Recent Progress in Cellulose Nanofibril Hydrogels for Biomedical Applications
Abstract
1. Introduction
2. Methods for Preparing CNF Hydrogels
2.1. Physical Crosslinking Methods
2.2. Chemical Crosslinking Methods
3. Biomedical Applications of CNF Hydrogels
3.1. Drug Delivery Systems
3.1.1. Stimuli-Responsive Hydrogels
3.1.2. Composite and Nanocomposite Hydrogels

3.1.3. Injectable and Localized CNF Hydrogels
3.1.4. Sustained and Sequential Release Hydrogels
3.2. Tissue Engineering
3.2.1. Scaffold Materials
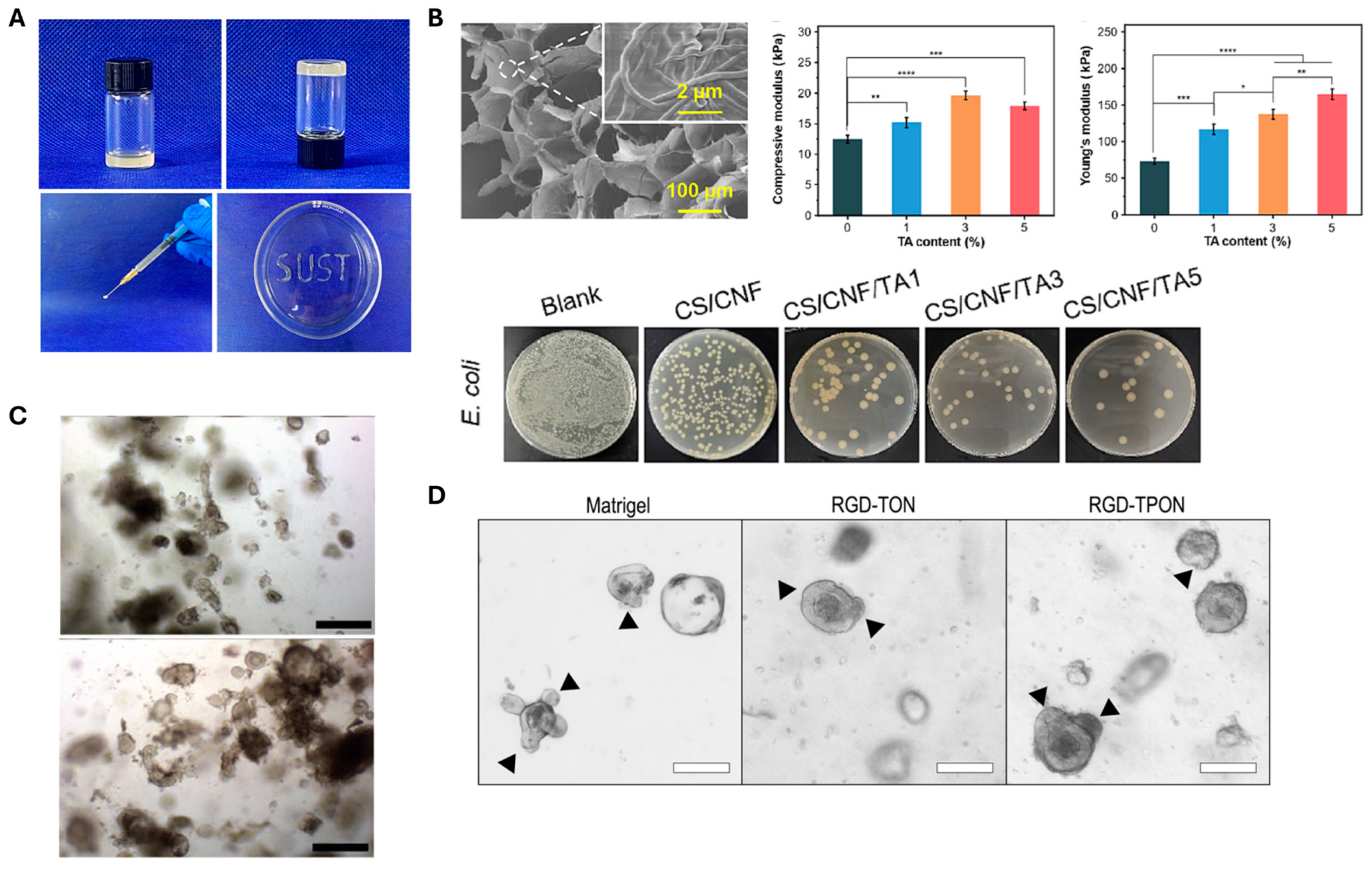
3.2.2. Wound Dressing
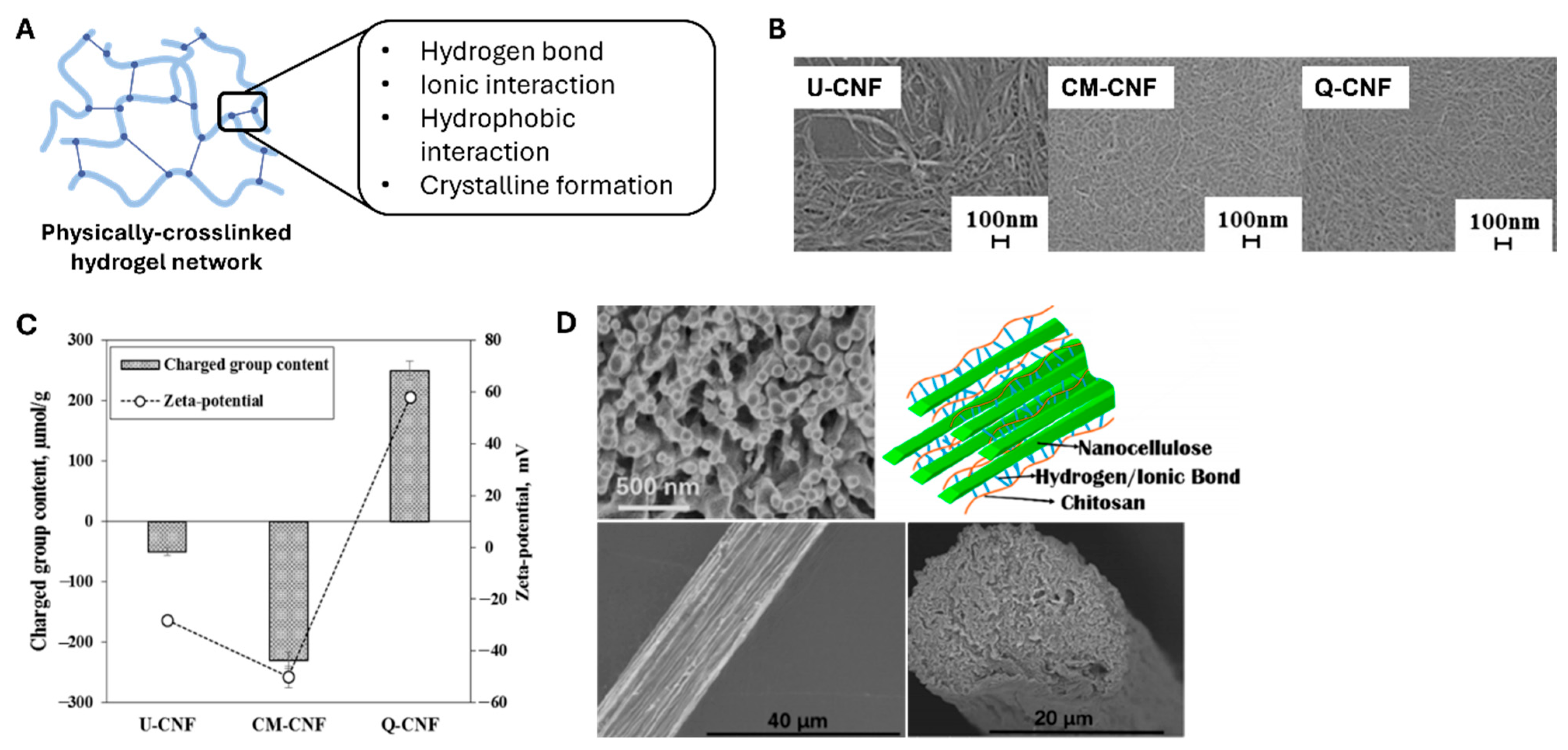
3.2.3. Three-Dimensional Cell Culture Platforms
| Hydrogel Composition/Type | Formation Method | Functionalization | Cell Type | References |
|---|---|---|---|---|
| TEMPO-oxidized CNF/injectable thixogel | Self-assembly (without added crosslinker) | - | Human breast cancer (MCF-7) and mouse embryonic stem cells (mESC; E14TG2A) | [90] |
| TEMPO-oxidized CNF/Injectable thixogel | Self-assembly (without added crosslinker) | - | Human liver organoid | [78] |
| TEMPO-oxidized nanofibrillated cellulose (NFC)/bulk gel | Self-assembly (without added crosslinker) | - | Mesenchymal stem cells (MSCs) | [92] |
| TEMPO-oxidized CNF/bulk gel | Calcium ion (Ca2+) crosslinking | - | Pre-osteoblast cells (MC3T3-E1) | [91] |
| TEMPO-oxidized CNF/bulk gel | Calcium ion (Ca2+) crosslinking | Fibronectin-derived moieties (RGD peptides), laminin-1, insulin-like growth factor (IGF-1) | Small intestinal organoids | [93] |
| TEMPO-oxidized (TON) and TEMPO/periodate-oxidized (TPON) CNF/bulk gel | Magnesium (Mg2+) and calcium ion (Ca2+) crosslinking | Fibronectin-derived moieties (RGD peptides) | Intestinal organoids | [79] |
| TEMPO-oxidized CNF/microgel | Calcium ion (Ca2+) crosslinking | Hyaluronic acid (HA) | Human adipose-derived stem cell (hADSC) | [94] |
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNF | Cellulose nanofibril |
| CNC | Cellulose nanocrystal |
| TEMPO | 2,2,6,6-tetramethyl-1-piperidinyloxy |
| SEM | Scanning electron microscope |
| U-CNF | Untreated CNF |
| CM-CNF | Carboxymethylated CNF |
| Q-CNF | Quaternized CNF |
| PVP | Polyvinylpyrrolidone |
| PEG | Poly (ethylene glycol) |
| PNIPAm | Poly(N-isopropylacrylamide) |
| SA | Sodium alginate |
| TH | Tetracycline hydrochloride |
| MPDA | Mesoporous polydopamine |
| GO | Graphene oxide |
| TOCN | TEMPO-oxidized CNF |
| PPy | Polypyrrole |
References
- Khan, M.I.; An, X.; Dai, L.; Li, H.; Khan, A.; Ni, Y. Chitosan-based polymer matrix for pharmaceutical excipients and drug delivery. Curr. Med. Chem. 2019, 26, 2502–2513. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect European. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: A review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828. [Google Scholar] [CrossRef]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent strategies in preparation of cellulose nanocrystals and cellulose nanofibrils derived from raw cellulose materials. Int. J. Polym. Sci. 2018, 2018, 7923068. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Wågberg, L.; Decher, G.; Norgren, M.; Lindström, T.; Ankerfors, M.; Axnäs, K. The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 2008, 24, 784–795. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Aulin, C.; Ahola, S.; Josefsson, P.; Nishino, T.; Hirose, Y.; Osterberg, M.; Wagberg, L. Nanoscale Cellulose Films with Different Crystallinities and Mesostructures—Their Surface Properties and Interaction with Water. Langmuir 2009, 25, 7675–7685. [Google Scholar] [CrossRef]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Green, J.J.; Elisseeff, J.H. Mimicking biological functionality with polymers for biomedical applications. Nature 2016, 540, 386–394. [Google Scholar] [CrossRef]
- Haycock, J.W. 3D cell culture: A review of current approaches and techniques. 3D Cell Cult. Methods Protoc. 2010, 695, 1–15. [Google Scholar]
- Fu, N.; Zhang, X.; Sui, L.; Liu, M.; Lin, Y. Application of Scaffold Materials in Cartilage Tissue Engineering. In Cartilage Regeneration. Stem Cell Biology and Regenerative Medicine; Lin, Y., Ed.; Humana Press: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Mendoza, L.; Batchelor, W.; Tabor, R.F.; Garnier, G. Gelation mechanism of cellulose nanofibre gels: A colloids and interfacial perspective. J. Colloid. Interface Sci. 2018, 509, 39–46. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Im, W.; Park, S.Y.; Goo, S.; Yook, S.; Lee, H.L.; Yang, G.; Youn, H.J. Incorporation of CNF with different charge property into PVP hydrogel and its characteristics. Nanomaterials 2021, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Geng, L.; Chen, S.; Shi, S.; Hsiao, B.S.; Peng, X. Hierarchical assembly of nanocellulose into filaments by flow-assisted alignment and interfacial complexation: Conquering the conflicts between strength and toughness. ACS Appl. Mater. Interfaces 2020, 12, 32090–32098. [Google Scholar] [CrossRef]
- Fall, A.B.; Lindstrom, S.B.; Sundman, O.; Odberg, L.; Wagberg, L. Colloidal stability of aqueous nanofibrillated cellulose dispersions. Langmuir 2011, 27, 11332–11338. [Google Scholar] [CrossRef]
- Ureña-Benavides, E.E.; Ao, G.; Davis, V.A.; Kitchens, C.L. Rheology and phase behavior of lyotropic cellulose nanocrystal suspensions. Macromolecules 2011, 44, 8990–8998. [Google Scholar] [CrossRef]
- Håkansson, K.M.; Fall, A.B.; Lundell, F.; Yu, S.; Krywka, C.; Roth, S.V.; Santoro, G.; Kvick, M.; Wittberg, L.P.; Wågberg, L. Hydrodynamic alignment and assembly of nanofibrils resulting in strong cellulose filaments. Nat. Commun. 2014, 5, 4018. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green. Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Navarra, M.A.; Bosco, C.D.; Moreno, J.S.; Vitucci, F.M.; Paolone, A.; Panero, S. Synthesis and characterization of cellulose-based hydrogels to be used as gel electrolytes. Membranes 2015, 5, 810–823. [Google Scholar] [CrossRef]
- Del Valle, L.J.; Díaz, A.; Puiggalí, J. Hydrogels for biomedical applications: Cellulose, chitosan, and protein/peptide derivatives. Gels 2017, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.; Medronho, B.; Alves, L.; Costa, C.; Edlund, H.; Norgren, M. The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys. Chem. Chem. Phys. 2017, 19, 23704–23718. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Cohen, Y.; Garnier, G.; Garvey, C.J.; Russell, R.A.; Darwish, T.; Garnier, G. Cellulose dissolution in ionic liquid: Ion binding revealed by neutron scattering. Macromolecules 2018, 51, 7649–7655. [Google Scholar] [CrossRef]
- Mohd, N.; Draman, S.; Salleh, M.; Yusof, N. Dissolution of cellulose in ionic liquid: A review. AIP Conf. Proc. 2017, 1809, 020035. [Google Scholar]
- Heinze, T.; Koschella, A. Solvents applied in the field of cellulose chemistry: A mini review. Polímeros 2005, 15, 84–90. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid dissolution of cellulose in LiOH/urea and NaOH/urea aqueous solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, M.; Zhang, W.; Guo, W.; Zhang, X.; Zhang, B. Facile preparation of irradiated poly (vinyl alcohol)/cellulose nanofiber hydrogels with ultrahigh mechanical properties for artificial joint cartilage. Materials 2024, 17, 4125. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, C.; Yong, L.C.; Sun, N.; Liu, F.G. Flexible and Transparent PVA/CNF Hydrogel with Ultrahigh Dielectric Constant. ACS Appl. Polym. Mater. 2024, 6, 5706–5713. [Google Scholar] [CrossRef]
- Takeno, H.; Inoguchi, H.; Hsieh, W.-C. Mechanical and structural properties of cellulose nanofiber/poly (vinyl alcohol) hydrogels cross-linked by a freezing/thawing method and borax. Cellulose 2020, 27, 4373–4387. [Google Scholar] [CrossRef]
- Hobzova, R.; Hrib, J.; Sirc, J.; Karpushkin, E.; Michalek, J.; Janouskova, O.; Gatenholm, P. Embedding of bacterial cellulose nanofibers within PHEMA hydrogel matrices: Tunable stiffness composites with potential for biomedical applications. J. Nanomater. 2018, 2018, 5217095. [Google Scholar] [CrossRef]
- Syverud, K.; Kirsebom, H.; Hajizadeh, S.; Chinga-Carrasco, G. Cross-linking cellulose nanofibrils for potential elastic cryo-structured gels. Nanoscale Res. Lett. 2011, 6, 626. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef]
- Plackett, D.; Letchford, K.; Jackson, J.; Burt, H. A review of nanocellulose as a novel vehicle for drug delivery. Nord. Pulp Pap. Res. J. 2014, 29, 105–118. [Google Scholar] [CrossRef]
- Xue, Y.; Mou, Z.; Xiao, H. Nanocellulose as a sustainable biomass material: Structure, properties, present status and future prospects in biomedical applications. Nanoscale 2017, 9, 14758–14781. [Google Scholar] [CrossRef] [PubMed]
- Masruchin, N.; Park, B.-D.; Causin, V. Dual-responsive composite hydrogels based on TEMPO-oxidized cellulose nanofibril and poly (N-isopropylacrylamide) for model drug release. Cellulose 2018, 25, 485–502. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, C.; Zhou, W.; Luan, Q.; Li, W.; Deng, Q.; Dong, X.; Tang, H.; Huang, F. A pH-responsive gel macrosphere based on sodium alginate and cellulose nanofiber for potential intestinal delivery of probiotics. ACS Sustain. Chem. Eng. 2018, 6, 13924–13931. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, H.; Wang, X.; Liu, J.; Liu, G.; Meng, X.; Lin, S. Oxidized cellulose-filled double thermo/pH-sensitive hydrogel for local chemo-photothermal therapy in breast cancer. Carbohydr. Polym. 2024, 332, 121931. [Google Scholar] [CrossRef] [PubMed]
- Lem, O.; Gangurde, P.; Koivuniemi, A.; Keskinen, A.; Efimov, A.; Durandin, N.; Laaksonen, T. Far-red light-triggered cargo release from liposomes b ound to a photosensitizer-cellulose nanofiber hydrogel. Carbohydr. Polym. 2024, 336, 122134. [Google Scholar] [CrossRef]
- Park, D.; Kim, J.W.; Shin, K.; Kim, J.W. Bacterial cellulose nanofibrils-reinforced composite hydrogels for mechanical compression-responsive on-demand drug release. Carbohydr. Polym. 2021, 272, 118459. [Google Scholar] [CrossRef]
- Osman, N.; Devnarain, N.; Omolo, C.A.; Fasiku, V.; Jaglal, Y.; Govender, T. Surface modification of nano-drug delivery systems for enhancing antibiotic delivery and activity. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2022, 14, e1758. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Q.; Huo, Y.; Liu, C.; Li, B.; Li, Y. Construction of a mesoporous polydopamine@ GO/cellulose nanofibril composite hydrogel with an encapsulation structure for controllable drug release and toxicity shielding. ACS Appl. Mater. Interfaces 2020, 12, 57410–57420. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Yan, C.; Wu, J.; Wang, Y.; Jiang, M.; Wang, Y. Yunnan Baiyao-enhanced cellulose nanofiber composite hydrogel wearable patch for transdermal drug delivery and anti-freezing applications. Int. J. Biol. Macromol. 2025, 315, 144684. [Google Scholar] [CrossRef]
- Bora, A.; Sarmah, D.; Rather, M.A.; Mandal, M.; Karak, N. Nanocomposite of starch, gelatin and itaconic acid-based biodegradable hydrogel and ZnO/cellulose nanofiber: A pH-sensitive sustained drug delivery vehicle. Int. J. Biol. Macromol. 2024, 256, 128253. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, R.; Zhao, C.; Quan, Z.; Zhu, H.; Wang, L.; Bu, Q.; He, Y.; He, H. A novel medically imageable intelligent cellulose nanofibril-based injectable hydrogel for the chemo-photothermal therapy of tumors. Chem. Eng. J. 2022, 431, 133255. [Google Scholar] [CrossRef]
- Bai, W.; Chen, H.; Li, J.; Cai, W.; Kong, Y.; Zuo, X. Calcium carbonate hollow microspheres encapsulated cellulose nanofiber/sodium alginate hydrogels as a sequential delivery system. Int. J. Biol. Macromol. 2025, 309, 142839. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H.; Wei, H.; Yu, C.-Y. Injectable hydrogels as emerging drug-delivery platforms for tumor therapy. Biomater. Sci. 2024, 12, 1151–1170. [Google Scholar] [CrossRef] [PubMed]
- Laurén, P.; Lou, Y.-R.; Raki, M.; Urtti, A.; Bergström, K.; Yliperttula, M. Technetium-99m-labeled nanofibrillar cellulose hydrogel for in vivo drug release. Eur. J. Pharm. Sci. 2014, 65, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zelikin, A.N.; Ehrhardt, C.; Healy, A.M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016, 8, 997–1007. [Google Scholar] [CrossRef]
- Paukkonen, H.; Kunnari, M.; Laurén, P.; Hakkarainen, T.; Auvinen, V.-V.; Oksanen, T.; Koivuniemi, R.; Yliperttula, M.; Laaksonen, T. Nanofibrillar cellulose hydrogels and reconstructed hydrogels as matrices for controlled drug release. Int. J. Pharm. 2017, 532, 269–280. [Google Scholar] [CrossRef]
- Lin, N.; Gèze, A.; Wouessidjewe, D.; Huang, J.; Dufresne, A. Biocompatible double-membrane hydrogels from cationic cellulose nanocrystals and anionic alginate as complexing drugs codelivery. ACS Appl. Mater. Interfaces 2016, 8, 6880–6889. [Google Scholar] [CrossRef]
- Liu, H.; Liu, K.; Han, X.; Xie, H.; Si, C.; Liu, W.; Bae, Y. Cellulose nanofibrils-based hydrogels for biomedical applications: Progresses and challenges. Curr. Med. Chem. 2020, 27, 4622–4646. [Google Scholar] [CrossRef]
- Govindarasu, M.; Palanisamy, S.; Joy, J.G.; Sharma, G.; You, S.; Kim, J.-C. Advances of nanocellulose and cellulose-based derivatives for biomedical applications. Cellulose 2025, 32, 5735–5762. [Google Scholar] [CrossRef]
- Bazghaleh, A.A.; Dogolsar, M.A.; Salehi, R.; Barzin, J. Synthesis and characterization of an injectable, self-healing hydrogel based on succinyl chitosan, oxidized pectin, and cellulose nanofiber for biomedical applications. J. Polym. Res. 2025, 32, 63. [Google Scholar] [CrossRef]
- Shanto, P.C.; Park, S.; Park, M.; Lee, B.-T. Physico-biological evaluation of 3D printed dECM/TOCN/alginate hydrogel based scaffolds for cartilage tissue regeneration. Biomater. Adv. 2023, 145, 213239. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, S.; Coseri, S. An injectable and self-healing cellulose nanofiber-reinforced alginate hydrogel for bone repair. Carbohydr. Polym. 2023, 300, 120243. [Google Scholar] [CrossRef]
- Liu, X.; Hu, H.; Ma, J.; Wang, B. Mineralized cellulose nanofibers reinforced bioactive hydrogel remodels the osteogenic and angiogenic microenvironment for enhancing bone regeneration. Carbohydr. Polym. 2025, 357, 123480. [Google Scholar] [CrossRef] [PubMed]
- Doench, I.; Torres-Ramos, M.E.; Montembault, A.; de Oliveira, P.N.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.M.; Osorio-Madrazo, A. Injectable and gellable chitosan formulations filled with cellulose nanofibers for intervertebral disc tissue engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Doench, I.; Tran, T.A.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Sudre, G.; Cachon, T.; Louback-Mohamed, M.; Horbelt, N. Cellulose nanofiber-reinforced chitosan hydrogel composites for intervertebral disc tissue repair. Biomimetics 2019, 4, 19. [Google Scholar] [CrossRef]
- Jeencham, R.; Tawonsawatruk, T.; Numpaisal, P.-O.; Ruksakulpiwat, Y. Reinforcement of injectable hydrogel for meniscus tissue engineering by using cellulose nanofiber from cassava pulp. Polymers 2023, 15, 2092. [Google Scholar] [CrossRef] [PubMed]
- Sinna, J.; Jeencham, R.; Mueangkhot, P.; Sophon, S.; Noralak, P.; Raksapakdee, R.; Numpaisal, P.-O.; Ruksakulpiwat, Y. Development of Poly (vinyl alcohol) Grafted Glycidyl Methacrylate/Cellulose Nanofiber Injectable Hydrogels for Meniscus Tissue Engineering. Polymers 2023, 15, 4230. [Google Scholar] [CrossRef]
- Tohidi, H.; Maleki-Jirsaraei, N.; Simchi, A.; Mohandes, F.; Emami, Z.; Fassina, L.; Naro, F.; Conti, B.; Barbagallo, F. An electroconductive; thermosensitive, and injectable chitosan/pluronic/gold-decorated cellulose nanofiber hydrogel as an efficient carrier for regeneration of cardiac tissue. Materials 2022, 15, 5122. [Google Scholar] [CrossRef]
- Hou, R.; Xie, Y.; Song, R.; Bao, J.; Shi, Z.; Xiong, C.; Yang, Q. Nanocellulose/polypyrrole hydrogel scaffolds with mechanical strength and electrical activity matching native cardiac tissue for myocardial tissue engineering. Cellulose 2024, 31, 4247–4262. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Rafat, M.; Moustardas, P.; Mukwaya, A.; Tabe, S.; Bellisario, M.; Peebo, B.; Lagali, N. A double-crosslinked nanocellulose-reinforced dexamethasone-loaded collagen hydrogel for corneal application and sustained anti-inflammatory activity. Acta Biomater. 2023, 172, 234–248. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Huang, C.-F.; Wei, Y.; Hsu, S.-H. Novel chitosan–cellulose nanofiber self-healing hydrogels to correlate self-healing properties of hydrogels with neural regeneration effects. NPG Asia Mater. 2019, 11, 25. [Google Scholar] [CrossRef]
- Li, Y.; Xun, X.; Duan, L.; Gao, E.; Li, J.; Lin, L.; Li, X.; He, A.; Ao, H.; Xu, Y. Cartilage structure-inspired nanofiber-hydrogel composite with robust proliferation and stable chondral lineage-specific differentiation function to orchestrate cartilage regeneration for artificial tracheal construction. Bioact. Mater. 2025, 47, 136–151. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Dong, X.; Liu, X.; Lin, H.; Yang, D.; Shi, X.; Chen, C.; Tao, F.; Jiang, L.; Deng, H. Cellulose nanofibers embedded chitosan/tannin hydrogel with high antibacterial activity and hemostatic ability for drug-resistant bacterial infected wound healing. Carbohydr. Polym. 2024, 329, 121687. [Google Scholar] [CrossRef]
- Krüger, M.; Oosterhoff, L.A.; van Wolferen, M.E.; Schiele, S.A.; Walther, A.; Geijsen, N.; De Laporte, L.; van der Laan, L.J.; Kock, L.M.; Spee, B. Cellulose nanofibril hydrogel promotes hepatic differentiation of human liver organoids. Adv. Healthc. Mater. 2020, 9, 1901658. [Google Scholar] [CrossRef]
- Curvello, R.; Garnier, G. Cationic cross-linked nanocellulose-based matrices for the growth and recovery of intestinal organoids. Biomacromolecules 2020, 22, 701–709. [Google Scholar] [CrossRef]
- Goh, M.; Hwang, Y.; Tae, G. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr. Polym. 2016, 147, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; De Angelis, B.; Pea, F.; Scalise, A.; Stefani, S.; Tasinato, R.; Zanetti, O.; Paola, L.D. Challenges in the management of chronic wound infections. J. Glob. Antimicrob. Resist. 2021, 26, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- Masoud, A.R.; Velisdeh, Z.J.; Bappy, M.J.P.; Pandey, G.; Saberian, E.; Mills, D.K. Cellulose-Based Nanofibers in Wound Dressing. Biomimetics 2025, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jin, L.; Xu, Q. Nanocellulose strengthened glucose-responsive, antioxidant and antibacterial hydrogels for wound dressings. Cellulose 2025, 32, 5575–5593. [Google Scholar] [CrossRef]
- Zhong, Y.; Seidi, F.; Li, C.; Wan, Z.; Jin, Y.; Song, J.; Xiao, H. Antimicrobial/biocompatible hydrogels dual-reinforced by cellulose as ultrastretchable and rapid self-healing wound dressing. Biomacromolecules 2021, 22, 1654–1663. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Z.; Liu, K.; Ji, X.; Fatehi, P.; Chen, J. A cellulose nanofibril-reinforced hydrogel with robust mechanical, self-healing, pH-responsive and antibacterial characteristics for wound dressing applications. J. Nanobiotechnol. 2022, 20, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gatsi, B.; Yao, X.; Jin, Y.; Amhal, H. Cellulose nanofiber-reinforced antimicrobial and antioxidant multifunctional hydrogel with self-healing, adhesion for enhanced wound healing. Carbohydr. Polym. 2025, 352, 123189. [Google Scholar] [CrossRef]
- Hong, G.; Li, J.; Wei, W.; Wu, Y.; Li, L.; Chen, Y.; Xie, D.; Qu, Q.; Rojas, O.J.; Hu, G. Starfish-Inspired Synergistic Reinforced Hydrogel Wound Dressing: Dual Responsiveness and Enhanced Bioactive Compound Delivery for Advanced Skin Regeneration and Management. ACS Nano 2025, 19, 10180–10198. [Google Scholar] [CrossRef]
- Zhang, B.; Duan, W.; Wang, Y.; Dai, L.; Cai, B.; Kong, L.; Fan, J.; Zhang, G.; Wang, L.; Wu, W. Recent advances of cellulose nanofiber-based materials in cell culture: From population to single-cell. TrAC Trends Anal. Chem. 2023, 166, 117159. [Google Scholar] [CrossRef]
- Sanandiya, N.D.; Vasudevan, J.; Das, R.; Lim, C.T.; Fernandez, J.G. Stimuli-responsive injectable cellulose thixogel for cell encapsulation. Int. J. Biol. Macromol. 2019, 130, 1009–1017. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, D.X.; Choy, S.; Nguyen, H.-L.; Cha, H.J.; Hwang, D.S. 3D cellulose nanofiber scaffold with homogeneous cell population and long-term proliferation. Cellulose 2018, 25, 7299–7314. [Google Scholar] [CrossRef]
- Nikolits, I.; Radwan, S.; Liebner, F.; Dietrich, W.; Egger, D.; Chariyev-Prinz, F.; Kasper, C. Hydrogels from TEMPO-oxidized nanofibrillated cellulose support in vitro cultivation of encapsulated human mesenchymal stem cells. ACS Appl. Bio Mater. 2023, 6, 543–551. [Google Scholar] [CrossRef]
- Curvello, R.; Kerr, G.; Micati, D.J.; Chan, W.H.; Raghuwanshi, V.S.; Rosenbluh, J.; Abud, H.E.; Garnier, G. Engineered plant-based nanocellulose hydrogel for small intestinal organoid growth. Adv. Sci. 2021, 8, 2002135. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.; Tae, G. Mesenchymal stem cell-encapsulated cellulose nanofiber microbeads and enhanced biological activities by hyaluronic acid incorporation. Carbohydr. Polym. 2022, 280, 119026. [Google Scholar] [CrossRef]
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Mäkitie, A.; Luukko, K. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 2016, 244, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Bernardes, J.S.; Fávaro, W.J.; Paula, A.J.; Durán, N. Cellulose nanocrystals as carriers in medicine and their toxicities: A review. Carbohydr. Polym. 2018, 181, 514–527. [Google Scholar] [CrossRef]
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.-J.; Han, C.-R.; Duan, J.-F.; Xu, F.; Sun, R.-C. Tough nanocomposite hydrogels from cellulose nanocrystals/poly (acrylamide) clusters: Influence of the charge density, aspect ratio and surface coating with PEG. Cellulose 2014, 21, 541–551. [Google Scholar] [CrossRef]
- Sun, B.; Hou, Q.; Liu, Z.; Ni, Y. Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 2015, 22, 1135–1146. [Google Scholar] [CrossRef]


| Hydrogel Composition/Type | Formation Method | Tissue Type | Role of CNF | Key Properties/Application | References |
|---|---|---|---|---|---|
| Poly(vinyl alcohol) (PVA), CNF | γ-ray irradiation, annealing, and rehydration | Cartilage |
|
| [35] |
| TEMPO-oxidized cellulose nanofiber (TOCN), Decellularized extracellular matrix (dECM), Sodium alginate (SA)/3D printable | Two-step calcium ion (Ca2+) crosslinking |
|
| [64] | |
| Oxidized alginate (OSA), Gelatin (Gel), and CNF/Injectable and self-healing | One-step Schiff base reaction (aldehyde–amine) | Bone |
|
| [65] |
| Enzymatically mineralized TEMPO-oxidized bacterial cellulose nanofibers (m-TOBC), Mesoporous silica nanoparticles (MSNs) loaded with the angiogenic drug dimethyloxalylglycine (DMOG), Gelatin methacryloyl (GelMA)/3D printable | Visible light (405 nm) crosslinking after 3D printing |
|
| [66] | |
| Viscous chitosan (CHI), CNF/Injectable | Physical mixing, no crosslinker (rheology-based structuring) | Intervertebral Disc (IVD) |
|
| [67] |
| Viscous chitosan (CHI), CNF | Physical mixing and further neutralized with sodium hydroxide (NaOH) |
|
| [68] | |
| Poly (ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer-diacrylate (PEO-PPO-PEO-DA), CNF, Gelatin methacryloyl (GelMA)/Injectable | UV crosslinking (365 nm at 6 mW/cm2) | Meniscus |
|
| [69] |
| Poly (vinyl alcohol) (PVA), Glycidyl Methacrylate (GMA), CNF/Injectable | UV crosslinking (365 nm at 6 mW/cm2) |
|
| [70] | |
| Pluronic® F-127 (PEO99–PPO65–PEO99), Chitosan, Gold nanoparticles attached on TEMPO-oxidized bacterial cellulose nanofibers (Au@OBC) | Physical gelation via thermo-responsive behavior | Cardiac |
|
| [71] |
| TEMPO-oxidized cellulose nanofibers (TOCN), Pyrrole monomer (PPy) | Iron ion (Fe3+) crosslinking followed by in situ polymerization of PPy in the presence of Fe3+ |
|
| [72] | |
| Porcine skin collagen, TEMPO-oxidized cellulose nanofibers (CNF), Dexamethasone | First linking collagen and CNFs via carbodiimide chemistry, followed by photo crosslinking | Corneal |
|
| [73] |
| TEMPO-oxidized cellulose nanofibers (CNF), Telechelic difunctional PEG (DF-PEG), Glycol chitosan (CS)/Injectable and self-healing | Schiff base reaction between aldehyde-functionalized telechelic difunctional PEG (DF-PEG-CHO) and amino groups on chitosan (CS) | Neural |
|
| [74] |
| Fragmented short-length TEMPO-oxidized bacterial cellulose nanofibers (sOBC), Gelatin methacryloyl (GelMA), Transforming growth factor beta (TGF-β), and Fibroblast growth factor (FGF) | UV crosslinking | Tracheal |
|
| [75] |
| Hydrogel Composition/Type | Formation Method | Antimicrobial or Therapeutic Agents | Role of CNF | Key Findings | References |
|---|---|---|---|---|---|
| Chitosan (CS), CNF, Tannic acid (TA) | Both chemical (amino group of the CS chain and the ester group of genipin) and physical crosslinking (hydrophobic and hydrogen bond formed between CS, CNF, and TA) | Tannic acid (TA) |
|
| [77] |
| CNFs, Tannin (TA), 3-acrylamidophenyl boronic acid (AAPBA), Acrylamide (AM)/Self-healing and glucose responsiveness | AAPBA was copolymerized with AM using APS and MBA as crosslinker, CNFs and TA were crosslinked with poly(AM– AAPBA) through the formation of dynamic borate ester bonds between the boronic acid groups in AAPBA and the o-dihydroxy groups in TA and CNFs | Tannin (TA) |
|
| [84] |
| Poly(vinyl alcohol) (PVA), Borax, Dopamine-grafted oxidized carboxymethyl cellulose (OCMC-DA), CNF, Neomycin (NEO)/Self-healing and pH responsive | Dynamic reversible borate ester linkages and hydrogen bonds between OCMC-DA, PVA, and CNF, along with dynamic crosslinking imine linkages between NEO and OCMC-DA | Neomycin (NEO) |
|
| [85] |
| Poly(vinyl alcohol) (PVA), Borax, Resveratrol-grafted cellulose nanofibrils (RPC)/Self-healing and pH-responsive | Dynamic reversible borate ester linkages and hydrogel bond between PVA, borax and RPC | Resveratrol |
|
| [86] |
| CNF, polyvinyl alcohol (PVA), and curcumin-modified silver nanoparticles (cAg), Borax | One-step polymerization (hydrogen bonds between CNF and PVA, dynamic boronic ester bonds between borate ion, CNF and PVA, and coordinate covalent bonds between Ag and CNF) | Curcumin-modified silver nanoparticles (cAg) |
|
| [87] |
| Dopamine-modified Tempo-oxidized cellulose nanofibers (DA-TCNF), Chitosan, (3-aminobenzeneboronic acid)- grafted oxidized dextran (POD), and Poly(vinyl alcohol) (PVA)/ROS and pH responsiveness | POD and DA-TCNF form dynamic Schiff base, boronic ester linkages and hydrogen bond with PVA and chitosan | Mangiferin and Vitamin C |
|
| [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, T.; Goh, M.; Lim, C.; Moon, J.; Lee, K.; Park, J.; Chung, K.; Kim, Y.; Lee, S.; Hong, H.J.; et al. Recent Progress in Cellulose Nanofibril Hydrogels for Biomedical Applications. Polymers 2025, 17, 2272. https://doi.org/10.3390/polym17172272
Won T, Goh M, Lim C, Moon J, Lee K, Park J, Chung K, Kim Y, Lee S, Hong HJ, et al. Recent Progress in Cellulose Nanofibril Hydrogels for Biomedical Applications. Polymers. 2025; 17(17):2272. https://doi.org/10.3390/polym17172272
Chicago/Turabian StyleWon, Taeyen, MeeiChyn Goh, Chaewon Lim, Jieun Moon, Kyueui Lee, Jaehyeung Park, Kyeongwoon Chung, Younghee Kim, Seonhwa Lee, Hye Jin Hong, and et al. 2025. "Recent Progress in Cellulose Nanofibril Hydrogels for Biomedical Applications" Polymers 17, no. 17: 2272. https://doi.org/10.3390/polym17172272
APA StyleWon, T., Goh, M., Lim, C., Moon, J., Lee, K., Park, J., Chung, K., Kim, Y., Lee, S., Hong, H. J., & Gwon, K. (2025). Recent Progress in Cellulose Nanofibril Hydrogels for Biomedical Applications. Polymers, 17(17), 2272. https://doi.org/10.3390/polym17172272






