Facile Preparation of a Cellulose-Based Thermoresponsive Gel for Rapid Water Harvesting from the Atmosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PNIPAM/TO-CNF/LiCl Gel
2.3. Characterizations
2.4. Hygroscopicity and Desorption Testing of Gel
3. Results and Discussion
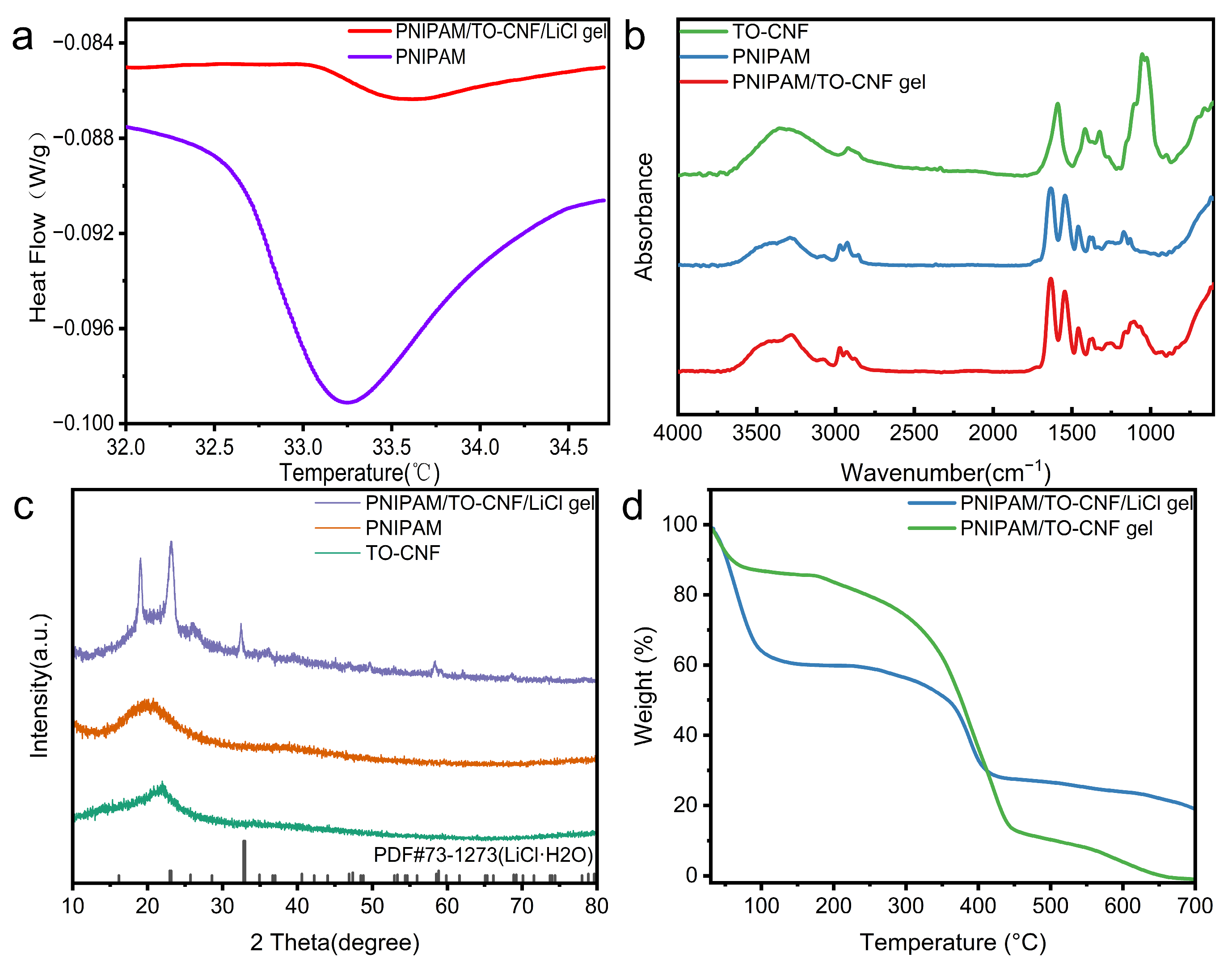
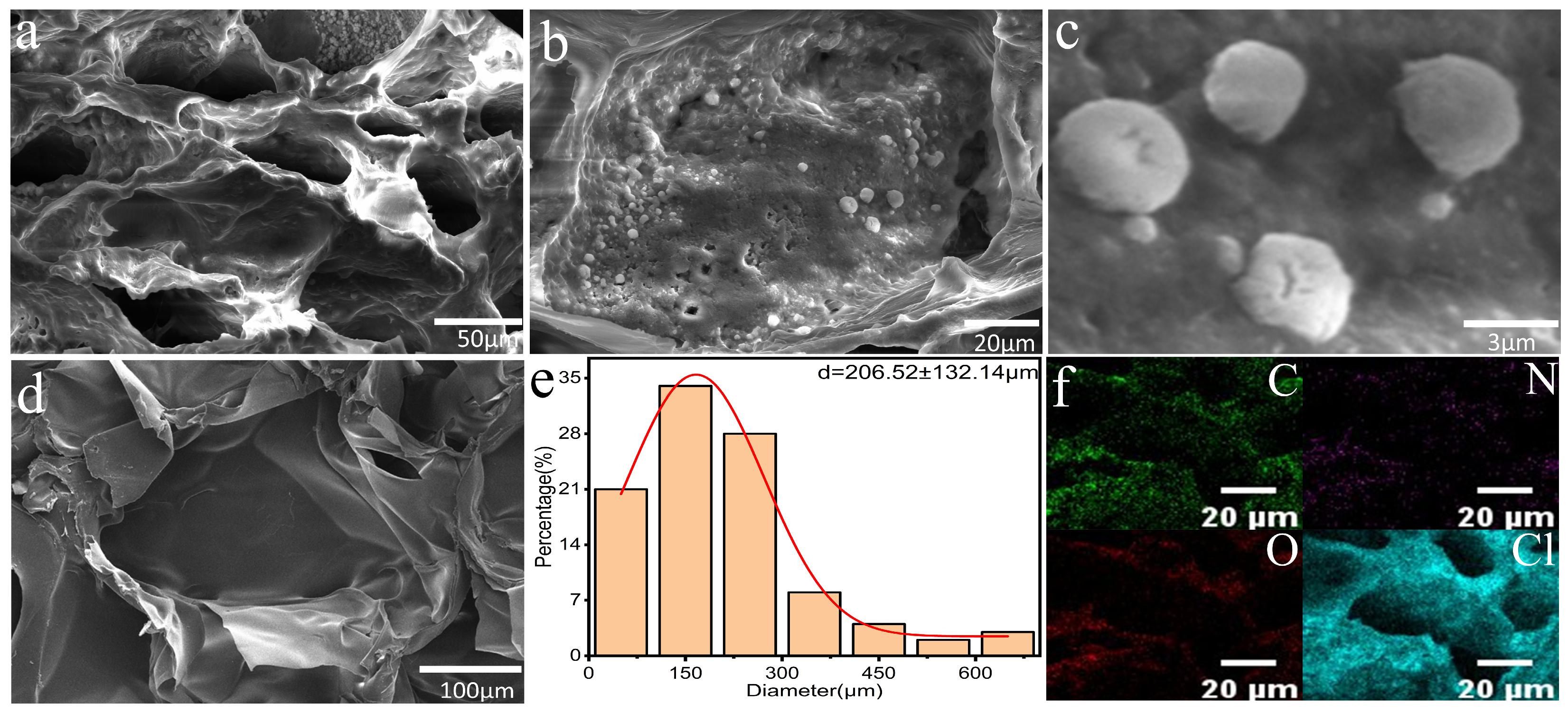
3.1. Characterization of PNIPAM/TO-CNF/LiCl Gel
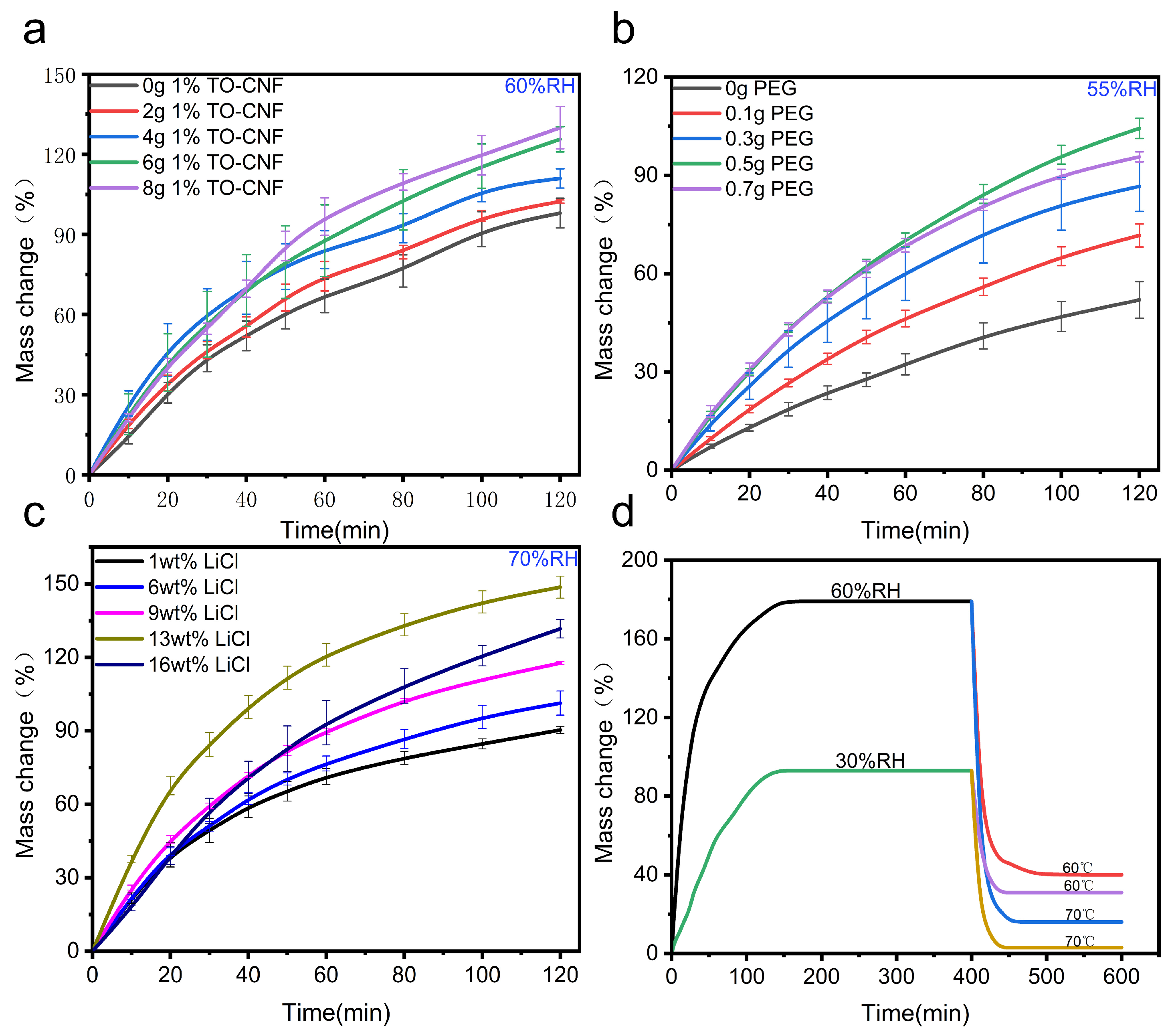
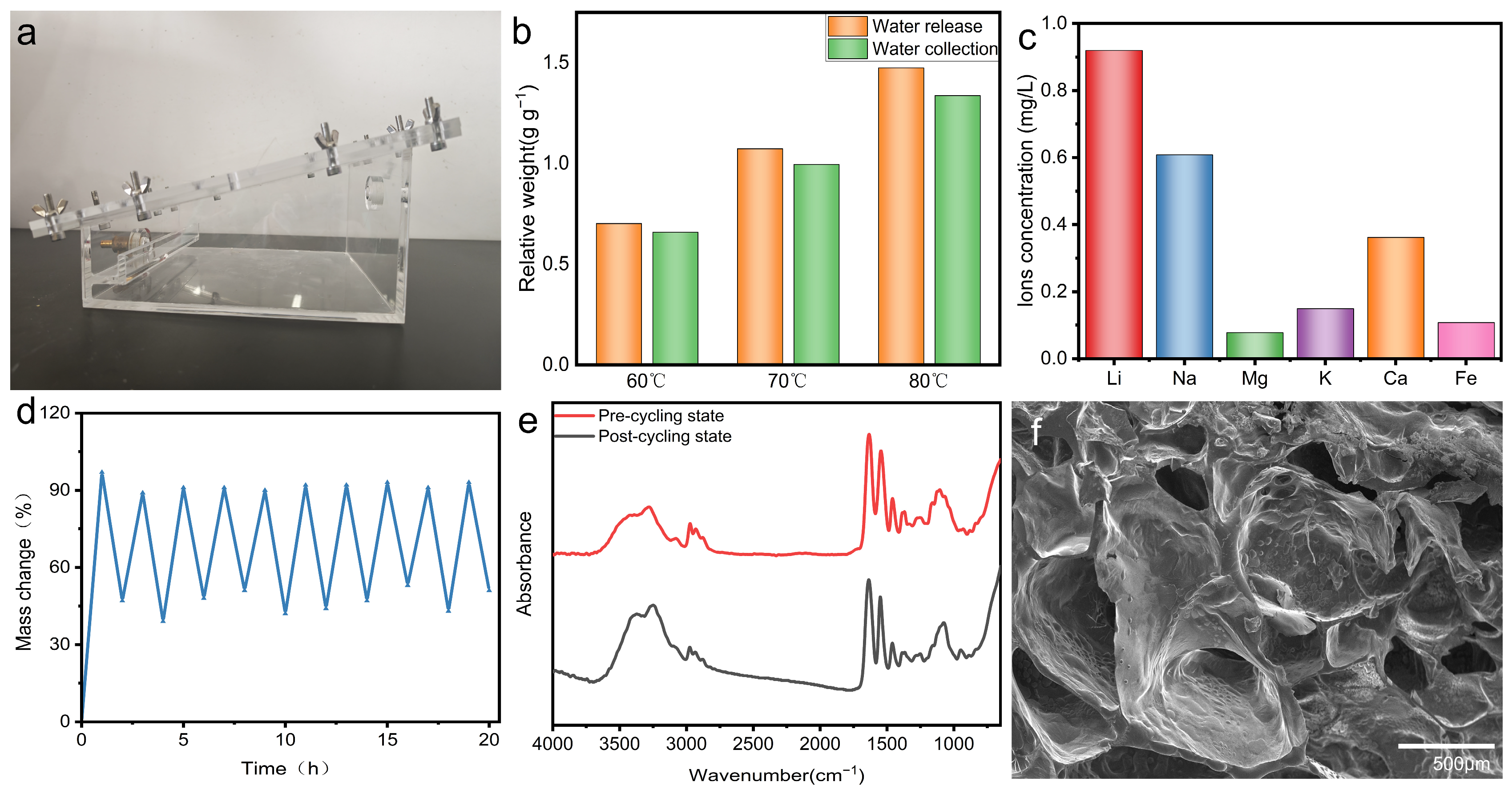
3.2. The Atmospheric Water Harvesting Performance of PNIPAM/TO-CNF/LiCl Gel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- McDonald, R.I.; Weber, K.; Padowski, J.; Flörke, M.; Schneider, C.; Green, P.A.; Gleeson, T.; Eckman, S.; Lehner, B.; Balk, D.; et al. Water on an Urban Planet: Urbanization and the Reach of Urban Water Infrastructure. Glob. Environ. Change 2014, 27, 96–105. [Google Scholar] [CrossRef]
- Garrick, D.; De Stefano, L.; Yu, W.; Jorgensen, I.; O’Donnell, E.; Turley, L.; Aguilar-Barajas, I.; Dai, X.; De Souza Leão, R.; Punjabi, B.; et al. Rural Water for Thirsty Cities: A Systematic Review of Water Reallocation from Rural to Urban Regions. Environ. Res. Lett. 2019, 14, 043003. [Google Scholar] [CrossRef]
- Flörke, M.; Schneider, C.; McDonald, R.I. Water Competition between Cities and Agriculture Driven by Climate Change and Urban Growth. Nat. Sustain. 2018, 1, 51–58. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future Global Urban Water Scarcity and Potential Solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bodirsky, B.L.; Rijneveld, R.; Beier, F.; Bak, M.P.; Batool, M.; Droppers, B.; Popp, A.; Van Vliet, M.T.H.; Strokal, M. A Triple Increase in Global River Basins with Water Scarcity Due to Future Pollution. Nat. Commun. 2024, 15, 880. [Google Scholar] [CrossRef]
- Schneider, S.H. Encyclopedia of Weather and Climate; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- Lu, H.; Shi, W.; Guo, Y.; Guan, W.; Lei, C.; Yu, G. Materials Engineering for Atmospheric Water Harvesting: Progress and Perspectives. Adv. Mater. 2022, 34, 2110079. [Google Scholar] [CrossRef]
- Chen, H.; Ran, T.; Gan, Y.; Zhou, J.; Zhang, Y.; Zhang, L.; Zhang, D.; Jiang, L. Ultrafast Water Harvesting and Transport in Hierarchical Microchannels. Nat. Mater. 2018, 17, 935–942. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, R.; Zhang, Y.; Wang, J. Progress and Expectation of Atmospheric Water Harvesting. Joule 2018, 2, 1452–1475. [Google Scholar] [CrossRef]
- Cho, H.J.; Preston, D.J.; Zhu, Y.; Wang, E.N. Nanoengineered Materials for Liquid–Vapour Phase-Change Heat Transfer. Nat. Rev. Mater. 2016, 2, 16092. [Google Scholar] [CrossRef]
- Entezari, A.; Ejeian, M.; Wang, R. Modifying Water Sorption Properties with Polymer Additives for Atmospheric Water Harvesting Applications. Appl. Therm. Eng. 2019, 161, 114109. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Li, Q.; Wu, T.; Zhang, M.; Shi, Q. Water Sorption on Composite Material “Zeolite 13X Modified by LiCl and CaCl2”. Microporous Mesoporous Mater. 2020, 299, 110109. [Google Scholar] [CrossRef]
- Wang, J.; Dang, Y.; Meguerdichian, A.G.; Dissanayake, S.; Kankanam-Kapuge, T.; Bamonte, S.; Tobin, Z.M.; Achola, L.A.; Suib, S.L. Water Harvesting from the Atmosphere in Arid Areas with Manganese Dioxide. Environ. Sci. Technol. Lett. 2020, 7, 48–53. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Shi, L.; Alsaedi, M.; Wang, P. Harvesting Water from Air: Using Anhydrous Salt with Sunlight. Environ. Sci. Technol. 2018, 52, 5398–5406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-N.; Zhao, L.-J.; Chen, S.-H.; Guo, X.; Luan, Y.-M.; Zhang, Y.-H. Hygroscopic Property of Inorganic Salts in Atmospheric Aerosols Measured with Physisorption Analyzer. Atmos. Environ. 2021, 247, 118171. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Chao, J.; Wu, S.; Yan, T.; Li, W.; Cao, B.; Wang, R. Efficient Solar-Driven Water Harvesting from Arid Air with Metal–Organic Frameworks Modified by Hygroscopic Salt. Angew. Chem. Int. Ed. 2020, 59, 5202–5210. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water Harvesting from Air with Metal-Organic Frameworks Powered by Natural Sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef]
- Hu, Y.; Fang, Z.; Wan, X.; Ma, X.; Wang, S.; Fan, S.; Dong, M.; Ye, Z.; Peng, X. Carbon Nanotubes Decorated Hollow Metal–Organic Frameworks for Efficient Solar-Driven Atmospheric Water Harvesting. Chem. Eng. J. 2022, 430, 133086. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Alsaedi, M.; Wu, M.; Shi, L.; Wang, P. Hybrid Hydrogel with High Water Vapor Harvesting Capacity for Deployable Solar-Driven Atmospheric Water Generator. Environ. Sci. Technol. 2018, 52, 11367–11377. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, X.; Liu, Y.; Shi, Y.; Dai, Y.; Yu, G. Super Moisture-Absorbent Gels for All-Weather Atmospheric Water Harvesting. Adv. Mater. 2019, 31, 1806446. [Google Scholar] [CrossRef]
- Entezari, A.; Ejeian, M.; Wang, R. Super Atmospheric Water Harvesting Hydrogel with Alginate Chains Modified with Binary Salts. ACS Mater. Lett. 2020, 2, 471–477. [Google Scholar] [CrossRef]
- Jin, S.; Liu, M.; Chen, S.; Gao, C. Synthesis, Characterization and the Rapid Response Property of the Temperature Responsive PVP-g-PNIPAM Hydrogel. Eur. Polym. J. 2008, 44, 2162–2170. [Google Scholar] [CrossRef]
- Zhang, H.; Niu, Q.; Wang, N.; Nie, J.; Ma, G. Thermo-Sensitive Drug Controlled Release PLA Core/PNIPAM Shell Fibers Fabricated Using a Combination of Electrospinning and UV Photo-Polymerization. Eur. Polym. J. 2015, 71, 440–450. [Google Scholar] [CrossRef]
- Yilmaz, G.; Meng, F.L.; Lu, W.; Abed, J.; Peh, C.K.N.; Gao, M.; Sargent, E.H.; Ho, G.W. Autonomous Atmospheric Water Seeping MOF Matrix. Sci. Adv. 2020, 6, eabc8605. [Google Scholar] [CrossRef]
- Wang, X.; Yang, D.; Zhang, M.; Hu, Q.; Gao, K.; Zhou, J.; Yu, Z.-Z. Super-Hygroscopic Calcium Chloride/Graphene Oxide/Poly(N-Isopropylacrylamide) Gels for Spontaneous Harvesting of Atmospheric Water and Solar-Driven Water Release. ACS Appl. Mater. Interfaces 2022, 14, 33881–33891. [Google Scholar] [CrossRef]
- Maity, D.; Teixeira, A.P.; Fussenegger, M. Hydratable Core–Shell Polymer Networks for Atmospheric Water Harvesting Powered by Sunlight. Small 2023, 19, 2301427. [Google Scholar] [CrossRef]
- Ni, F.; Qiu, N.; Xiao, P.; Zhang, C.; Jian, Y.; Liang, Y.; Xie, W.; Yan, L.; Chen, T. Tillandsia-Inspired Hygroscopic Photothermal Organogels for Efficient Atmospheric Water Harvesting. Angew. Chem. Int. Ed. 2020, 59, 19237–19246. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Liu, H.; Du, C.; Yu, H.; Zhou, Z. Thermo-Responsive and Compression Properties of TEMPO-Oxidized Cellulose Nanofiber-Modified PNIPAm Hydrogels. Carbohydr. Polym. 2016, 147, 201–207. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, H.; Li, Y.; Kuang, Y.; Xu, X.; Chen, C.; Huang, H.; Jia, C.; Zhao, X.; Hitz, E.; et al. Lightweight, Mesoporous, and Highly Absorptive All-Nanofiber Aerogel for Efficient Solar Steam Generation. ACS Appl. Mater. Interfaces 2018, 10, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Ruoko, T.; Gladisch, J.; Erlandsson, J.; Wågberg, L.; Crispin, X.; Fabiano, S. Cellulose-Conducting Polymer Aerogels for Efficient Solar Steam Generation. Adv. Sustain. Syst. 2020, 4, 2000004. [Google Scholar] [CrossRef]
- Sun, F.; Liu, W.; Dong, Z.; Deng, Y. Underwater Superoleophobicity Cellulose Nanofibril Aerogel through Regioselective Sulfonation for Oil/Water Separation. Chem. Eng. J. 2017, 330, 774–782. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, W.; Lei, C.; Lu, H.; Shi, W.; Yu, G. Scalable Super Hygroscopic Polymer Films for Sustainable Moisture Harvesting in Arid Environments. Nat. Commun. 2022, 13, 2761. [Google Scholar] [CrossRef]
- Guan, W.; Lei, C.; Guo, Y.; Shi, W.; Yu, G. Hygroscopic-Microgels-Enabled Rapid Water Extraction from Arid Air. Adv. Mater. 2024, 36, 2207786. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, T.; Wan, D.; Dai, M.; Ling, S.; Wang, J.; Liu, Y.; Fang, Y.; Xu, S.; Yeo, J.; et al. Solar-Powered Nanostructured Biopolymer Hygroscopic Aerogels for Atmospheric Water Harvesting. Nano Energy 2021, 80, 105569. [Google Scholar] [CrossRef]
- Sun, J.; An, B.; Zhang, K.; Xu, M.; Wu, Z.; Ma, C.; Li, W.; Liu, S. Moisture-Indicating Cellulose Aerogels for Multiple Atmospheric Water Harvesting Cycles Driven by Solar Energy. J. Mater. Chem. A 2021, 9, 24650–24660. [Google Scholar] [CrossRef]
- Han, X.; Zhang, W.; Che, X.; Long, L.; Li, M.; Li, C. Synergetic and Persistent Harvesting of Electricity and Potable Water from Ambient Moisture with Biohybrid Fibrils. J. Mater. Chem. A 2022, 10, 8356–8363. [Google Scholar] [CrossRef]
- Zhu, P.; Yu, Z.; Sun, H.; Zheng, D.; Zheng, Y.; Qian, Y.; Wei, Y.; Lee, J.; Srebnik, S.; Chen, W.; et al. 3D Printed Cellulose Nanofiber Aerogel Scaffold with Hierarchical Porous Structures for Fast Solar-Driven Atmospheric Water Harvesting. Adv. Mater. 2024, 36, 2306653. [Google Scholar] [CrossRef]
- Graeber, G.; Díaz-Marín, C.D.; Gaugler, L.C.; Zhong, Y.; El Fil, B.; Liu, X.; Wang, E.N. Extreme Water Uptake of Hygroscopic Hydrogels through Maximized Swelling-Induced Salt Loading. Adv. Mater. 2024, 36, 2211783. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. Cellulose-Nanofiber-Based Materials. In Cellulose Based Composites; Wiley: Hoboken, NJ, USA, 2014; pp. 1–25. ISBN 978-3-527-32719-5. [Google Scholar]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose Nanocrystals vs. Cellulose Nanofibrils: A Comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-Oxidized Cellulose Nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Cha, R.; He, Z.; Ni, Y. Preparation and Characterization of Thermal/pH-Sensitive Hydrogel from Carboxylated Nanocrystalline Cellulose. Carbohydr. Polym. 2012, 88, 713–718. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Tu, J.; Shi, R.; Luo, Z.; Xiong, C.; Jiang, M. Shape Stability of Polyethylene Glycol/Acetylene Black Phase Change Composites for Latent Heat Storage. Adv. Mater. Sci. Eng. 2018, 2018, 3954163. [Google Scholar] [CrossRef]
- Shan, H.; Poredoš, P.; Ye, Z.; Qu, H.; Zhang, Y.; Zhou, M.; Wang, R.; Tan, S.C. All-Day Multicyclic Atmospheric Water Harvesting Enabled by Polyelectrolyte Hydrogel with Hybrid Desorption Mode. Adv. Mater. 2023, 35, 2302038. [Google Scholar] [CrossRef]
- Lei, C.; Guo, Y.; Guan, W.; Lu, H.; Shi, W.; Yu, G. Polyzwitterionic Hydrogels for Efficient Atmospheric Water Harvesting. Angew. Chem. Int. Ed. 2022, 61, e202200271. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Yan, T.; Wu, S.; Wu, M.; Chao, J.; Huo, X.; Wang, P.; Wang, R. Ultrahigh Solar-Driven Atmospheric Water Production Enabled by Scalable Rapid-Cycling Water Harvester with Vertically Aligned Nanocomposite Sorbent. Energy Environ. Sci. 2021, 14, 5979–5994. [Google Scholar] [CrossRef]
- Zhou, H.; Yan, L.; Tang, D.; Xu, T.; Dai, L.; Li, C.; Chen, W.; Si, C. Solar-Driven Drum-Type Atmospheric Water Harvester Based on Bio-Based Gels with Fast Adsorption/Desorption Kinetics. Adv. Mater. 2024, 36, 2403876. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating First Addendum, 4th ed.; 1st add.; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]

| Name | Sorption Water Capacity (g g−1) [RH] | Adsorption Time [min] | Desorption Water Capacity (g g−1) [°C] | Desorption Time [min] | Ref. |
|---|---|---|---|---|---|
| Bina/FCNT | 5.6 [70%] | 660 | 5.10 [80 °C] | 40 | [21] |
| PAMPS-CNT-LiCl | 1.87 [60%] | 600 | 1.72 [90 °C] | 200 | [44] |
| CNF/LiCl scaffolds | 1.15 [57%] | 720 | 0.98 [60 °C] | 120 | [37] |
| PAM 75%sat LiCl | 3.93 [70%] | 600 | 2.91 [70 °C] | 120 | [38] |
| PDMAPS-LiCl | 0.62 [30%] | 120 | 0.5 [80 °C] | 30 | [45] |
| LiCl@rGO–SA | 2.85 [60%] | 480 | 2.73 [90 °C] | 200 | [46] |
| CAL gel | 0.79 [30%] | 120 | 0.51 [65 °C] | 60 | [47] |
| PNIPAM/TO-CNF/LiCl gel | 0.93 [30%] 178 [60%] | 140 | 0.62 [30%] [60 °C] 1.37 [60%] [60 °C] | 80 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, H.; Liu, X.; Du, J.; Xu, Y. Facile Preparation of a Cellulose-Based Thermoresponsive Gel for Rapid Water Harvesting from the Atmosphere. Polymers 2025, 17, 2253. https://doi.org/10.3390/polym17162253
Wang X, Zhang H, Liu X, Du J, Xu Y. Facile Preparation of a Cellulose-Based Thermoresponsive Gel for Rapid Water Harvesting from the Atmosphere. Polymers. 2025; 17(16):2253. https://doi.org/10.3390/polym17162253
Chicago/Turabian StyleWang, Xiaoyu, Hui Zhang, Xinxin Liu, Jie Du, and Yingguang Xu. 2025. "Facile Preparation of a Cellulose-Based Thermoresponsive Gel for Rapid Water Harvesting from the Atmosphere" Polymers 17, no. 16: 2253. https://doi.org/10.3390/polym17162253
APA StyleWang, X., Zhang, H., Liu, X., Du, J., & Xu, Y. (2025). Facile Preparation of a Cellulose-Based Thermoresponsive Gel for Rapid Water Harvesting from the Atmosphere. Polymers, 17(16), 2253. https://doi.org/10.3390/polym17162253








