and in-situ polymerization. Ultrason. Sonochem. 2018, 48, 188–198. [Google Scholar] [CrossRef]
- Kherroub, D.E.; Belbachir, M.; Lamouri, S. Nylon 6/clay nanocomposites prepared with Algerian modified clay (12-maghnite). Res. Chem. Int. 2014, 41, 5217–5228. [Google Scholar] [CrossRef]
- Francucci, G.; Rodriguez, E.; Rodriguez, M.E. Ultrasound-Assisted Extrusion Compounding of Nano Clay/Polypropylene Nano Compounds. Polymers 2024, 16, 2426. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.; Jeon, B.B.; Song, E.; Lee, H. Terpene Compound Composition and Antioxidant Activity of Essential Oils from Needles of Pinus densiflora, Pinus koraiensis, Abies holophylla, and Juniperus chinensis by Harvest Period. Forests 2024, 15, 566. [Google Scholar] [CrossRef]
- Hamid, S.; Shahdadi, F.; Sardoei, A.S.; Hatami, M.; Ghorbanpour, M. Investigation of physio-mechanical, antioxidant and antimicrobial properties of starch–zinc oxide nanoparticles active films reinforced with FerulagummosaBoiss essential oil. Sci. Rep. 2024, 14, 5789. [Google Scholar]
- Mondal, K.; Bhattacharjee, S.K.; Mudenur, C.; Ghosh, T.; Goud, V.V.; Katiyar, V. Development of antioxidant-rich edible active films and coatings incorporated with de-oiled ethanolic green algae extract: A candidate for prolonging the shelf life of fresh produce. RSC Adv. 2022, 12, 13295–13313. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, Z.; Liu, R.; Jiang, Y. Effect of powdery mildew on interleaf microbial communities and leaf antioxidant enzyme systems. J. For. Res. 2023, 34, 1535–1547. [Google Scholar] [CrossRef]
- Omnia, T.N.; Abdel-wahab, M.S.; Hamza, Z.S.; Ahmed, S.A.; El-Bassuony, A.A.; Abdel-Gawad, O.F.; Mohamed, H.S. Investigating the anticancer and antioxidant potentials of a polymer-grafted sodium alginate composite embedded with CuO and TiO2 nanoparticles. J. Polym. Environ. 2024, 32, 2713–2728. [Google Scholar]




| Samples | α-co-β-P | Nano-Clay | Time | Frequency | Yield |
|---|---|---|---|---|---|
| α-co-β-P/clay 2% | 0.5 g | 2% (wt) | 3 h | 20 KHz | 100% |
| α-co-β-P/clay 5% | 0.5 g | 5% (wt) | 3 h | 20 KHz | 100% |
| α-co-β-P/clay 8% | 0.5 g | 8% (wt) | 3 h | 20 KHz | 100% |
| α-co-β-P/clay 8% | 0.5 g | 10% (wt) | 3 h | 20 KHz | 100% |
| Samples | Onset Degradation Tem °C | Max Degradation Tem °C | Residue at 700 °C (g) |
|---|---|---|---|
| Copolymer | 220 | 380 | 0 |
| Nanocomposites 2% | 300 | 430 | 0.2 |
| Nanocomposites 5% | 310 | 420 | 0.5 |
| Nanocomposites 8% | 350 | 450 | 0.6 |
| Nanocomposites 10% | 330 | 470 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derdar, H.; Cherifi, Z.; Mitchell, G.R.; Mateus, A.; Zerrouki, M.; Hammoudi, N.; Bachari, K.; Chebout, R.; Touahra, F.; Bouchama, A.; et al. Nanocomposites from β-Pinene and α-Pinene Copolymer: Synthesis, Characterization, and Antioxidant Evaluation. Polymers 2025, 17, 2378. https://doi.org/10.3390/polym17172378
Derdar H, Cherifi Z, Mitchell GR, Mateus A, Zerrouki M, Hammoudi N, Bachari K, Chebout R, Touahra F, Bouchama A, et al. Nanocomposites from β-Pinene and α-Pinene Copolymer: Synthesis, Characterization, and Antioxidant Evaluation. Polymers. 2025; 17(17):2378. https://doi.org/10.3390/polym17172378
Chicago/Turabian StyleDerdar, Hodhaifa, Zakaria Cherifi, Geoffrey Robert Mitchell, Artur Mateus, Meziane Zerrouki, Naima Hammoudi, Khaldoun Bachari, Redouane Chebout, Fouzia Touahra, Abdelghani Bouchama, and et al. 2025. "Nanocomposites from β-Pinene and α-Pinene Copolymer: Synthesis, Characterization, and Antioxidant Evaluation" Polymers 17, no. 17: 2378. https://doi.org/10.3390/polym17172378
APA StyleDerdar, H., Cherifi, Z., Mitchell, G. R., Mateus, A., Zerrouki, M., Hammoudi, N., Bachari, K., Chebout, R., Touahra, F., Bouchama, A., Harrane, A., & Meghabar, R. (2025). Nanocomposites from β-Pinene and α-Pinene Copolymer: Synthesis, Characterization, and Antioxidant Evaluation. Polymers, 17(17), 2378. https://doi.org/10.3390/polym17172378








