Effect of Temperature and Relative Humidity on CO2 Adsorption Performance of Biomass-Derived Aerogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Aerogel Preparation
2.3. Physicochemical Characterization of Biomass Aerogels
- (1)
- Fourier transform infrared spectroscopy (FTIR)
- (2)
- Microstructural morphology testing
- (3)
- Specific surface area measurement (BET)
- (4)
- Thermal stability testing (TG-DSC)
- (5)
- Porosity (ε) cellulose and chitosan of biomass aerogels
2.4. CO2 Adsorption of CNF-C and CS Biomass Aerogels Testing
2.4.1. Experimental Setup
2.4.2. Experimental Procedure
3. Results and Discussion
3.1. Structural Characterization and Performance of Biomass Aerogels
3.1.1. FTIR Analysis
3.1.2. Microstructure and Morphology Analysis
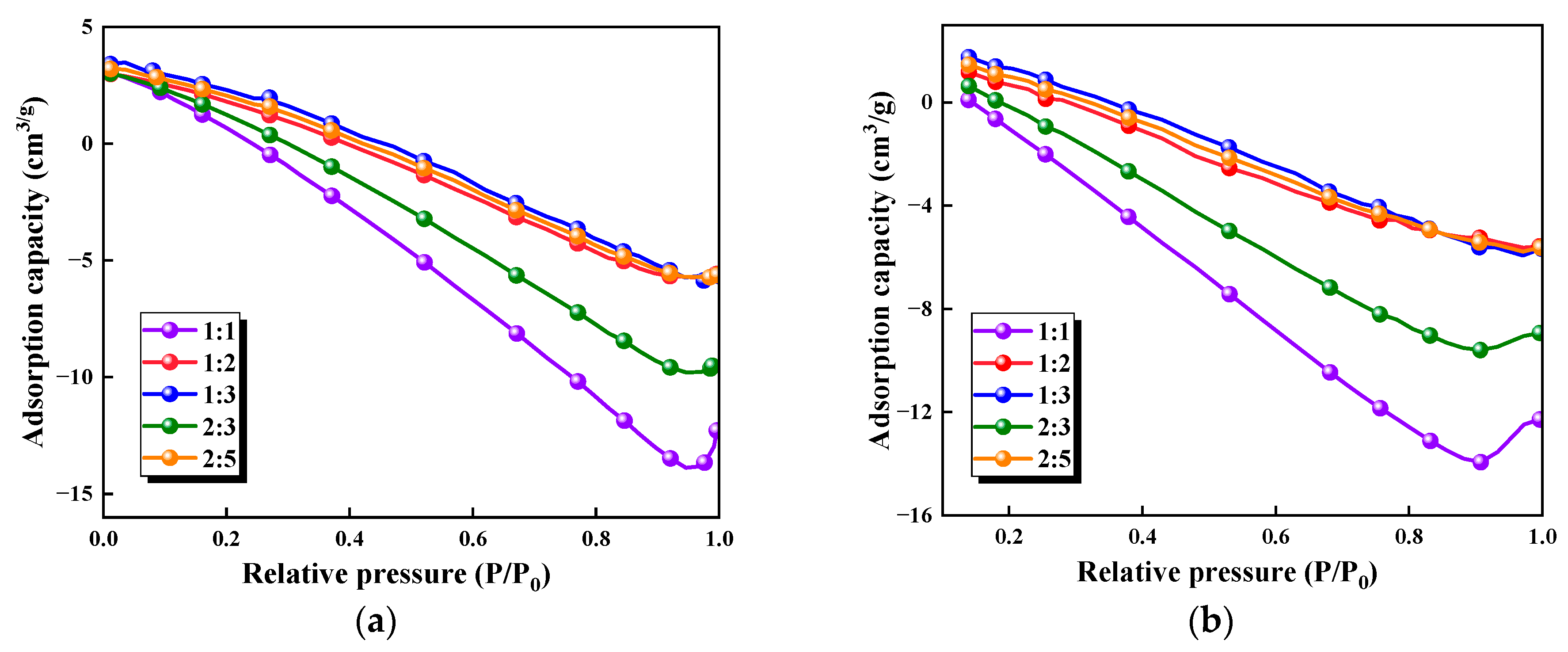
3.1.3. BET Analysis of Biomass Aerogels
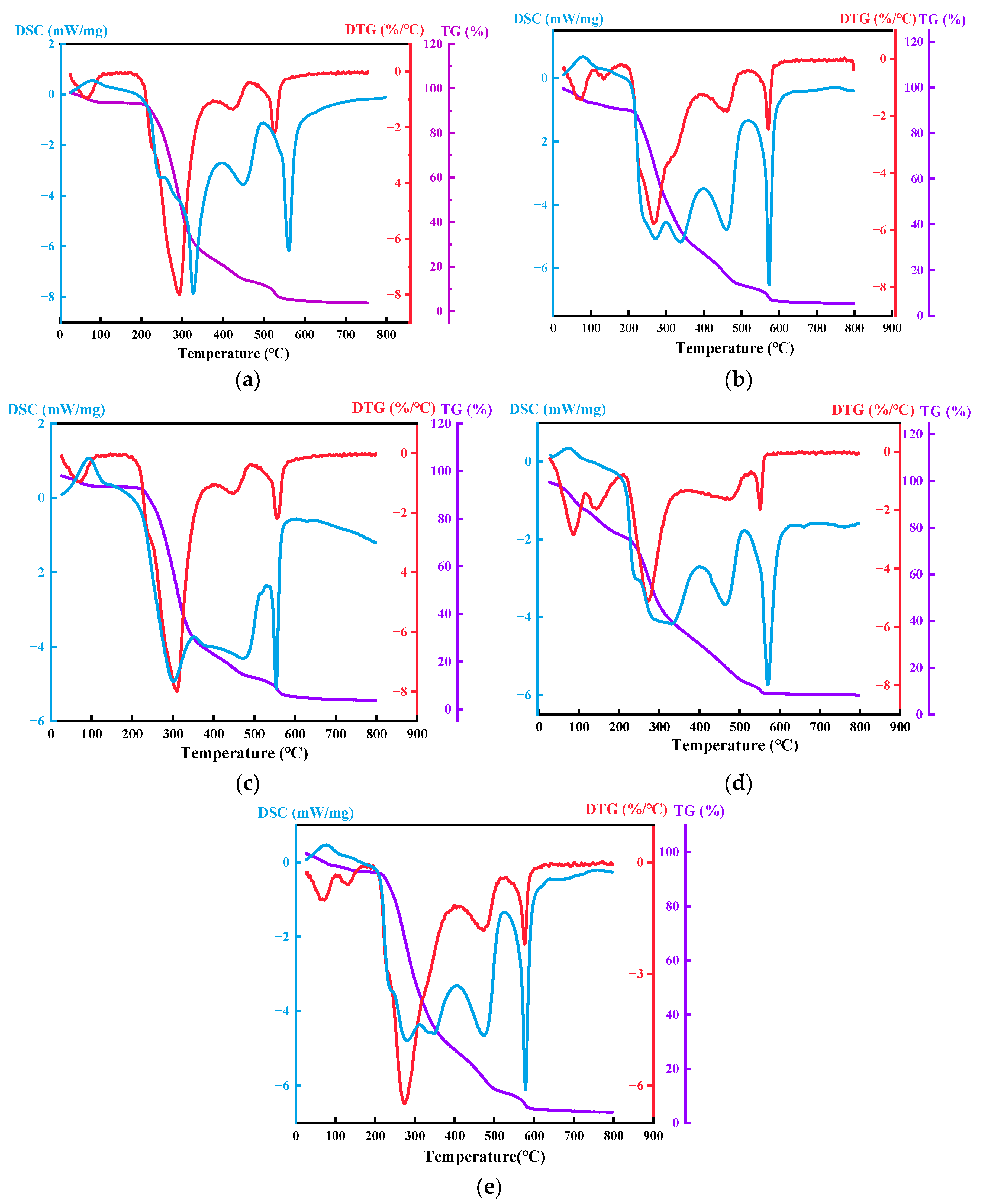
3.1.4. Thermal Stability Analysis
3.1.5. Porosity Characteristic Analysis
3.2. CO2 Adsorption Performance of CNF-C/CS Biomass Aerogels
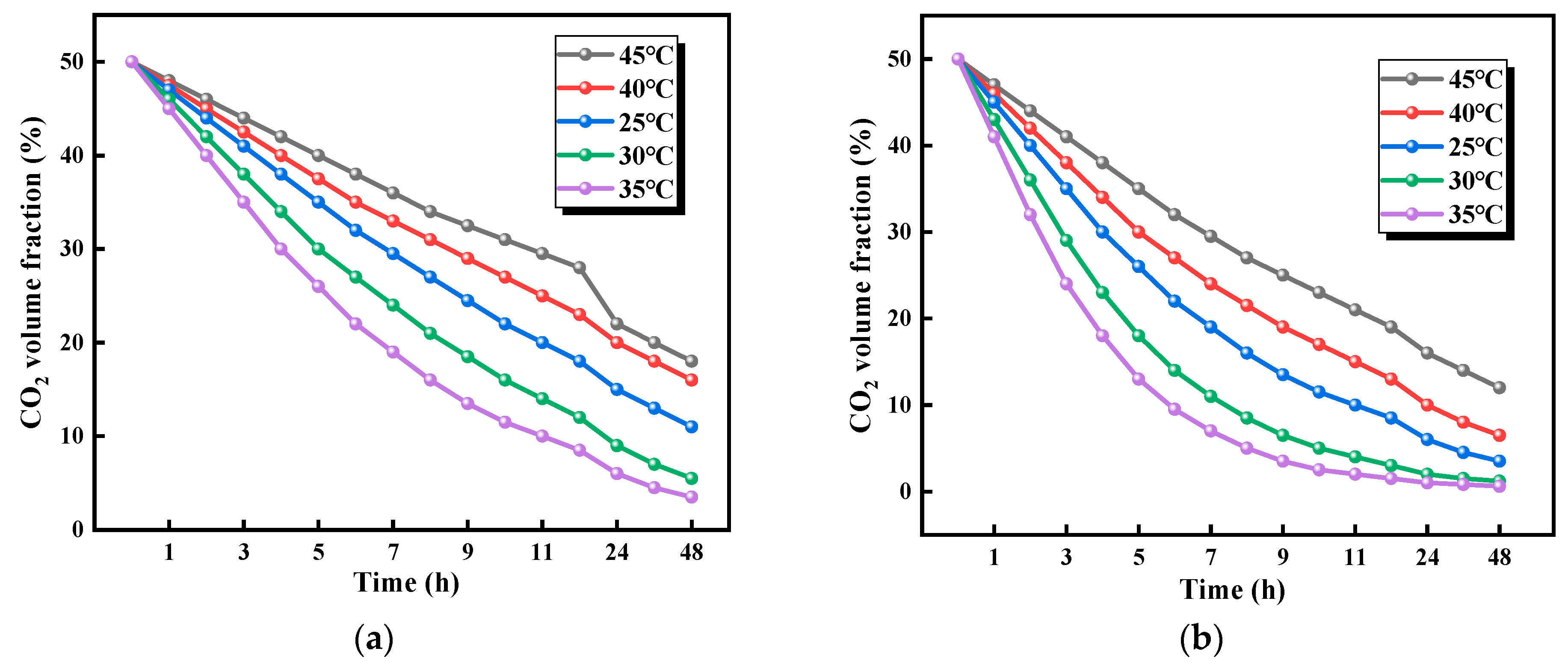
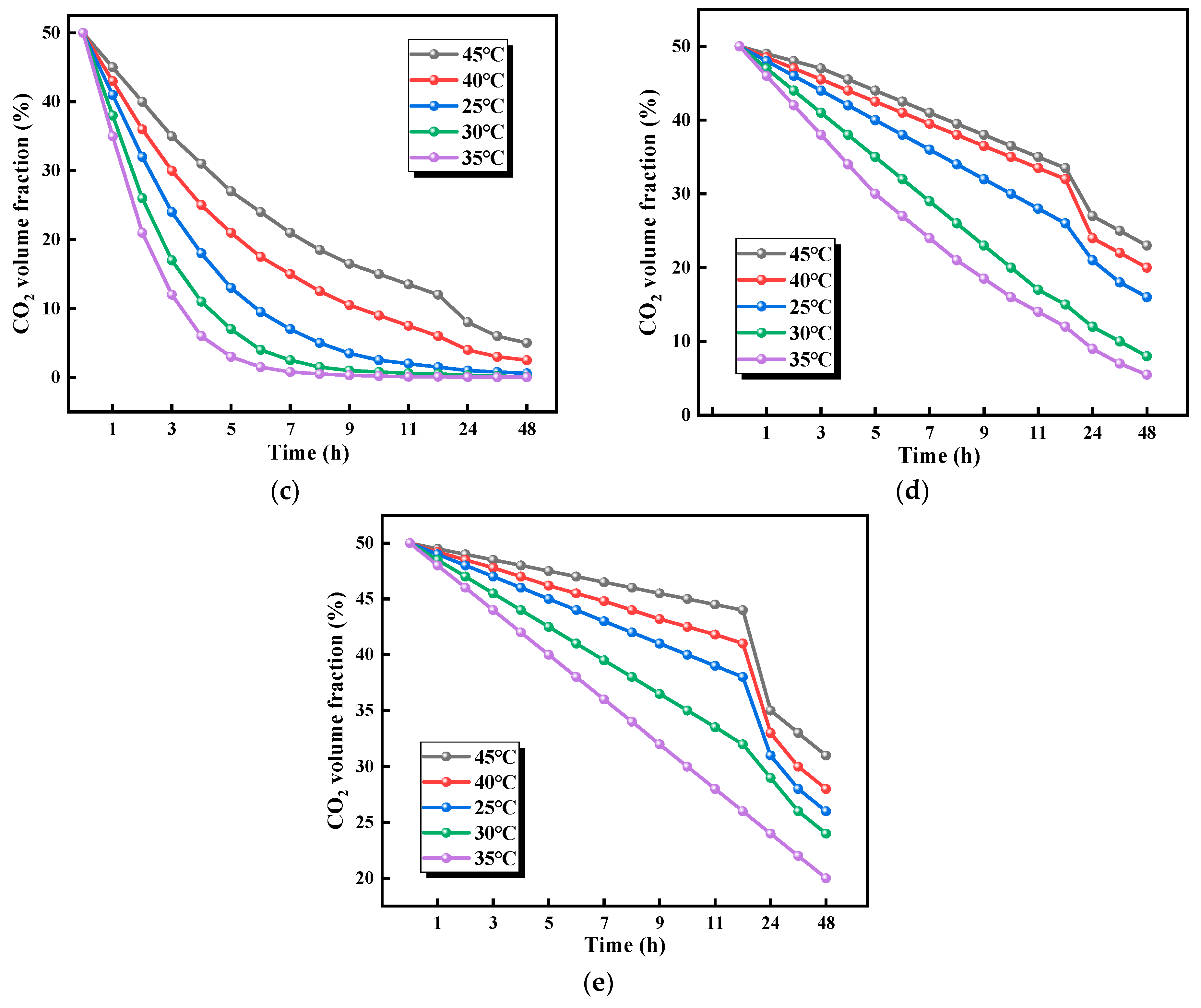
3.2.1. Effects of Temperature on CO2 Adsorption Performance
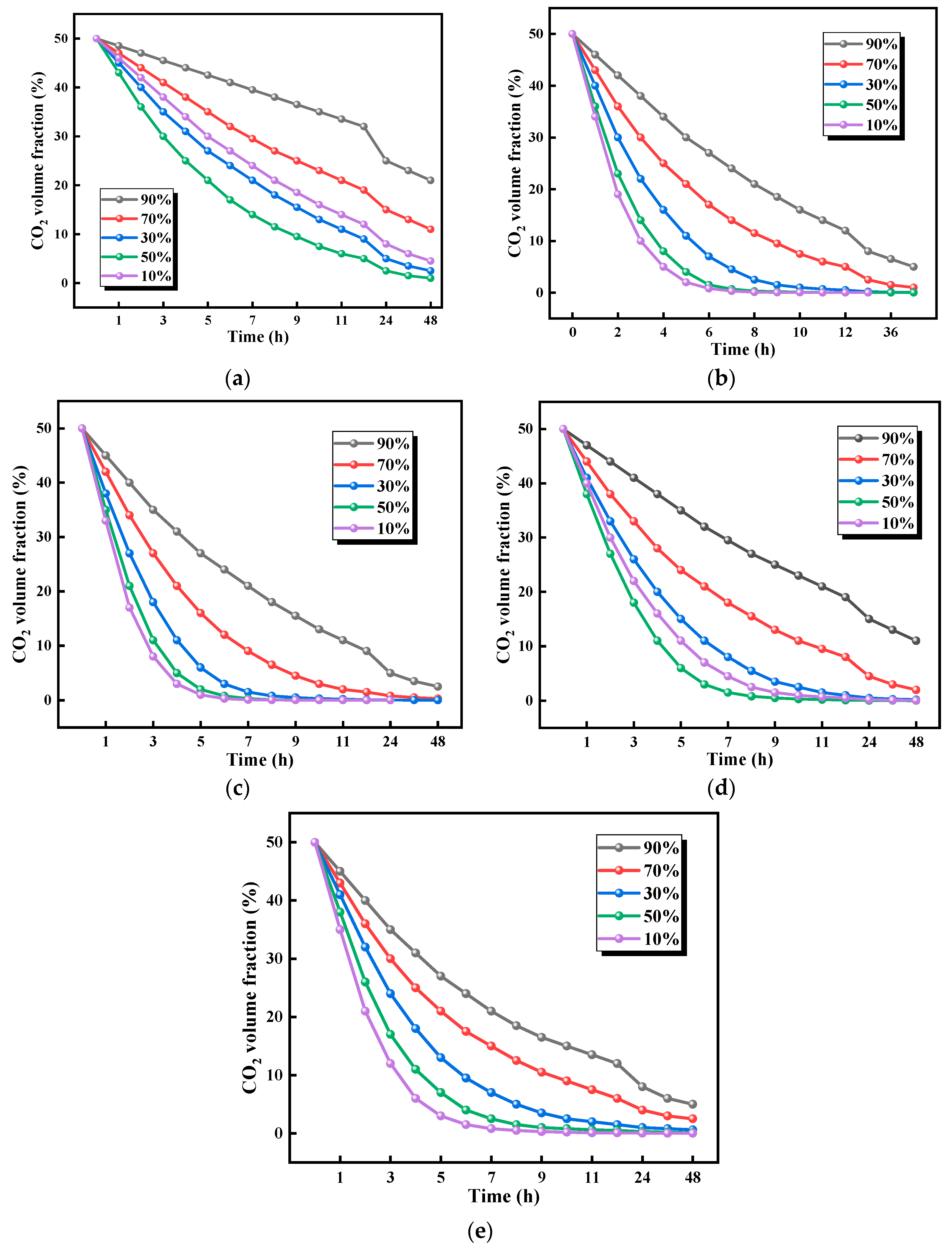
3.2.2. Effects of Humidity on CO2 Adsorption Performance
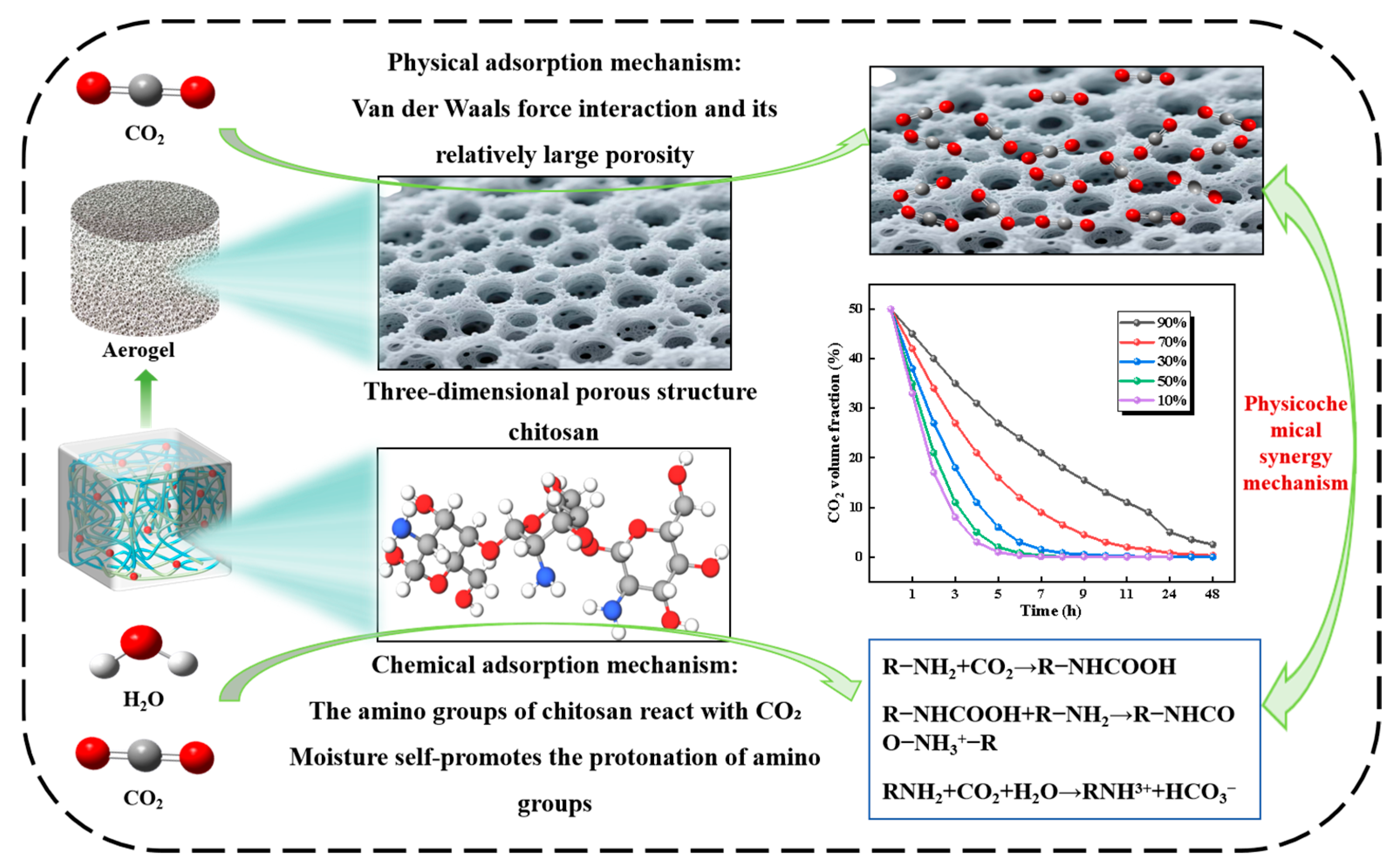
3.3. Adsorption Mechanism Analysis
4. Conclusions
- (1)
- FTIR analysis confirmed that -NH2 functional groups on the biomass aerogel surface served as primary active sites for CO2 adsorption, with concentration showing positive correlation with chitosan content. BET and SEM characterization revealed increased specific surface area with higher chitosan ratios. Though accompanied by progressive pore densification, excessive chitosan caused structural collapse. The sample with a ratio of 1:3 has the superior thermal stability before 350.0 °C, while the 1:1 ratio exhibited the poorest. The maximum void fraction is 80.74% in the ratio of 1:1, and sample and pore architecture varied substantially across different compositions. These structural characteristics directly governed the capture performance of CO2 of biomass aerogel. Current SEM analysis is qualitative; future work should quantitatively correlate image-based morphological features with BET/BJH pore structure parameters.
- (2)
- Within the 25.0–35.0 °C range, CO2 adsorption capacity increased with temperature, reaching optimal performance at 35.0 °C. Beyond 35.0 °C, adsorption capacity declined due to thermally induced structural degradation of the material. The optimal adsorption efficiency was achieved at 50% relative humidity, and maximum adsorption efficiency at 50% relative humidity. CO2 uptake showed a positive correlation with humidity in the 10–50% relative humidity range but decreased above 50% relative humidity due to competitive occupation of active sites by water molecules. Notably, distinct composition-dependent behaviors were observed. The cellulose-rich 1:1 ratio exhibited predominantly physical adsorption, demonstrating greater sensitivity to temperature and humidity variations. In contrast, the chitosan-rich 1:3 ratio displayed chemical adsorption-dominated behavior with superior environmental stability.
- (3)
- The key finding reveals that the biomass aerogel achieves efficient CO2 capture through a synergistic physicochemical mechanism. Physical adsorption relies on the three-dimensional porous structure, where the 80.74% porosity of the 1:1 ratio provides abundant van der Waals adsorption sites. Increased chitosan content constructs a crosslinked network, reducing pore wall thickness to 20–50 μm while significantly enhancing structural rigidity. Chemical adsorption occurs through carbamate formation between the -NH2 groups of chitosan and CO2 at 50% humidity, with the 1:3 ratio exhibiting 28% higher amino group density. Water molecules demonstrate dual functionality. Moderate humidity promotes amino protonation, while excessive humidity induces competitive hydrogen bonding that inhibits adsorption. This synergistic mechanism optimizes the balance between pore architecture and amino group density, ultimately enhancing CO2 adsorption efficiency.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | Analytical Reagent |
| GR | Guaranteed Reagent |
| BET | Specific surface area measurement |
| HAc | acetic acid |
| PAMAM | Polyamidoamine |
| CNF-C | Carboxylated Cellulose Nanofibers |
| PEGDGE | Polyethylene glycol diglycidyl ether |
| SEM | Scanning electron microscopy |
| TG | thermogravimetry |
| CNT | Carbon Nanotubes |
| TG-DSC | Thermal stability testing |
| CS | Chitosan |
| DTG | Derivative thermogravimetry |
| DSC | Differential scanning calorimetry |
| FTIR | Fourier transform infrared spectroscopy |
| ε | Porosity |
References
- Deng, J.; Li, X.; Wang, K.; Wang, C.; Ren, L.; Qu, G.; Li, Q. Advances and Prospects in the Risk Prediction and Early Warning Technology for the compound disaster of coal spontaneous combustion and gas explosion. Process Saf. Environ. Prot. 2025, 201 Pt A, 107489. [Google Scholar] [CrossRef]
- Kang, F.; Song, J.; Zhang, J.; Pan, C.; Wang, D.; Bai, Z.; Fan, S.; Deng, J. Research on the variation of CO concentration and temperature in the directional drilling process. J. Loss Prev. Process Ind. 2025, in press. 2025. [Google Scholar] [CrossRef]
- Zou, C.; Yang, Z.; Huang, S.; Ma, F.; Sun, Q.; Pan, S.; Tian, W. Resource types, formation, distribution and prospects of coal–measure gas. Petrol. Explor. Dev. 2019, 46, 451–462. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Shao, Z. A statistical analysis of coalmine fires and explosions in China. Process Saf. Environ. Prot. Eng. 2019, 121, 357–366. [Google Scholar] [CrossRef]
- Li, Y.; Pan, S.; Ning, S.; Shao, L.; Jing, Z.; Wang, Z. Coal measure metallogeny: Metallogenic system and implication for resource and environment. Sci. China Earth Sci. 2022, 65, 1211–1228. Available online: https://api.semanticscholar.org/CorpusID:249285085 (accessed on 3 August 2025). [CrossRef]
- Tcvetkov, P.; Cherepovitsyn, A.; Fedoseev, S. The changing role of CO2 in the transition to a circular economy: Review of carbon sequestration projects. Sustainability 2019, 11, 5834. [Google Scholar] [CrossRef]
- Wang, C.P.; Duan, X.D.; Deng, J.; Bai, Z.J.; Chen, W.L.; Deng, Y.; Qu, G.Y. Characteristics of antioxidant temperature-sensitive hydrogel inhibiting coal spontaneous combustion. Fuel 2025, 394, 135089. [Google Scholar] [CrossRef]
- Wang, J.R.; Deng, J.; Ren, S.J.; Qu, G.Y.; Wang, C.P.; Guo, R.Q.; Zhao, X.Q. Acoustic wave propagation characteristics and spontaneous combustion warning of coal during oxidative warming of loose coal. Fuel 2025, 398, 135528. [Google Scholar] [CrossRef]
- Si, J.; Li, L.; Cheng, G.; Shao, H.; Wang, Y.; Li, Z. Characteristics and safety of CO2 for the fire prevention technology with gob-side entry retaining in goaf. ACS Omega 2021, 6, 185118526. [Google Scholar] [CrossRef]
- Qi, X.; Chen, L.; Zhang, L. In situ FTIR study on real-time changes of active groups during lignite reaction under low oxygen concentration conditions. J. Energy Inst. 2019, 92, 1557–1566. [Google Scholar] [CrossRef]
- Ren, L.F.; Yu, X.; Li, J.B.; Li, Q.W.; Kong, X.Z.; Zhai, X.W.; Ma, T.; Tang, Z.Q. Microscopic characteristics and gas generation patterns of spontaneous combustion oxidation in deep high-temperature coal seams of the Juye mining area. Fuel 2026, 403, 136124. [Google Scholar] [CrossRef]
- Xiang, Z.; Si, G.; Wang, Y.; Belle, B.; Webb, D. Goaf gas drainage and its impact on coal oxidation behaviour: A conceptual model. Int. J. Coal Geol. 2024, 248, 103878. [Google Scholar] [CrossRef]
- Shuangming, W.; Lang, L.; Yujiao, Z.; Bo, Z.; Jingyu, W.; Mengbo, Z. New energy exploitation in coal-endowed areas under the target of “double carbon”: A new path for transformation and upgrading of coal mines in the future. Int. J. Coal Sci. Technol. 2023, 51, 59–79. [Google Scholar] [CrossRef]
- Blanchard, L.; Hancu, D.; Beckman, E. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Niu, H.; Sun, Q.; Bu, Y. Review and prospects of research on materials to prevent and extinguish mine fires. Fire Mater. 2023, 47, 739–757. [Google Scholar] [CrossRef]
- Doyo, A.N.; Kumar, R.; Barakat, M.A. Recent advances in cellulose, chitosan, and alginate based biopolymeric composites for adsorption of heavy metals from wastewater. J. Taiwan Inst. Chem. Eng. 2023, 151, 105095. [Google Scholar] [CrossRef]
- Sabarish, R.; Jasila, K.; Aswathy, J. Recent advances in cellulose- and alginate-based hydrogels for water and wastewater treatment: A review. Carbohydr. Polym. 2024, 323, 121339. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Strnad, S.; Zemljič, L.F. Cellulose–chitosan functional biocomposites. Polymers 2023, 15, 425. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Adnan, A.S.; Nurul Fazita, M.R.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar] [CrossRef]
- Shih, C.M.; Shieh, Y.T.; Twu, Y.K. Preparation and characterization of cellulose/chitosan blend films. Carbohydr. Polym. 2009, 78, 169–174. [Google Scholar] [CrossRef]
- Wörmeyer, K.; Smirnova, I. Adsorption of CO2, moisture and ethanol at low partial pressure using aminofunctionalised silica aerogels. Chem. Eng. J. 2013, 225, 350–357. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.; Fan, M. Dynamic capture of low-concentration CO2 on amine hybrid silsesquioxane aerogel. Chem. Eng. J. 2016, 283, 1059–1068. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, C.; Sun, J. Facile synthesis of silica aerogel supported K2CO3 sorbents with enhanced CO2 capture capacity for ultra-dilute flue gas treatment. Fuel 2018, 215, 735–743. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, Y.; Li, C. Removal of low concentration CO2 at ambient temperature using several potassium-based sorbents. Appl. Energy 2014, 124, 241–247. [Google Scholar] [CrossRef]
- Rodríguez–Mata, V.; Hernández–Ferrer, J.; Carrera, C.; Benito, A.M.; Maser, W.K.l.; García-Bordejé, E. Towards high-efficient microsupercapacitors based on reduced graphene oxide with optimized reduction degree. Energy Storage Mater. 2025, 25, 740–749. [Google Scholar] [CrossRef]
- Barwich, S.; de Araujo, J.M.; Rafferty, A.; da Rocha, C.G.; Ferreira, M.S.; Coleman, J.N. On the relationship between morphology and conductivity in nanosheet networks. Carbon 2021, 171, 306–319. [Google Scholar] [CrossRef]
- Yu, D.X.; Wang, A.J.; He, L.L.; Yuan, J.; Wu, L.; Chen, J.R.; Feng, J.J. Facile synthesis of uniform AuPd@ Pd nanocrystals supported on three-dimensional porous N-doped reduced graphene oxide hydrogels as highly active catalyst for methanol oxidation reaction. Electrochim. Acta 2016, 213, 565–573. [Google Scholar] [CrossRef]
- Panahi, S.; Ghaemi, A.; Rahimpour, F. A Sustainable Aminated Cellulose-Based Adsorbent for CO2 Capture: Synthesis, Characterization, and Adsorption Performance. J. Hazard. Mater. Adv. 2025, 19, 100812. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, D.; Feng, Y.; Liu, H.; Li, S.; Qiu, Y.; Ren, J. The preparation and characterization of gel-mixed matrix membranes (g-MMMs) with high CO2 permeability and stability performance. J. Membr. Sci. 2022, 652, 120471. [Google Scholar] [CrossRef]
- Sam, D.K.; Sam, E.K.; Durairaj, A.; Lv, X.; Zhou, Z.; Liu, J. Synthesis of biomass–based carbon aerogels in energy and sustainability. Carbohydr. Res. 2020, 491, 107986. [Google Scholar] [CrossRef] [PubMed]
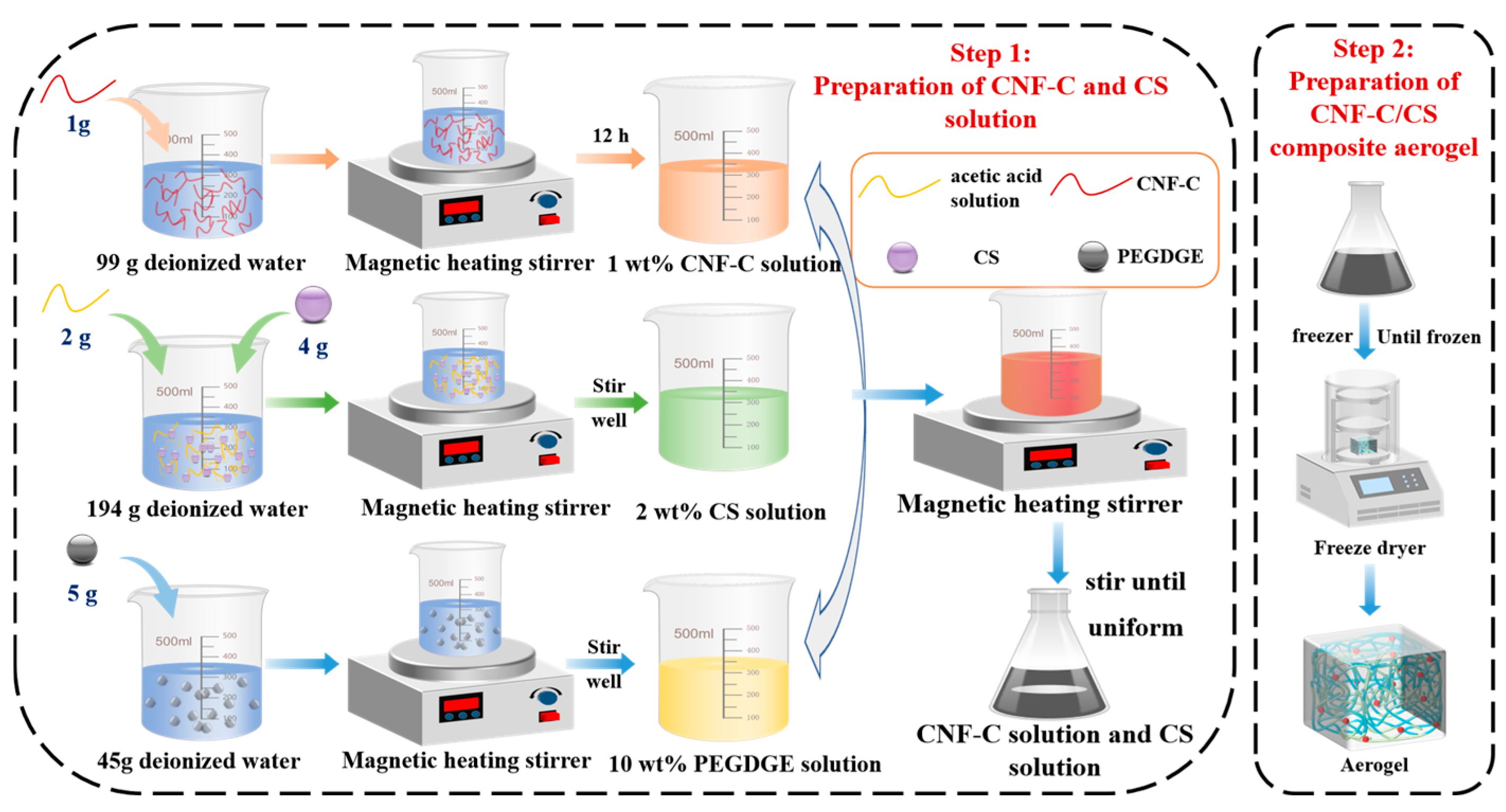
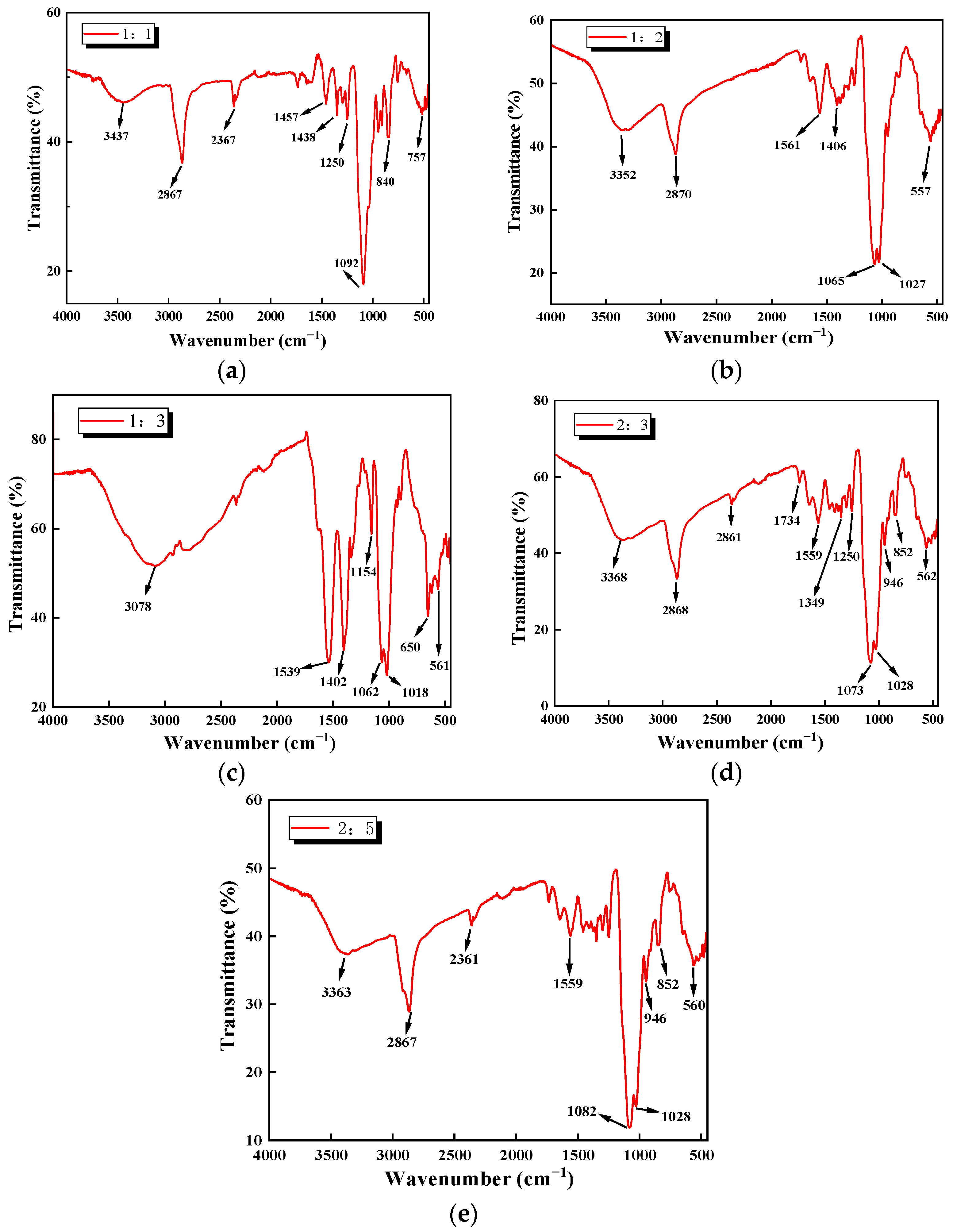

| Cellulose/Chiosan Ratios | Functional Groups |
|---|---|
| 1:1 | -NH2, C-H, -C-C-, C-X, -OH |
| 1:2 | -NH2, C-H, -C-C-, C-X |
| 1:3 | -NH2, C-H, -C-C-, C-X, =C-H |
| 2:3 | -NH2, C-H, -C-C-, C-X, C=O |
| 2:5 | -NH2, C-H, -C-C-, C-X |
| Cellulose/Chitosan Ratios | BET Specific Surface Area (m2/g) | t-Plot Hole Capacity (cm3/g) |
|---|---|---|
| 1:1 | 2.2568 | 0.015006 |
| 2:3 | 3.3319 | 0.012112 |
| 1:2 | 5.3976 | 0.010670 |
| 2:5 | 6.1235 | 0.010603 |
| 1:3 | 7.0515 | 0.010067 |
| Ratios | 1:2 | 1:3 | 2:5 | 2:3 | 1:1 |
|---|---|---|---|---|---|
| Temperature | 266.1 °C | 272.5 °C | 273.5 °C | 281.7 °C | 310.1 °C |
| Cellulose/Chitosan Ratios | ρa (g/cm3) | ρt (g/cm3) | (%) |
|---|---|---|---|
| 1:1 | 0.26 | 1.35 | 80.74 |
| 1:2 | 0.33 | 1.34 | 75.37 |
| 1:3 | 0.29 | 1.32 | 78.03 |
| 2:3 | 0.37 | 1.35 | 72.59 |
| 2:5 | 0.34 | 1.31 | 74.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Z.; Ren, S.; Deng, J.; Su, C.; Kang, F.; Zhang, Y. Effect of Temperature and Relative Humidity on CO2 Adsorption Performance of Biomass-Derived Aerogels. Polymers 2025, 17, 2375. https://doi.org/10.3390/polym17172375
Bai Z, Ren S, Deng J, Su C, Kang F, Zhang Y. Effect of Temperature and Relative Humidity on CO2 Adsorption Performance of Biomass-Derived Aerogels. Polymers. 2025; 17(17):2375. https://doi.org/10.3390/polym17172375
Chicago/Turabian StyleBai, Zujin, Shuyao Ren, Jun Deng, Chang Su, Furu Kang, and Yifan Zhang. 2025. "Effect of Temperature and Relative Humidity on CO2 Adsorption Performance of Biomass-Derived Aerogels" Polymers 17, no. 17: 2375. https://doi.org/10.3390/polym17172375
APA StyleBai, Z., Ren, S., Deng, J., Su, C., Kang, F., & Zhang, Y. (2025). Effect of Temperature and Relative Humidity on CO2 Adsorption Performance of Biomass-Derived Aerogels. Polymers, 17(17), 2375. https://doi.org/10.3390/polym17172375





