Chitosan Films Loaded with Alginate Nanoparticles for Gentamicin Release on Demand
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticles Preparation
2.3. Films Preparation
2.4. Gentamicin Quantification
2.5. Nanoparticles Characterization
2.5.1. Dynamic Light Scattering
2.5.2. Transmission Electron Microscopy
2.5.3. Gentamicin Incorporation
2.5.4. Gentamicin Release from Nanoparticles over Time
2.6. Films Characterization
2.6.1. Morphology
2.6.2. Thickness
2.6.3. Swelling and Mass Loss
2.6.4. Mechanical Properties
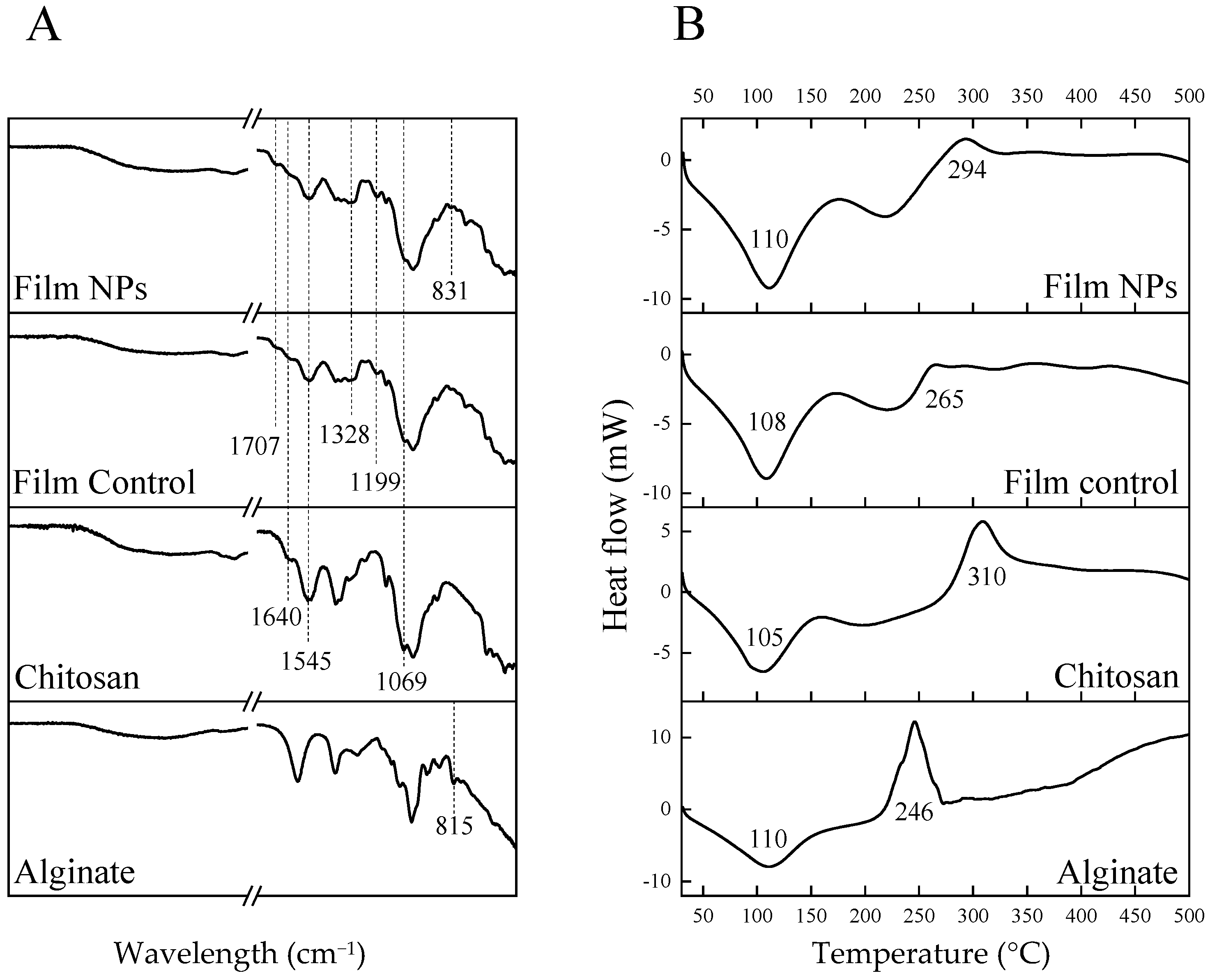
2.6.5. Fourier Transformed Infrared Spectroscopy-Attenuated Total Reflectance (FTIR-ATR)
2.6.6. Differential Scanning Calorimetry (DSC)
2.6.7. Gentamicin Release from Films over Time
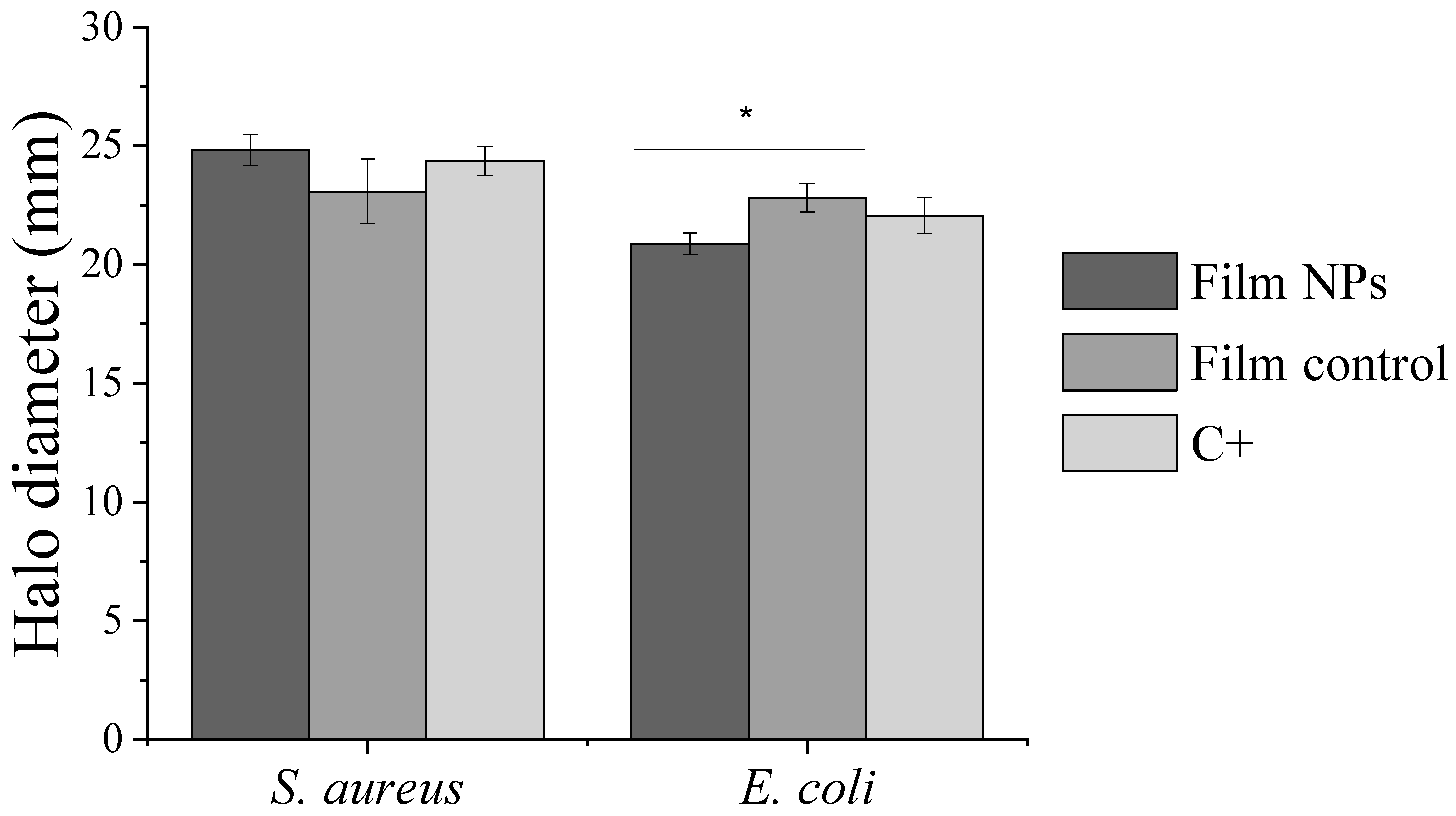
2.7. Antibacterial Assays
2.7.1. Bacteria Stock Preparation
2.7.2. Antibacterial Activity
2.8. Biocompatibility
2.8.1. Cell Culture
2.8.2. Indirect Cytotoxicity Test
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers 2022, 14, 1010. [Google Scholar] [CrossRef]
- Michalicha, A.; Belcarz, A.; Giannakoudakis, D.A.; Staniszewska, M.; Barczak, M. Designing Composite Stimuli-Responsive Hydrogels for Wound Healing Applications: The State-of-the-Art and Recent Discoveries. Materials 2024, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, Z.; Chen, X.; He, C. Tissue-adhesive, Antibacterial, Naturally-derived Polymer Hydrogels as Wound Dressings for Infected and Chronic Wound Healing. J. Polym. Sci. 2024, 62, 2320–2339. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. J. Compos. Sci. 2025, 9, 133. [Google Scholar] [CrossRef]
- Das, I.J.; Bal, T. PH Factors in Chronic Wound and PH-Responsive Polysaccharide-Based Hydrogel Dressings. Int. J. Biol. Macromol. 2024, 279, 135118. [Google Scholar] [CrossRef]
- Xia, Y.; Ma, Z.; Wu, X.; Wei, H.; Zhang, H.; Li, G.; Qian, Y.; Shahriari-Khalaji, M.; Hou, K.; Cao, R.; et al. Advances in Stimuli-Responsive Chitosan Hydrogels for Drug Delivery Systems. Macromol. Biosci. 2024, 24, 2300399. [Google Scholar] [CrossRef]
- Maity, C.; Das, N. Alginate-Based Smart Materials and Their Application: Recent Advances and Perspectives. Top. Curr. Chem. 2022, 380, 3. [Google Scholar] [CrossRef]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and Challenges in the Use of Chitosan and Its Derivatives in Biomedical Fields: A Review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100323. [Google Scholar] [CrossRef]
- Haider, A.; Khan, S.; Iqbal, D.N.; Shrahili, M.; Haider, S.; Mohammad, K.; Mohammad, A.; Rizwan, M.; Kanwal, Q.; Mustafa, G. Advances in Chitosan-Based Drug Delivery Systems: A Comprehensive Review for Therapeutic Applications. Eur. Polym. J. 2024, 210, 112983. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Aderibigbe, B.A. Polymer-Based Hydrogels Enriched with Essential Oils: A Promising Approach for the Treatment of Infected Wounds. Polymers 2022, 14, 3772. [Google Scholar] [CrossRef]
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Jadhav, S.R. Encapsulation of Essential Oils and Their Application in Antimicrobial Active Packaging. Food Control. 2022, 136, 108883. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Yan, Z.; Ji, S.; Xiao, S.; Gao, J. Metal Nanoparticle Hybrid Hydrogels: The State-of-the-Art of Combining Hard and Soft Materials to Promote Wound Healing. Theranostics 2024, 14, 1534–1560. [Google Scholar] [CrossRef]
- Ahmad, N.; Bukhari, S.N.A.; Hussain, M.A.; Ejaz, H.; Munir, M.U.; Amjad, M.W. Nanoparticles Incorporated Hydrogels for Delivery of Antimicrobial Agents: Developments and Trends. RSC Adv. 2024, 14, 13535–13564. [Google Scholar] [CrossRef]
- Chevallier, P.; Wiggers, H.J.; Copes, F.; Zorzi Bueno, C.; Mantovani, D. Prolonged Antibacterial Activity in Tannic Acid–Iron Complexed Chitosan Films for Medical Device Applications. Nanomaterials 2023, 13, 484. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Sodium Alginate W201502. Available online: https://www.sigmaaldrich.com/CA/en/product/aldrich/w201502 (accessed on 1 April 2025).
- Rampim, I.T.; Wiggers, H.J.; Bueno, C.Z.; Chevallier, P.; Copes, F.; Mantovani, D. Sourcing Interchangeability in Commercial Chitosan: Focus on the Physical–Chemical Properties of Six Different Products and Their Impact on the Release of Antibacterial Agents. Polymers 2025, 17, 884. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2002.
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. Am. Soc. Microbiol. 2009, 15, 1–23. Available online: https://asm.org/protocols/kirby-bauer-disk-diffusion-susceptibility-test-pro (accessed on 3 September 2024).
- Microbiologics SCA. Microbiologics Dilutions Guide. Available online: https://www.microbiologics.com/Microbiologics-Dilutions-Guide (accessed on 15 May 2025).
- Cockerill, F.R.; Wikler, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferraro, M.J.; Hardy, D.J.; Hecht, D.W.; Hindler, J.A.; Patel, J.B.; et al. M07-A9; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- ISO 10993-5; Biological Evaluation of Medical Device-Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Heriot, M.; Nottelet, B.; Garric, X.; D’Este, M.; Richards, G.R.; Moriarty, F.T.; Eglin, D.; Guillaume, O. Interaction of Gentamicin Sulfate with Alginate and Consequences on the Physico-Chemical Properties of Alginate-Containing Biofilms. Int. J. Biol. Macromol. 2019, 121, 390–397. [Google Scholar] [CrossRef]
- Park, K.H.; Choi, Y.W.; Ryu, H.; Lee, H.J.; Moon, J.-H.; Song, H.-J.; Park, Y.-J. Controlled Drug Release Using Chitosan-Alginate-Gentamicin Multi-Component Beads. Materials 2022, 15, 7682. [Google Scholar] [CrossRef] [PubMed]
- Madian, N.G.; El-Ashmanty, B.A.; Abdel-Rahim, H.K. Improvement of Chitosan Films Properties by Blending with Cellulose, Honey and Curcumin. Polymers 2023, 15, 2587. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and Properties of Chitosan Films: Effect of the Type of Solvent Acid. LWT 2021, 135, 109984. [Google Scholar] [CrossRef]
- Ogawa, K.; Yui, T.; Miya, M. Dependence on the Preparation Procedure of the Polymorphism and Crystallinity of Chitosan Membranes. Biosci. Biotechnol. Biochem. 1992, 56, 858–862. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications. Adv. Sci. News 2023, 10, 2303326. [Google Scholar] [CrossRef]
- Minsart, M.; Van Vlierberghe, S.; Dubruel, P.; Mignon, A. Commercial Wound Dressings for the Treatment of Exuding Wounds: An in-Depth Physico-Chemical Comparative Study. Burn. Trauma 2022, 10, tkac024. [Google Scholar] [CrossRef]
- Kondaveeti, S.; de Assis Bueno, P.V.; Carmona-Ribeiro, A.M.; Esposito, F.; Lincopan, N.; Sierakowski, M.R.; Petri, D.F.S. Microbicidal Gentamicin-Alginate Hydrogels. Carbohydr. Polym. 2018, 186, 159–167. [Google Scholar] [CrossRef]
- Belattmania, Z.; Kaidi, S.; El Atouani, S.; Katif, C.; Bentiss, F.; Jama, C.; Reani, A.; Sabour, B.; Vasconcelos, V. Isolation and FTIR-ATR and 1H NMR Characterization of Alginates from the Main Alginophyte Species of the Atlantic Coast of Morocco. Molecules 2020, 25, 4335. [Google Scholar] [CrossRef] [PubMed]
- Bayir, E. Development of a Three-Dimensional in Vitro Blood-Brain Barrier Using the Chitosan-Alginate Polyelectrolyte Complex as the Extracellular Matrix. J. Bioact. Compat. Polym. 2023, 38, 252–269. [Google Scholar] [CrossRef]
- Brahmi, M.; Essifi, K.; Bakirhan, N.K.; El Bachiri, A.; Ouldriane, S.D.; Tahani, A. New Insights into Physicochemical Aspects Involved in the Formation of Chitosan-Alginate Biobased Polyelectrolyte Complexes on Natural Montmorillonite Clay Surface. J. Mol. Liq. 2023, 387, 122635. [Google Scholar] [CrossRef]
- Xia, Z.; Singh, A.; Kiratitanavit, W.; Mosurkal, R.; Kumar, J.; Nagarajan, R. Unraveling the Mechanism of Thermal and Thermo-Oxidative Degradation of Tannic Acid. Thermochim. Acta 2015, 605, 77–85. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Muhamadejevs, R.; Haldimann, K.; Gysin, M.; Crich, D.; Jaudzems, K.; Hobbie, S.N. Experimental Determination of the PKa Values of Clinically Relevant Antibiotics: Toward Establishing PKa-Activity. ACS Omega 2024, 9, 5876–5887. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of PH on Wound-Healing: A New Perspective for Wound-Therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Bennison, L.; Miller, C.; Summers, R.; Minnis, A.; Sussman, G.; McGuiness, W. The PH of Wounds during Healing and Infection: A Descriptive Literature Review. Wound Pract. Res. 2017, 25, 63–69. [Google Scholar]
- Chowdhury, F.; Ahmed, S.; Rahman, M.; Ahmed, M.A.; Hossain, M.D.; Reza, H.M.; Park, S.Y.; Sharker, S.M. Chronic Wound-Dressing Chitosan-Polyphenolic Patch for PH Responsive Local Antibacterial Activity. Mater. Today Commun. 2022, 31, 103310. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Khatoon, F. In Vitro Study of Temperature and PH-Responsive Gentamycin Sulphate-Loaded Chitosan-Based Hydrogel Films for Wound Dressing Applications. Polym. Plast. Technol. Eng. 2015, 54, 573–580. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Iqbal, I.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Jabeen, F.; Riduan Mohamad, M.; Jusoh, N. Development of Antibacterial, Degradable and PH-Responsive Chitosan/Guar Gum/Polyvinyl Alcohol Blended Hydrogels for Wound Dressing. Molecules 2021, 26, 5937. [Google Scholar] [CrossRef]
- Wiggers, H.J.; Chevallier, P.; Copes, F.; Simch, F.H.; da Silva Veloso, F.; Genevro, G.M.; Mantovani, D. Quercetin-Crosslinked Chitosan Films for Controlled Release of Antimicrobial Drugs. Front. Bioeng. Biotechnol. 2022, 10, 814162. [Google Scholar] [CrossRef]
- Sun, P.; Jiao, J.; Wang, X.; Chen, L.; Chen, Z.; Zhang, K.; Qu, K.; Qin, X.; Yang, Z.; Zhong, J.L.; et al. Nanomedicine Hybrid and Catechol Functionalized Chitosan as PH-Responsive Multi-Function Hydrogel to Efficiently Promote Infection Wound Healing. Int. J. Biol. Macromol. 2023, 238, 124106. [Google Scholar] [CrossRef]
- Lu, B.; Han, X.; Zou, D.; Luo, X.; Liu, L.; Wang, J.; Maitz, M.F.; Yang, P.; Huang, N.; Zhao, A. Catechol-Chitosan/Polyacrylamide Hydrogel Wound Dressing for Regulating Local Inflammation. Mater. Today Bio. 2022, 16, 100392. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Nie, H.; Wang, J.; Lin, J.; Wang, Q.; Sun, J.; Zhang, W.; Wang, J. Enhanced Functional Properties of Chitosan Films Incorporated with Curcumin-Loaded Hollow Graphitic Carbon Nitride Nanoparticles for Bananas Preservation. Food Chem. 2022, 366, 130539. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.A.; Ur.Rehman, A.; Howari, H.; Alhodaib, A.; Ullah, F.; Mustafa, Z.u.; Elaissari, A.; Ahmed, N. Hydrogel Containing Solid Lipid Nanoparticles Loaded with Argan Oil and Simvastatin: Preparation, In Vitro and Ex Vivo Assessment. Gels 2022, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Buntum, T.; Kongprayoon, A.; Mungyoi, W.; Charoenram, P.; Kiti, K.; Thanomsilp, C.; Supaphol, P.; Suwantong, O. Wound-Aided Semi-Solid Poly(Vinyl Alcohol) Hydrogels Inc. Essent. Oil-Loaded Chitosan Nanoparticles. Int. J. Biol. Macromol. 2021, 189, 135–141. [Google Scholar] [CrossRef] [PubMed]





| Gentamicin (% w/w) | 30 | 40 | 60 |
|---|---|---|---|
| Size (nm) | 387.0 ± 138.2 a* | 285.2 ± 6.0 ab | 86.2 ± 4.4 b |
| PDI | 0.28 ± 0.02 a | 0.21 ± 0.02 b | 0.15 ± 0.00 c |
| Zeta (mV) | −44.9 ± 1.2 a | −42.0 ± 0.7 b | −35.0 ± 0.8 c |
| LC (μg/mg) | 296.2 ± 3.2 a | 398.6 ± 2.0 b | 599.3 ± 1.0 c |
| LE (%) | 98.7 ± 1.1 a | 99.6 ± 0.5 b | 99.9 ± 0.2 b |
| Property | Film Control | Film NPs |
|---|---|---|
| Thickness (µm) | 14.6 ± 4.2 a | 18.4 ± 3.4 a |
| Swelling (%) | 167.3 ± 21.4 a | 103.0 ± 11.4 b |
| Mass loss (%) | 25.0 ± 1.6 a | 21.9 ± 2.6 a |
| Tensile strenght (MPa) | 32.6 ± 7.9 a | 24.6 ± 4.9 b |
| Elongation at break (%) | 1.9 ± 0.9 a | 1.5 ± 0.7 a |
| Young’s modulus (MPa) | 38.4 ± 8.4 a | 27.6 ± 5.8 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorzi Bueno, C.; Wiggers, H.J.; Chevallier, P.; Copes, F.; Mantovani, D. Chitosan Films Loaded with Alginate Nanoparticles for Gentamicin Release on Demand. Polymers 2025, 17, 2261. https://doi.org/10.3390/polym17162261
Zorzi Bueno C, Wiggers HJ, Chevallier P, Copes F, Mantovani D. Chitosan Films Loaded with Alginate Nanoparticles for Gentamicin Release on Demand. Polymers. 2025; 17(16):2261. https://doi.org/10.3390/polym17162261
Chicago/Turabian StyleZorzi Bueno, Cecilia, Helton José Wiggers, Pascale Chevallier, Francesco Copes, and Diego Mantovani. 2025. "Chitosan Films Loaded with Alginate Nanoparticles for Gentamicin Release on Demand" Polymers 17, no. 16: 2261. https://doi.org/10.3390/polym17162261
APA StyleZorzi Bueno, C., Wiggers, H. J., Chevallier, P., Copes, F., & Mantovani, D. (2025). Chitosan Films Loaded with Alginate Nanoparticles for Gentamicin Release on Demand. Polymers, 17(16), 2261. https://doi.org/10.3390/polym17162261







