Next-Generation Wound Healing Materials: Role of Biopolymers and Their Composites
Abstract
1. Introduction
2. Biopolymers for Wound Healing
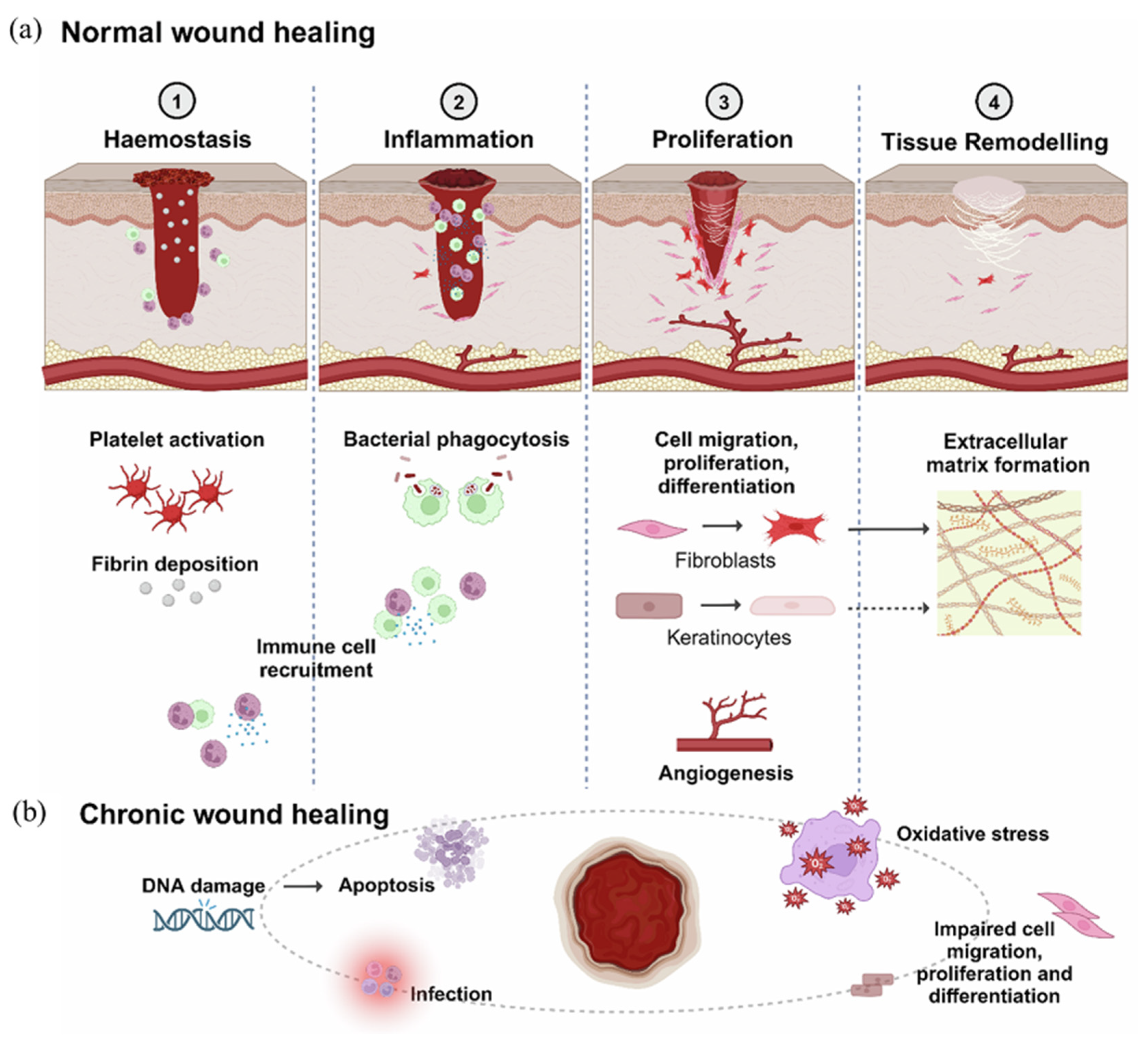
2.1. Natural Biopolymers and Their Significance in Wound Healing
2.1.1. Alginate for Multifunctional and Biocompatible Wound Healing Material
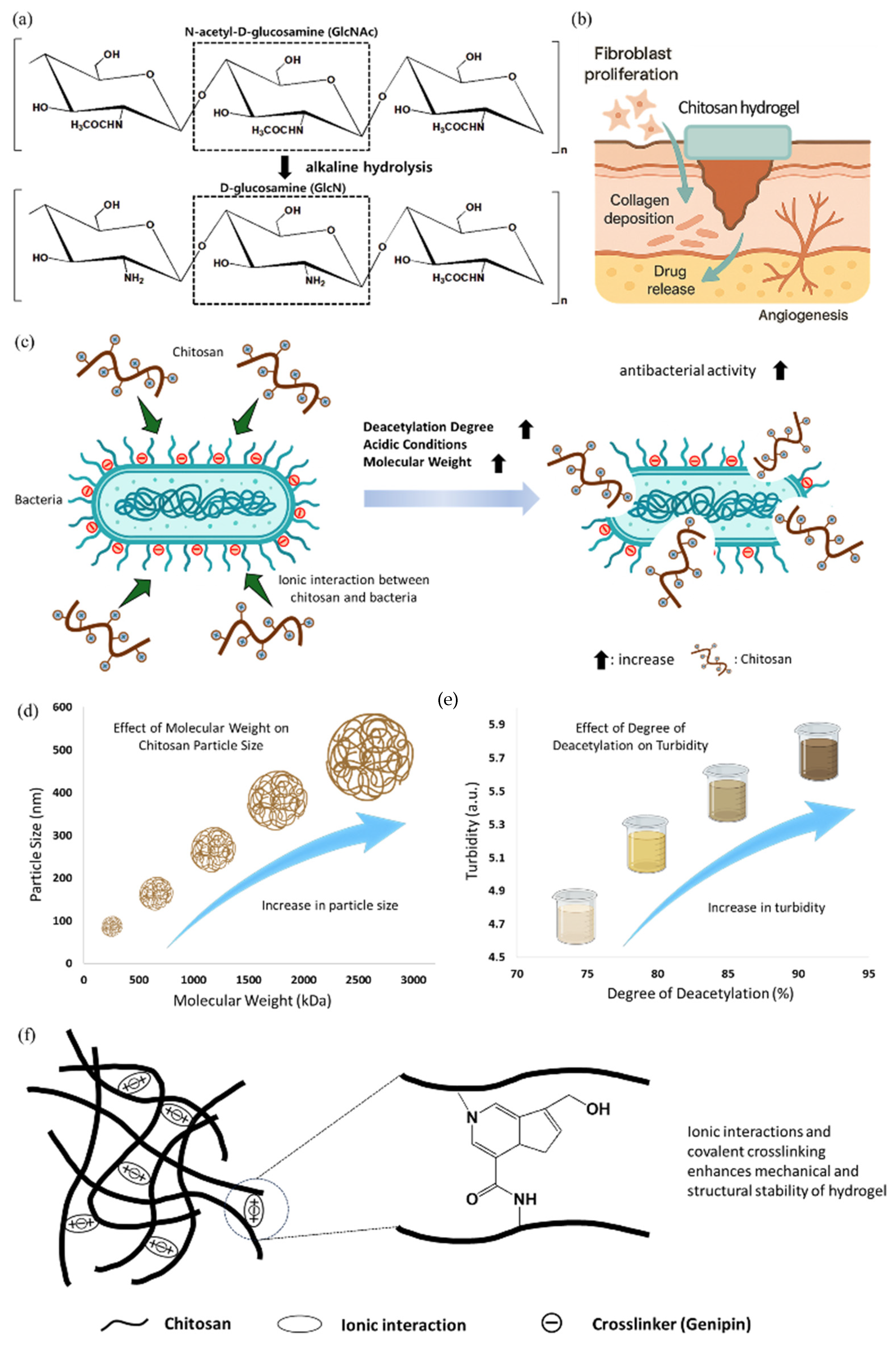
2.1.2. Chitosan for Antibacterial Protection and Bio-Responsive Healing
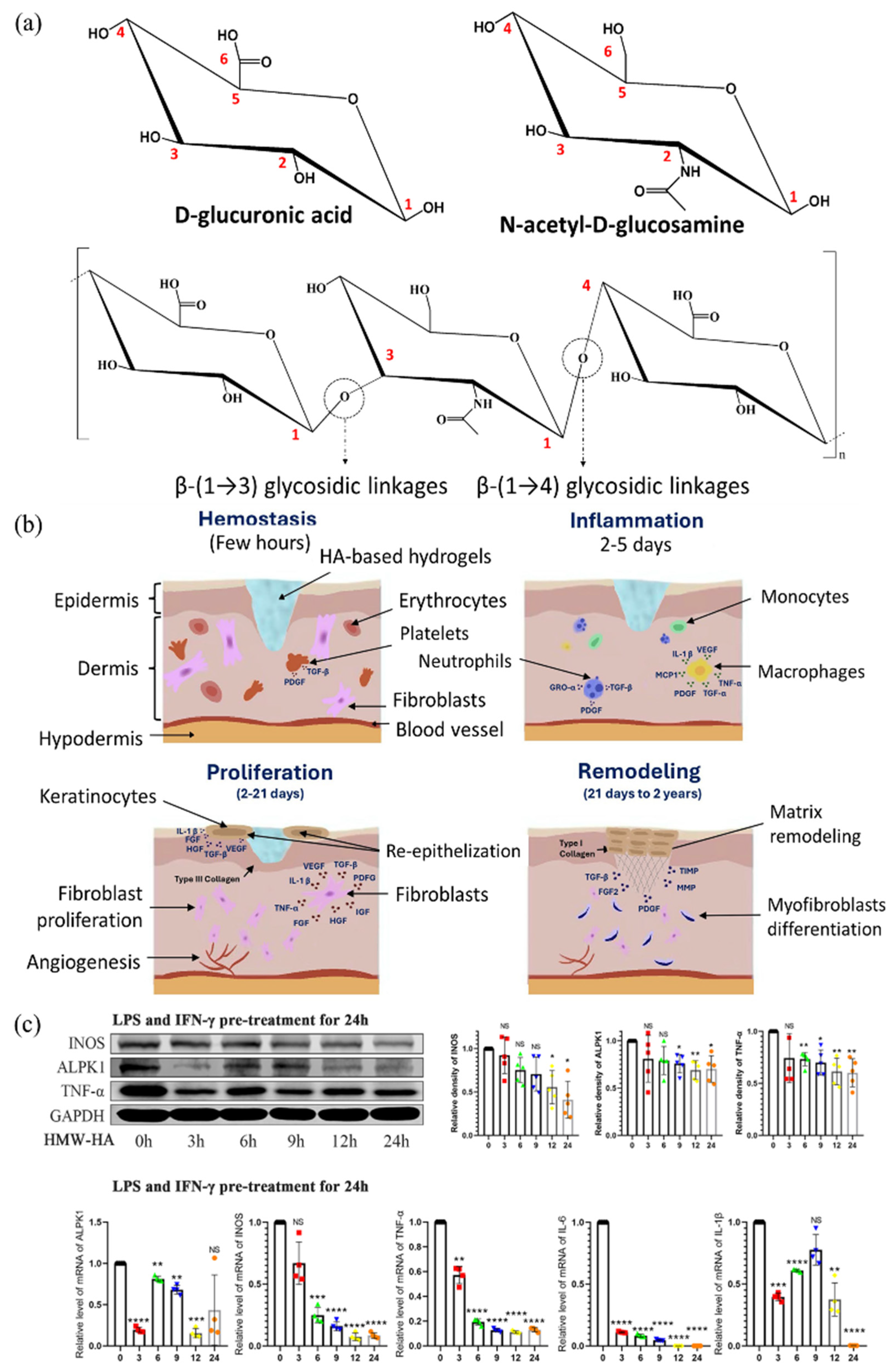
2.1.3. Hyaluronic Acid for Gel Design with Excellent Water Retention Capacity
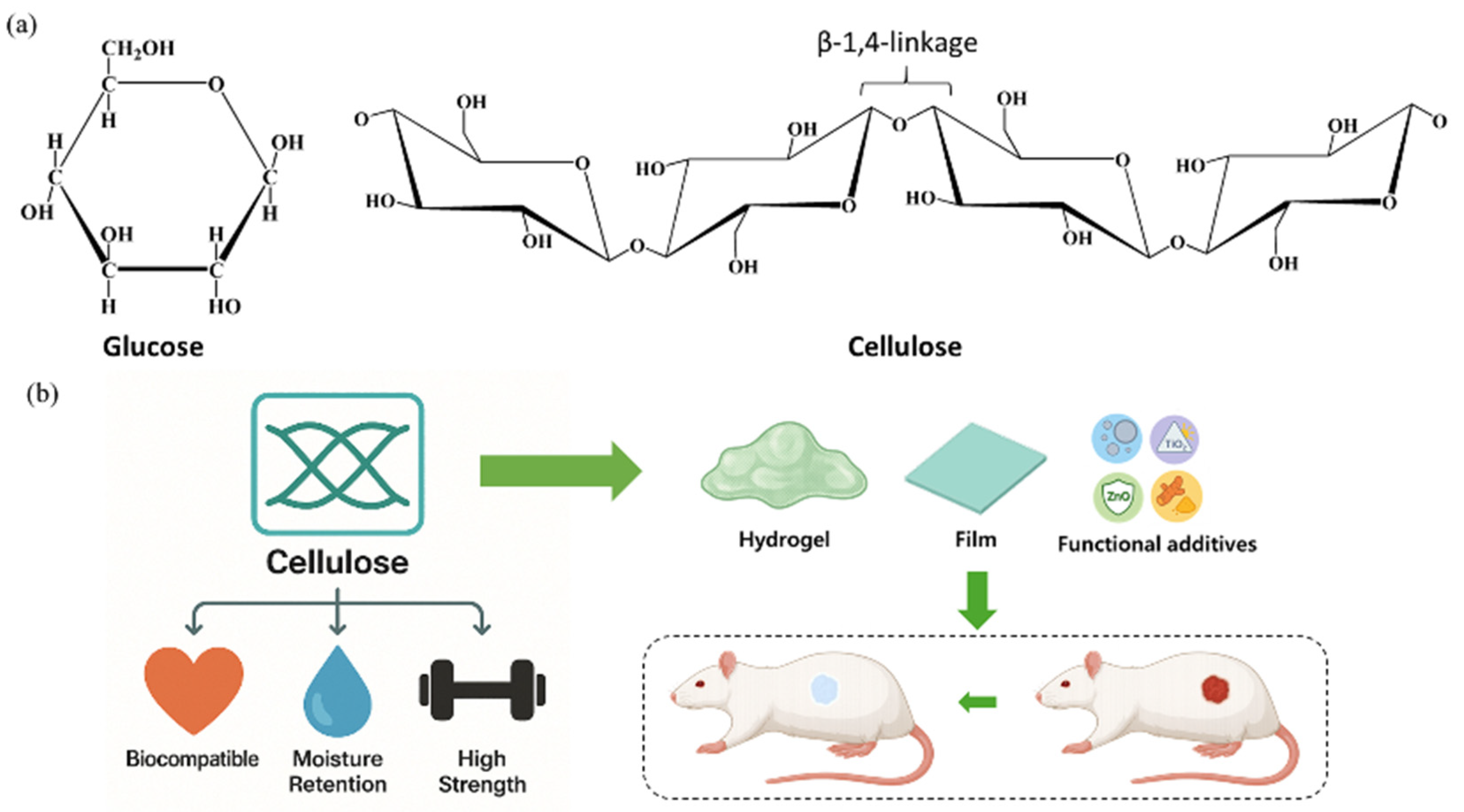
2.1.4. Cellulose for Hydrogel with Improved Mechanical Stability
2.2. Synthetic Biopolymers: Tunable Platforms for Biomedical and Therapeutic Applications
2.2.1. Polylactic Acid: Tunable for Antibacterial and Immunomodulatory Healing
2.2.2. Polyglycolic Acid: Crystallinity-Driven Biodegradability for Biomedical Scaffolds
2.2.3. Poly (Lactic-co-Glycolic Acid): Tunable Copolymer for Controlled Drug Delivery
2.2.4. Polycaprolactone: Durable Polymer for Tissue Support and Drug Delivery
3. Biopolymeric Composites in Wound Healing
3.1. Types of Biopolymeric Composites and Their Applications in Wound Healing
3.1.1. Classification of Biopolymeric Composites by Matrix Type: Natural and Synthetic
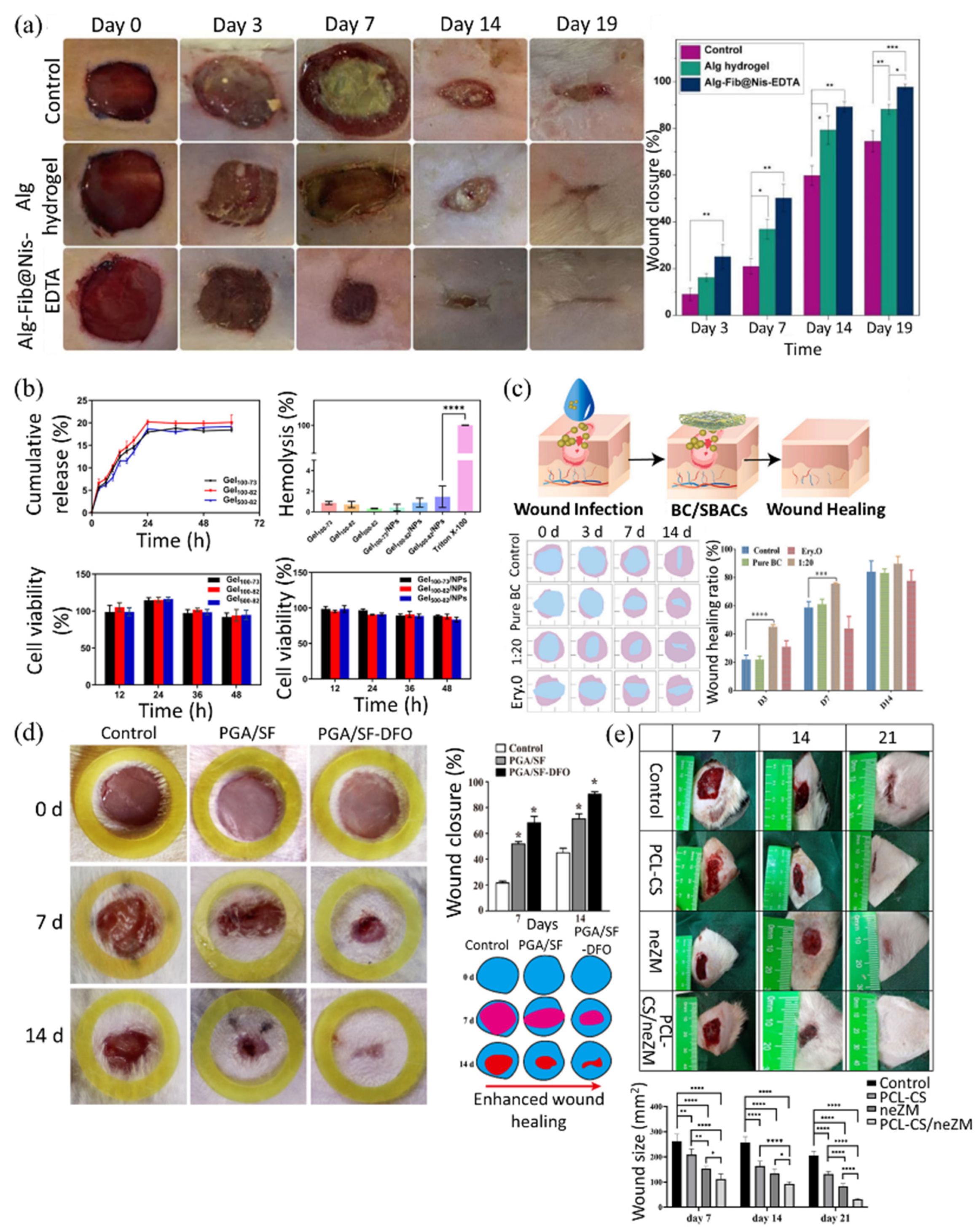
3.1.2. Classification of Biopolymeric Composites According to the Reinforcement Type: Nanoparticle-Incorporated Composites, Fiber-Reinforced Composites, and Drug-Loaded Composites
4. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagle, S.M.; Stevens, K.A.; Wilbraham, S.C. Wound Assessment; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Buganza Tepole, A.; Kuhl, E. Systems-Based Approaches toward Wound Healing. Pediatr. Res. 2013, 73, 553–563. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Hunt, M.; Torres, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Cellular and Molecular Roles of Reactive Oxygen Species in Wound Healing. Commun. Biol. 2024, 7, 1534. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Sousa, D.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Polymeric Biomaterials for Wound Healing. Front. Bioeng. Biotechnol. 2023, 11, 1136077. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ullah, S.; Ullah, S.; Shakeel, M.; Afsar, T.; Husain, F.M.; Amor, H.; Razak, S. Innovative Biopolymers Composite Based Thin Film for Wound Healing Applications. Sci. Rep. 2024, 14, 27415. [Google Scholar] [CrossRef] [PubMed]
- Aslam Khan, M.U.; Abd Razak, S.I.; Al Arjan, W.S.; Nazir, S.; Sahaya Anand, T.J.; Mehboob, H.; Amin, R. Recent Advances in Biopolymeric Composite Materials for Tissue Engineering and Regenerative Medicines: A Review. Molecules 2021, 26, 619. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and Molecular Mechanisms of Skin Wound Healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Kathawala, M.H.; Ng, W.L.; Liu, D.; Naing, M.W.; Yeong, W.Y.; Spiller, K.L.; Van Dyke, M.; Ng, K.W. Healing of Chronic Wounds: An Update of Recent Developments and Future Possibilities. Tissue Eng. Part B Rev. 2019, 25, 429–444. [Google Scholar] [CrossRef]
- Singh, V.; Marimuthu, T.; Makatini, M.M.; Choonara, Y.E. Biopolymer-Based Wound Dressings with Biochemical Cues for Cell-Instructive Wound Repair. Polymers 2022, 14, 5371. [Google Scholar] [CrossRef]
- Qin, J.; Chen, F.; Wu, P.; Sun, G. Recent Advances in Bioengineered Scaffolds for Cutaneous Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 841583. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Desimone, M.F. Biopolymers Take Center Stage in Wound Healing Advancements. Pharmaceutics 2024, 16, 755. [Google Scholar] [CrossRef] [PubMed]
- Gobi, R.; Ravichandiran, P.; Babu, R.S.; Yoo, D.J. Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymers 2021, 13, 1962. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Hybrid-Based Wound Dressings: Combination of Synthetic and Biopolymers. Polymers 2022, 14, 3806. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Jung, M.; Lee, H. Designing Microparticle-Impregnated Polyelectrolyte Composite: The Combination of ATRP, Fast Azidation, and Click Reaction Using a Single-Catalyst, Single-Pot Strategy. Int. J. Mol. Sci. 2019, 20, 5582. [Google Scholar] [CrossRef]
- Xin, H.; Al Maruf, D.S.A.; Akin-Ige, F.; Amin, S. Stimuli-Responsive Hydrogels for Skin Wound Healing and Regeneration. Emergent Mater. 2024, 8, 1339–1356. [Google Scholar] [CrossRef]
- Jia, X.; Dou, Z.; Zhang, Y.; Li, F.; Xing, B.; Hu, Z.; Li, X.; Liu, Z.; Yang, W.; Liu, Z. Smart Responsive and Controlled-Release Hydrogels for Chronic Wound Treatment. Pharmaceutics 2023, 15, 2735. [Google Scholar] [CrossRef]
- Yuan, T.; Tan, M.; Xu, Y.; Xiao, Q.; Wang, H.; Wu, C.; Li, F.; Peng, L. All-in-One Smart Dressing for Simultaneous Angiogenesis and Neural Regeneration. J. Nanobiotechnol. 2023, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Nifontova, G.; Safaryan, S.; Khristidis, Y.; Smirnova, O.; Vosough, M.; Shpichka, A.; Timashev, P. Advancing Wound Healing by Hydrogel-Based Dressings Loaded with Cell-Conditioned Medium: A Systematic Review. Stem Cell Res. Ther. 2024, 15, 371. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Zhao, C.; Li, J.; Huang, R.; Zhang, Q.; Li, Y.; Li, X. An Integrated Smart Sensor Dressing for Real-Time Wound Microenvironment Monitoring and Promoting Angiogenesis and Wound Healing. Front. Cell Dev. Biol. 2021, 9, 701525. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Song, Y.H.; Mahata, M.K.; Lee, K.T. PH-Responsive Polyelectrolyte Complexation on Upconversion Nanoparticles: A Multifunctional Nanocarrier for Protection, Delivery, and 3D-Imaging of Therapeutic Protein. J. Mater. Chem. B 2022, 10, 3420–3433. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic Polymeric Biomaterials for Wound Healing: A Review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Would Exudate and the Role of Dressings. Int. Wound J. 2008, 5, iii-12. [CrossRef]
- Seijo-Rabina, A.; Paramés-Estevez, S.; Concheiro, A.; Pérez-Muñuzuri, A.; Alvarez-Lorenzo, C. Effect of Wound Dressing Porosity and Exudate Viscosity on the Exudate Absorption: In Vitro and in Silico Tests with 3D Printed Hydrogels. Int. J. Pharm. X 2024, 8, 100288. [Google Scholar] [CrossRef]
- Upton, P.; Dunk, A.M.; Upton, D. Complications Associated with Postoperative Dressings: A Clinician’s Perspective. Wound Pract. Res. 2019, 27, 158. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Flórez, M.; Cazón, P.; Vázquez, M. Selected Biopolymers’ Processing and Their Applications: A Review. Polymers 2023, 15, 641. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Tyagi, N.; Song, Y.H.; De, R. Recent Progress on Biocompatible Nanocarrier-Based Genistein Delivery Systems in Cancer Therapy. J. Drug Target. 2019, 27, 394–407. [Google Scholar] [CrossRef]
- Kaur, R.; Pathak, L.; Vyas, P. Biobased Polymers of Plant and Microbial Origin and Their Applications—A Review. Biotechnol. Sustain. Mater. 2024, 1, 13. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(Butylene Succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef]
- Aaliya, B.; Sunooj, K.V.; Lackner, M. Biopolymer Composites: A Review. Int. J. Biobased Plast. 2021, 3, 40–84. [Google Scholar] [CrossRef]
- Kuperkar, K.; Atanase, L.; Bahadur, A.; Crivei, I.; Bahadur, P. Degradable Polymeric Bio(Nano)Materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, P.L. Biopolymers and Composites: Properties, Characterization and Their Applications in Food, Medical and Pharmaceutical Industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Bucciol, F.; Chaji, S.; Cravotto, G. Mechanochemical Degradation of Biopolymers. Molecules 2023, 28, 8031. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Biopolymer Composites: Synthesis, Properties, and Applications. Int. J. Mol. Sci. 2022, 23, 2257. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Akula, B.; Enyioma, H.; Novak, M.; Amin, V.; Liang, H. Biodegradable Biobased Polymers: A Review of the State of the Art, Challenges, and Future Directions. Polymers 2024, 16, 2262. [Google Scholar] [CrossRef]
- McKay, I.; Vargas, J.; Yang, L.; Felfel, R.M. A Review of Natural Fibres and Biopolymer Composites: Progress, Limitations, and Enhancement Strategies. Materials 2024, 17, 4878. [Google Scholar] [CrossRef]
- Laycock, B.; Pratt, S.; Halley, P. A Perspective on Biodegradable Polymer Biocomposites—From Processing to Degradation. Funct. Compos. Mater. 2023, 4, 10. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A.; Sukumaran, N.P.; Haponiuk, J.T.; Thomas, S. Biopolymers and Their Composites for Drug Delivery: A Brief Review. Macromol. Symp. 2018, 380, 1800114. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Zheng, L.; Tseomashko, N.; Voronova, A.; Vasil’kov, A.; Hu, X.; Wang, X. Recent Advances of Collagen Composite Biomaterials for Biomedical Engineering: Antibacterial Functionalization and 3D-Printed Architecturalization. Collagen Leather 2024, 6, 22. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Jorgensen, A.M.; Mahajan, N.; Atala, A.; Murphy, S.V. Advances in Skin Tissue Engineering and Regenerative Medicine. J. Burn. Care Res. 2023, 44, S33–S41. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.; Soker, S.; Murphy, S.V. Regenerative Medicine Approaches for Skin Wound Healing: From Allografts to Engineered Skin Substitutes. Curr. Transplant. Rep. 2024, 11, 207–221. [Google Scholar] [CrossRef]
- Abdul Kareem, N.; Aijaz, A.; Jeschke, M.G. Stem Cell Therapy for Burns: Story so Far. Biologics 2021, 15, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, J.; Wang, Y.; Li, X.; Yang, L.; Zhao, M.; Chen, C. Development of Biomaterials to Modulate the Function of Macrophages in Wound Healing. Bioengineering 2024, 11, 1017. [Google Scholar] [CrossRef]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing From Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Biomaterial Wound Dressing Market Size, Share & Trends Analysis Report By Product (Natural, Synthetic), By Application (Chronic Wounds, Acute Wounds), By End-Use, By Region, And Segment Forecasts, 2024–2030. Available online: https://www.grandviewresearch.com/industry-analysis/biomaterial-wound-dressing-market-report (accessed on 29 July 2025).
- El Sayed Hassan, M.; Bai, J.; Dou, D.-Q. Biopolymers; Definition, Classification and Applications. Egypt. J. Chem. 2019, 62, 1725–1737. [Google Scholar] [CrossRef]
- Yadav, P. Biomedical Biopolymers, Their Origin and Evolution in Biomedical Sciences: A Systematic Review. J. Clin. Diagn. Res. 2015, 9, ZE21. [Google Scholar] [CrossRef] [PubMed]
- Alaswad, S.O.; Mahmoud, A.S.; Arunachalam, P. Recent Advances in Biodegradable Polymers and Their Biological Applications: A Brief Review. Polymers 2022, 14, 4924. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivasatva, A.K.; Singh, A.; Singh, P.; Verma, S.; Vats, M.; Sagadevan, S. Biopolymers as Renewable Polymeric Materials for Sustainable Development—An Overview. Polimery 2022, 67, 185–196. [Google Scholar] [CrossRef]
- Kurowiak, J.; Klekiel, T.; Będziński, R. Biodegradable Polymers in Biomedical Applications: A Review—Developments, Perspectives and Future Challenges. Int. J. Mol. Sci. 2023, 24, 16952. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Abdan, K.; Kaneko, T. A Concise Review on the Physicochemical Properties of Biopolymer Blends Prepared in Ionic Liquids. Molecules 2021, 26, 216. [Google Scholar] [CrossRef]
- He, B.; Gao, Y.; Lou, W.; Wang, J.; Xin, S.; Ma, K.; Li, L. Biodegradable Food Packaging Films: Material Source, Mechanical and Water Vapor Barrier Properties, and Improvement Strategies—A Comprehensive Review. Food Res. Int. 2025, 211, 116431. [Google Scholar] [CrossRef]
- Sabu Mathew, S.; Jaiswal, A.K.; Jaiswal, S. A Comprehensive Review on Hydrophobic Modification of Biopolymer Composites for Food Packaging Applications. Food Packag. Shelf Life 2025, 48, 101464. [Google Scholar] [CrossRef]
- Jalil, M.A.; Repon, M.R.; Halim, A.F.M.F.; Alim, M.A. Environmental Impact of Chemically Modified Natural Biopolymers. In Handbook of Natural Polymers, Volume 2; Elsevier: Amsterdam, The Netherlands, 2024; pp. 523–541. [Google Scholar]
- Abdullah; Cai, J.; Hafeez, M.A.; Wang, Q.; Farooq, S.; Huang, Q.; Tian, W.; Xiao, J. Biopolymer-Based Functional Films for Packaging Applications: A Review. Front. Nutr. 2022, 9, 1000116. [Google Scholar] [CrossRef]
- Chandrababu, V.; Parameswaranpillai, J.; Gopi, J.A.; Pathak, C.; Midhun Dominic, C.D.; Feng, N.L.; Krishnasamy, S.; Muthukumar, C.; Hameed, N.; Ganguly, S. Progress in Food Packaging Applications of Biopolymer-Nanometal Composites—A Comprehensive Review. Biomater. Adv. 2024, 162, 213921. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Kaidi, S.; Bentiss, F.; Jama, C.; Khaya, K.; Belattmania, Z.; Reani, A.; Sabour, B. Isolation and Structural Characterization of Alginates from the Kelp Species Laminaria ochroleuca and Saccorhiza polyschides from the Atlantic Coast of Morocco. Colloids Interfaces 2022, 6, 51. [Google Scholar] [CrossRef]
- Stender, E.G.P.; Dybdahl Andersen, C.; Fredslund, F.; Holck, J.; Solberg, A.; Teze, D.; Peters, G.H.J.; Christensen, B.E.; Aachmann, F.L.; Welner, D.H.; et al. Structural and Functional Aspects of Mannuronic Acid–Specific PL6 Alginate Lyase from the Human Gut Microbe Bacteroides cellulosilyticus. J. Biol. Chem. 2019, 294, 17915–17930. [Google Scholar] [CrossRef]
- Jiao, W.; Chen, W.; Mei, Y.; Yun, Y.; Wang, B.; Zhong, Q.; Chen, H.; Chen, W. Effects of Molecular Weight and Guluronic Acid/Mannuronic Acid Ratio on the Rheological Behavior and Stabilizing Property of Sodium Alginate. Molecules 2019, 24, 4374. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.L.; Fernandez, J.M.; Dellatorre, F.G.; Cortizo, A.M.; Oberti, T.G. Purification of Alginate Improves Its Biocompatibility and Eliminates Cytotoxicity in Matrix for Bone Tissue Engineering. Algal Res. 2019, 40, 101499. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Yang, J.; Steck, J.; Suo, Z. Gelation Kinetics of Alginate Chains through Covalent Bonds. Extreme Mech. Lett. 2020, 40, 100898. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; deClaville Christiansen, J. Mechanical Properties of Alginate Hydrogels Cross-Linked with Multivalent Cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef]
- Kostenko, A.; Swioklo, S.; Connon, C.J. Effect of Calcium Sulphate Pre-Crosslinking on Rheological Parameters of Alginate Based Bio-Inks and on Human Corneal Stromal Fibroblast Survival in 3D Bio-Printed Constructs. Front. Mech. Eng. 2022, 8, 867685. [Google Scholar] [CrossRef]
- Nützl, M.; Schrottenbaum, M.; Müller, T.; Müller, R. Mechanical Properties and Chemical Stability of Alginate-Based Anisotropic Capillary Hydrogels. J. Mech. Behav. Biomed. Mater. 2022, 134, 105397. [Google Scholar] [CrossRef]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked Alginate Hydrogels with Tunable Biodegradation Rates and Mechanical Properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef]
- Araiza-Verduzco, F.; Rodríguez-Velázquez, E.; Cruz, H.; Rivero, I.A.; Acosta-Martínez, D.R.; Pina-Luis, G.; Alatorre-Meda, M. Photocrosslinked Alginate-Methacrylate Hydrogels with Modulable Mechanical Properties: Effect of the Molecular Conformation and Electron Density of the Methacrylate Reactive Group. Materials 2020, 13, 534. [Google Scholar] [CrossRef]
- Petrusic, S.; Lewandowski, M.; Giraud, S.; Jovancic, P.; Bugarski, B.; Ostojic, S.; Koncar, V. Development and Characterization of Thermosensitive Hydrogels Based on Poly(N-isopropylacrylamide) and Calcium Alginate. J. Appl. Polym. Sci. 2012, 124, 890–903. [Google Scholar] [CrossRef]
- Aderibigbe, B.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-Isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, X. Alginate Hydrogel Dressings for Advanced Wound Management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and Characterization of Alginate Hydrogels with High Water-Retaining Capacity. Polymers 2023, 15, 2592. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.-W.; Kim, S.-K.; Jeon, Y.-J.; Je, J.-Y.; Ahn, C.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Chitooligosaccharide and Its Derivatives: Preparation and Biological Applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef]
- Terkula Iber, B.; Azman Kasan, N.; Torsabo, D.; Wese Omuwa, J. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2022, 10, 1097–1123. [Google Scholar] [CrossRef]
- Olaru, M.; Simionescu, N.; Doroftei, F.; David, G. Strategy Based on Michael Addition Reaction for the Development of Bioinspired Multilayered and Multiphasic 3D Constructs. Polymers 2023, 15, 1635. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Amor, I.B.; Hemmami, H.; Laouini, S.E.; Abdelaziz, A.G.; Barhoum, A. Influence of Chitosan Source and Degree of Deacetylation on Antibacterial Activity and Adsorption of AZO Dye from Water. Biomass Convers. Biorefinery 2024, 14, 16245–16255. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial Properties of Chitosan from Different Developmental Stages of the Bioconverter Insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Egorov, A.R.; Kirichuk, A.A.; Rubanik, V.V.; Rubanik, V.V.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan and Its Derivatives: Preparation and Antibacterial Properties. Materials 2023, 16, 6076. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, X.; Lu, Y.; Dong, S.; Zhao, Y.; Zeng, M. Effects of Chitosan Molecular Weight and Degree of Deacetylation on the Properties of Gelatine-Based Films. Food Hydrocoll. 2012, 26, 311–317. [Google Scholar] [CrossRef]
- Wang, H.; Roman, M. Effects of Chitosan Molecular Weight and Degree of Deacetylation on Chitosan−Cellulose Nanocrystal Complexes and Their Formation. Molecules 2023, 28, 1361. [Google Scholar] [CrossRef] [PubMed]
- Dutta, J.; Mohini, P. Effect of Degree of Deacetylation and Molecular Weight on Physicochemical Properties Ofchitosan Films. J. Indian Chem. Soc. 2020, 97, 731–735. [Google Scholar]
- Muzzarelli, R.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite Hydrogels Based on Gelatin, Chitosan and Polyvinyl Alcohol to Biomedical Applications: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial Production of Hyaluronic Acid: Current State, Challenges, and Perspectives. Microb. Cell Fact. 2011, 10, 99. [Google Scholar] [CrossRef]
- Prestwich, G.D.; Marecak, D.M.; Marecek, J.F.; Vercruysse, K.P.; Ziebell, M.R. Controlled Chemical Modification of Hyaluronic Acid: Synthesis, Applications, and Biodegradation of Hydrazide Derivatives. J. Control. Release 1998, 53, 93–103. [Google Scholar] [CrossRef]
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical Modification of Hyaluronan and Their Biomedical Applications. Front. Chem. 2022, 10, 830671. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Chylińska, N.; Maciejczyk, M. Hyaluronic Acid and Skin: Its Role in Aging and Wound-Healing Processes. Gels 2025, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sun, Y.; Yang, H.; Qiu, P.; Cong, Z.; Zou, Y.; Song, L.; Guo, J.; Anastassiades, T.P. A Low Molecular Weight Hyaluronic Acid Derivative Accelerates Excisional Wound Healing by Modulating Pro-Inflammation, Promoting Epithelialization and Neovascularization, and Remodeling Collagen. Int. J. Mol. Sci. 2019, 20, 3722. [Google Scholar] [CrossRef]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 4, 622. [Google Scholar] [CrossRef]
- Ramanathan, G.; Hassan, M.; Rochev, Y. Optimising the Viscoelastic Properties of Hyaluronic Acid Hydrogels through Colloidal Particle Interactions: A Response Surface Methodology Approach. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703, 135320. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 458280. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.-L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Triggs-Raine, B. Biology of Hyaluronan: Insights from Genetic Disorders of Hyaluronan Metabolism. World J. Biol. Chem. 2015, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Žádníková, P.; Šínová, R.; Pavlík, V.; Šimek, M.; Šafránková, B.; Hermannová, M.; Nešporová, K.; Velebný, V. The Degradation of Hyaluronan in the Skin. Biomolecules 2022, 12, 251. [Google Scholar] [CrossRef]
- Hejran, A.B.; Ashrafi, H.; Baseer, A.Q.; Sarwari, A.; Monib, A.W.; Hassand, M.H.; Sediqi, S.; Kakar, U.M.; Niazi, P.; Rahime, M. The Importance of Hyaluronic Acid in Biological Systems. Eur. J. Theor. Appl. Sci. 2024, 2, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Glinkowski, W.; Śladowski, D.; Tomaszewski, W. Molecular Mechanisms and Therapeutic Role of Intra-Articular Hyaluronic Acid in Osteoarthritis: A Precision Medicine Perspective. J. Clin. Med. 2025, 14, 2547. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Chiang, C.-F.; Kuo, F.-C.; Su, S.-C.; Huang, C.-L.; Liu, J.-S.; Lu, C.-H.; Hsieh, C.-H.; Wang, C.-C.; Lee, C.-H.; et al. High-Molecular-Weight Hyaluronic Acid Inhibits IL-1β-Induced Synovial Inflammation and Macrophage Polarization through the GRP78-NF-ΚB Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 11917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Feng, Y.; Liu, X.; Li, H.; Guo, H.; Ke, J.; Long, X. The Relationship of alpk1, Hyaluronic Acid and m1 Macrophage Polarization in the Temporomandibular Joint Synovitis. J. Cell. Mol. Med. 2024, 28, e18172. [Google Scholar] [CrossRef]
- Loqué, D.; Scheller, H.V.; Pauly, M. Engineering of Plant Cell Walls for Enhanced Biofuel Production. Curr. Opin. Plant Biol. 2015, 25, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Production and Characteristics of Cellulose from Different Sources; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–38. [Google Scholar]
- de Souza, K.C.; dos Santos, G.R.; Trindade, F.C.S.; de S Costa, A.F.; de Almeida, Y.M.; Sarubbo, L.A.; Vinhas, G.M. Production of Bacterial Cellulose Biopolymers in Media Containing Rice and Corn Hydrolysate as Carbon Sources. Polym. Polym. Compos. 2021, 29, S1466–S1474. [Google Scholar] [CrossRef]
- De Silva, A.G.S.D.; Hashim, Z.K.; Solomon, W.; Zhao, J.-B.; Kovács, G.; Kulmány, I.M.; Molnár, Z. Unveiling the Role of Edaphic Microalgae in Soil Carbon Sequestration: Potential for Agricultural Inoculants in Climate Change Mitigation. Agriculture 2024, 14, 2065. [Google Scholar] [CrossRef]
- Nobles, D.R.; Brown, R.M. The Pivotal Role of Cyanobacteria in the Evolution of Cellulose Synthases and Cellulose Synthase-like Proteins. Cellulose 2004, 11, 437–448. [Google Scholar] [CrossRef]
- Carpita, N.C. Update on Mechanisms of Plant Cell Wall Biosynthesis: How Plants Make Cellulose and Other (1→4)-β-d-Glycans. Plant Physiol. 2011, 155, 171–184. [Google Scholar] [CrossRef]
- Álvarez-Martínez, I.; Pfrengle, F. On the Structure, Conformation and Reactivity of β-1,4-Linked Plant Cell Wall Glycans: Why Are Xylan Polysaccharides or Furanosyl Substituents Easier to Hydrolyze than Cellulose? Cellulose 2025, 32, 2145–2165. [Google Scholar] [CrossRef]
- Wohlert, M.; Benselfelt, T.; Wågberg, L.; Furó, I.; Berglund, L.A.; Wohlert, J. Cellulose and the Role of Hydrogen Bonds: Not in Charge of Everything. Cellulose 2022, 29, 1–23. [Google Scholar] [CrossRef]
- Zangiabadi, M.; Zhao, Y. Synergistic Hydrolysis of Cellulose by a Blend of Cellulase-Mimicking Polymeric Nanoparticle Catalysts. J. Am. Chem. Soc. 2022, 144, 17110–17119. [Google Scholar] [CrossRef]
- Erdal, N.B.; Hakkarainen, M. Degradation of Cellulose Derivatives in Laboratory, Man-Made, and Natural Environments. Biomacromolecules 2022, 23, 2713–2729. [Google Scholar] [CrossRef] [PubMed]
- Brunšek, R.; Kopitar, D.; Schwarz, I.; Marasović, P. Biodegradation Properties of Cellulose Fibers and PLA Biopolymer. Polymers 2023, 15, 3532. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Daystar, J.S.; Venditti, R.A.; Pawlak, J.J.; Zambrano, M.C.; Barlaz, M.; Ankeny, M.; Pires, S. Biodegradability of Cellulose Fibers, Films, and Particles: A Review. Bioresources 2025, 20, 2391–2458. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Perdoch, W.; Treu, A.; Mazela, B.; Majka, J.; Czajkowski, Ł.; Olek, W. Hydrophobic and Hygroscopic Properties of Cellulose Treated with Silicone Agents. Eur. J. Wood Wood Prod. 2024, 82, 821–832. [Google Scholar] [CrossRef]
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836. [Google Scholar] [CrossRef]
- Grzybek, P.; Dudek, G.; van der Bruggen, B. Cellulose-Based Films and Membranes: A Comprehensive Review on Preparation and Applications. Chem. Eng. J. 2024, 495, 153500. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose Nanocomposites: Fabrication and Biomedical Applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A Comprehensive Review on Chemical Properties and Applications of Biopolymers and Their Composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic Acid (PLA): Properties, Synthesis, and Biomedical Applications—A Review of the Literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Shiva, S.; Asuwin Prabu, R.G.; Bajaj, G.; John, A.E.; Chandran, S.; Kumar, V.V.; Ramakrishna, S. A Review on the Recent Applications of Synthetic Biopolymers in 3D Printing for Biomedical Applications. J. Mater. Sci. Mater. Med. 2023, 34, 62. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Fang, Y.; Zhang, Z.; Cao, Y.; Yan, Y.; Gan, L.; Xu, J.; Zhou, G. Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing. Materials 2023, 16, 5459. [Google Scholar] [CrossRef]
- Twaites, B.; de las Heras Alarcón, C.; Alexander, C. Synthetic Polymers as Drugs and Therapeutics. J. Mater. Chem. 2005, 15, 441–455. [Google Scholar] [CrossRef]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- de França, J.O.C.; da Silva Valadares, D.; Paiva, M.F.; Dias, S.C.L.; Dias, J.A. Polymers Based on PLA from Synthesis Using D,L-Lactic Acid (or Racemic Lactide) and Some Biomedical Applications: A Short Review. Polymers 2022, 14, 2317. [Google Scholar] [CrossRef]
- Castañeda-Rodríguez, S.; González-Torres, M.; Ribas-Aparicio, R.M.; Del Prado-Audelo, M.L.; Leyva-Gómez, G.; Gürer, E.S.; Sharifi-Rad, J. Recent Advances in Modified Poly (Lactic Acid) as Tissue Engineering Materials. J. Biol. Eng. 2023, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, K.; Bindhu, B.; Veluraja, K. Perspectives of Polylactic Acid from Structure to Applications. Polym. Renew. Resour. 2021, 12, 60–74. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(Lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, A.; Raptis, V.; Baltzaki, C.I.M.; Manios, T.; Harmandaris, V.; Velonia, K. Synthesis and Modeling of Poly(L-Lactic Acid) via Polycondensation of L-Lactic Acid. Polymers 2023, 15, 4569. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Shang, X.; Wu, X.; Ma, L.; Xing, C.; Zhu, J. Epoxy-Based Chain Extenders in Polylactic Acid (PLA): A Comprehensive Review of Structure, Performance, and Challenges. J. Mater. Sci. Chem. Eng. 2025, 13, 20–44. [Google Scholar] [CrossRef]
- Alkan Goksu, Y. Enhancing the Sustainability of Poly(Lactic Acid) (PLA) Through Ketene-Based Chain Extension. J. Polym. Environ. 2024, 32, 3640–3653. [Google Scholar] [CrossRef]
- Liu, C.; Jia, Y.; He, A. Preparation of Higher Molecular Weight Poly (L-Lactic Acid) by Chain Extension. Int. J. Polym. Sci. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Horváth, T.; Marossy, K.; Szabó, T.J. Ring-Opening Polymerization and Plasticization of Poly(L-Lactic)Acid by Adding of Glycerol-Dioleate. J. Therm. Anal. Calorim. 2022, 147, 2221–2227. [Google Scholar] [CrossRef]
- Viamonte-Aristizábal, S.; García-Sancho, A.; Arrabal Campos, F.M.; Martínez-Lao, J.A.; Fernández, I. Synthesis of High Molecular Weight L-Polylactic Acid (PLA) by Reactive Extrusion at a Pilot Plant Scale: Influence of 1,12-Dodecanediol and Di(Trimethylol Propane) as Initiators. Eur. Polym. J. 2021, 161, 110818. [Google Scholar] [CrossRef]
- Litauszki, K.; Petrény, R.; Haramia, Z.; Mészáros, L. Combined Effects of Plasticizers and D-Lactide Content on the Mechanical and Morphological Behavior of Polylactic Acid. Heliyon 2023, 9, e14674. [Google Scholar] [CrossRef] [PubMed]
- Tábi, T.; Wacha, A.F.; Hajba, S. Effect of D-lactide Content of Annealed Poly(Lactic Acid) on Its Thermal, Mechanical, Heat Deflection Temperature, and Creep Properties. J. Appl. Polym. Sci. 2019, 136, 47103. [Google Scholar] [CrossRef]
- Feng, L.; Bian, X.; Li, G.; Chen, X. Thermal Properties and Structural Evolution of Poly(l-Lactide)/Poly(d-Lactide) Blends. Macromolecules 2021, 54, 10163–10176. [Google Scholar] [CrossRef]
- Pölöskei, K.; Csézi, G.; Hajba, S.; Tábi, T. Investigation of the Thermoformability of Various d-Lactide Content Poly(Lactic Acid) Films by Ball Burst Test. Polym. Eng. Sci. 2020, 60, 1266–1277. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent Advances in Electrospun Nanofibers for Wound Healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef]
- Heydari, P.; Zargar Kharazi, A.; Shariati, L. Enhanced Wound Regeneration by PGS/PLA Fiber Dressing Containing Platelet-Rich Plasma: An in Vitro Study. Sci. Rep. 2024, 14, 12019. [Google Scholar] [CrossRef] [PubMed]
- Fatahian, R.; Erfani, R. Surrogate Modeling of Electrospun PVA/PLA Nanofibers Using Artificial Neural Network for Biomedical Applications. Sci. Rep. 2025, 15, 12886. [Google Scholar] [CrossRef] [PubMed]
- Parangusan, H.; Karuppasamy, K.; Bhadra, J. Fenugreek-Enriched Electrospun PLA Scaffold: Revolutionizing Tissue Engineering Solutions. J. Polym. Res. 2025, 32, 175. [Google Scholar] [CrossRef]
- Yu, C.; Bao, J.; Xie, Q.; Shan, G.; Bao, Y.; Pan, P. Crystallization Behavior and Crystalline Structural Changes of Poly(Glycolic Acid) Investigated via Temperature-Variable WAXD and FTIR Analysis. CrystEngComm 2016, 18, 7894–7902. [Google Scholar] [CrossRef]
- Little, A.; Ma, S.; Haddleton, D.M.; Tan, B.; Sun, Z.; Wan, C. Synthesis and Characterization of High Glycolic Acid Content Poly(Glycolic Acid- Co -Butylene Adipate-Co-Butylene Terephthalate) and Poly(Glycolic Acid-Co-Butylene Succinate) Copolymers with Improved Elasticity. ACS Omega 2023, 8, 38658–38667. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Weidner, S.M.; Meyer, A. Syntheses of Polyglycolide via Polycondensation: A Reinvestigation. Macromol. Chem. Phys. 2024, 225, 2300397. [Google Scholar] [CrossRef]
- Sanko, V.; Sahin, I.; Aydemir Sezer, U.; Sezer, S. A Versatile Method for the Synthesis of Poly(Glycolic Acid): High Solubility and Tunable Molecular Weights. Polym. J. 2019, 51, 637–647. [Google Scholar] [CrossRef]
- Zhang, P.; Ladelta, V.; Hadjichristidis, N. Living/Controlled Anionic Polymerization of Glycolide in Fluoroalcohols: Toward Sustainable Bioplastics. J. Am. Chem. Soc. 2023, 145, 14756–14765. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(Glycolic Acid) (PGA): A Versatile Building Block Expanding High Performance and Sustainable Bioplastic Applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Fu, Y.; Jin, Y.; Weng, Y.; Bian, X.; Chen, X. Degradation Behaviors of Polylactic Acid, Polyglycolic Acid, and Their Copolymer Films in Simulated Marine Environments. Polymers 2024, 16, 1765. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, Q.; Shi, X.; Niu, H.; Guo, P.; Li, Z.; Lyu, M.; Lei, M. A Theoretical and Experimental Study on the Degradation Mechanism of Polyglycolic Acid under Acidic, Neutral, and Basic Conditions. J. Appl. Polym. Sci. 2024, 141, e55947. [Google Scholar] [CrossRef]
- Ceonzo, K.; Gaynor, A.; Shaffer, L.; Kojima, K.; Vacanti, C.A.; Stahl, G.L. Polyglycolic Acid-Induced Inflammation: Role of Hydrolysis and Resulting Complement Activation. Tissue Eng. 2006, 12, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Hajiali, H.; Shahgasempour, S.; Naimi-Jamal, M.R.; Peirovi, H. Electrospun PGA/Gelatin Nanofibrous Scaffolds and Their Potential Application in Vascular Tissue Engineering. Int. J. Nanomed. 2011, 6, 2133–2141. [Google Scholar] [CrossRef]
- Kakei, Y.; Hashikawa, K.; Uryu, K.; Funahara, R.; Shigeoka, M.; Akashi, M. Evaluation of the Effects of Covering With Polyglycolic Acid Sheet on Wound Healing: A Pilot Histopathological Study. Cureus 2022, 14, e27209. [Google Scholar] [CrossRef]
- Lanao, R.P.F.; Jonker, A.M.; Wolke, J.G.C.; Jansen, J.A.; van Hest, J.C.M.; Leeuwenburgh, S.C.G. Physicochemical Properties and Applications of Poly(Lactic-Co-Glycolic Acid) for Use in Bone Regeneration. Tissue Eng. Part B Rev. 2013, 19, 380–390. [Google Scholar] [CrossRef]
- Ayyoob, M.; Kim, Y. Effect of Chemical Composition Variant and Oxygen Plasma Treatments on the Wettability of PLGA Thin Films, Synthesized by Direct Copolycondensation. Polymers 2018, 10, 1132. [Google Scholar] [CrossRef]
- Little, A.; Wemyss, A.M.; Haddleton, D.M.; Tan, B.; Sun, Z.; Ji, Y.; Wan, C. Synthesis of Poly(Lactic Acid-Co-Glycolic Acid) Copolymers with High Glycolide Ratio by Ring-Opening Polymerisation. Polymers 2021, 13, 2458. [Google Scholar] [CrossRef]
- Sriyai, M.; Chaiwon, T.; Molloy, R.; Meepowpan, P.; Punyodom, W. Efficiency of Liquid Tin(ii) n-Alkoxide Initiators in the Ring-Opening Polymerization of l-Lactide: Kinetic Studies by Non-Isothermal Differential Scanning Calorimetry. RSC Adv. 2020, 10, 43566–43578. [Google Scholar] [CrossRef]
- Kaihara, S.; Matsumura, S.; Mikos, A.G.; Fisher, J.P. Synthesis of Poly(L-Lactide) and Polyglycolide by Ring-Opening Polymerization. Nat. Protoc. 2007, 2, 2767–2771. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Tavtorkin, A.N.; Shlyakhtin, A.V.; Ivchenko, P.V. Chemical Features of the Synthesis, Degradation, Molding and Performance of Poly(Lactic-Co-Glycolic) Acid (PLGA) and PLGA-Based Articles. Eur. Polym. J. 2024, 215, 113250. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, Z.; Huang, Q. Enhancing Degradation Resistance of Polyglycolic Acid through Stereocomplex Polylactic Acid Integration: A Novel “Stereo-Lock” Approach. Polymer 2025, 321, 128088. [Google Scholar] [CrossRef]
- Ma, S.; Feng, X.; Liu, F.; Wang, B.; Zhang, H.; Niu, X. The Pro-inflammatory Response of Macrophages Regulated by Acid Degradation Products of Poly(Lactide-co-glycolide) Nanoparticles. Eng. Life Sci. 2021, 21, 709–720. [Google Scholar] [CrossRef]
- Jang, H.J.; Park, S.-B.; Bedair, T.M.; Oh, M.-K.; Ahn, D.-J.; Park, W.; Joung, Y.K.; Han, D.K. Effect of Various Shaped Magnesium Hydroxide Particles on Mechanical and Biological Properties of Poly(Lactic-Co-Glycolic Acid) Composites. J. Ind. Eng. Chem. 2018, 59, 266–276. [Google Scholar] [CrossRef]
- Jusu, S.M.; Obayemi, J.D.; Salifu, A.A.; Nwazojie, C.C.; Uzonwanne, V.; Odusanya, O.S.; Soboyejo, W.O. Drug-Encapsulated Blend of PLGA-PEG Microspheres: In Vitro and in Vivo Study of the Effects of Localized/Targeted Drug Delivery on the Treatment of Triple-Negative Breast Cancer. Sci. Rep. 2020, 10, 14188. [Google Scholar] [CrossRef] [PubMed]
- Kaluzynski, K.; Pretula, J.; Lewinski, P.; Kaźmierski, S.; Penczek, S. Synthesis and Properties of Functionalized Poly(ε-Caprolactone); Chain Polymerization Followed by Polycondensation in One Pot with Initiator and Catalyst in One Molecule. Synthesis and Molecular Structures. Macromolecules 2022, 55, 2210–2221. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Polycaprolactone Composites/Blends and Their Applications Especially in Water Treatment. ChemEngineering 2023, 7, 104. [Google Scholar] [CrossRef]
- Wu, D.; Lv, Y.; Guo, R.; Li, J.; Habadati, A.; Lu, B.; Wang, H.; Wei, Z. Kinetics of Sn(Oct)2-Catalyzed Ring Opening Polymerization of ε-Caprolactone. Macromol. Res. 2017, 25, 1070–1075. [Google Scholar] [CrossRef]
- Punyodom, W.; Limwanich, W.; Meepowpan, P.; Thapsukhon, B. Ring-Opening Polymerization of ε-Caprolactone Initiated by Tin(II) Octoate/n-Hexanol: DSC Isoconversional Kinetics Analysis and Polymer Synthesis. Des. Monomers Polym. 2021, 24, 89–97. [Google Scholar] [CrossRef]
- Lozano, D.; Leiva, B.; Gómez-Escalonilla, I.; Portal-Núñez, S.; de Górtazar, A.; Manzano, M.; Vallet-Regí, M. Pleiotrophin-Loaded Mesoporous Silica Nanoparticles as a Possible Treatment for Osteoporosis. Pharmaceutics 2023, 15, 658. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tena, A.; Pérez-Camargo, R.A.; Coulembier, O.; Sangroniz, L.; Aranburu, N.; Guerrica-Echevarria, G.; Liu, G.; Wang, D.; Cavallo, D.; Müller, A.J. Effect of Molecular Weight on the Crystallization and Melt Memory of Poly(ε-Caprolactone) (PCL). Macromolecules 2023, 56, 4602–4620. [Google Scholar] [CrossRef]
- Blackwell, C.J.; Haernvall, K.; Guebitz, G.M.; Groombridge, M.; Gonzales, D.; Khosravi, E. Enzymatic Degradation of Star Poly(ε-Caprolactone) with Different Central Units. Polymers 2018, 10, 1266. [Google Scholar] [CrossRef]
- Suzuki, M.; Tachibana, Y.; Kasuya, K. Biodegradability of Poly(3-Hydroxyalkanoate) and Poly(ε-Caprolactone) via Biological Carbon Cycles in Marine Environments. Polym. J. 2021, 53, 47–66. [Google Scholar] [CrossRef]
- Sebag, S.C.; Hao, M.; Qian, Q.; Upara, C.; Ding, Q.; Zhu, M.; Banas, J.A.; Cao, H.; Hong, L.; Yang, L. A Medium Chain Fatty Acid, 6-Hydroxyhexanoic Acid (6-HHA), Protects against Obesity and Insulin Resistance. Acta Pharm. Sin. B 2024, 14, 1892–1894. [Google Scholar] [CrossRef]
- Asaduzzaman, F.; Salmon, S. Controllable Water-Triggered Degradation of PCL Solution-Blown Nanofibrous Webs Made Possible by Lipase Enzyme Entrapment. Fibers 2023, 11, 49. [Google Scholar] [CrossRef]
- Kamath, S.M.; Sridhar, K.; Jaison, D.; Gopinath, V.; Ibrahim, B.K.M.; Gupta, N.; Sundaram, A.; Sivaperumal, P.; Padmapriya, S.; Patil, S.S. Fabrication of Tri-Layered Electrospun Polycaprolactone Mats with Improved Sustained Drug Release Profile. Sci. Rep. 2020, 10, 18179. [Google Scholar] [CrossRef] [PubMed]
- Mtibe, A.; Motloung, M.P.; Bandyopadhyay, J.; Ray, S.S. Synthetic Biopolymers and Their Composites: Advantages and Limitations—An Overview. Macromol. Rapid Commun. 2021, 42, 2100130. [Google Scholar] [CrossRef]
- Shiroud Heidari, B.; Ruan, R.; Vahabli, E.; Chen, P.; De-Juan-Pardo, E.M.; Zheng, M.; Doyle, B. Natural, Synthetic and Commercially-Available Biopolymers Used to Regenerate Tendons and Ligaments. Bioact. Mater. 2023, 19, 179–197. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, Y.; Yang, W.; Bu, K.; Tanveer, S.K.; Hai, J. Global Trends in Natural Biopolymers in the 21st Century: A Scientometric Review. Front. Chem. 2022, 10, 915648. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Z.; Sun, J.; Yan, Y.; Bu, M.; Huo, Y.; Li, Y.-F.; Hu, N. Recent Advances in Natural Functional Biopolymers and Their Applications of Electronic Skins and Flexible Strain Sensors. Polymers 2021, 13, 813. [Google Scholar] [CrossRef]
- Kalpana Manivannan, R.; Sharma, N.; Kumar, V.; Jayaraj, I.; Vimal, S.; Umesh, M. A Comprehensive Review on Natural Macromolecular Biopolymers for Biomedical Applications: Recent Advancements, Current Challenges, and Future Outlooks. Carbohydr. Polym. Technol. Appl. 2024, 8, 100536. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Martinez-Rodriguez, S.; Md Fadilah, N.I.; Looi Qi Hao, D.; Markey, G.; Shukla, P.; Fauzi, M.B.; Panetsos, F. Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers 2024, 16, 1280. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Banerjee, A.; Okoro, P.D.; Masoumi, A.; Kanjilal, B.; Akbari, M.; Martins-Green, M.; Armstrong, D.G.; Noshadi, I. Integration of Functional Polymers and Biosensors to Enhance Wound Healing. Adv. Healthc. Mater. 2024, 13, 2401461. [Google Scholar] [CrossRef] [PubMed]
- Brooker, C.; Tronci, G. A Collagen-Based Theranostic Wound Dressing with Visual, Long-Lasting Infection Detection Capability. Int. J. Biol. Macromol. 2023, 236, 123866. [Google Scholar] [CrossRef]
- Khalid, A.; Bai, D.; Abraham, A.; Jadhav, A.; Linklater, D.; Matusica, A.; Nguyen, D.; Murdoch, B.J.; Zakhartchouk, N.; Dekiwadia, C.; et al. Electrospun Nanodiamond-Silk Fibroin Membranes: A Multifunctional Platform for Biosensing and Wound Healing Applications. ACS Appl. Mater. Interfaces 2020, 12, 48408–48419. [Google Scholar] [CrossRef]
- Han, H.-R. Antibiotic Action, Drug Delivery, Biodegradability, and Wound Regeneration Characteristics of Surgical Sutures and Cutting-Edge Surgical Suture Manufacturing Technologies. J. Funct. Biomater. 2025, 16, 135. [Google Scholar] [CrossRef]
- Yadav, P.D.; Londhe, P.V.; Chavan, S.S.; Mohite, D.D.; Firame, G.B.; Kadam, S.S.; Patil, M.J.; Ansari, M.I. Electrospun Composite Nanofibers for Wound Healing: Synthesis, Characterization, and Clinical Potential of Biopolymer-Based Materials. Discov. Mater. 2024, 4, 99. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Arcentales-Vera, B.; Estrella-Nuñez, J.; Yánez-Vega, H.; Bucio, E. Antimicrobial Activity of Composites-Based on Biopolymers. Macromol 2022, 2, 258–283. [Google Scholar] [CrossRef]
- Ho, T.T.; Tran, H.A.; Doan, V.K.; Maitz, J.; Li, Z.; Wise, S.G.; Lim, K.S.; Rnjak-Kovacina, J. Natural Polymer-Based Materials for Wound Healing Applications. Adv. NanoBiomed Res. 2024, 4, 2300131. [Google Scholar] [CrossRef]
- Ansari, M.; Darvishi, A. A Review of the Current State of Natural Biomaterials in Wound Healing Applications. Front. Bioeng. Biotechnol. 2024, 12, 1309541. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Kirar, N. Swelling and Drug Release Behavior of Calcium Alginate/Poly (Sodium Acrylate) Hydrogel Beads. Des. Monomers Polym. 2016, 19, 89–98. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef]
- Soleimanpour, M.; Mirhaji, S.S.; Jafari, S.; Derakhshankhah, H.; Mamashli, F.; Nedaei, H.; Karimi, M.R.; Motasadizadeh, H.; Fatahi, Y.; Ghasemi, A.; et al. Designing a New Alginate-Fibrinogen Biomaterial Composite Hydrogel for Wound Healing. Sci. Rep. 2022, 12, 7213. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Kumar, M.; Ahmad, N.; Ge, Y.; Mahmood, S. Recent Applications of Chitosan and Its Derivatives in Antibacterial, Anticancer, Wound Healing, and Tissue Engineering Fields. Polymers 2024, 16, 1351. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, S.; Chen, J.; Wang, M.; Lin, Z.; Liu, Y. Synthesis and Characterization of a Novel Chitosan-Based Nanoparticle–Hydrogel Composite System Promising for Skin Wound Drug Delivery. Mar. Drugs 2024, 22, 428. [Google Scholar] [CrossRef]
- Aya, K.L.; Stern, R. Hyaluronan in Wound Healing: Rediscovering a Major Player. Wound Repair Regen. 2014, 22, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Li, C.; Li, J.; Qiu, M.; Wang, Y.; Han, W. Effects of Molecular Weights on the Bioactivity of Hyaluronic Acid: A Review. Carbohydr. Res. 2025, 552, 109472. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef]
- Galindo, A.N.; Chi, A.K.; Liashenko, I.; O’Neill, K.L.; Sharma, R.; Khachatourian, J.D.; Hajarizadeh, A.; Dalton, P.D.; Hettiaratchi, M.H. Hyaluronic Acid-Coated Melt Electrowritten Scaffolds Promote Myoblast Attachment, Alignment, and Differentiation. bioRxiv 2025. [Google Scholar] [CrossRef]
- Dogaru, A.-I.; Oprea, O.-C.; Isopencu, G.-O.; Banciu, A.; Jinga, S.-I.; Busuioc, C. Bacterial Cellulose-Based Nanocomposites for Wound Healing Applications. Polymers 2025, 17, 1225. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q.; Hu, Y.; Guo, Q.; Zhu, H.; Wang, H.; Hu, H.; Wang, S.; Ye, J. Production of Bacterial Cellulose with High Active Components Loading Capacity for Skin Wound Repair. Int. J. Biol. Macromol. 2025, 311, 143963. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-Co-Glycolic Acid) and Progress of Poly (Lactic-Co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and Biocompatible Polymers for Tissue Engineering Application: A Review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable Polylactic Acid and Its Composites: Characteristics, Processing, and Sustainable Applications in Sports. Polymers 2023, 15, 3096. [Google Scholar] [CrossRef]
- Cheng, K.; Yuan, M.; Zhang, Y.; Sun, N.; Peng, B. Application and Properties of Polyglycolic Acid as a Degradation Agent in MPU/HNBR Degradable Elastomer Composites for Dissolvable Frac Plugs. Polymers 2024, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Utomo, Y.K.S.; Yang, L.; Liang, G.; Liu, W. Mechanic-Driven Biodegradable Polyglycolic Acid/Silk Fibroin Nanofibrous Scaffolds Containing Deferoxamine Accelerate Diabetic Wound Healing. Pharmaceutics 2022, 14, 601. [Google Scholar] [CrossRef]
- Peng, H.; Du, F.; Wang, J.; Wu, Y.; Wei, Q.; Chen, A.; Duan, Y.; Shi, S.; Zhang, J.; Yu, S. Adipose-Derived Stem-Cell-Membrane-Coated PLGA-PEI Nanoparticles Promote Wound Healing via Efficient Delivery of MiR-21. Pharmaceutics 2024, 16, 1113. [Google Scholar] [CrossRef]
- Ruan, C.; Hu, N.; Ma, Y.; Li, Y.; Liu, J.; Zhang, X.; Pan, H. The Interfacial PH of Acidic Degradable Polymeric Biomaterials and Its Effects on Osteoblast Behavior. Sci. Rep. 2017, 7, 6794. [Google Scholar] [CrossRef]
- Osanloo, M.; Noori, F.; Varaa, N.; Tavassoli, A.; Goodarzi, A.; Moghaddam, M.T.; Ebrahimi, L.; Abpeikar, Z.; Farmani, A.R.; Safaei, M.; et al. The Wound Healing Effect of Polycaprolactone-Chitosan Scaffold Coated with a Gel Containing Zataria Multiflora Boiss. Volatile Oil Nanoemulsions. BMC Complement. Med. Ther. 2024, 24, 56. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; De Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Wiesmann, N.; Mendler, S.; Buhr, C.R.; Ritz, U.; Kämmerer, P.W.; Brieger, J. Zinc Oxide Nanoparticles Exhibit Favorable Properties to Promote Tissue Integration of Biomaterials. Biomedicines 2021, 9, 1462. [Google Scholar] [CrossRef]
- Nosrati, H.; Heydari, M. Titanium Dioxide Nanoparticles: A Promising Candidate for Wound Healing Applications. Burns Trauma 2025, 13, tkae069. [Google Scholar] [CrossRef]
- Tyagi, N.; De, R.; Begun, J.; Popat, A. Cancer Therapeutics with Epigallocatechin-3-Gallate Encapsulated in Biopolymeric Nanoparticles. Int. J. Pharm. 2017, 518, 220–227. [Google Scholar] [CrossRef]
- Yao, C.-J.; Yang, S.-J.; Shieh, M.-J.; Young, T.-H. Development of a Chitosan-Silver Nanocomposite/β-1,3-Glucan/Hyaluronic Acid Composite as an Antimicrobial System for Wound Healing. Polymers 2025, 17, 350. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Alasiri, A.S.; Ahmad, J.; Alqahtani, A.A.; Abdullah, M.M.; Abdel-Wahab, B.A.; Pathak, K.; Saikia, R.; Das, A.; Sarma, H.; et al. Green Synthesis of Titanium Dioxide Nanoparticles Using Ocimum Sanctum Leaf Extract: In Vitro Characterization and Its Healing Efficacy in Diabetic Wounds. Molecules 2022, 27, 7712. [Google Scholar] [CrossRef]
- Gizaw, M.; Thompson, J.; Faglie, A.; Lee, S.-Y.; Neuenschwander, P.; Chou, S.-F. Electrospun Fibers as a Dressing Material for Drug and Biological Agent Delivery in Wound Healing Applications. Bioengineering 2018, 5, 9. [Google Scholar] [CrossRef]
- Ashraf, S.S.; Parivar, K.; Hayati Roodbari, N.; Mashayekhan, S.; Amini, N. Fabrication and Characterization of Biaxially Electrospun Collagen/Alginate Nanofibers, Improved with Rhodotorula mucilaginosa Sp. GUMS16 Produced Exopolysaccharides for Wound Healing Applications. Int. J. Biol. Macromol. 2022, 196, 194–203. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, W.; Lei, X.; Luo, X.; Xiang, Q.; Zhu, X.; Pan, Q.; Jin, P.; Cheng, B. Treatment of Acute Wounds With Recombinant Human-Like Collagen and Recombinant Human-Like Fibronectin in C57BL/6 Mice Individually or in Combination. Front. Bioeng. Biotechnol. 2022, 10, 908585. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef]
- Serpico, L.; Dello Iacono, S.; Cammarano, A.; De Stefano, L. Recent Advances in Stimuli-Responsive Hydrogel-Based Wound Dressing. Gels 2023, 9, 451. [Google Scholar] [CrossRef]
- De, R.; Mahata, M.K.; Kim, K. Structure-Based Varieties of Polymeric Nanocarriers and Influences of Their Physicochemical Properties on Drug Delivery Profiles. Adv. Sci. 2022, 9, 2105373. [Google Scholar] [CrossRef]
- Song, Y.H.; De, R.; Lee, K.T. Emerging Strategies to Fabricate Polymeric Nanocarriers for Enhanced Drug Delivery across Blood-Brain Barrier: An Overview. Adv. Colloid Interface Sci. 2023, 320, 103008. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, H.; Song, T.; Liu, X.; Gao, Y.; Zhou, J.; Li, Y. Sustainable Dual Release of Antibiotic and Growth Factor from PH-Responsive Uniform Alginate Composite Microparticles to Enhance Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 22730–22744. [Google Scholar] [CrossRef]
- Psarrou, M.; Mitraki, A.; Vamvakaki, M.; Kokotidou, C. Stimuli-Responsive Polysaccharide Hydrogels and Their Composites for Wound Healing Applications. Polymers 2023, 15, 986. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, J.; Wu, H.; Lin, X.; Cai, L. Stimuli-Responsive Hydrogel Dressing for Wound Healing. APL Mater. 2025, 13, 010601. [Google Scholar] [CrossRef]
- Faal, M.; Faal, M.; Ahmadi, T.; Dehgan, F. Fabrication and Evaluation of Polylactic Acid-Curcumin Containing Carbon Nanotubes (CNTs) Wound Dressing Using Electrospinning Method with Experimental and Computational Approaches. Sci. Rep. 2025, 15, 13398. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.; Muñana-González, S.; Lanceros-Mendez, S.; Ruiz-Rubio, L.; Alvarez, L.P.; Vilas-Vilela, J.L. Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation. Polymers 2024, 16, 2599. [Google Scholar] [CrossRef]
- Li, Y.; Gong, Q.; Liu, X.; Xia, Z.; Yang, Y.; Chen, C.; Qian, C. Wide Temperature-Tolerant Polyaniline/Cellulose/Polyacrylamide Hydrogels for High-Performance Supercapacitors and Motion Sensors. Carbohydr. Polym. 2021, 267, 118207. [Google Scholar] [CrossRef]
- Velasco-Rodriguez, B.; Diaz-Vidal, T.; Rosales-Rivera, L.C.; García-González, C.A.; Alvarez-Lorenzo, C.; Al-Modlej, A.; Domínguez-Arca, V.; Prieto, G.; Barbosa, S.; Soltero Martínez, J.F.A.; et al. Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 6758. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Mechanically Viscoelastic Nanoreinforced Hybrid Hydrogels Composed of Polyacrylamide, Sodium Carboxymethylcellulose, Graphene Oxide, and Cellulose Nanocrystals. Carbohydr. Polym. 2018, 193, 228–238. [Google Scholar] [CrossRef]
- Qian, L. Cellulose-Based Composite Hydrogels: Preparation, Structures, and Applications. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M., Ed.; Polymers and Polymeric Composites: A Reference Series; Springer: Cham, Switzerland, 2019; pp. 655–704. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063. [Google Scholar] [CrossRef]
- Xu, T.O.; Kim, H.S.; Stahl, T.; Nukavarapu, S.P. Self-Neutralizing PLGA/Magnesium Composites as Novel Biomaterials for Tissue Engineering. Biomed. Mater. 2018, 13, 035013. [Google Scholar] [CrossRef]
- Lee, M.-W.; Hung, C.-L.; Cheng, J.-C.; Wang, Y.-J. A New Anti-Adhesion Film Synthesized from Polygalacturonic Acid with 1-Ethyl-3-(3-Dimethylaminopropyl)Carbodiimide Crosslinker. Biomaterials 2005, 26, 3793–3799. [Google Scholar] [CrossRef]
- Mania, S.; Banach-Kopeć, A.; Staszczyk, K.; Kulesza, J.; Augustin, E.; Tylingo, R. An Influence of Molecular Weight, Deacetylation Degree of Chitosan Xerogels on Their Antimicrobial Activity and Cytotoxicity. Comparison of Chitosan Materials Obtained Using Lactic Acid and CO2 Saturation. Carbohydr. Res. 2023, 534, 108973. [Google Scholar] [CrossRef]
- Dutta, D.; Sit, N. A Comprehensive Review on Types and Properties of Biopolymers as Sustainable Bio-based Alternatives for Packaging. Food Biomacromolecules 2024, 1, 58–87. [Google Scholar] [CrossRef]
- Serra, M.; Casas, A.; Toubarro, D.; Barros, A.N.; Teixeira, J.A. Microbial Hyaluronic Acid Production: A Review. Molecules 2023, 28, 2084. [Google Scholar] [CrossRef]
- Cruz, M.A.; Flor-Unda, O.; Avila, A.; Garcia, M.D.; Cerda-Mejía, L. Advances in Bacterial Cellulose Production: A Scoping Review. Coatings 2024, 14, 1401. [Google Scholar] [CrossRef]
- Beheshtizadeh, N.; Gharibshahian, M.; Pazhouhnia, Z.; Rostami, M.; Zangi, A.R.; Maleki, R.; Azar, H.K.; Zalouli, V.; Rajavand, H.; Farzin, A.; et al. Commercialization and Regulation of Regenerative Medicine Products: Promises, Advances and Challenges. Biomed. Pharmacother. 2022, 153, 113431. [Google Scholar] [CrossRef]
- Mathur, V.; Dsouza, V.; Srinivasan, V.; Vasanthan, K.S. Volumetric Additive Manufacturing for Cell Printing: Bridging Industry Adaptation and Regulatory Frontiers. ACS Biomater. Sci. Eng. 2025, 11, 156–181. [Google Scholar] [CrossRef] [PubMed]


| Name | Chemical Structure | Properties | Ref. |
|---|---|---|---|
| Polylactic acid (PLA) | 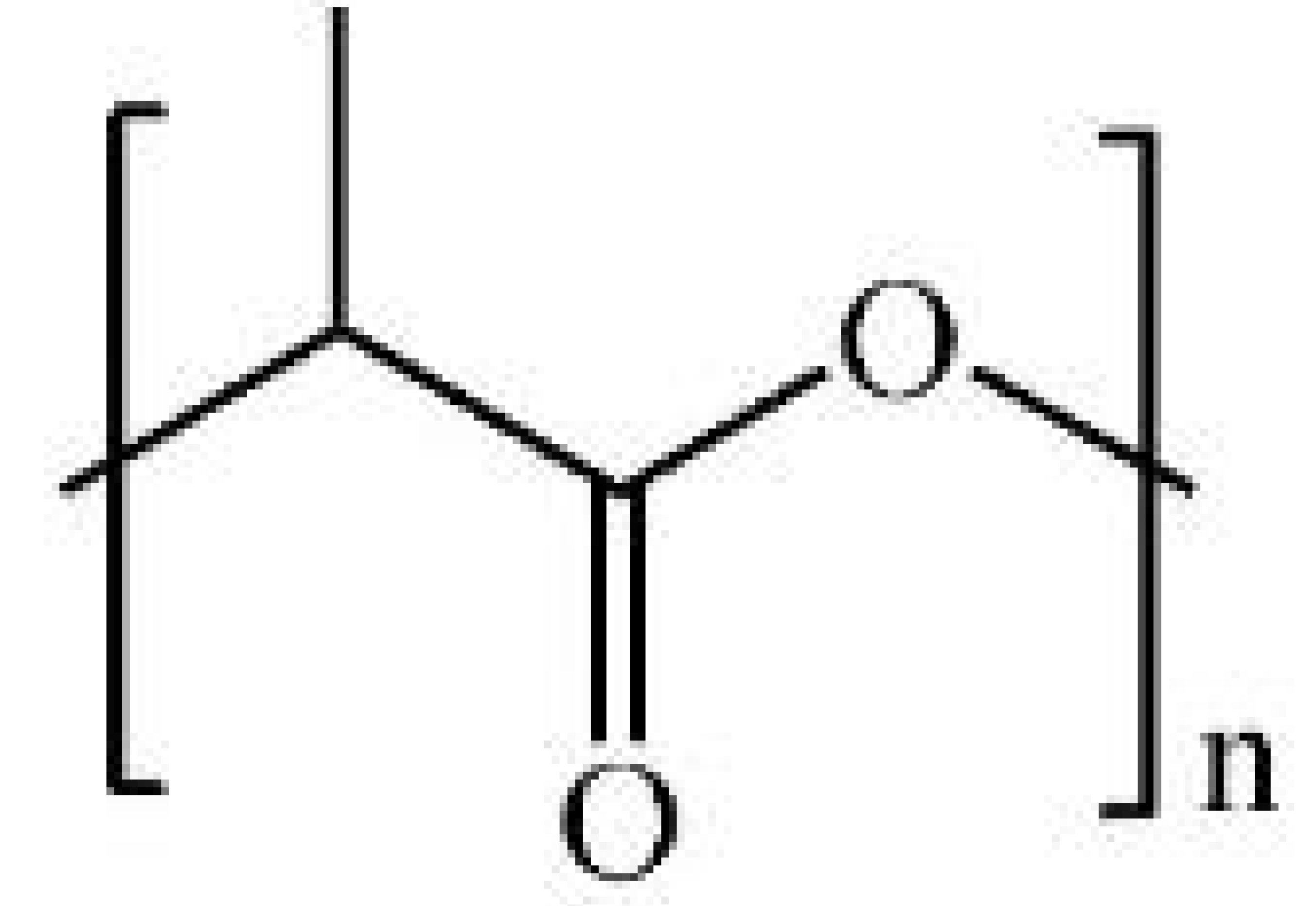 |
| [122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140] |
| Polyglycolic acid (PGA) | 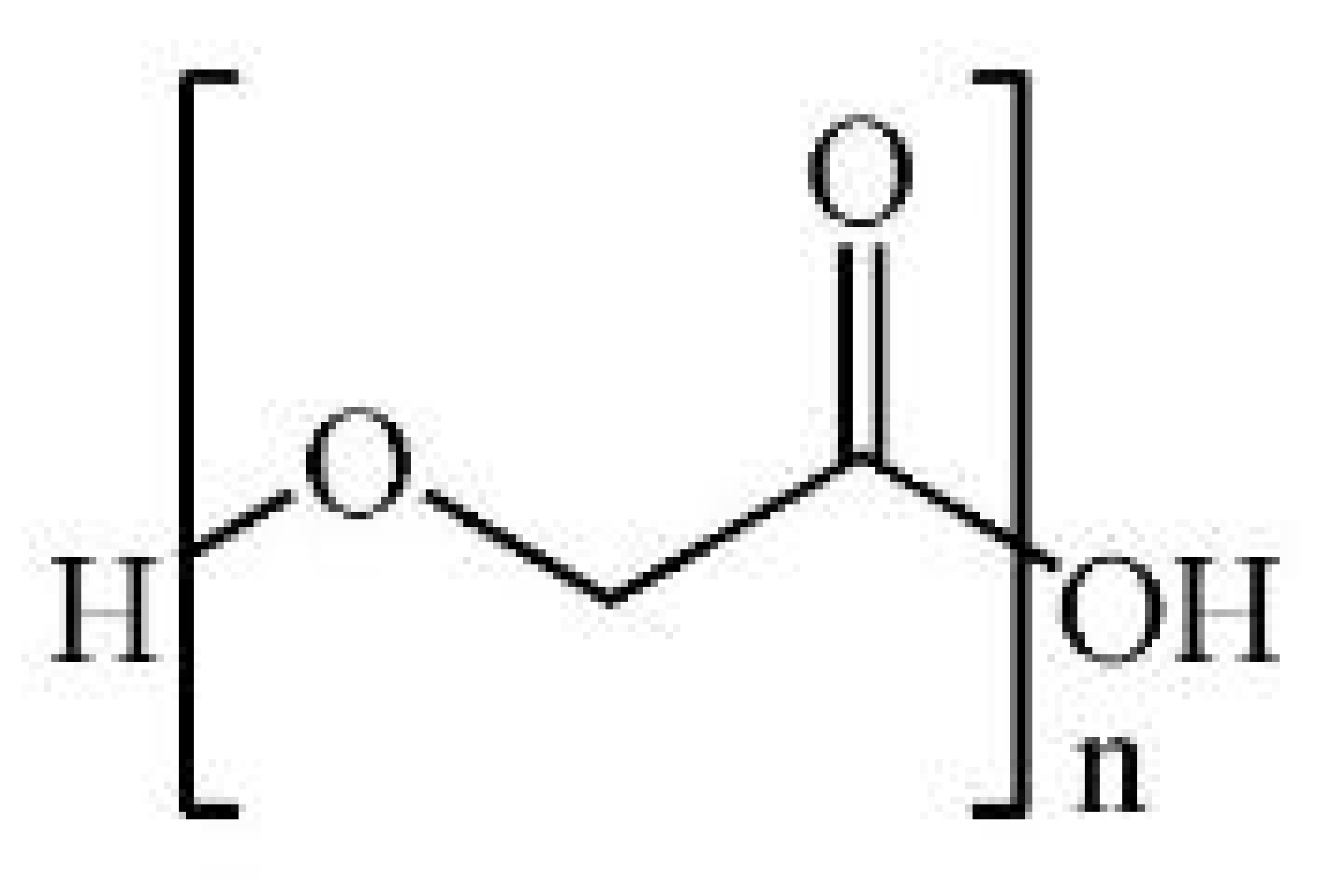 |
| [141,142,143,144,145,146,147,148,149,150,151,152] |
| Poly(lactic-co-glycolic acid) (PLGA) | 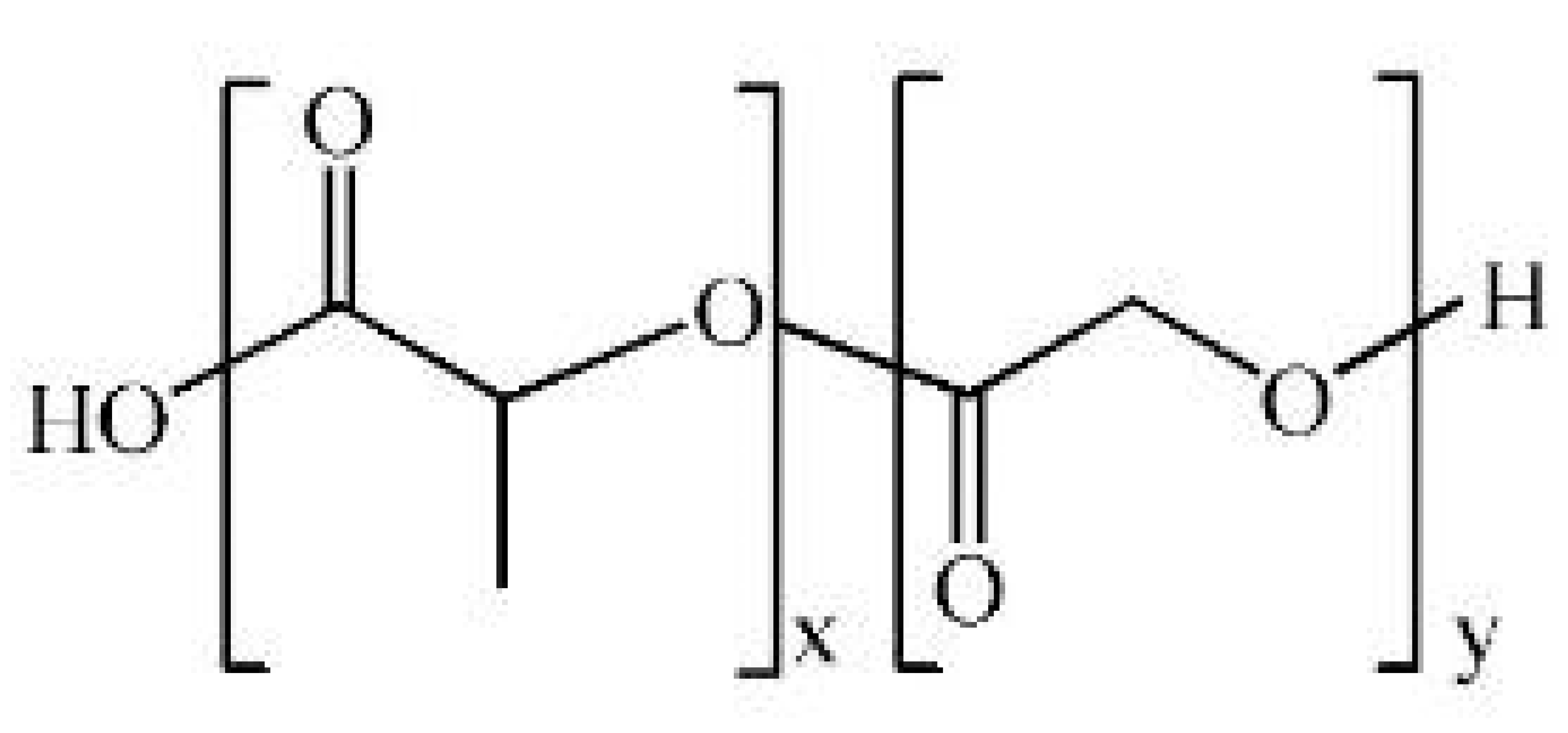 |
| [153,154,155,156,157,158,159,160,161,162] |
| Polycaprolactone (PCL) |  |
| [163,164,165,166,167,168,169,170,171,172,173] |
| Type | Examples | Advantages | Ref. |
|---|---|---|---|
| Synthetic biopolymers |
|
| [45,190,191] |
| Natural biopolymers |
|
| [192,193,194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; De, R. Next-Generation Wound Healing Materials: Role of Biopolymers and Their Composites. Polymers 2025, 17, 2244. https://doi.org/10.3390/polym17162244
Park J, De R. Next-Generation Wound Healing Materials: Role of Biopolymers and Their Composites. Polymers. 2025; 17(16):2244. https://doi.org/10.3390/polym17162244
Chicago/Turabian StylePark, Jonghyuk, and Ranjit De. 2025. "Next-Generation Wound Healing Materials: Role of Biopolymers and Their Composites" Polymers 17, no. 16: 2244. https://doi.org/10.3390/polym17162244
APA StylePark, J., & De, R. (2025). Next-Generation Wound Healing Materials: Role of Biopolymers and Their Composites. Polymers, 17(16), 2244. https://doi.org/10.3390/polym17162244








