One-Pot Synthesis of Silicone–Urethane Hybrid Foam and Comparison of Flame Retardant, Rheological, and Mechanical Properties with Polyurethane Foam
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of SUF
2.3. Characterization
2.4. Mechanical Properties of SUF
2.5. Thermal Properties of SUF
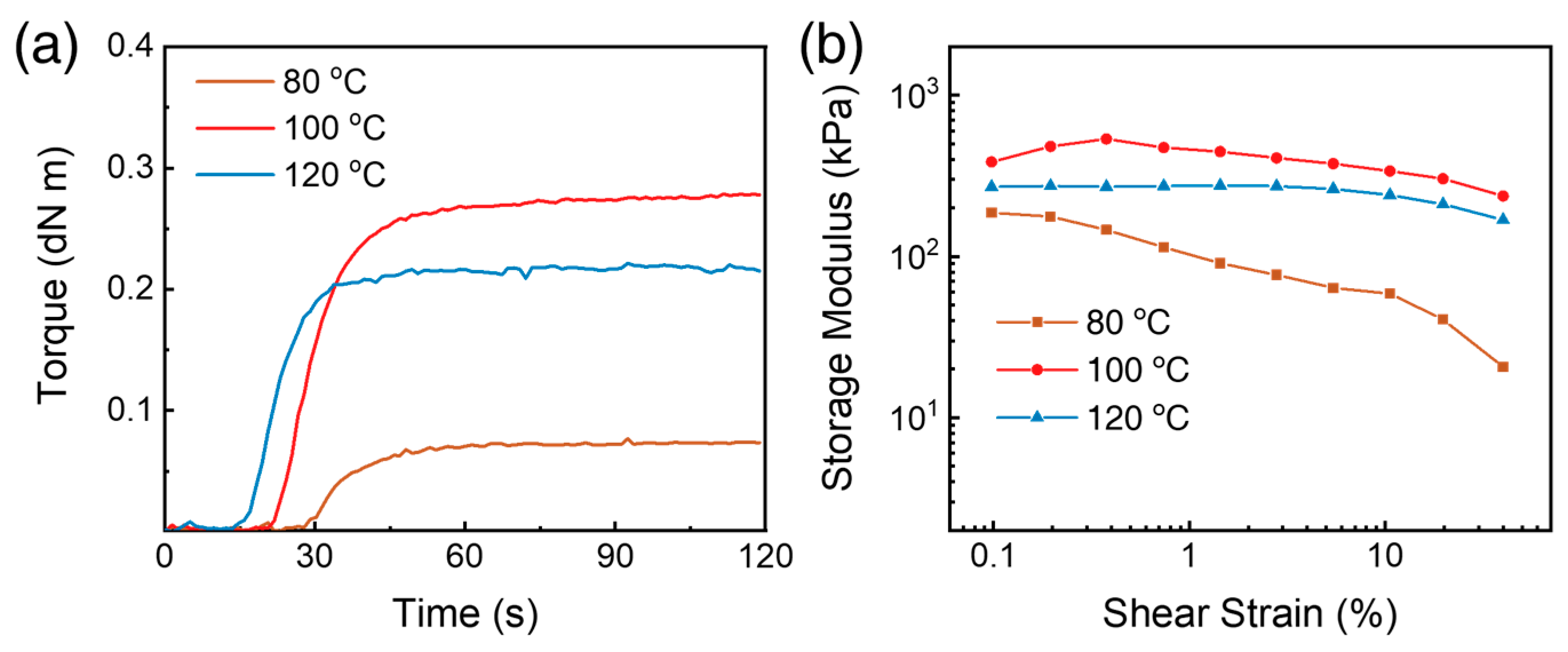
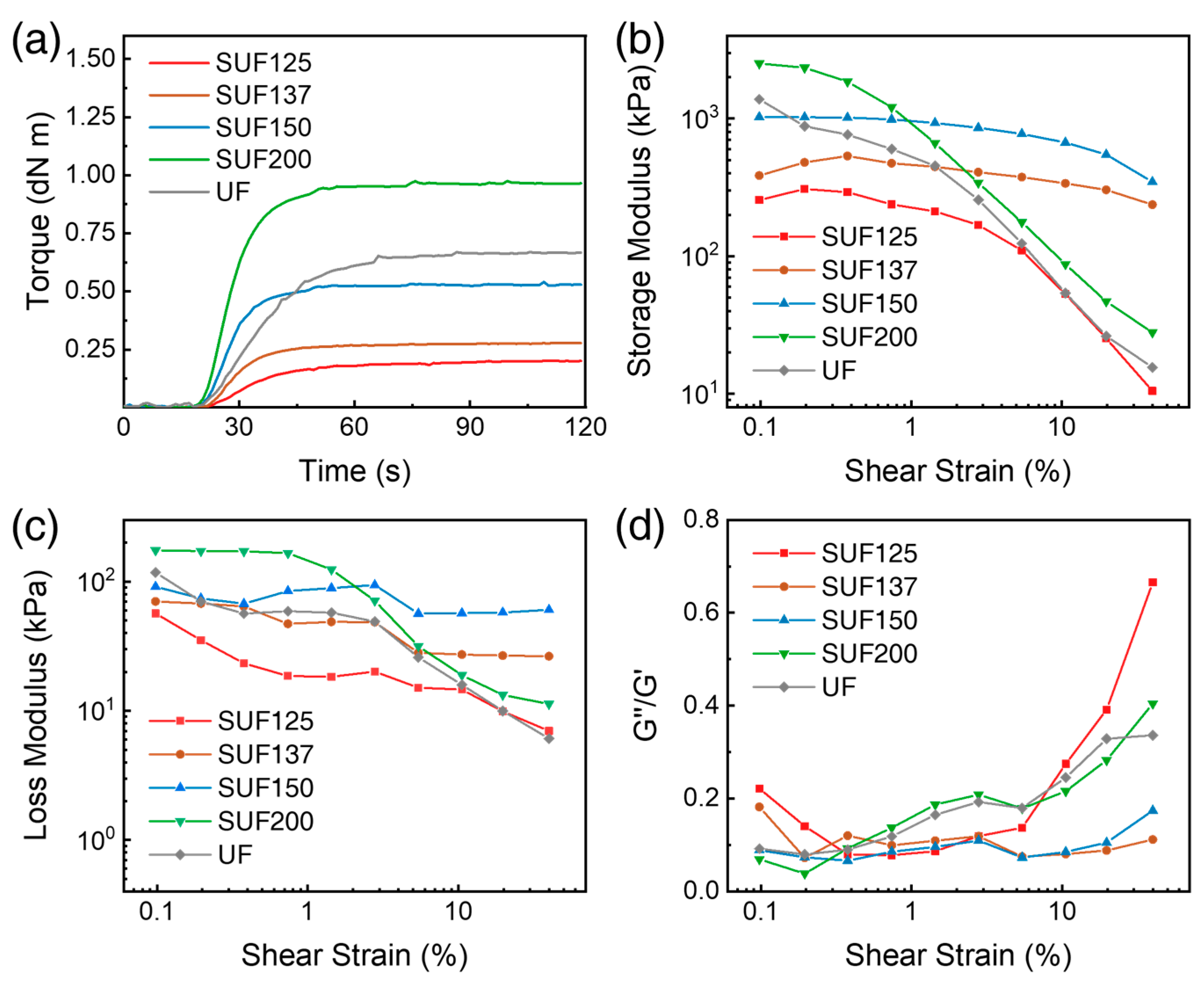
2.6. Viscoelastic Properties of SUF
3. Results and Discussion
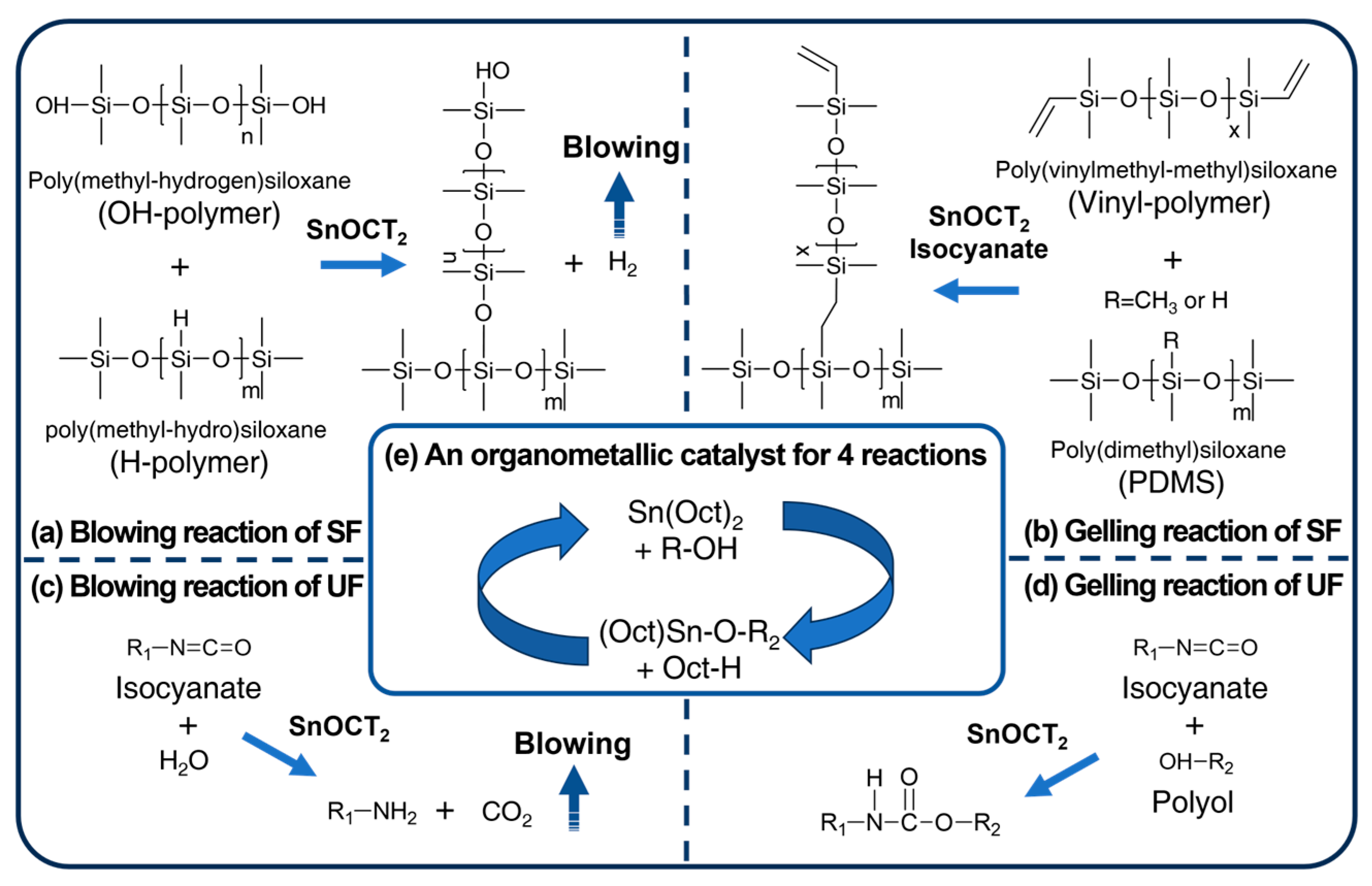
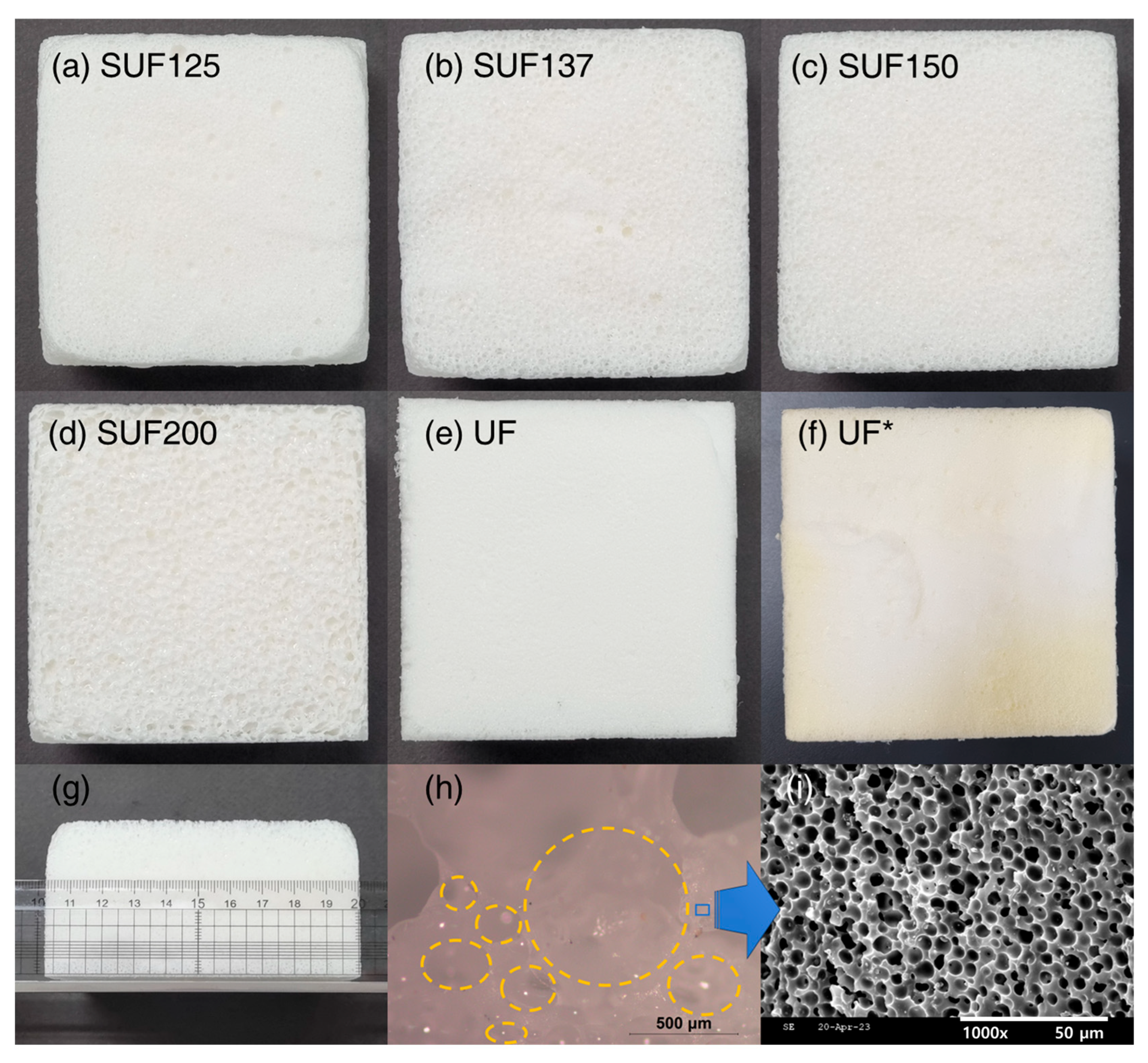
3.1. Preparation of SUFs
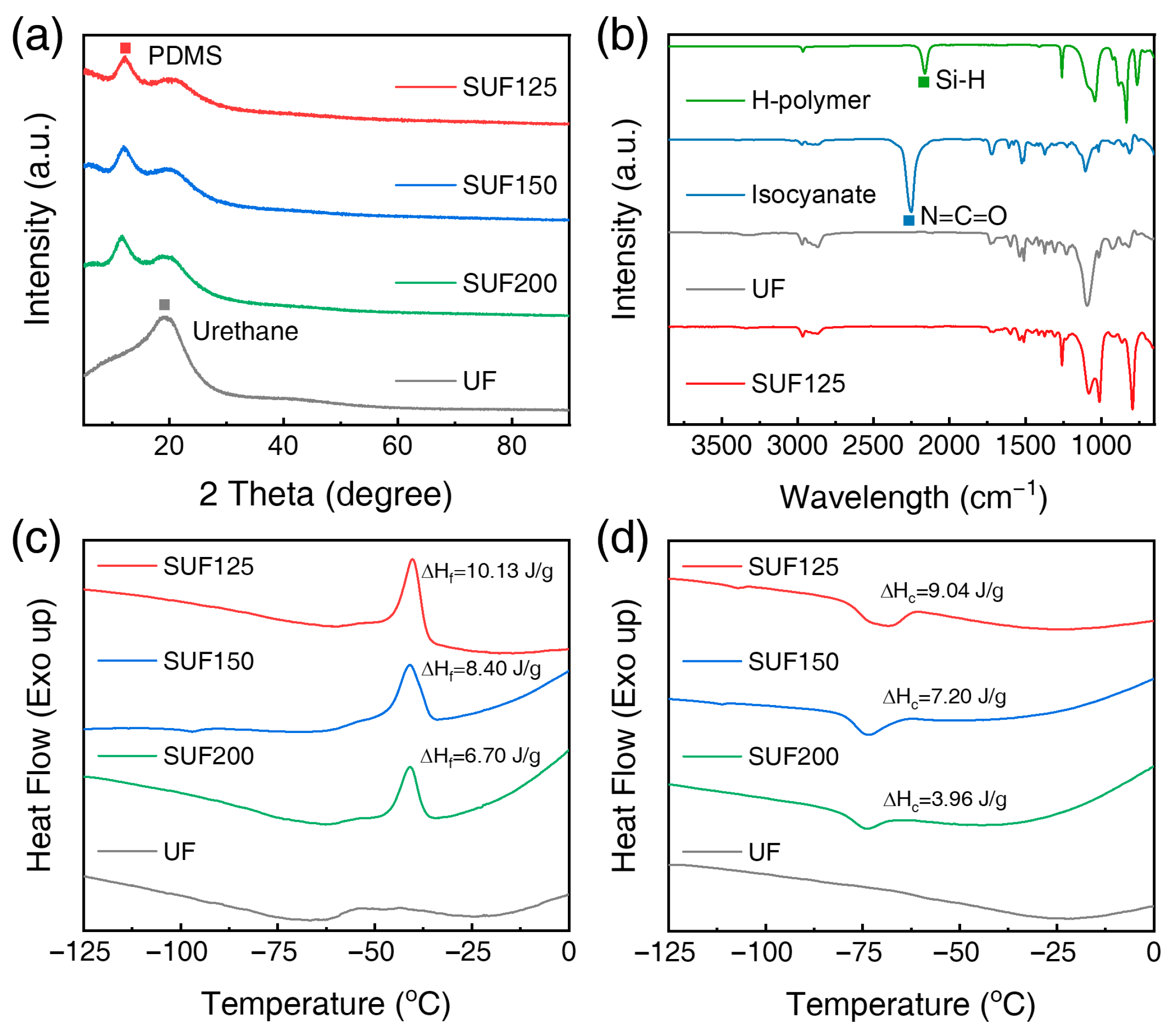
3.2. Characterization of SUFs
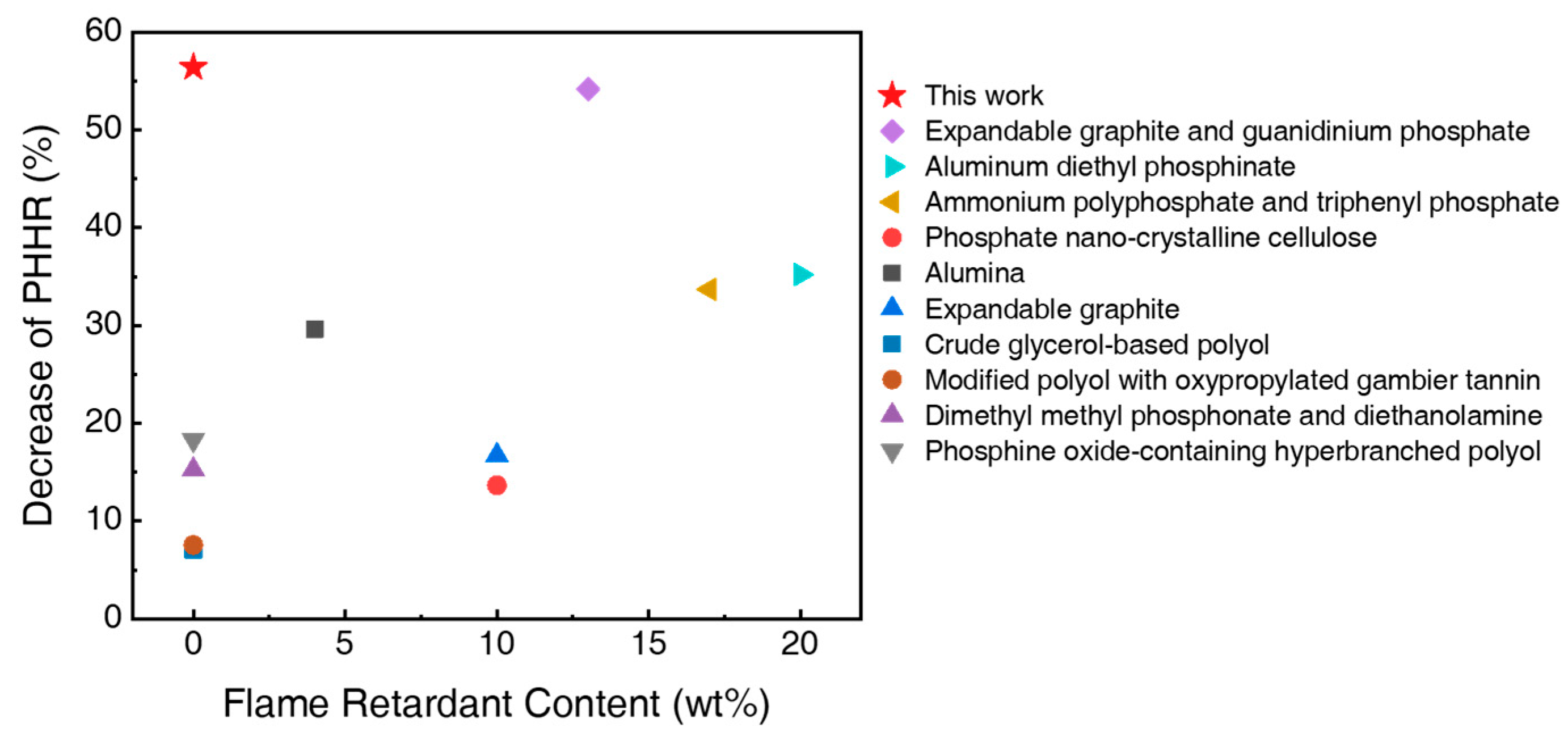
3.3. Flammability and Characteristics of the Char Residues
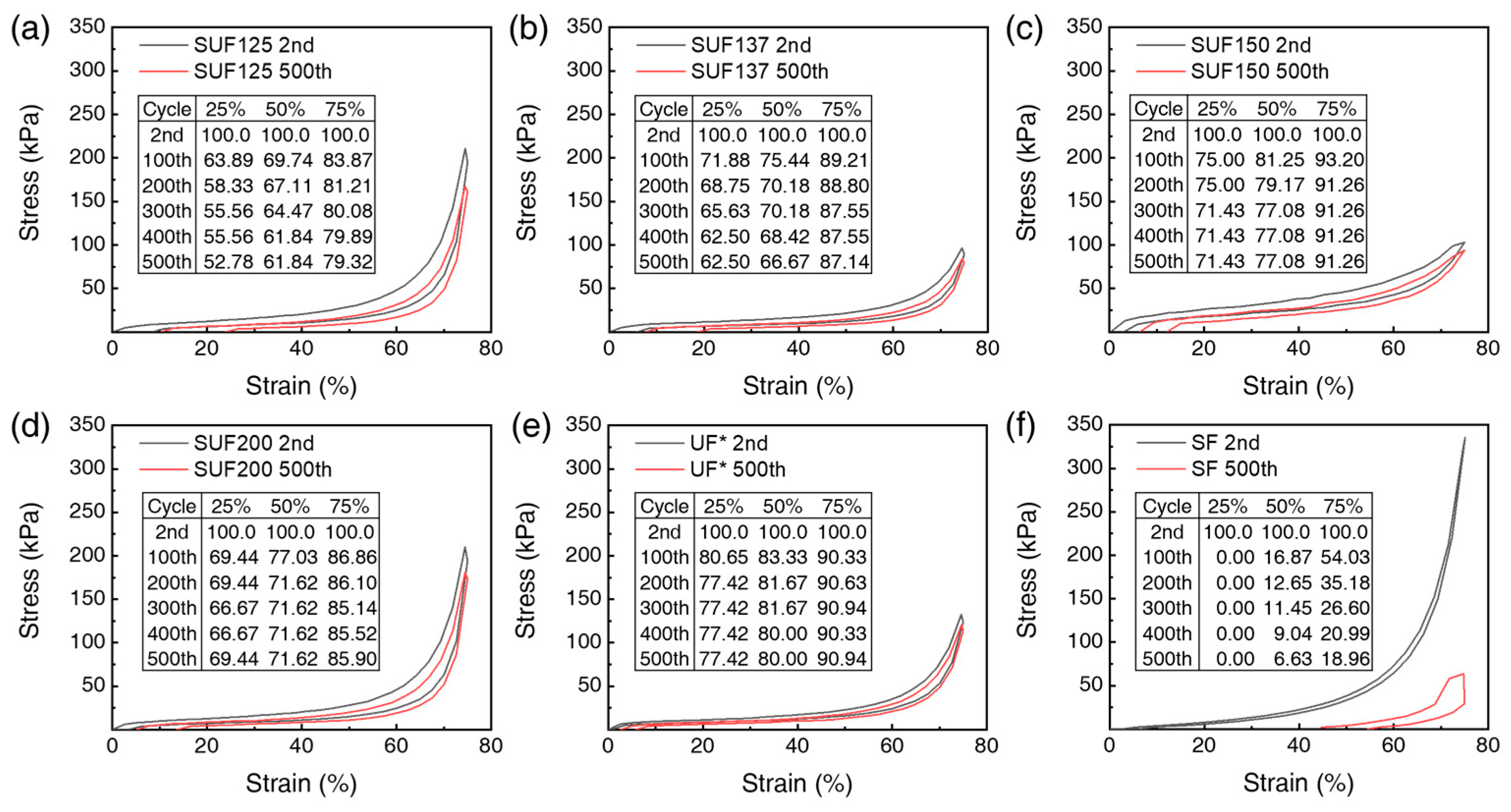
3.4. Activation Energy and Mechanical Properties of SUFs
3.5. Rheological Properties of SUF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John, J.; Bhattacharya, M.; Turner, R.B. Characterization of polyurethane foams from soybean oil. J. App. Polym. Sci. 2002, 86, 3097–3107. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Effect of foam density on the properties of water blown rigid polyurethane foam. J. App. Polym. Sci. 2008, 108, 1810–1817. [Google Scholar] [CrossRef]
- Hepburn, C. Polyurethane Elastomers; Springer: Berlin/Heidelberg, Germany, 1992; p. 444. [Google Scholar] [CrossRef]
- Braun, T.; Navratil, J.D.; Farag, A. Polyurethane Foam Sorbents in Separation Science; CRC Press: Boca Raton, FL, USA, 2008; p. 228. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, X.; Xu, X.; Liu, L.; Yu, B.; Maluk, C.; Huang, G.; Wang, H.; Song, P. Bioinspired, Highly Adhesive, Nanostructured Polymeric Coatings for Superhydrophobic Fire-Extinguishing Thermal Insulation Foam. ACS Nano 2021, 15, 11667–11680. [Google Scholar] [CrossRef]
- Wang, S.; Yang, X.; Li, Z.; Xu, X.; Liu, H.; Wang, D.; Min, H.; Shang, S. Novel eco-friendly maleopimaric acid based polysiloxane flame retardant and application in rigid polyurethane foam. Compos. Sci. Technol. 2020, 198, 108272. [Google Scholar] [CrossRef]
- Lu, S.; Feng, Y.; Zhang, P.; Hong, W.; Chen, Y.; Fan, H.; Yu, D.; Chen, X. Preparation of Flame-Retardant Polyurethane and Its Applications in the Leather Industry. Polymers 2021, 13, 1730. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Xi, X.; Li, J.; Fan, X.; Wang, Z. Synergistic effect of carbon and phosphorus flame retardants in rigid polyurethane foams. Fire Mater. 2018, 42, 447–453. [Google Scholar] [CrossRef]
- Duquesne, S.; Bras, M.L.; Bourbigot, S.; Delobel, R.; Vezin, H.; Camino, G.; Eling, B.; Lindsay, C.; Roels, T. Expandable graphite: A fire retardant additive for polyurethane coatings. Fire Mater. 2003, 27, 103–117. [Google Scholar] [CrossRef]
- Chao, C.Y.; Wang, J.H. Comparison of the thermal decomposition behavior of a non-fire retarded and a fire retarded flexible polyurethane foam with phosphorus and brominated additives. J. Fire Sci. 2001, 19, 137–156. [Google Scholar] [CrossRef]
- Ravey, M.; Pearce, E.M. Flexible polyurethane foam. III. Phosphoric acid as a flame retardant. J. App. Polym. Sci. 1999, 74, 1317–1319. [Google Scholar] [CrossRef]
- Ravey, M.; Keidar, I.; Weil, E.D.; Pearce, E.M. Flexible polyurethane foam. II. Fire retardation by tris (1, 3-dichloro-2-propyl) phosphate part A. Examination of the vapor phase (the flame). J. App. Polym. Sci. 1998, 68, 217–229. [Google Scholar] [CrossRef]
- Ravey, M.; Keidar, I.; Weil, E.D.; Pearce, E.M. Flexible polyurethane foam. II. Fire retardation by tris (1, 3-dichloro-2-propyl) phosphate. Part B. Examination of the condensed phase (the pyrolysis zone). J. App. Polym. Sci. 1998, 68, 231–254. [Google Scholar] [CrossRef]
- Wang, X.; Yang, K.; Wang, Y. Physical and chemical effects of diethyl N,N’-diethanolaminomethylphosphate on flame retardancy of rigid polyurethane foam. J. App. Polym. Sci. 2001, 82, 276–282. [Google Scholar] [CrossRef]
- Price, D.; Liu, Y.; John Milnes, G.; Hull, R.; Baljinder, K.; Kandola, A.; Horrocks, R. An investigation into the mechanism of flame retardancy and smoke suppression by melamine in flexible polyurethane foam. Fire Mater. 2002, 26, 201–206. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Huang, Z.; Xing, W.; Song, L.; Yuan, H. Effect of aluminum diethylphosphinate on the thermal stability and flame retardancy of flexible polyurethane foams. Fire Saf. J. 2019, 106, 72–79. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, G.; Xu, W. Roles of organically-modified montmorillonite and phosphorous flame retardant during the combustion of rigid polyurethane foam. Polym. Degrad. Stab. 2014, 101, 32–39. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Li, C.; Yan, M.; Wu, Y.; Cao, J.; He, S. Fabrication of nano-crystalline cellulose with phosphoric acid and its full application in a modified polyurethane foam. Polym. Degrad. Stab. 2013, 98, 1940–1944. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Singh Walia, R. Flame retardancy of ceramic-based rigid polyurethane foam composites. J. App. Polym. Sci. 2019, 136, 48250. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Wang, X.; Li, H.; Sun, J.; Sun, W.; Yao, Y.; Gu, X.; Zhang, S. Surface coated rigid polyurethane foam with durable flame retardancy and improved mechanical property. Chem. Eng. J. 2020, 385, 123755. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Arbenz, A.; Frache, A.; Cuttica, F.; Avérous, L. Advanced biobased and rigid foams, based on urethane-modified isocyanurate from oxypropylated gambier tannin polyol. Polym. Degrad. Stab. 2016, 132, 62–68. [Google Scholar] [CrossRef]
- Rao, W.; Xu, H.; Xu, Y.; Qi, M.; Liao, W.; Xu, S.; Wang, Y. Persistently flame-retardant flexible polyurethane foams by a novel phosphorus-containing polyol. Chem. Eng. J. 2018, 343, 198–206. [Google Scholar] [CrossRef]
- Ma, C.; Qiu, S.; Xiao, Y.; Zhang, K.; Zheng, Y.; Xing, W.; Hu, Y. Fabrication of fire safe rigid polyurethane foam with reduced release of CO and NOx and excellent physical properties by combining phosphine oxide-containing hyperbranched polyol and expandable graphite. Chem. Eng. J. 2022, 431, 133347. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Jana, R.N.; Nando, G.B.; Khastgir, D. Compatibilised blends of LDPE and PDMS rubber as effective cable insulants. Plast. Rubber Compos. 2013, 32, 11–20. [Google Scholar] [CrossRef]
- Zhuo, J.L.; Dong, J.; Jiao, C.M.; Chen, X.L. Synergistic effects between red phosphorus and alumina trihydrate in flame retardant silicone rubber composites. Plast. Rubber Compos. 2013, 42, 239–243. [Google Scholar] [CrossRef]
- Kim, J.K.; Hwang, H.N.; Kang, D.W.; Kang, H.J. Physical Characteristics of Silicone Modified Epoxy as a Undercoating Materials. Polymers 2014, 38, 371–377. [Google Scholar] [CrossRef]
- Liu, S.; Shen, M.; Kuan, C.; Kuan, H.; Ke, C.; Chiang, C. Improving Thermal Stability of Polyurethane through the Addition of Hyperbranched Polysiloxane. Polymers 2019, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Nath, M. Toxicity and the cardiovascular activity of organotin compounds: A review. Appl. Organometal. Chem. 2008, 22, 598–612. [Google Scholar] [CrossRef]
- Gerard, E.; Verstraete, H.; Maas, W.; Schlenter, B. DBTDL replacement in high resilience slabstock foams. J. Cell. Plast. 2002, 38, 451–470. [Google Scholar] [CrossRef]
- Lee, C.L.; Niemi, R.G.; Kelly, K.M. New silicone RTVfoam. J. Cell. Plast. 1977, 13, 62–67. [Google Scholar] [CrossRef]
- Gupta, N.S.; Lee, K.S.; Labouriau, A. Tuning Thermal and Mechanical Properties of Polydimethylsiloxane with Carbon Fibers. Polymers 2021, 13, 1141. [Google Scholar] [CrossRef]
- ISO 5660-1:2015; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). Available online: https://www.iso.org/standard/57957.html (accessed on 1 July 2025).
- Hwang, S.; Jin, S.H.; Kim, Y.; Seo, J.S.; So, J.; Kim, J.; Lee, Y.; Baeck, S.; Shim, S.E.; Qian, Y. Deciphering van der Waals interaction between polypropylene and carbonated fly ash from experimental and molecular simulation. J. Hazard. Mater. 2022, 421, 126725. [Google Scholar] [CrossRef]
- Bhattacharya, A.B.; Chatterjee, T.; Naskar, K. Automotive applications of thermoplastic vulcanizates. J. App. Polym. Sci. 2020, 137, 49181. [Google Scholar] [CrossRef]
- Goff, J.; Sulaiman, S.; Arkles, B.; Lewicki, P. Soft Materials with Recoverable Shape Factors from Extreme Distortion States. Adv. Mater. 2016, 28, 2393–2398. [Google Scholar] [CrossRef] [PubMed]
- Haan, J.L.; Stafford, K.M.; Masel, R.I. Effects of the addition of antimony, tin, and lead to palladium catalyst formulations for the direct formic acid fuel cell. J. Phys. Chem. C 2010, 114, 11665–11672. [Google Scholar] [CrossRef]
- Kim, Y.; Joeng, H.; Lee, K.W.; Hwang, S.; Shim, S.E. Mechanical and Thermal Properties of Environmentally Benign Silicone Foam Filled with Wollastonite. Elastom. Compos. 2020, 55, 300–305. [Google Scholar] [CrossRef]
- Becker, K.H.; Geiger, H.; Schmidt, F.; Wiesen, P. Kinetic investigation of NCO radicals reacting with selected hydrocarbons. Phys. Chem. Chem. Phys. 1999, 1, 5305–5309. [Google Scholar] [CrossRef]
- Silva, A.L.; Bordado, J.C. Recent Developments in Polyurethane Catalysis: Catalytic Mechanisms Review. Catal. Rev. 2004, 46, 31–51. [Google Scholar] [CrossRef]
- Sardon, H.; Pascual, A.; Mecerreyes, D.; Taton, D.; Cramail, H.; Hedrick, J.L. Synthesis of Polyurethanes Using Organocatalysis: A Perspective. Macromolecules 2015, 48, 3153–3165. [Google Scholar] [CrossRef]
- DiBenedetto, A.T. Prediction of the glass transition temperature of polymers: A model based on the principle of corresponding states. J. Polym. Sci. B Polym. Phys. 1987, 25, 1949–1969. [Google Scholar] [CrossRef]
- Abdullah, M.; Ramtani, S.; Yagoubi, N. Mechanical properties of polyurethane foam for potential application in the prevention and treatment of pressure ulcers. Results Eng. 2023, 19, 101237. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Z.; Jiang, L.; Jin, X.; Yang, H.; Jiang, Q.; Xue, X.; Jiang, B.; Huang, W. A facile method for the synthesis of foamed silicone rubber. J. Porous. Mat. 2024, 31, 1143–1153. [Google Scholar] [CrossRef]
- Lancastre, J.J.H.; Fernandes, N.; Margaça, F.M.A.; Miranda Salvado, I.M.; Ferreira, L.M.; Falcão, A.N.; Casimiro, M.H. Study of PDMS conformation in PDMS-based hybrid materials prepared by gamma irradiation. Radiat. Phys. Chem. 2012, 81, 1336–1340. [Google Scholar] [CrossRef]
- Yang, W.; Luo, S.; Zhang, B.; Huang, Z.; Tang, X. Electroless preparation and characterization of magnetic Ni–P plating on polyurethane foam. App. Surf. Sci. 2008, 254, 7427–7430. [Google Scholar] [CrossRef]
- Rutnakornpituk, M.; Ngamdee, P.; Phinyocheep, P. Preparation and properties of polydimethylsiloxane-modified chitosan. Carbohydr. Polym. 2006, 63, 229–237. [Google Scholar] [CrossRef]
- Reinerte, S.; Avotina, L.; Zarins, A.; Cabulis, U.; Viksna, A. TG/DTA-FTIR as a method for analysis of tall oil based rigid polyurethane foam decomposition gaseous products in a low oxygen environment. Polym. Degrad. Stab. 2020, 180, 109313. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahman, W.; Rahmat, A.R.; Khan, M.I. Detection of synergistic interactions of polyvinyl alcohol–cassava starch blends through DSC. Carbohydr. Polym. 2010, 79, 224–226. [Google Scholar] [CrossRef]
- Mojsiewicz-Pienkowska, K.; Jamrógiewicz, M.; Zebrowska, M.; Sznitowska, M.; Centkowska, K. Technology of an adhesive silicone film as drug carrier in transdermal therapy. I: Analytical methods used for characterization and design of the universal elastomer layers. J. Pharm. Biomed. Anal. 2011, 56, 131–138. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.; Jaszcz, K.; Chladek, G.; Grabowska, P.; Okseniuk, A.; Szpot, M.; Zawadzka, M.; Sokotowska, A.; Tarkiewicz, A. Characterization of changes in structural, physicochemical and mechanical properties of rigid polyurethane building insulation after thermal aging in air and seawater. Polym. Bull. 2022, 79, 3061–3083. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, G. Preparation of Bisphenol-A and Polydimethylsiloxane (PDMS) Block Copolycarbonates by Melt Polycondensation: Effects of PDMS Chain Length on Conversion and Miscibility. Polymers 2021, 13, 2660. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Pang, M.; Zhu, L.; Zhang, Y.; Feng, S. Mechanical properties of silicone rubber composed of diverse vinyl content silicone gums blending. Mater. Des. 2010, 31, 4083–4087. [Google Scholar] [CrossRef]
- Schweitzer, J.; Merad, S.; Schrodj, G.; Gall, F.B.; Vonna, L. Determination of the Crosslinking Density of a Silicone Elastomer. J. Chem. Educ. 2019, 96, 1472–1478. [Google Scholar] [CrossRef]
- Lee, C.; Park, J. The Properties of DSC and DMA for Epoxy Nano-and-Micro Mixture Composites. Trans. Electr. Electron. Mater. 2010, 11, 69–72. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Doi, Y.; Uneyama, T. Entanglement Molecular Weight. Nihon Reoroji Gakk. 2020, 48, 177–183. [Google Scholar] [CrossRef]
- So, J.I.; Lee, C.S.; Kim, B.S.; Jeong, H.W.; Seo, J.S.; Baeck, S.H.; Shim, S.E. Improvement of Heat Resistance of Fluorosilicone Rubber Employing Vinyl-Functionalized POSS as a Chemical Crosslinking Agent. Polymers 2023, 15, 1300. [Google Scholar] [CrossRef] [PubMed]
- Scala, J.L.; Wool, R.P. Property analysis of triglyceride-based thermosets. Polymer 2005, 46, 61–69. [Google Scholar] [CrossRef]
- Kamiński, M. Homogenization with uncertainty in Poisson ratio for polymers with rubber particles. Compos. B Eng. 2015, 69, 267–277. [Google Scholar] [CrossRef]



| Samples | PDMS | Catalyst | Polyurethane (FlexFoam-iT!™ III) | |||
|---|---|---|---|---|---|---|
| Vinyl Polymer | OH Polymer | H Polymer | Sn(OCT)2 | Part A | Part B | |
| SUF125 | 30 | 65 | 5 | 0.1 | 46 | 79 |
| SUF137 | 30 | 65 | 5 | 0.1 | 50 | 87 |
| SUF150 | 30 | 65 | 5 | 0.1 | 55 | 95 |
| SUF200 | 30 | 65 | 5 | 0.1 | 73 | 127 |
| Tf (°C) | Tc (°C) | (J/g) | (J/g) | Xc (%) | |
|---|---|---|---|---|---|
| SUF125 | −40.24 | −68.52 | 10.13 | 9.04 | 37.3 |
| SUF137 | −40.61 | −70.75 | 9.28 | 8.75 | 35.9 |
| SUF150 | −40.94 | −73.75 | 8.40 | 7.20 | 34.3 |
| SUF200 | −40.94 | −73.78 | 6.70 | 3.96 | 32.8 |
| TCOP (g) | TCO2P (g) | |
|---|---|---|
| SUF125 | 1.08 | 80.45 |
| SUF137 | 1.02 | 82.22 |
| SUF150 | 1.10 | 75.63 |
| SUF200 | 1.69 | 88.41 |
| UF* | 2.26 | 100.24 |
| Tensile Strength (kPa) | Elongation at Break (%) | Density (g/cm3) | |
|---|---|---|---|
| SUF125 | 146.6 (10.1) | 94.7 (13.1) | 0.157 (0.0022) |
| SUF137 | 156.6 (.9) | 109.8 (7.3) | 0.146 (0.0026) |
| SUF150 | 180.5 (10.3) | 86.9 (.7) | 0.135 (0.0045) |
| SUF200 | 136.6 (5.3) | 91.3 (.5) | 0.156 (0.0041) |
| UF | 86.4 (5.9) | 101.1 (3.4) | 0.060 (0.0015) |
| UF* | 166.5 (.7) | 189.2 (.3) | 0.120 (0.0032) |
| ML (dN m) | MH (dN m) | M (dN m) | t90 (s) | |
|---|---|---|---|---|
| SUF125-100 °C | 0.001 | 0.204 | 0.203 | 63.8 |
| SUF137-100 °C | 0.001 | 0.279 | 0.278 | 44.6 |
| SUF150-100 °C | 0.001 | 0.544 | 0.543 | 43.4 |
| SUF200-100 °C | 0.001 | 0.974 | 0.975 | 42.2 |
| UF-100 °C | 0.002 | 0.669 | 0.667 | 59.0 |
| SUF137-80 °C | 0.001 | 0.077 | 0.076 | 53.0 |
| SUF137-120 °C | 0.001 | 0.222 | 0.221 | 33.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Jeong, H.W.; Kim, A.; Kwan, T.S.; Jeoung, S.K.; Baeck, S.-H.; Shim, S.E.; Qian, Y. One-Pot Synthesis of Silicone–Urethane Hybrid Foam and Comparison of Flame Retardant, Rheological, and Mechanical Properties with Polyurethane Foam. Polymers 2025, 17, 2352. https://doi.org/10.3390/polym17172352
Hwang S, Jeong HW, Kim A, Kwan TS, Jeoung SK, Baeck S-H, Shim SE, Qian Y. One-Pot Synthesis of Silicone–Urethane Hybrid Foam and Comparison of Flame Retardant, Rheological, and Mechanical Properties with Polyurethane Foam. Polymers. 2025; 17(17):2352. https://doi.org/10.3390/polym17172352
Chicago/Turabian StyleHwang, Sosan, Hyeon Woo Jeong, Asell Kim, Tae Soon Kwan, Sun Kyoung Jeoung, Sung-Hyeon Baeck, Sang Eun Shim, and Yingjie Qian. 2025. "One-Pot Synthesis of Silicone–Urethane Hybrid Foam and Comparison of Flame Retardant, Rheological, and Mechanical Properties with Polyurethane Foam" Polymers 17, no. 17: 2352. https://doi.org/10.3390/polym17172352
APA StyleHwang, S., Jeong, H. W., Kim, A., Kwan, T. S., Jeoung, S. K., Baeck, S.-H., Shim, S. E., & Qian, Y. (2025). One-Pot Synthesis of Silicone–Urethane Hybrid Foam and Comparison of Flame Retardant, Rheological, and Mechanical Properties with Polyurethane Foam. Polymers, 17(17), 2352. https://doi.org/10.3390/polym17172352







