An Overview of Bio-Based Polymers with Potential for Food Packaging Applications
Abstract
1. Introduction
2. Bio-Based Polymers for Food Packaging
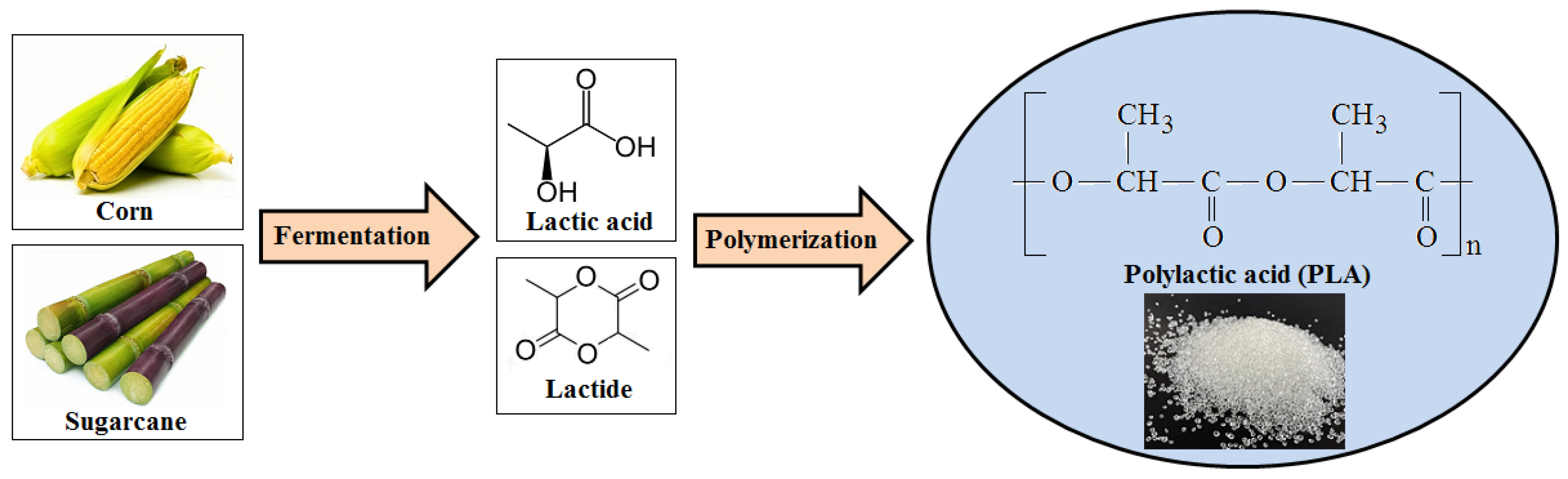
2.1. Polylactic Acid (PLA)
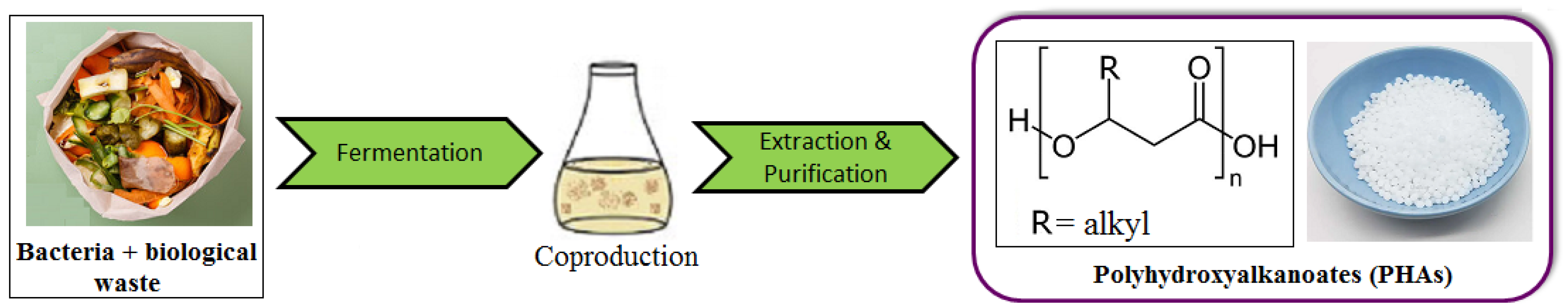
2.2. Polyhydroxyalkanoates (PHAs)
2.3. Starch-Based Polymers
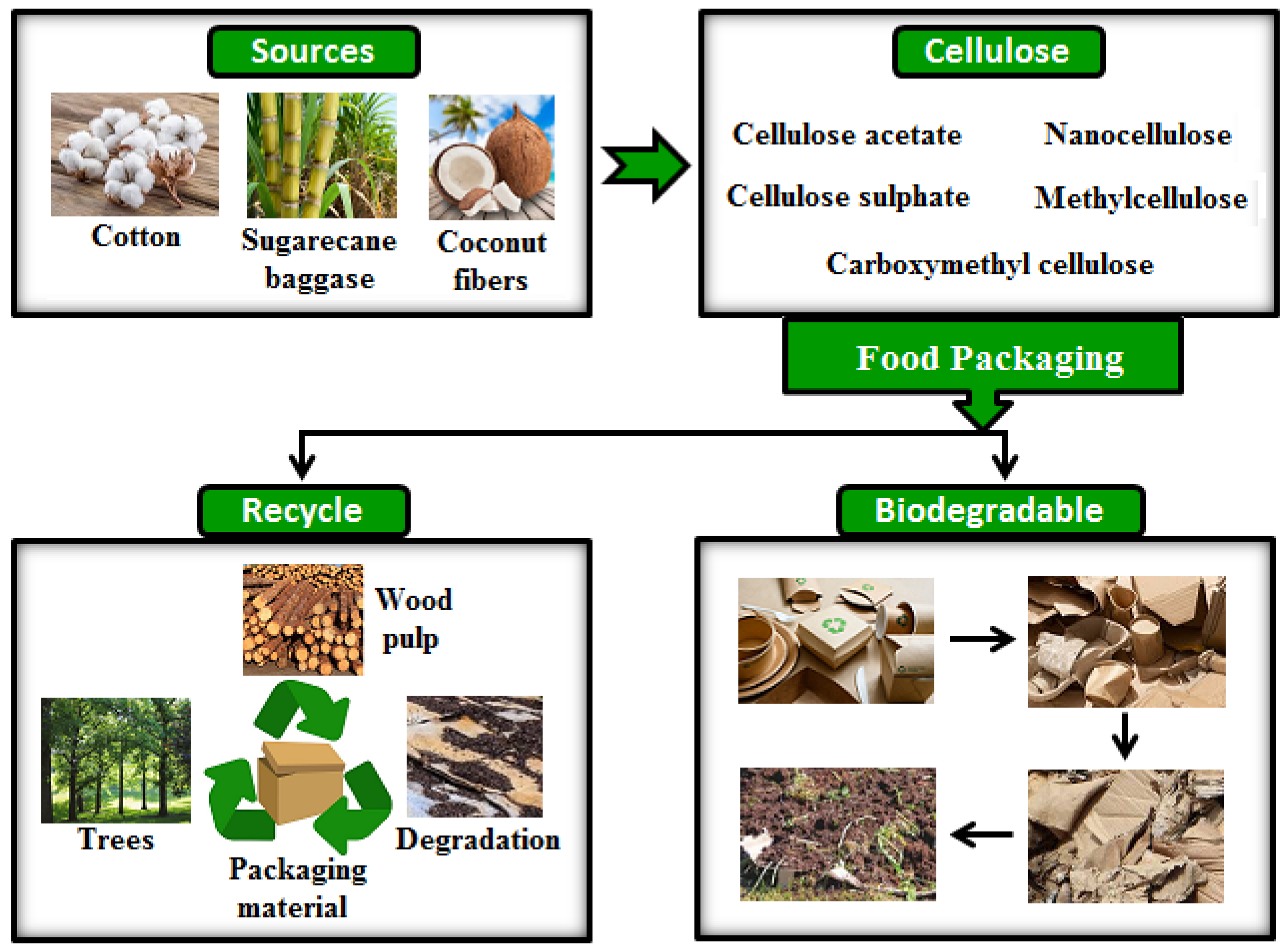
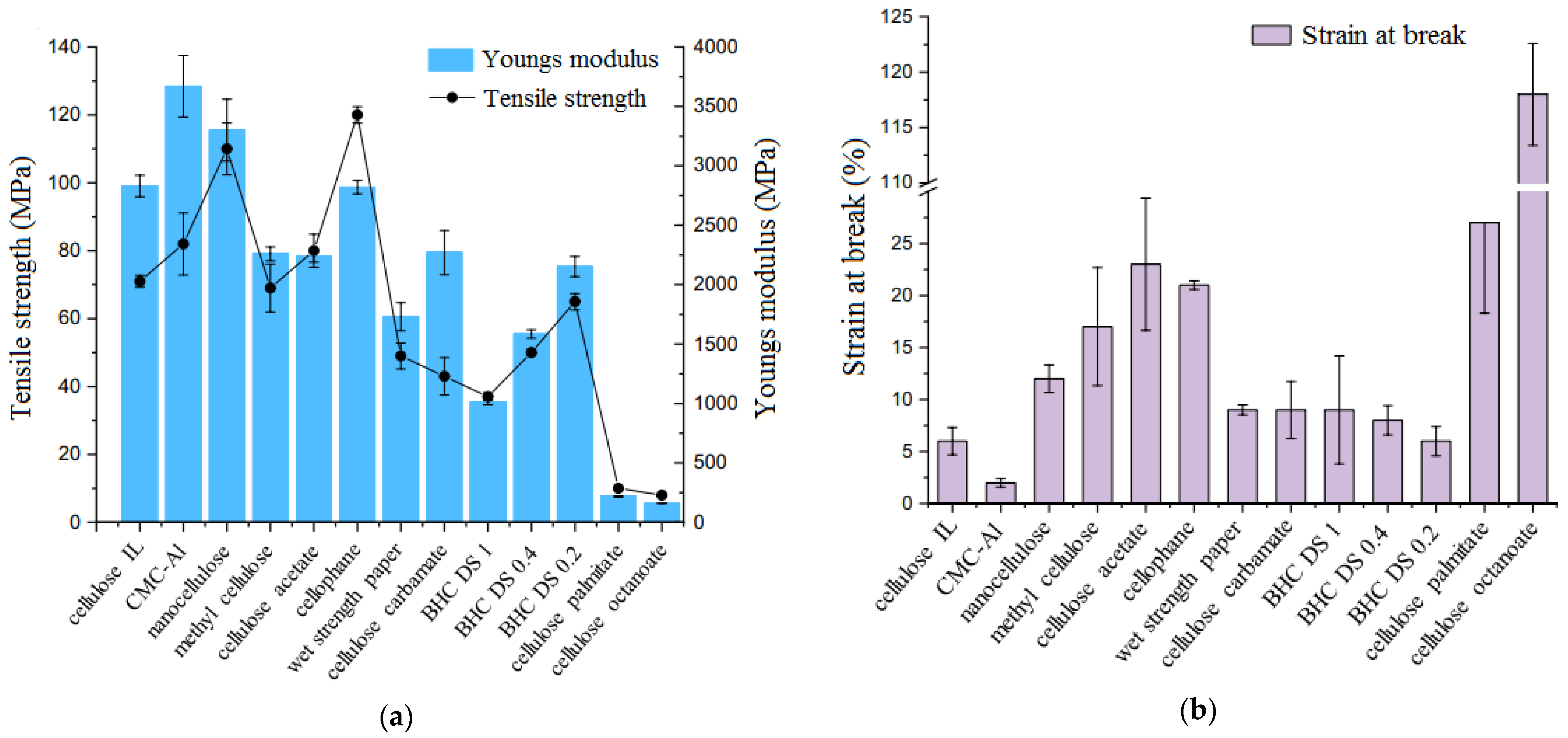
2.4. Cellulose-Based Polymers
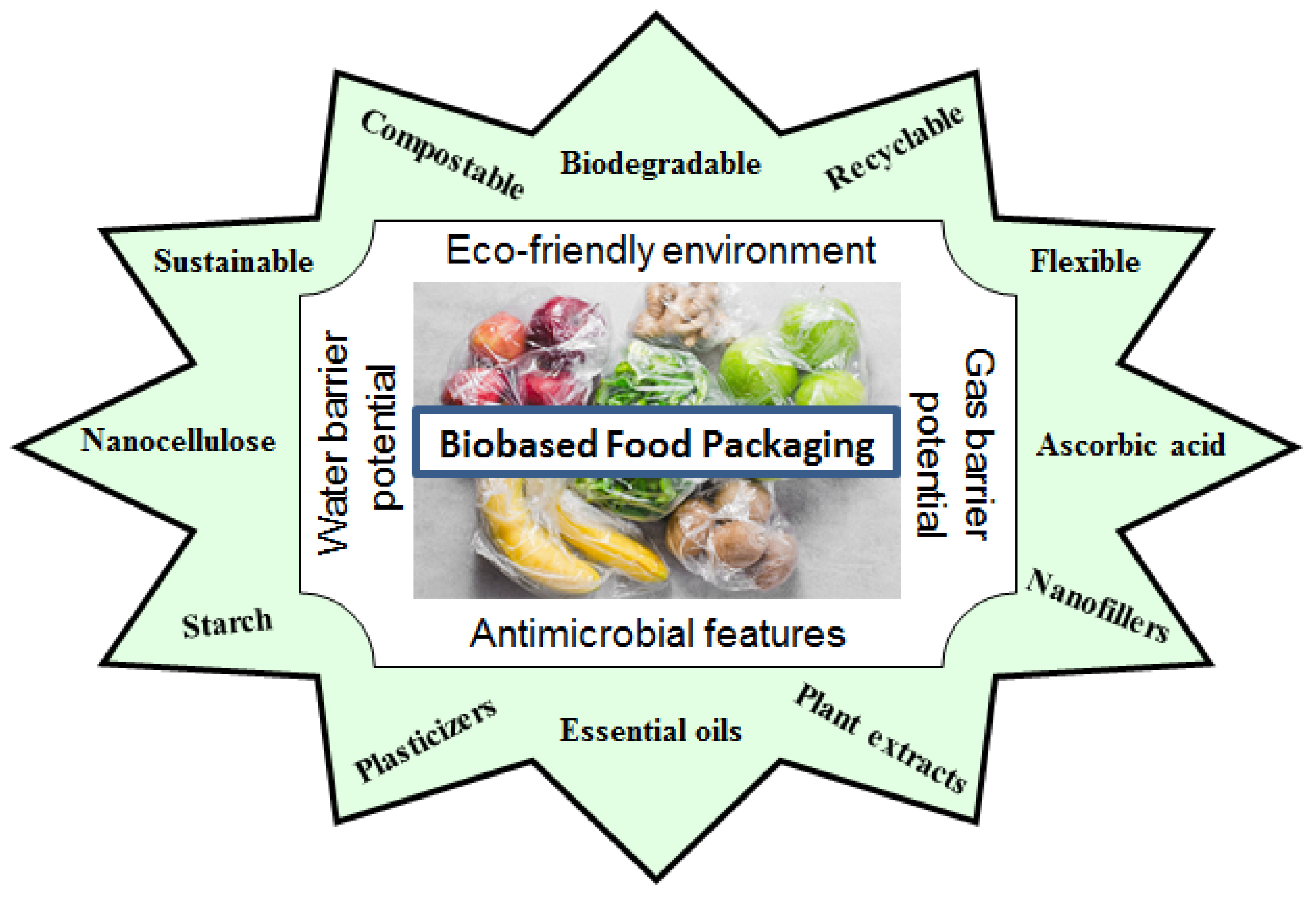
3. Advantages of Bio-Based Polymers Used in Food Packaging
4. Properties of Bio-Based Polymers
4.1. Mechanical Properties
4.2. Barrier Properties
4.3. Antimicrobial Property
4.4. Eco-Friendly Behavior
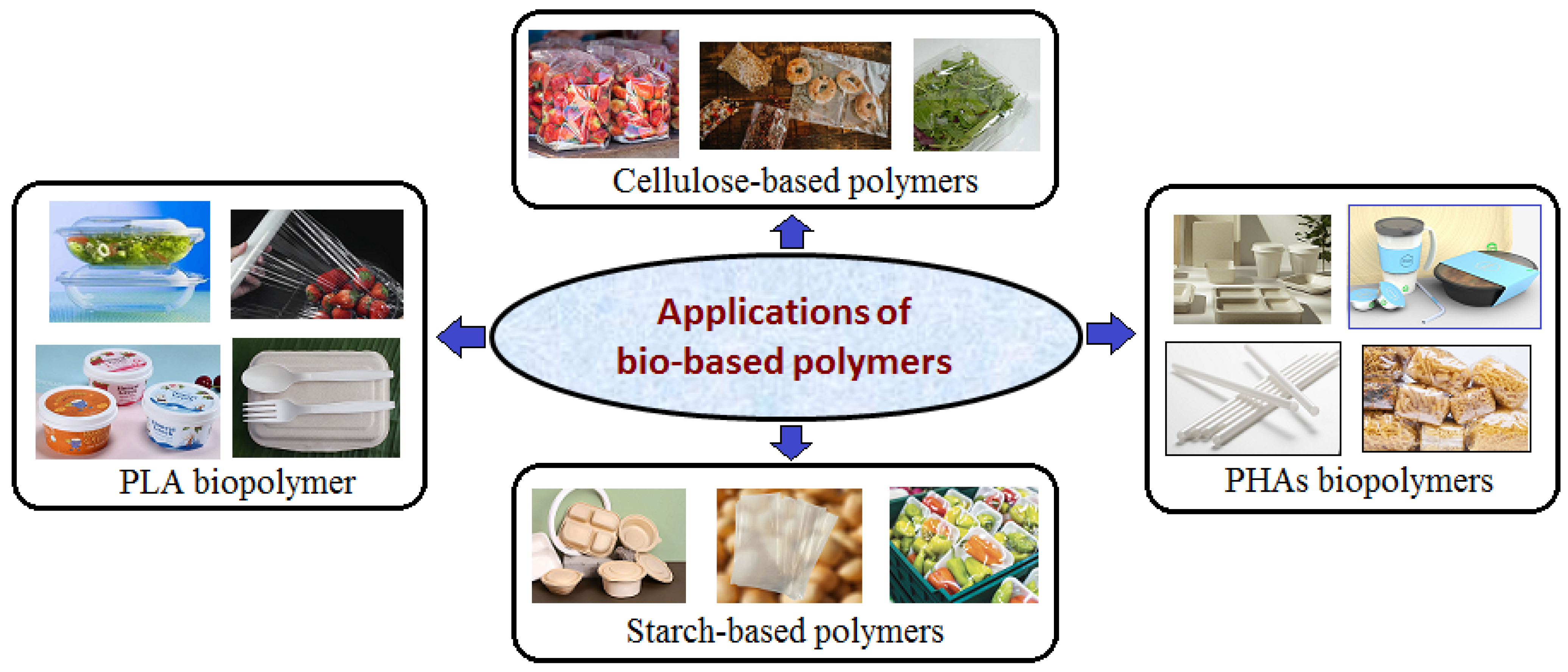
5. Applications of Bio-Based Polymers
6. Disadvantages of Bio-Based Polymers Used in Food Packaging
7. Current Challenges and Future Research Directions
- ➢
- Cost
- ➢
- End-of-life Considerations
- ➢
- Resource Competition
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mukhi, S.; Rukmini, M.S.; Reghupathi, I.; Manjrekar, P.; Sindhu, H.; Hegde, A. Effect of Food Packaging on Human Health—A Review. J. Chem. Health Risks 2024, 14, 2089–2097. [Google Scholar]
- Adly, H.M.; Saati, A.A.; Obaid, M.S.; Saleh, S.A.K. Chemical Migration of Polycyclic Aromatic Hydrocarbons and Other Compounds from Plastic Food Packaging: Assessment of Food Safety Risks and Health Impacts. Foods 2025, 14, 1013. [Google Scholar] [CrossRef]
- Chinglenthoiba, C.; Lani, M.N.; Anuar, S.T.; Amesho, K.T.T.; Priya, K.L.; Santos, J.H. Microplastics in food packaging: Analytical methods, health risks, and sustainable alternatives. J. Hazard. Mater. Adv. 2025, 18, 100746. [Google Scholar] [CrossRef]
- Khandeparkar, A.S.; Paul, R.; Sridhar, A.; Lakshmaiah, V.V.; Nagella, P. Eco-friendly innovations in food packaging: A sustainable revolution. Sustain. Chem. Pharm. 2024, 39, 101579. [Google Scholar] [CrossRef]
- Lunetta, E.; Cacciotti, I. Chapter 1—An overview of the packaging industry: State of the art, opportunities, challenges, criticisms, and solutions. In Nanostructured Materials for Food Packaging Applications; Jacob, J., Cacciotti, I., Thomas, S., Eds.; Elsevier Inc.: Edinburgh/London, UK, 2024; pp. 1–30. [Google Scholar]
- Donkor, L.; Kontoh, G.; Yaya, A.; Bediako, J.K.; Apalangya, V. Bio-based and sustainable food packaging systems: Relevance, challenges, and prospects. Appl. Food Res. 2023, 3, 100356. [Google Scholar] [CrossRef]
- D’Almeida, A.P.; de Albuquerque, T.L. Innovations in Food Packaging: From Bio-Based Materials to Smart Packaging Systems. Processes 2024, 12, 2085. [Google Scholar] [CrossRef]
- De Marchi, E.; Pigliafreddo, S.; Banterle, A.; Parolini, M.; Cavaliere, A. Plastic packaging goes sustainable: An analysis of consumer preferences for plastic water bottles. Environ. Sci. Policy. 2020, 114, 305–311. [Google Scholar] [CrossRef]
- Yaris, A.; Sezgin, A.C. Chapter 81 Food Packaging: Glass And Plastic. In Researches on Science and Art in 21st Century; GECE Publishing: Boston, MA, USA, 2017; pp. 735–740. [Google Scholar]
- Akram, N.; Saeed, M.; Mansha, A.; Bokhari, T.H.; Ali, A. Chapter 8—Metal packaging for food items advantages, disadvantages and applications. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Altalhi, T., Cruz, J.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 129–141. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R.; Alam, T. An overview of paper and paper based food packaging materials: Health safety and environmental concerns. J. Food Sci. Technol. 2019, 56, 4391–4403. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Gao, R.; Zhu, Y.; Lin, Q. Applications of biodegradable materials in food packaging: A review. Alex. Eng. J. 2024, 91, 70–83. [Google Scholar] [CrossRef]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Recent advances and future prospects of cellulose, starch, chitosan, polylactic acid and polyhydroxyalkanoates for sustainable food packaging applications. Int. J. Biol. Macromol. 2022, 221, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Helanto, K.; Matikainen, L.; Talja, R.; Rojas, O.J. Bio-based Polymers for Sustainable Packaging and Biobarriers: A Critical Review. BioRes.. 2019, 14, 4902–4951. [Google Scholar] [CrossRef]
- Stoica, D.; Alexe, P.; Ivan, A.S.; Stanciu, S.; Tatu, D.M.; Stoica, M. Bioplastics from Biomass. In Biopolymers: Recent Updates, Challenges and Opportunities; Nadda, A.K., Sharma, S., Bhat, R., Eds.; Springer Series on Polymer and Composite Materials; Springer: Cham, Switzerland, 2022; pp. 353–372. [Google Scholar]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.H. An overview of biodegradable poly (lactic acid) production from fermentative lactic acid for biomedical and bioplastic applications. Biomass Convers. Biorefinery. 2024, 14, 3057–3076. [Google Scholar] [CrossRef]
- Ragaert, P.; Buntinx, M.; Maes, C.; Vanheusden, C.; Peeters, R.; Wang, S.; D’hooge, D.R.; Cardon, L. Polyhydroxyalkanoates for Food Packaging Applications. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Garcia-Garcia, D.; Dominici, F.; Torre, L.; Sanchez-Nacher, L.; Balart, R. PLA Films with Improved Flexibility Properties by Using Maleinized Cottonseed Oil. Eur. Polym. J. 2017, 91, 248–259. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods 2023, 12, 2422. [Google Scholar] [CrossRef] [PubMed]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-sourced polymers as alternatives to conventional food packaging materials: A review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Babaremu, K.; Oladijo, O.P.; Akinlabi, E. Biopolymers: A suitable replacement for plastics in product packaging. Adv. Ind. Eng. Polym. Res. 2023, 6, 333–340. [Google Scholar] [CrossRef]
- Parente, A.G.; de Oliveira, H.P.; Cabrera, M.P.; de Morais Neri, D.F. Bio-based polymer films with potential for packaging applications: A systematic review of the main types tested on food. Polym. Bull. 2023, 80, 4689–4717. [Google Scholar] [CrossRef]
- Rajendran, D.S.; Venkataraman, S.; Jha, S.K.; Chakrabarty, D.; Kumar, V.V. A review on bio-based polymer polylactic acid potential on sustainable food packaging. Food Sci. Biotechnol. 2024, 33, 1759–1788. [Google Scholar] [CrossRef]
- Mou, L.; Li, J.; Lu, Y.; Li, G.; Li, J. Polylactic acid: A future universal biobased polymer with multifunctional performance—From monomer synthesis, and processing to applications: A review. J. Hazard. Mater. Adv. 2025, 18, 100757. [Google Scholar] [CrossRef]
- Swetha, T.A.; Ananthi, V.; Bora, A.; Sengottuvelan, N.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A review on biodegradable polylactic acid (PLA) production from fermentative food waste—Its applications and degradation. Int. J. Biol. Macromol. 2023, 234, 123703. [Google Scholar] [CrossRef]
- Chafran, L.S.; Paiva, M.F.; França, J.O.C.; Sales, M.J.A.; Dias, S.C.L.; Dias, J.A. Preparation of PLA blends by polycondensation of D,L-lactic acid using supported 12-tungstophosphoric acid as a heterogeneous catalyst. Heliyon 2019, 5, e01810. [Google Scholar] [CrossRef]
- Tone, A.M.; Herranz Solana, N.; Khan, M.R.; Borriello, A.; Torrieri, E.; Sánchez Reig, C.; Monedero Prieto, F.M. Study on the Properties of PLA- and PP-Based Films for Food Applications Incorporating Orange Peel Extract from Agricultural by-Products. Polymers 2024, 16, 1245. [Google Scholar] [CrossRef]
- Moraczewski, K.; Stepczyńska, M.; Raszkowska-Kaczor, A.; Szymańska, L.; Rytlewski, P. PLA/PCL Polymer Material for Food Packaging with Enhanced Antibacterial Properties. Polymers 2025, 17, 1134. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.; Rahman, A.; Rehman, A.U.; Walsh, M.K.; Miller, C.D. Food waste conversion to microbial polyhydroxyalkanoates. Microb. Biotechnol. 2017, 10, 1751–7915. [Google Scholar] [CrossRef] [PubMed]
- Javaid, H.; Nawaz, A.; Riaz, N.; Mukhtar, H.; -Ul-Haq, I.; Shah, K.A.; Khan, H.; Naqvi, S.M.; Shakoor, S.; Rasool, A.; et al. Biosynthesis of Polyhydroxyalkanoates (PHAs) by the Valorization of Biomass and Synthetic Waste. Molecules 2020, 25, 5539. [Google Scholar] [CrossRef]
- Vicente, D.; Proença, D.N.; Morais, P.V. The Role of Bacterial Polyhydroalkanoate (PHA) in a Sustainable Future: A Review on the Biological Diversity. Int. J. Environ. Res. Public Health 2023, 20, 2959. [Google Scholar] [CrossRef]
- Chouhan, A.; Tiwari, A. Production of polyhydroxyalkanoate (PHA) biopolymer from crop residue using bacteria as an alternative to plastics: A review. RSC Adv. 2025, 15, 11845–11862. [Google Scholar] [CrossRef] [PubMed]
- Atarés, L.; Chiralt, A.; González-Martínez, C.; Vargas, M. Production of Polyhydroxyalkanoates for Biodegradable Food Packaging Applications Using Haloferax mediterranei and Agrifood Wastes. Foods 2024, 13, 950. [Google Scholar] [CrossRef]
- Zhou, W.; Bergsma, S.; Colpa, D.I.; Euverink, G.-J.W.; Krooneman, J. Polyhydroxyalkanoates (PHAs) synthesis and degradation by microbes and applications towards a circular economy. J. Environ. Manag. 2023, 341, 118033. [Google Scholar] [CrossRef]
- Soto, L.R.; Byrne, E.; van Niel, E.W.; Sayed, M.; Villanueva, C.C.; Hatti-Kaul, R. Hydrogen and polyhydroxybutyrate production from wheat straw hydrolysate using Caldicellulosiruptor species and Ralstonia eutropha in a coupled process. Bioresour. Technol. 2019, 272, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Hathi, Z.; Haque, M.A.; Kumar, S.; Kumar, A.; Singh, E.; Lin, C.S. Effect of levulinic acid on production of polyhydroxyalkanoates from food waste by Haloferax mediterranei. Environ. Res. 2022, 214, 114001. [Google Scholar] [CrossRef] [PubMed]
- Stublić, K.; Ranilović, J.; Ocelić Bulatović, V.; Kučić Grgić, D. Advancing Sustainability: Utilizing Bacterial Polyhydroxyalkanoate for Food Packaging. Processes 2024, 12, 1886. [Google Scholar] [CrossRef]
- Sedničková, M.; Pekařová, S.; Kucharczyk, P.; Bočkaj, J.; Janigová, I.; Kleinová, A.; Jochec-Mošková, D.; Omaníková, L.; Perďochová, D.; Koutný, M.; et al. Changes of physical properties of PLA-based blends during early stage of biodegradation in compost. Int. J. Biol. Macromol. 2018, 113, 434–442. [Google Scholar] [CrossRef]
- Bonnenfant, C.; Gontard, N.; Aouf, C. PHBV-based polymers as food packaging: Physical-chemical and structural stability under reuse conditions. Polymer 2023, 270, 125784. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.E.; Hernandez-Landaverde, M.A.; Delgado, J.M.; Ramirez-Gutierrez, C.F.; Ramirez-Cardona, M.; MillanMalo, B.M.; Londoño-Restrepo, S.M. Crystalline structures of the main components of starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar] [CrossRef]
- Tanetrungroj, Y.; Prachayawarakorn, J. Effect of Starch Types on Properties of Biodegradable Polymer Based on Thermoplastic Starch Process by Injection Molding Technique. Songklanakarin J. Sci. Technol. 2015, 37, 193–199. [Google Scholar]
- García-Guzmán, L.; Cabrera-Barjas, G.; Soria-Hernández, C.G.; Castaño, J.; Guadarrama-Lezama, A.Y.; Rodríguez Llamazares, S. Progress in Starch-Based Materials for Food Packaging Applications. Polysaccharides 2022, 3, 136–177. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-Based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Andrade, L.A.; Barbosa, N.A.; Pereira, J. Extraction and properties of starches from the non-traditional vegetables Yam and Taro. Polímeros 2017, 27, 151–157. [Google Scholar] [CrossRef]
- De Paola, M.G.; Mammolenti, D.; Lupi, F.R.; De Santo, M.P.; Gabriele, D.; Calabrò, V. Formulation and process investigation of glycerol/starch suspensions for edible films production by tape casting. Chem. Pap. 2021, 76, 1–14. [Google Scholar] [CrossRef]
- Mileti, O.; Baldino, N.; Filice, F.; Lupi, F.R.; Sinicropi, M.S.; Gabriele, D. Formulation Study on Edible Film from Waste Grape and Red Cabbage. Foods 2023, 12, 2804. [Google Scholar] [CrossRef]
- Onyeaka, H.; Obileke, K.; Makaka, G.; Nwokolo, N. Current Research and Applications of Starch-Based Biodegradable Films for Food Packaging. Polymers 2022, 14, 1126. [Google Scholar] [CrossRef]
- Parra, D.; Tadini, C.; Ponce, P.; Lugão, A. Mechanical properties and water vapor transmission in some blends of cassava starch edible films. Carbohydr. Polym. 2004, 58, 475–481. [Google Scholar] [CrossRef]
- Garavito, J.; Peña-Venegas, C.P.; Castellanos, D.A. Production of Starch-Based Flexible Food Packaging in Developing Countries: Analysis of the Processes, Challenges, and Requirements. Foods 2024, 13, 4096. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Whiteside, W.S.; Suri, S.; Barua, S.; Phimolsiripol, Y. Native and modified biodegradable starch-based packaging for shelf-life extension and safety of fruits/vegetables. Int. J. Food Sci. Technol. 2023, 58, 862–870. [Google Scholar] [CrossRef]
- Carneiro da Silva, L.R.; Rios, A.d.O.; Campomanes Santana, R.M. Polymer blends of poly(lactic acid) and starch for the production of films applied in food packaging: A brief review. Polym. Renew. Res. 2023, 14, 108–153. [Google Scholar] [CrossRef]
- Chaleat, C.; Halley, P.; Truss, R. Chapter 7. Mechanical Properties of Starch-Based Plastics. In Starch Polymers: From Genetic Engineering to Green Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 187–205. ISBN 978-0-444-53730-0. [Google Scholar]
- Zhang, J.M.; Luo, N.; Zhang, X.Y.; Xu, L.L.; Wu, J. All-cellulose nanocomposites reinforced with in situ retained cellulose nanocrystals during selective dissolution of cellulose in an ionic liquid. ACS Sustain. Chem. Eng. 2016, 4, 4417–4423. [Google Scholar] [CrossRef]
- Qu, T.; Wang, X.; Zhang, F. Antibacterial Food Packaging with Chitosan and Cellulose Blends for Food Preservation. Polymers 2025, 17, 1850. [Google Scholar] [CrossRef]
- Manikanika, L.C. Extraction of cellulosic fibers from the natural resources: A short review. Mater. Today Proc. 2022, 48, 1265–1270. [Google Scholar] [CrossRef]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent Advances in Biopolymers and Biopolymer-Based Nanocomposites for Food Packaging Materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef]
- Tapadar, S.H.; Kemisetti, D.P. A Review on Biodegradable Cellulose Based Bio films as a Viable Packaging Material in Pharmaceutical and Food Industry. Indian J. Nat. Sci. 2024, 14, 67321–67328. [Google Scholar]
- Lai, M.; Zhao, L.; Ren, J.; Li, K. Highly tough, extensible, and bio-based films composed of cellulose and phenolic acids. Int. J. Biol. Macromol. 2025, 320, 145942. [Google Scholar] [CrossRef]
- He, X.; Lu, W.; Sun, C.; Khalesi, H.; Mata, A.; Andaleeb, R.; Fang, Y. Cellulose and cellulose derivatives: Different colloidal states and food-related applications. Carbohydr. Polym. 2020, 255, 117334. [Google Scholar] [CrossRef]
- Nath, P.C.; Sharma, R.; Mahapatra, U.; Mohanta, Y.K.; Rustagi, S.; Sharma, M.; Mahajan, S.; Nayak, P.K.; Sridhar, K. Sustainable production of cellulosic biopolymers for enhanced smart food packaging: An up-to-date review. Int. J. Biol. Macromol. 2024, 273, 133090. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Davoudpour, Y.; Saurabh, C.K.; Hossain, M.S.; Adnan, A.S.; Dungani, R.; Paridah, M.T.; Sarker, M.Z.I.; Fazita, M.R.N.; Syakir, M.I.; et al. A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Jiang, Z.; Ngai, T. Recent Advances in Chemically Modified Cellulose and Its Derivatives for Food Packaging Applications: A Review. Polymers 2022, 14, 1533. [Google Scholar] [CrossRef]
- Jayakrishnan, A.; Shahana, S.; Ayswaria, R. Preparation and evaluation of bioactive cellulose acetate films from Musa acuminate. RSC Sustain. 2024, 2, 2335–2347. [Google Scholar] [CrossRef]
- Rajeswari, A.; Christy, E.J.S.; Swathi, E.; Pius, A. Fabrication of improved cellulose acetate-based biodegradable films for food packaging applications. Environ. Chem. Ecotoxicol. 2020, 2, 107–114. [Google Scholar] [CrossRef]
- Siew, Z.Z.; Chan, E.W.C.; Wong, C.W. Enhancing the Tearability and Barrier Properties of Cellulose Acetate Bioplastic Film with Polyethylene Glycol 1450 as an LDPE Replacement for Food Packaging. Food Bioprocess. Technol. 2024, 17, 2265–2276. [Google Scholar] [CrossRef]
- Sultan, M.; Ibrahim, S.; Hafez, O.M.; Saleh, M.A. Cellulose Sulfate Active Packaging Material with Treatments on Orange Shelf Life. Int. J. Chemtech Res. 2016, 9, 79–90. [Google Scholar]
- Thiangtham, S.; Runt, J.; Manuspiya, H. Sulfonation of dialdehyde cellulose extracted from sugarcane bagasse for synergistically enhanced water solubility. Carbohydr. Polym. 2019, 208, 314–322. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kim, J.T.; Roy, S.; Jayakumar, A. Recent advances in carboxymethyl cellulose-based active and intelligent packaging materials: A comprehensive review. Int. J. Biol. Macromol. 2024, 259, 129194. [Google Scholar] [CrossRef]
- Lawal, K.G.; Riaz, A.; Mostafa, H.; Stathopoulos, C.; Manikas, I.; Maqsood, S. Development of Carboxymethylcellulose Based Active and Edible Food Packaging Films Using Date Seed Components as Reinforcing Agent: Physical, Biological, and Mechanical Properties. Food Biophys. 2023, 18, 497–509. [Google Scholar] [CrossRef]
- Dey, P.; Bhattacharjee, S.; Yadav, D.K.; Hmar, B.Z.; Gayen, K.; Bhowmick, T.K. Valorization of waste biomass for synthesis of carboxy-methyl-cellulose as a sustainable edible coating on fruits: A review. Int. J. Biol. Macromol. 2023, 253, 127412. [Google Scholar] [CrossRef]
- Hussain, R.; Batool, S.A.; Aizaz, A.; Abbas, M.; Rehman, M.A.U. Biodegradable Packaging Based on Poly(vinyl Alcohol) and Carboxymethyl Cellulose Films Incorporated with Ascorbic Acid for Food Packaging Applications. ACS Omega 2023, 8, 42301–42310. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Rosnan, S.M.; Enomae, T. Carboxymethyl cellulose-chitosan edible films for food packaging: A review of recent advances. Carbohydr. Polym. 2024, 346, 122612. [Google Scholar] [CrossRef]
- Wang, F.; Hu, Z.; Ouyang, S.; Wang, S.; Liu, Y.; Li, M.; Wu, Y.; Li, Z.; Qian, J.; Wu, Z.; et al. Application progress of nanocellulose in food packaging: A review. Int. J. Biol. Macromol. 2024, 268, 131936. [Google Scholar] [CrossRef]
- Esmaeili, A.; Fazel, M.E. Optimization and preparation of Methylcellulose edible film combined with of Ferulago angulata essential oil (FEO) nanocapsules for food packaging applications. Flavour Fragr. J. 2016, 31, 341–349. [Google Scholar] [CrossRef]
- Karim, S.F.A.; Hamzah, N.A.N.; Aziz, R.A.A.; Ibrahim, U.K. The Effect of Plasticizers towards the Characteristics of Methylcellulose Film Packaging. IOP Conf. Ser. Mater. Sci. Eng. 2020, 845, 012017. [Google Scholar] [CrossRef]
- Du, J.; Zhu, Q.; Guo, J.; Gu, J.; Guo, J.; Wu, Y.; Ren, L.; Yang, S.; Jiang, J. Preparation and characterization of edible films from gelatin and hydroxypropyl methyl cellulose/sodium carboxymethyl cellulose. Heliyon 2024, 11, e41613. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Shi, W.; Valencak, T.G.; Zhang, Y.; Liu, G.; Ren, D. Biodegradation of conventional plastics: Candidate organisms and potential mechanisms. Sci. Total Environ. 2023, 885, 163908. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Elsamahy, T.; Al-Tohamy, R.; Zhu, D.; Mahmoud, Y.A.; Koutra, E.; Metwally, M.A.; Kornaros, M.; Sun, J. Plastic wastes biodegradation: Mechanisms, challenges and future prospects. Sci. Total Environ. 2021, 780, 146590. [Google Scholar] [CrossRef]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A comprehensive review on polylactic acid (PLA)—Synthesis, processing and application in food packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef]
- Gunti, R.; Ratna Prasad, A.V.; Gupta, A.V.S.S.K.S. Mechanical and degradation properties of natural fiber-reinforced PLA composites: Jute, sisal, and elephant grass. Polym. Compos. 2018, 39, 1125–1136. [Google Scholar] [CrossRef]
- Moutinho, L.G.; Soares, E.; Oliveira, M. Thermoforming of bio-based polylactic acid (PLA) sheets reinforced with cork powder. Mater. Today Commun. 2025, 46, 112867. [Google Scholar] [CrossRef]
- De Luca, S.; Milanese, D.; Gallichi-Nottiani, D.; Cavazza, A.; Sciancalepore, C. Poly(lactic acid) and Its Blends for Packaging Application: A Review. Clean Technol. 2023, 5, 1304–1343. [Google Scholar] [CrossRef]
- Ninago, M.D.; López, O.V.; Lencina, M.M.S.; García, M.A.; Andreucetti, N.A.; Ciolino, A.E.; Villar, M.A. Enhancement of thermoplastic starch final properties by blending with poly(ε-caprolactone). Carbohydr. Polym. 2015, 134, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Arruda, T.R.; Machado, G.d.O.; Marques, C.S.; Souza, A.L.d.; Pelissari, F.M.; Oliveira, T.V.d.; Silva, R.R.A. An Overview of Starch-Based Materials for Sustainable Food Packaging: Recent Advances, Limitations, and Perspectives. Macromol 2025, 5, 19. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, P.; Chen, J.; Yan, Y.; Wu, Z. Recent Advances and Applications in Starch for Intelligent Active Food Packaging: A Review. Foods 2022, 11, 2879. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhao, Z.; Yang, M.; Lu, X.; Fu, L.; Jiang, G. Preparation and characterization of sodium cellulose sulfate/chitosan composite films loaded with curcumin for monitoring pork freshness. Curr. Res. Food Sci. 2022, 5, 1475–1483. [Google Scholar] [CrossRef]
- Anusha, G.D.; Meghana, V.G.; Devamani, M. A review on applications of cellulose and cellulose derivatives in food packaging. Int. J. Adv. Biochem. Res. 2025, SP-9, 805–813. [Google Scholar]
- Kasle, P.; Bains, A.; Goksen, G.; Dhull, S.B.; Ali, N.; Nagarik, R.; Fareed, M.; Chawla, P. Exploring the Properties of Carboxymethyl Cellulose Blended With Other Polymeric Compounds for the Formulation of Biodegradable Packaging Films and Edible Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70215. [Google Scholar] [CrossRef]
- Lino, R.C.; Matos de Carvalho, S.; Noronha, C.M.; Sganzerla, W.G.; Gonçalves da Rosa, C.; Nunes, M.R.; D’Avila, R.F.; Zambiazi, R.C.; Barreto, P.L.M. Production of methylcellulose films functionalized with poly-ε-caprolactone nanocapsules entrapped β-carotene for food packaging application. Food Res. Int. 2022, 160, 111750. [Google Scholar] [CrossRef]
- Irimia, A.; Grigoraș, V.C.; Popescu, C.-M. Active Cellulose-Based Food Packaging and Its Use on Foodstuff. Polymers 2024, 16, 389. [Google Scholar] [CrossRef]
- Santos, M.R.d.; Durval, I.J.B.; Medeiros, A.D.M.d.; Silva Júnior, C.J.G.d.; Converti, A.; Costa, A.F.d.S.; Sarubbo, L.A. Biotechnology in Food Packaging Using Bacterial Cellulose. Foods 2024, 13, 3327. [Google Scholar] [CrossRef]
- Indrayani, Y.; Suryanegara, L.; Sagiman, S.; Roslinda, E.; Marwanto, M. Short Communication: Biodegradable of bio-composites made from Polylactid Acid (PLA) and cellulose fibers from oil palm empty fruit bunch. Nusant. Biosci. 2019, 11, 8–11. [Google Scholar] [CrossRef]
- Beukelaer, H.d.; Hilhorst, M.; Workala, Y.; Maaskant, E.; Post, W. Overview of the mechanical, thermal and barrier properties of biobased and/or biodegradable thermoplastic materials. Polym. Test. 2022, 116, 107803. [Google Scholar] [CrossRef]
- Moldovan, A.; Cuc, S.; Prodan, D.; Rusu, M.; Popa, D.; Taut, A.C.; Petean, I.; Bombo¸s, D.; Doukeh, R.; Nemes, O. Development and Characterization of Polylactic Acid (PLA)-Based Nanocomposites Used for Food Packaging. Polymers 2023, 15, 2855. [Google Scholar] [CrossRef]
- O’Loughlin, J.; Doherty, D.; Herward, B.; McGleenan, C.; Mahmud, M.; Bhagabati, P.; Boland, A.N.; Freeland, B.; Rochfort, K.D.; Kelleher, S.M.; et al. The Potential of Bio-Based Polylactic Acid (PLA) as an Alternative in Reusable Food Containers: A Review. Sustainability 2023, 15, 15312. [Google Scholar] [CrossRef]
- Öz, A.T.; Süfer, Ö.; Çelebi, S.Y. Poly (Lactic Acid) Films in Food Packaging Systems. Food Sci. Nutr. Technol. 2017, 2, 000131. [Google Scholar] [CrossRef]
- Samrot, A.V.; Samanvitha, S.K.; Shobana, N.; Renitta, E.R.; Senthilkumar, P.; Kumar, S.S.; Abirami, S.; Dhiva, S.; Bavanilatha, M.; Prakash, P.; et al. The Synthesis, Characterization and Applications of Polyhydroxyalkanoates (PHAs) and PHA-Based Nanoparticles. Polymers 2021, 13, 3302. [Google Scholar] [CrossRef] [PubMed]
- Carvalheira, M.; Marreiros, B.C.; Reis, M.A.M. Chapter 13—Acids (VFAs) and bioplastic (PHA) recovery. In Clean Energy and Resource, Recovery; An, A., Tyagi, V., Kumar, M., Cetecioglu, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 245–254. [Google Scholar] [CrossRef]
- Sykacek, E.; O’Connor, K.; Omann, M.; Mundigler, N.; Neureiter, M. Pilot scale production and evaluation of mechanical and thermal properties of P(3HB) from Bacillus megaterium cultivated on desugarized sugar beet molasses. J. Appl. Polym. Sci. 2022, 139, e51503. [Google Scholar] [CrossRef]
- Kaya, E.C.; Yucel, U. Advances in Cellulose-Based Packaging Films for Food Products. In Cellulose—Fundamentals and Conversion into Biofuel and Useful Chemicals; Jeyakumar, R.B., Sankarapandian, K., Ravi, Y.K., Eds.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Romão, S.; Bettencourt, A.; Ribeiro, I.A.C. Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers 2022, 14, 4968. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Dorgan, J.R.; Lehermeier, H.; Mang, M. Thermal and rheological properties of commercial-grade poly(lactic Acid)s. J. Polym. Environ. 2000, 8, 1–9. [Google Scholar] [CrossRef]
- Gamiz-Conde, A.K.; Burelo, M.; Franco-Urquiza, E.A.; Martínez-Franco, E.; Luna-Barcenas, G.; Bravo-Alfaro, D.A.; Trevino-Quintanilla, C.D. Development and properties of bio-based polymer composites using PLA and untreated agro-industrial residues. Polym. Test. 2024, 139, 108576. [Google Scholar] [CrossRef]
- Oksiuta, Z.; Jalbrzykowski, M.; Mystkowska, J.; Romanczuk, E.; Osiecki, T. Mechanical and Thermal Properties of Polylactide (PLA) Composites Modified with Mg, Fe, and Polyethylene (PE) Additives. Polymers 2020, 12, 2939. [Google Scholar] [CrossRef]
- Dana, H.R.; Ebrahimi, F. Synthesis, properties, and applications of polylactic acid-based polymers. Polym. Eng. Sci. 2023, 63, 22. [Google Scholar] [CrossRef]
- Ferri, J.M.; Fenollar, O.; Jorda-Vilaplana, A.; García-Sanoguera, D.; Balart, R. Effect of miscibility on mechanical and thermal properties of poly(lactic acid)/polycaprolactone blends. Polym. Int. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Domenek, S.; Fernandes-Nassr, S.; Ducruet, V. Rheology, mechanical properties, and barrier properties of poly(lactic acid). In Synthesis, Structure and Properties of Poly(lactic Acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer International: Cham, Switzerland, 2018; pp. 303–341. [Google Scholar]
- Min, K.-E.; Jang, J.-W.; Kim, C.; Yi, S. Enhancement of Mechanical Properties of PCL/PLA/DMSO2 Composites for Bone Tissue Engineering. Appl. Sci. 2024, 14, 6190. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Fei, X.; Wang, T.; Zhang, X.; Xiao, Y.; Thitsartarn, W.; Tanoto, H.; He, C.; Li, Z. Innovative biomaterials for food packaging: Unlocking the potential of polyhydroxyalkanoate (PHA) biopolymers. Biomat. Adv. 2024, 163, 213929. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bunster, G.; Pavez, P. Novel Production Methods of Polyhydroxyalkanoates and Their Innovative Uses in Biomedicine and Industry. Molecules 2022, 27, 8351. [Google Scholar] [CrossRef]
- Larrañaga, A.; Fernández, J.; Vega, A.; Etxeberria, A.; Ronchel, C.; Adrio, J.L.; Sarasua, J.R. Crystallization and its effect on the mechanical properties of a medium chain length polyhydroxyalkanoate. J. Mech. Behav. Biomed. Mater. 2014, 39, 87–94. [Google Scholar] [CrossRef]
- Zaafouri, I.; Laurent, H.; Zrida, M.; Hamzaoui, A.H. Mechanical characterization of polyhydroxyalkanoate reinforced with Alfa fibers: Influence of fiber content and strain rate. J. Reinf. Plast. Compos. 2024, 44, 792–808. [Google Scholar] [CrossRef]
- Jaffur, B.N.; Kumar, G.; Khadoo, P. Production and functionalization strategies for superior polyhydroxybutyrate blend performance. Int. J. Biol. Macromol. 2024, 278, 134907. [Google Scholar] [CrossRef]
- Jayakumar, A.; Prabhu, K.; Shah, L.; Radha, P. Biologically and environmentally benign approach for PHB-silver nanocomposite synthesis and its characterization. Polym. Test. 2020, 81, 106197. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Quiles-Carrillo, L.; Balart, R.; Torres-Giner, S.; Arrieta, M.P. Innovative solutions and challenges to increase the use of Poly(3-hydroxybutyrate) in food packaging and disposables. Eur. Polym. J. 2022, 178, 111505. [Google Scholar] [CrossRef]
- Varghese, S.A.; Pulikkalparambil, H.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Novel biodegradable polymer films based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Ceiba pentandra natural fibers for packaging applications. Food Packag. Shelf Life 2020, 25, 100538. [Google Scholar] [CrossRef]
- Dedieu, I.; Aouf, C.; Gaucel, S.; Peyron, S. Recycled Poly(hydroxybutyrate-co-valerate) as Food Packaging: Effect of Multiple Melt Processing on Packaging Performance and Food Contact Suitability. J. Polym. Environ. 2023, 31, 1019–1028. [Google Scholar] [CrossRef]
- Ojha, N.; Das, N. Fabrication and characterization of biodegradable PHBV/SiO2 nanocomposite for thermo-mechanical and antibacterial applications in food packaging. IET Nanobiotechnol. 2020, 14, 785–795. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Effect of various plasticizers and concentration on the physical, thermal, mechanical, and structural properties of cassava-starch-based films. Starch/Stärke 2016, 68, 1500366. [Google Scholar] [CrossRef]
- Fazeli, M.; Keley, M.; Biazar, E. Preparation and characterization of starchbased composite films reinforced by cellulose nanofibers. Int. J. Biol. Macromol. 2018, 116, 272–280. [Google Scholar] [CrossRef]
- González, K.; Iturriaga, L.; González, A.; Eceiza, A.; Gabilondo, N. Improving mechanical and barrier properties of thermoplastic starch and polysaccharide nanocrystals nanocomposites. Eur. Polym. J. 2020, 123, 109415. [Google Scholar] [CrossRef]
- Almeida, T.; Karamysheva, A.; Valente, B.F.A.; Silva, J.M.; Braz, M.; Almeida, A.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Biobased ternary films of thermoplastic starch, bacterial nanocellulose and gallic acid for active food packaging. Food Hydrocoll. 2023, 144, 108934. [Google Scholar] [CrossRef]
- Dominic, M.C.D.; dos Santos Rosa, D.; Camani, P.H.; Kumar, A.S.; Neenu, K.V.; Begum, P.M.S.; Dinakaran, D.; John, E.; Baby, D.; Thomas, M.M.; et al. Thermoplastic starch nanocomposites using cellulose-rich Chrysopogon zizanioides nanofibers. Int. J. Biol. Macromol. 2021, 191, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Mileti, O.; Mammolenti, D.; Baldino, N.; Lupi, F.R.; Gabriele, D. Starch films loaded with tannin: The study of rheological and physical properties. Int. J. Biol. Macromol. 2024, 254, 127973. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, M.K.; Navasingh, R.J.H.; Selvam, J.D.R.; Čep, R. Development and characterization of starch bioplastics as a sustainable alternative for packaging. Sci. Rep. 2025, 15, 15264. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- De Moura, M.R.; Mattoso, L.H.C.; Zucolotto, V. Development of cellulose-based bactericidal nanocomposites containing silver nanoparticles and their use as active food packaging. J. Food Eng. 2012, 109, 520–524. [Google Scholar] [CrossRef]
- Jorda, J.; Kain, G.; Barbu, M.-C.; Köll, B.; Petutschnigg, A.; Král, P. Mechanical Properties of Cellulose and Flax Fiber Unidirectional Reinforced Plywood. Polymers 2022, 14, 843. [Google Scholar] [CrossRef]
- Hussain, S.A.; Yadav, M.P.; Sharma, B.K.; Qi, P.X.; Jin, T.Z. Biodegradable Food Packaging Films Using a Combination of Hemicellulose and Cellulose Derivatives. Polymers 2024, 16, 3171. [Google Scholar] [CrossRef]
- Sridhara, P.K.; Vilaseca, F. Assessment of Fiber Orientation on the Mechanical Properties of PA6/Cellulose Composite. Appl. Sci. 2020, 10, 5565. [Google Scholar] [CrossRef]
- Chen, G.; Liu, B. Cellulose sulfate based film with slow-release antimicrobial properties prepared by incorporation of mustard essential oil and β-cyclodextrin. Food Hydrocoll. 2016, 55, 100–107. [Google Scholar] [CrossRef]
- Vidal, O.L.; Tsukui, A.; Garrett, R.; Rocha-Leão, M.H.M.; Carvalho, C.W.P.; Freitas, S.P.; Moraes de Rezende, C.; Ferreira, M.S.L. Production of bioactive films of carboxymethyl cellulose enriched with green coffee oil and its residues. Int. J. Biol. Macromol. 2020, 146, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, I.; Vikman, M.; Harlin, A.; Orelma, H. Enzymatic Degradation and Pilot Scale Composting of Cellulose Based Films with Diferent Chemical Structures. J. Polym. Environ. 2020, 28, 458–470. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Michelin, M.; Vicente, A.A.; Teixeira, J.A.; Cerqueira, M.Â. Processing, Production Methods and Characterization of Bio-Based Packaging Materials. In Lignocellulosic Materials and Their Use in Bio-Based Packaging. Springer Briefs in Molecular Science; Springer: Cham, Switzerland, 2018; pp. 49–63. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to Polylactic Acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Singha, S.; Hedenqvist, M.S. A Review on Barrier Properties of Poly(Lactic Acid)/Clay Nanocomposites. Polymers 2020, 12, 1095. [Google Scholar] [CrossRef]
- Li, F.; Zhang, C.; Weng, Y. Improvement of the Gas Barrier Properties of PLA/OMMT Films by Regulating the Interlayer Spacing of OMMT and the Crystallinity of PLA. ACS Omega 2020, 5, 18675–18684. [Google Scholar] [CrossRef]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the Barrier Properties of PLA: A State-of-the-Art Review for Food Packaging Applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef] [PubMed]
- Sonchaeng, U.; Iñiguez-Franco, F.; Auras, R.; Selke, S.; Rubino, M.; Lim, L.T. Poly(lactic acid) mass transfer properties. Prog. Polym. Sci. 2018, 86, 85–121. [Google Scholar] [CrossRef]
- Mattioli, S.; Peltzer, M.; Fortunati, E.; Armentano, I.; Jiménez, A.; Kenny, J.M. Structure, gas-barrier properties and overall migration of poly(lactic acid) films coated with hydrogenated amorphous carbon layers. Carbon 2013, 63, 274–282. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Q.; Guo, C.; Guo, H. Comparative Study on Water Vapour Resistance of Poly(lactic acid) Films Prepared by Blending, Filling and Surface Deposit. Membranes 2021, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Pens, C.J.d.S.; Klug, T.V.; Stoll, L.; Izidoro, F.; Flores, S.H.; Rios, A.d.O. Poly(lactic acid) and its improved properties by some modifications for food packaging applications: A review. Food Packag. Shelf Life 2024, 41, 101230. [Google Scholar] [CrossRef]
- Stoll, L.; Domenek, S.; Flôres, S.H.; Nachtigall, S.M.B.; Rios, A.d.O. Polylactide films produced with bixin and acetyl tributyl citrate: Functional properties for active packaging. J. Appl. Polym. Sci. 2021, 138, 50302. [Google Scholar] [CrossRef]
- Mulla, M.Z.; Rahman, M.R.T.; Marcos, B.; Tiwari, B.; Pathania, S. Poly Lactic Acid (PLA) Nanocomposites: Effect of Inorganic Nanoparticles Reinforcement on Its Performance and Food Packaging Applications. Molecules 2021, 26, 1967. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.-F.; Rhim, J.-W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable biopolymers for active packaging: Demand, development and directions. Sustainable Food Technol. 2023, 1, 50–72. [Google Scholar] [CrossRef]
- Jahangiri, F.; Mohanty, A.K.; Misra, M. Sustainable biodegradable coatings for food packaging: Challenges and opportunities. Green Chem. 2024, 26, 4934–4974. [Google Scholar] [CrossRef]
- Keskin, G.; Kızıl, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of polyhydroxyalkanoate (PHA) polymers family as substitutes of petroleum based polymers for packaging applications and solutions brought by their composites to form barrier materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- European Bioplastics Association. Bioplastics Facts and Figures; European Bioplastics Association: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Papadopoulou, E.L.; Basnett, P.; Paul, U.C.; Marras, S.; Ceseracciu, L.; Roy, I.; Athanassiou, A. Green Composites of Poly(3-hydroxybutyrate) Containing Graphene Nanoplatelets with Desirable Electrical Conductivity and Oxygen Barrier Properties. ACS Omega 2019, 4, 19746–19755. [Google Scholar] [CrossRef]
- Chaiyaso, T.; Rachtanapun, P.; Thajai, N.; Kiattipornpithak, K.; Jantrawut, P.; Ruksiriwanich, W.; Seesuriyachan, P.; Leksawasdi, N.; Phimolsiripol, Y.; Techapun, C.; et al. Sericin cocoon bio-compatibilizer for reactive blending of thermoplastic cassava starch. Sci. Rep. 2021, 11, 945. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Ma, H.; Li, J.; Cao, D.; Xu, J.; Zeng, J.; Gao, W.; Chen, K. Enhancing the mechanical and barrier properties of starch-based green packaging films via acetalization reaction and hydrogen bond dual crosslinking. Carbohydr. Polym. 2025, 364, 123787. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Yang, B.; Fan, B.; Zhang, H.; Weng, Y. Enhancement of mechanical and barrier property of hemicellulose film via crosslinking with sodium trimetaphosphate. Polymers 2021, 13, 927. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xue, R. Nanostructured materials for smart food packaging: Integrating preservation and antimicrobial properties. Alex. Eng. J. 2025, 124, 446–461. [Google Scholar] [CrossRef]
- Dejene, B.K.; Gudayu, A.D.; Abtew, M.A. Development and optimization of sustainable and functional food packaging using false banana (Enset) fiber and zinc-oxide (ZnO) nanoparticle-reinforced polylactic acid (PLA) biocomposites: A case of injera preservation. Int. J. Biol. Macromol. 2024, 279, 135092. [Google Scholar] [CrossRef]
- Ignatova, L.; Brazhnikova, Y.; Omirbekova, A.; Usmanova, A. Polyhydroxyalkanoates (PHAs) from Endophytic Bacterial Strains as Potential Biocontrol Agents against Postharvest Diseases of Apples. Polymers 2023, 15, 2184. [Google Scholar] [CrossRef]
- Romero-Castelán, E.; Rodríguez-Hernández, A.-I.; Chavarría-Hernández, N.; López-Ortega, M.-A.; del Rocio López-Cuellar, M. Natural antimicrobial systems protected by complex polyhydroxyalkanoate matrices for food biopackaging applications—A review. Int. J. Biol. Macromol. 2023, 233, 123418. [Google Scholar] [CrossRef]
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.Ö.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From production to nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar] [CrossRef] [PubMed]
- Buntinx, M.; Vanheusden, C.; Hermans, D. Processing and Properties of Polyhydroxyalkanoate/ZnO Nanocomposites: A Review of Their Potential as Sustainable Packaging Materials. Polymers 2024, 16, 3061. [Google Scholar] [CrossRef]
- Ibrahim, M.I.; Alsafadi, D.; Alamry, K.A.; Oves, M.; Alosaimi, A.M.; Hussein, M.A. A promising antimicrobial bionanocomposite based poly(3-hydroxybutyrate-co-3-hydroxyvalerate) reinforced silver doped zinc oxide nanoparticles. Sci. Rep. 2022, 12, 14299. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Cerqueira, M.A. Active Carboxymethyl Cellulose-Based Edible Coatings for the Extension of Fresh Goldenberries Shelf-Life. Horticulturae 2022, 8, 936. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Liu, B.; Wang, K.; Li, H.; Peng, L. Carboxymethyl cellulose-based multifunctional film integrated with polyphenol-rich extract and carbon dots from coffee husk waste for active food packaging applications. Food Chem. 2024, 448, 139143. [Google Scholar] [CrossRef]
- Santos, D.C.; Ribeiro-Santos, R.; Ventura, L.A.F.; Melo, N.R.; Costa, B.S.; Rojas, E.E.G.; Salgado, N.L. Antimicrobial activity studies and characterization of cellulose acetate films containing essential oils. Ital. J. Food Sci. 2016, 8, 248–257. [Google Scholar]
- Barron, A.; Sparks, T.D. Commercial Marine-Degradable Polymers for Flexible Packaging. iScience 2020, 23, 101353. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, A.; Sarosi, I.; Cuc, S.; Prodan, D.; Taut, A.C.; Petean, I.; Bombos, D.; Doukeh, R.; Nemes, O.; Man, S.C. Development and characterization of PLA food packaging composite. J. Therm. Anal. Calorim. 2025, 150, 2469–2481. [Google Scholar] [CrossRef]
- Pinyaphong, P.; Sriburi, P. Optimum Condition for Polyhydroxyalkanoate Production from Crude Glycerol by Bacillus sp. Isolated from Lipid-Containing Wastewater. Trends Sci. 2022, 19, 2588. [Google Scholar] [CrossRef]
- Forfora, N.; Azuaje, I.; Kanipe, T.; Gonzalez, J.A.; Lendewig, M.; Urdaneta, I.; Venditti, R.; Gonzalez, R.; Argyropoulos, D. Are starch-based materials more eco-friendly than fossil-based? A critical assessment. Clean. Environ. Syst. 2024, 13, 100177. [Google Scholar] [CrossRef]
- Torre-Celeizabal, A.; Russo, F.; Galiano, F.; Alberto Figoli, A.; Casado-Coterillo, C.; Garea, A. Green Synthesis of Cellulose Acetate Mixed Matrix Membranes: Structure-Function Characterization. ACS Sustainable Chem. Eng. 2025, 13, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Majdi, H.; Gholizadeh, P.; Kafil, H.S.; Hamishehkar, H.; Zarchi, A.A.K.; Khoddami, A. An eco-friendly chitosan/cellulose acetate hybrid nanostructure containing Ziziphora clinopodioides essential oils for active food packaging applications. Int. J. Biol. Macromol. 2023, 235, 123885. [Google Scholar] [CrossRef]
- Sari, N.H.; Suteja, S.; Sapuan, S.M.; Ilyas, R.A. Chapter 14 Properties and Food Packaging Application of Poly-(Lactic) Acid; Sapuan, S.M., Ilyas, R.A., Eds.; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Mavai, S.; Bains, A.; Sridhar, K.; Rashid, S.; Elossaily, G.M.; Ali, N.; Chawla, P.; Sharma, M. Formulation and application of poly lactic acid, gum, and cellulose-based ternary bioplastic for smart food packaging: A review. Int. J. Biol. Macromol. 2024, 268, 131687. [Google Scholar] [CrossRef]
- Masood, F. Chapter 8—Polyhydroxyalkanoates in the Food Packaging Industry. In Nanotechnology Applications in Food. Flavor, Stability, Nutrition and Safety; Oprea, A.E., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 153–177. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Yazdian, F. Polyhydroxyalkanoates (PHAs) in Food Packaging. In Biodegradable Polymer-Based Food Packaging; Dutt Tripathi, A., Darani, K.K., Rai, D.C., Paul, V., Eds.; Springer: Singapore, 2022; pp. 115–122. [Google Scholar] [CrossRef]
- Agarwal, S.; Singhal, S.; Godiya, C.B.; Kumar, S. Prospects and Applications of Starch Based Biopolymers. Int. J. Environ. Anal. Chem. 2023, 103, 6907–6926. [Google Scholar] [CrossRef]
- Mallick, N.; Pattanayak, D.R.; Soni, A.B.; Pal, D. Starch based polymeric composite for food packaging applications. Int. J. Eng. Res. 2020, 10, 11–34. [Google Scholar] [CrossRef]
- Yudhistira, B.; Husnayain, N.; Punthi, F.; Gavahian, M.; Chang, C.K.; Hsieh, C.W. Progress in the application of emerging technology for the improvement of starch-based active packaging properties: A review. ACS Food Sci. Technol. 2024, 4, 1997–2012. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Ye, J.; Li, Y.; Wang, L.; Cao, R. Advances in Starch-Based Nanocomposites for Functional Food Systems: Harnessing AI and Nuclear Magnetic Resonance Technologies for Tailored Stability and Bioactivity. Foods 2025, 14, 773. [Google Scholar] [CrossRef]
- Das, P.P.; Kalyani, P.; Kumar, R.; Khandelwal, M. Cellulose-based natural nanofibers for fresh produce packaging: Current status, sustainability and future outlook. Sustain. Food Technol. 2023, 1, 528–544. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, Q.-Y.; Lou, C.-W.; Lin, J.-H.; Li, T.-T. Recent Advances of Cellulose-Based Hydrogels Combined with Natural Colorants in Smart Food Packaging. Gels 2024, 10, 755. [Google Scholar] [CrossRef]
- Boey, J.Y.; Mohamad, L.; Khok, Y.S.; Tay, G.S.; Baidurah, S. A Review of the Applications and Biodegradation of Polyhydroxyalkanoates and Poly(lactic acid) and Its Composites. Polymers 2021, 13, 1544. [Google Scholar] [CrossRef] [PubMed]
- Latos, M.; Masek, A.; Zaborski, M. The potential of juglone as natural dye and indicator for biodegradable polyesters. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2019, 233, 276–285. [Google Scholar] [CrossRef]
- Yuhana, N.Y.; Zaharah, E.; Zawawi, E. Review of bioplastics as food packaging materials. AIMS Mater. Sci. 2021, 8, 166–184. [Google Scholar] [CrossRef]
- Zarski, A.; Bajer, K.; Kapuśniak, J. Review of the Most Important Methods of Improving the Processing Properties of Starch toward Non-Food Applications. Polymers 2021, 13, 832. [Google Scholar] [CrossRef] [PubMed]
- Congli, C.; Na, J.; Yanfei, W.; Liu, X.; Sun, Q. Bioactive and intelligent starch-based films: A review. Trends Food Sci. Technol. 2021, 116, 854–869. [Google Scholar] [CrossRef]
- Meng, L.; Liu, H.; Yu, L.; Duan, Q.; Chen, L.; Liu, F.; Shao, Z.; Shi, K.; Lin, X. How water acting as both blowing agent and plasticizer affect on starch-based foam. Ind. Crops Prod. 2019, 134, 43–49. [Google Scholar] [CrossRef]
- Zehra Kuru, Z.; Kaya, M.A. Poly(Lactic Acid)/Polyester Blends: Review of Current and Future Applications. Eur. J. Dev. Res. 2023, 3, 175–199. [Google Scholar] [CrossRef]

| Bio-Based Polymer | Advantages |
|---|---|
| Polylactic Acid (PLA) |
|
| Polyhydroxyalkanoates (PHAs) |
|
| Starch-based polymers |
|
| Cellulose-based polymers |
|
| Bio-Based Polymer | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) | Refs. |
|---|---|---|---|---|
| PLA | 50–62 | 3453–3750 | 1.2–7.8 | [109,110,111] |
| PLA with PCL | 46–55 | 2687–3555 | 18–65 | [111,113] |
| PHA | 14–40 | 3500–4622 | 0.4–2 | [116,117] |
| PHB | 20–45 | 800–2600 | 2–8 | [118,119,120] |
| PHBV | 2–21 | 1100–1300 | 30–44 | [121,122,123] |
| TPS with CNFs | 10–18 | 53–144 | 5.3–8.7 | [125] |
| TPS with CNCs | 1.7–2.4 | 28–33 | 77–81 | [126] |
| TPS with BNC and gallic acid | 23–39 | 1200–2000 | 3.4–4.1 | [127] |
| Cellulose mixture with filler | 38–52 | 989–1020 | 4.8–8 | [132] |
| Cellulose pulp fibers with polyamide | 48–66 | 4400–4600 | 3.5–6 | [135] |
| Bio-Based Polymer | Disadvantages |
|---|---|
| Polylactic Acid (PLA) |
|
| Polyhydroxyalkanoates (PHAs) |
|
| Starch-based polymers |
|
| Cellulose-based polymers |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şomoghi, R.; Mihai, S.; Oancea, F. An Overview of Bio-Based Polymers with Potential for Food Packaging Applications. Polymers 2025, 17, 2335. https://doi.org/10.3390/polym17172335
Şomoghi R, Mihai S, Oancea F. An Overview of Bio-Based Polymers with Potential for Food Packaging Applications. Polymers. 2025; 17(17):2335. https://doi.org/10.3390/polym17172335
Chicago/Turabian StyleŞomoghi, Raluca, Sonia Mihai, and Florin Oancea. 2025. "An Overview of Bio-Based Polymers with Potential for Food Packaging Applications" Polymers 17, no. 17: 2335. https://doi.org/10.3390/polym17172335
APA StyleŞomoghi, R., Mihai, S., & Oancea, F. (2025). An Overview of Bio-Based Polymers with Potential for Food Packaging Applications. Polymers, 17(17), 2335. https://doi.org/10.3390/polym17172335







