Solvent-Free Processing of i-P3HB Blends: Enhancing Processability and Mechanical Properties for Sustainable Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. i-P3HB Blends Preparation by Extrusion
2.2. Preparing of Specimens for Tensile Tests
2.3. Differential Scanning Calorimetry (DSC)
2.4. Thermogravimetric Analysis (TGA)
2.5. Gel Permeation Chromatography (GPC)
2.6. Tensile Tests
3. Results and Discussion
3.1. Thermal Characterization
3.2. Thermal Stability of i-P3HB Blends
3.3. Mechanical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lemoigne, M. Products of dehydration and of polymerization of β-hydroxybutyric acid. Bull. Soc. Chem. Biol. 1926, 8, 770–782. [Google Scholar]
- Gahlawat, G. Polyhydroxyalkanoates Biopolymers; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-33896-1. [Google Scholar]
- Koller, M. (Ed.) The Handbook of Polyhydroxyalkanoates: Microbial Biosynthesis and Feedstocks; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Dill, S. Verarbeitung und Charakterisierung von Polyamid 6-Polyhydroxybutyrat-Blends: Verarbeitung und Charakterisierung von Polyamid 6-Polyhydroxybutyrat-Blends; Universitätsverlag der TU Berlin: Berlin, Germany, 2020. [Google Scholar]
- McAdam, B.; Fournet, M.B.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Türk, O. Stoffliche Nutzung Nachwachsender Rohstoffe; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2014; ISBN 978-3-8348-1763-1. [Google Scholar]
- Aoyagi, Y.; Yamashita, K.; Doi, Y. Thermal degradation of poly[(R)-3-hydroxybutyrate], poly[ε-caprolactone], and poly[(S)-lactide]. Polym. Degrad. Stab. 2002, 76, 53–59. [Google Scholar] [CrossRef]
- Grassie, N.; Murray, E.J.; Holmes, P.A. The thermal degradation of poly(-(d)-β-hydroxybutyric acid): Part 1—Identification and quantitative analysis of products. Polym. Degrad. Stab. 1984, 6, 47–61. [Google Scholar] [CrossRef]
- Ariffin, H.; Nishida, H.; Shirai, Y.; Hassan, M.A. Determination of multiple thermal degradation mechanisms of poly(3-hydroxybutyrate). Polym. Degrad. Stab. 2008, 93, 1433–1439. [Google Scholar] [CrossRef]
- Yousefi, A.M.; Wnek, G.E.; Gomez Jimenez, H.; Ghassemi, H.; Zhang, J. Investigating the Effects of Temperature, Azodicarbonamide, Boron Nitride, and Multilayer Film/Foam Coextrusion on the Properties of a Poly(Hydroxyalkanoate)/Poly(Lactic acid) Blend. J. Polym. Environ. 2024, 32, 6349–6374. [Google Scholar] [CrossRef]
- Janigová, I.; Lacćk, I.; Chodák, I. Thermal degradation of plasticized poly(3-hydroxybutyrate) investigated by DSC. Polym. Degrad. Stab. 2002, 77, 35–41. [Google Scholar] [CrossRef]
- Chodak, I. Polyhydroxyalkanoates: Properties and Modification for High Volume Applications. In Degradable Polymers; Scott, G., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 295–319. ISBN 978-90-481-6091-4. [Google Scholar]
- Luo, Z.; Wu, Y.L.; Li, Z.; Loh, X.J. Recent Progress in Polyhydroxyalkanoates-Based Copolymers for Biomedical Applications. Biotechnol. J. 2019, 14, e1900283. [Google Scholar] [CrossRef]
- Sun, Y.; Jia, Z.; Chen, C.; Cong, Y.; Mao, X.; Wu, J. Alternating Sequence Controlled Copolymer Synthesis of α-Hydroxy Acids via Syndioselective Ring-Opening Polymerization of O-Carboxyanhydrides Using Zirconium/Hafnium Alkoxide Initiators. J. Am. Chem. Soc. 2017, 139, 10723–10732. [Google Scholar] [CrossRef]
- Li, J.; Cheng, H.; Li, Y.; Wang, H.; Hu, H.; Liu, J. Effect of chain extender on the morphological, rheological and mechanical properties of biodegradable blends from PBAT and P34HB. J. Polym. Res. 2023, 30, 393. [Google Scholar] [CrossRef]
- Samui, A.B.; Kanai, T. Polyhydroxyalkanoates based copolymers. Int. J. Biol. Macromol. 2019, 140, 522–537. [Google Scholar] [CrossRef]
- Zhao, Q.; Cheng, G.X. Preparation of biodegradable poly(3-hydroxybutyrate) and poly(ethylene glycol) multiblock copolymers. J. Mater. Sci. 2004, 39, 3829–3831. [Google Scholar] [CrossRef]
- Roy, I.; Visakh, P.M. (Eds.) Polyhydroxyalkanoate (PHA) Based Blends, Composites and Nanocomposites; Royal Society of Chemistry: Cambridge, UK, 2014; ISBN 978-1-84973-946-7. [Google Scholar]
- Hamad, K.; Kaseem, M.; Ko, Y.G.; Deri, F. Biodegradable polymer blends and composites: An overview. Polym. Sci. Ser. A 2014, 56, 812–829. [Google Scholar] [CrossRef]
- Rebia, R.A.; Rozet, S.; Tamada, Y.; Tanaka, T. Biodegradable PHBH/PVA blend nanofibers: Fabrication, characterization, in vitro degradation, and in vitro biocompatibility. Polym. Degrad. Stab. 2018, 154, 124–136. [Google Scholar] [CrossRef]
- Mai, J.; Pratt, S.; Laycock, B.; Chan, C.M. Synthesis and Characterisation of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-b-poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Multi-Block Copolymers Produced Using Diisocyanate Chemistry. Polymers 2023, 15, 3257. [Google Scholar] [CrossRef] [PubMed]
- Gabirondo, E.; Sangroniz, A.; Etxeberria, A.; Torres-Giner, S.; Sardon, H. Poly(hydroxy acids) derived from the self-condensation of hydroxy acids: From polymerization to end-of-life options. Polym. Chem. 2020, 11, 4861–4874. [Google Scholar] [CrossRef]
- Nkrumah-Agyeefi, S.; Pella, B.J.; Singh, N.; Mukherjee, A.; Scholz, C. Modification of polyhydroxyalkanoates: Evaluation of the effectiveness of novel copper(II) catalysts in click chemistry. Int. J. Biol. Macromol. 2019, 128, 376–384. [Google Scholar] [CrossRef]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-6063-9. [Google Scholar]
- Parra, D.F.; Fusaro, J.; Gaboardi, F.; Rosa, D.S. Influence of poly (ethylene glycol) on the thermal, mechanical, morphological, physical–chemical and biodegradation properties of poly (3-hydroxybutyrate). Polym. Degrad. Stab. 2006, 91, 1954–1959. [Google Scholar] [CrossRef]
- Yang, H.; Ze-Sheng, L.; Qian, H.; Yang, Y.; Zhang, X.; Sun, C. Molecular dynamics simulation studies of binary blend miscibility of poly(3-hydroxybutyrate) and poly(ethylene oxide). Polymer 2004, 45, 453–457. [Google Scholar] [CrossRef]
- Alata, H.; Hexig, B.; Inoue, Y. Effect of poly(vinyl alcohol) fine particles as a novel biodegradable nucleating agent on the crystallization of poly(3-hydroxybutyrate). J. Polym. Sci. B Polym. Phys. 2006, 44, 1813–1820. [Google Scholar] [CrossRef]
- Chun, Y.S.; Kim, W.N. Thermal properties of poly(hydroxybutyrate-co-hydroxyvalerate) and poly(ϵ-caprolactone) blends. Polymer 2000, 41, 2305–2308. [Google Scholar] [CrossRef]
- Qiu, Z.; Yang, W.; Ikehara, T.; Nishi, T. Miscibility and crystallization behavior of biodegradable blends of two aliphatic polyesters. Poly(3-hydroxybutyrate-co-hydroxyvalerate) and poly(ε-caprolactone). Polymer 2005, 46, 11814–11819. [Google Scholar] [CrossRef]
- Qiu, Z.; Ikehara, T.; Nishi, T. Poly(hydroxybutyrate)/poly(butylene succinate) blends: Miscibility and nonisothermal crystallization. Polymer 2003, 44, 2503–2508. [Google Scholar] [CrossRef]
- Qiu, Z.; Ikehara, T.; Nishi, T. Miscibility and crystallization behaviour of biodegradable blends of two aliphatic polyesters. Poly(3-hydroxybutyrate-co-hydroxyvalerate) and poly(butylene succinate) blends. Polymer 2003, 44, 7519–7527. [Google Scholar] [CrossRef]
- Miao, L.; Qiu, Z.; Yang, W.; Ikehara, T. Fully biodegradable poly(3-hydroxybutyrate-co-hydroxyvalerate)/poly(ethylene succinate) blends: Phase behavior, crystallization and mechanical properties. React. Funct. Polym. 2008, 68, 446–457. [Google Scholar] [CrossRef]
- Javadi, A.; Kramschuster, A.J.; Pilla, S.; Lee, J.; Gong, S.; Turng, L.-S. Processing and characterization of microcellular PHBV/PBAT blends. Polym. Eng. Sci. 2010, 50, 1440–1448. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Lemstra, P.J.; Zhang, Y.; Wang, S. Toughening of PHBV/PBS and PHB/PBS Blends via In situ Compatibilization Using Dicumyl Peroxide as a Free-Radical Grafting Initiator. Macro Mater. Eng. 2012, 297, 402–410. [Google Scholar] [CrossRef]
- Mai, J.; Kockler, K.; Parisi, E.; Chan, C.M.; Pratt, S.; Laycock, B. Synthesis and physical properties of polyhydroxyalkanoate (PHA)-based block copolymers: A review. Int. J. Biol. Macromol. 2024, 263, 130204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ye, J.-W.; Zhang, X.; Huang, W.; Zhang, Z.; Lin, Y.; Zhang, G.; Wu, F.; Wang, Z.; Wu, Q.; et al. Effective production of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by engineered Halomonas bluephagenesis grown on glucose and 1,4-Butanediol. Bioresour. Technol. 2022, 355, 127270. [Google Scholar] [CrossRef]
- Policastro, G.; Panico, A.; Fabbricino, M. Improving biological production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) co-polymer: A critical review. Rev. Environ. Sci. Biotechnol. 2021, 20, 479–513. [Google Scholar] [CrossRef]
- de Macedo, M.A.; Oliveira-Filho, E.R.; Taciro, M.K.; Piccoli, R.A.M.; Gomez, J.G.C.; Silva, L.F. Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)] biotechnological production: Challenges and opportunities. Biomass Conv. Bioref. 2024, 14, 26631–26650. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, G.; So, Y.-M.; Pan, Y. Recent Advances in Zinc Complexes for Stereoselective Ring-Opening Polymerization and Copolymerization. Inorganics 2025, 13, 185. [Google Scholar] [CrossRef]
- Westlie, A.H.; Quinn, E.C.; Parker, C.R.; Chen, E.Y.-X. Synthetic biodegradable polyhydroxyalkanoates (PHAs): Recent advances and future challenges. Prog. Polym. Sci. 2022, 134, 101608. [Google Scholar] [CrossRef]
- Grillo, A.; Rusconi, Y.; D’Alterio, M.C.; De Rosa, C.; Talarico, G.; Poater, A. Ring Opening Polymerization of Six- and Eight-Membered Racemic Cyclic Esters for Biodegradable Materials. Int. J. Mol. Sci. 2024, 25, 1647. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Odian, G. Principles of Polymerization, 4th ed.; John Willey & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Rogers, M.E.; Long, T.E.; Turner, S.R. Introduction to Synthetic Methods in Step-Growth Polymers; John Willey & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Development of flexible materials based on plasticized electrospun PLA–PHB blends: Structural, thermal, mechanical and disintegration properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Vanovčanová, Z.; Alexy, P.; Feranc, J.; Plavec, R.; Bočkaj, J.; Kaliňáková, L.; Tomanová, K.; Perďochová, D.; Šariský, D.; Gálisová, I. Effect of PHB on the properties of biodegradable PLA blends. Chem. Pap. 2016, 70, 1408–1415. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Langford, A.; Chan, C.M.; Pratt, S.; Garvey, C.J.; Laycock, B. The morphology of crystallisation of PHBV/PHBV copolymer blends. Eur. Polym. J. 2019, 112, 104–119. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Raimo, M. Review Properties of blends and composites based on poly(3-hydroxy)butyrate (PHB) and poly(3-hydroxybutyrate-hydroxyvalerate) (PHBV) copolymers. J. Mater. Sci. 2000, 35, 523–545. [Google Scholar] [CrossRef]
- Zhao, H.; Cui, Z.; Wang, X.; Turng, L.S.; Peng, X. Processing and characterization of solid and microcellular poly(lactic acid)/polyhydroxybutyrate-valerate (PLA/PHBV) blends and PLA/PHBV/Clay nanocomposites. Compos. Part. B Eng. 2013, 51, 79–91. [Google Scholar] [CrossRef]
- Koller, M.; Braunegg, G. Polyhydroxyalkanoates: Experience of the past and prospects for the future. In Handbook of Biopolymer-Based Materials; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Costa, A.R.M.; Reul, L.T.; Sousa, F.M.; Ito, E.N.; Carvalho, L.H.; Canedo, E.L. Degradation during processing of vegetable fiber compounds based on PBAT/PHB blends. Polym. Test. 2018, 69, 266–275. [Google Scholar] [CrossRef]
- Gonzalez, A.; Irusta, L.; Fernández-Berridi, M.J.; Iriarte, M.; Iruin, J.J. Application of pyrolysis/gas chromatography/Fourier transform infrared spectroscopy and TGA techniques in the study of thermal degradation of poly (3-hydroxybutyrate). Polym. Degrad. Stab. 2005, 87, 347–354. [Google Scholar] [CrossRef]
- Roy, S.; Robert, J.W. Thermal Degradation of Bacterial Poly(hydroxybutyric acid): Mechanisms from the Dependence of Pyrolysis Yields on Sample Thickness. Macromolecules 1994, 27, 3782–3789. [Google Scholar] [CrossRef]
- Kabe, T.; Tsuge, T.; Hikima, T.; Takata, M.; Takemura, A.; Iwata, T. Processing, Mechanical Properties, and Structure Analysis of Melt-Spun Fibers of P(3HB)/UHMW-P(3HB) Identical Blend. In Green Polymer Chemistry: Biobased Materials and Biocatalysis; Cheng, H.N., Gross, R.A., Smith, P.B., Eds.; American Chemical Society: Washington, DC, USA, 2015; pp. 63–75. ISBN 9780841230651. [Google Scholar]
- Pachekoski, W.M.; Dalmolin, C.; Agnelli, J.A.M. The influence of the industrial processing on the degradation of poly(hidroxybutyrate)-PHB. Mat. Res. 2013, 16, 237–332. [Google Scholar] [CrossRef]
- Almustafa, W.; Grishchuk, S.; Sebastian, J.; Schubert, D.W.; Grun, G. Improving i-P3HB Processing Window: Solution-Casting Blends With a-P3HB and P34HB. J. Appl. Polym. Sci. 2025, 142, e56805. [Google Scholar] [CrossRef]
- Almustafa, W.; Schubert, D.W.; Grishchuk, S.; Sebastian, J.; Grun, G. Chemical Synthesis of Atactic Poly-3-hydroxybutyrate (a-P3HB) by Self-Polycondensation: Catalyst Screening and Characterization. Polymers 2024, 16, 1655. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Deutsches Instititut für Normung. DIN EN ISO 527-1: Plastics—Determination of Tensile Properties—Part 1: General Principles; Beuth Verlag: Berlin, Germany, 2019. [Google Scholar]
- Dawin, T.P.; Ahmadi, Z.; Taromi, F.A. Bio-based solution-cast blend films based on polylactic acid and polyhydroxybutyrate: Influence of pyromellitic dianhydride as chain extender on the morphology, dispersibility, and crystallinity. Prog. Org. Coat. 2018, 119, 23–30. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, J.; Smith, A.C.; Carvalho, C.; Wellner, N.; Yakimets, I. Characterization of polyhydroxybutyrate-hydroxyvalerate (PHB-HV)/maize starch blend films. J. Food Eng. 2008, 89, 361–369. [Google Scholar] [CrossRef]
- UZUN, G.; AYDEMIR, D. Biocomposites from polyhydroxybutyrate and bio-fillers by solvent casting method. Bull. Mater. Sci. 2017, 40, 383–393. [Google Scholar] [CrossRef]
- Adorna, J.A.; Ventura, R.L.G.; Dang, V.D.; Doong, R.; Ventura, J.S. Biodegradable polyhydroxybutyrate/cellulose/calcium carbonate bioplastic composites prepared by heat-assisted solution casting method. J. Appl. Polym. Sci. 2022, 139, 51645. [Google Scholar] [CrossRef]
- Bayarı, S.; Severcan, F. FTIR study of biodegradable biopolymers: P(3HB), P(3HB-co-4HB) and P(3HB-co-3HV). J. Mol. Struct. 2005, 744, 529–534. [Google Scholar] [CrossRef]
- Mascia, L.; Xanthos, M. An overview of additives and modifiers for polymer blends: Facts, deductions, and uncertainties. Adv. Polym. Technol. 1992, 11, 237–248. [Google Scholar] [CrossRef]
- Bodaghi, A. An overview on the recent developments in reactive plasticizers in polymers. Polym. Adv. Techs 2020, 31, 355–367. [Google Scholar] [CrossRef]
- Frone, A.N.; Nicolae, C.A.; Eremia, M.C.; Tofan, V.; Ghiurea, M.; Chiulan, I.; Radu, E.; Damian, C.M.; Panaitescu, D.M. Low Molecular Weight and Polymeric Modifiers as Toughening Agents in Poly(3-Hydroxybutyrate) Films. Polymers 2020, 12, 2446. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Buhk, M.; Trögner, G.; Rogausch, K.-D.; Petermann, J. Compatibility of syndiotactic with atactic polystyrene. Acta Polym. 1998, 49, 174–177. [Google Scholar] [CrossRef]
- Nishi, T.; Wang, T.T. Melting Point Depression and Kinetic Effects of Cooling on Crystallization in Poly(vinylidene fluoride)-Poly(methyl methacrylate) Mixtures. Macromolecules 1975, 8, 909–915. [Google Scholar] [CrossRef]
- Qiu, Z.; Ikehara, T.; Nishi, T. Melting behaviour of poly(butylene succinate) in miscible blends with poly(ethylene oxide). Polymer 2003, 44, 3095–3099. [Google Scholar] [CrossRef]
- Schawe, J. Analysis of melting processes using TOPEM: Analysis of melting processes using TOPEM. Anal. Melting Process. Using TOPEM 2007, 25, 13–17. [Google Scholar]
- Dong, T.; Mori, T.; Aoyama, T.; Inoue, Y. Rapid crystallization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer accelerated by cyclodextrin-complex as nucleating agent. Carbohydr. Polym. 2010, 80, 387–393. [Google Scholar] [CrossRef]
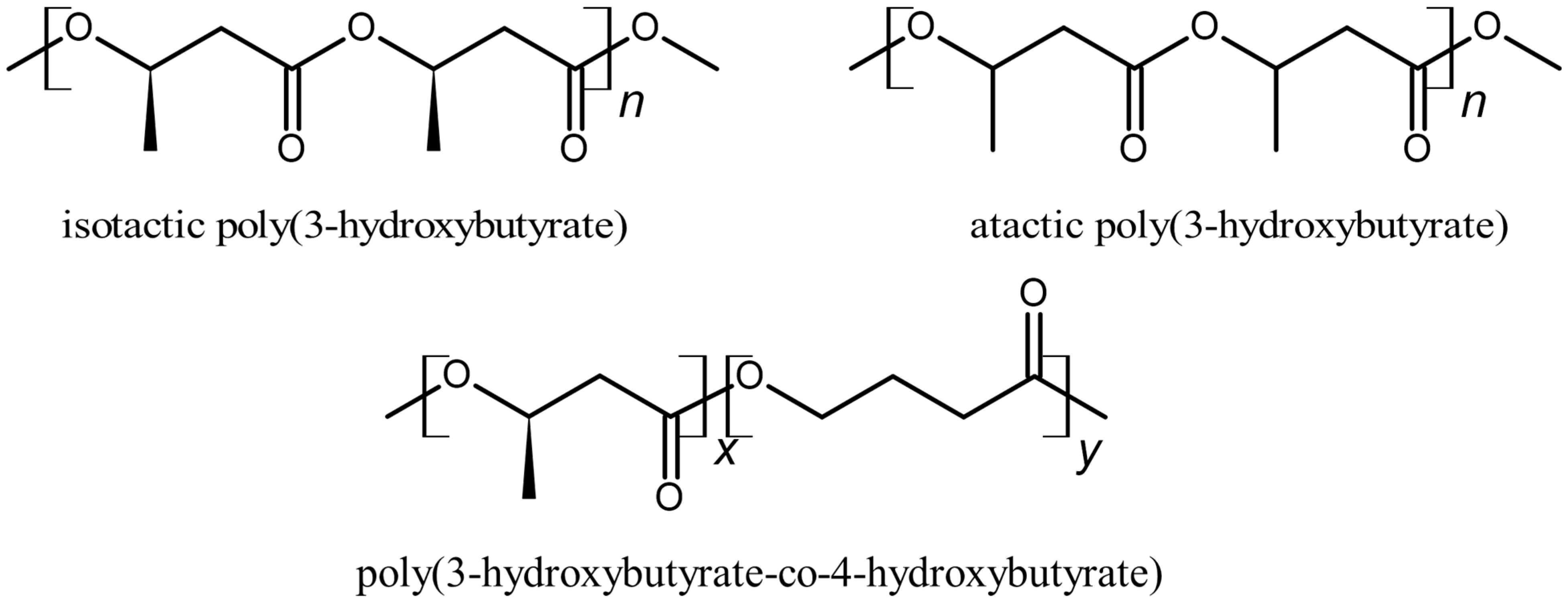



| i-P3HB | PP | LDPE | |
|---|---|---|---|
| Melting temperature [°C] | 175 | 176 | 110 |
| Glass transition temperature [°C] | 4 | −10 | −30 |
| Crystallinity [%] | 70 | 50 | 50 |
| E-modulus [GPa] | 3.5 | 1.5 | 0.2 |
| Tensile strength [MPa] | 40 | 38 | 10 |
| Elongation at break [%] | 5 | 400 | 600 |
| Sample Designation | Description |
|---|---|
| i-P3HB | Neat i-P3HB |
| P34HB | Neat P34HB |
| a-P3HB | Neat a-P3HB |
| a-P3HB-15 | Blend of i-P3HB with a-P3HB in 85:15 weight ratio |
| a-P3HB-30 | Blend of i-P3HB with a-P3HB in 70:30 weight ratio |
| a-P3HB-50 | Blend of i-P3HB with a-P3HB in 50:50 weight ratio |
| P34HB-15 | Blend of i-P3HB with P34HB in 85:15 weight ratio |
| P34HB-30 | Blend of i-P3HB with P34HB in 70:30 weight ratio |
| P34HB-50 | Blend of i-P3HB with P34HB in 50:50 weight ratio |
| a-P3HB-30-P34HB-15 | Blend of a-P3HB-30 with P34HB in 85:15 weight ratio |
| a-P3HB-30-P34HB-30 | Blend of a-P3HB-30 with P34HB in 70:30 weight ratio |
| a-P3HB-30-P34HB-50 | Blend of a-P3HB-30 with P34HB in 50:50 weight ratio |
| a-P3HB-50-P34HB-15 | Blend of a-P3HB-50 with P34HB in 85:15 weight ratio |
| a-P3HB-50-P34HB-30 | Blend of a-P3HB-50 with P34HB in 70:30 weight ratio |
| a-P3HB-50-P34HB-50 | Blend of a-P3HB-50 with P34HB in 50:50 weight ratio |
| Sample | Extrusion Temperature [°C] | Screw Speed [rpm] | |||
|---|---|---|---|---|---|
| Zone 1 | Zone 2 | Zone 3 | TM80 * | ||
| a-P3HB-15 | 70 | 160 | 160 | 165 | 40 |
| a-P3HB-30 | 70 | 155 | 155 | 160 | 40 |
| a-P3HB-50 | 70 | 150 | 150 | 155 | 40 |
| P34HB-15 | 70 | 165 | 170 | 175 | 50 |
| P34HB-30 | 70 | 165 | 170 | 175 | 50 |
| P34HB-50 | 70 | 165 | 170 | 175 | 55 |
| a-P3HB-50-P34HB-15 | 70 | 155 | 155 | 160 | 40 |
| a-P3HB-50-P34HB-30 | 70 | 155 | 150 | 155 | 40 |
| a-P3HB-50-P34HB-50 | 70 | 155 | 150 | 155 | 40 |
| a-P3HB-30-P34HB-15 | 70 | 155 | 155 | 160 | 40 |
| a-P3HB-30-P34HB-30 | 70 | 155 | 150 | 155 | 40 |
| a-P3HB-30-P34HB-50 | 70 | 155 | 150 | 155 | 40 |
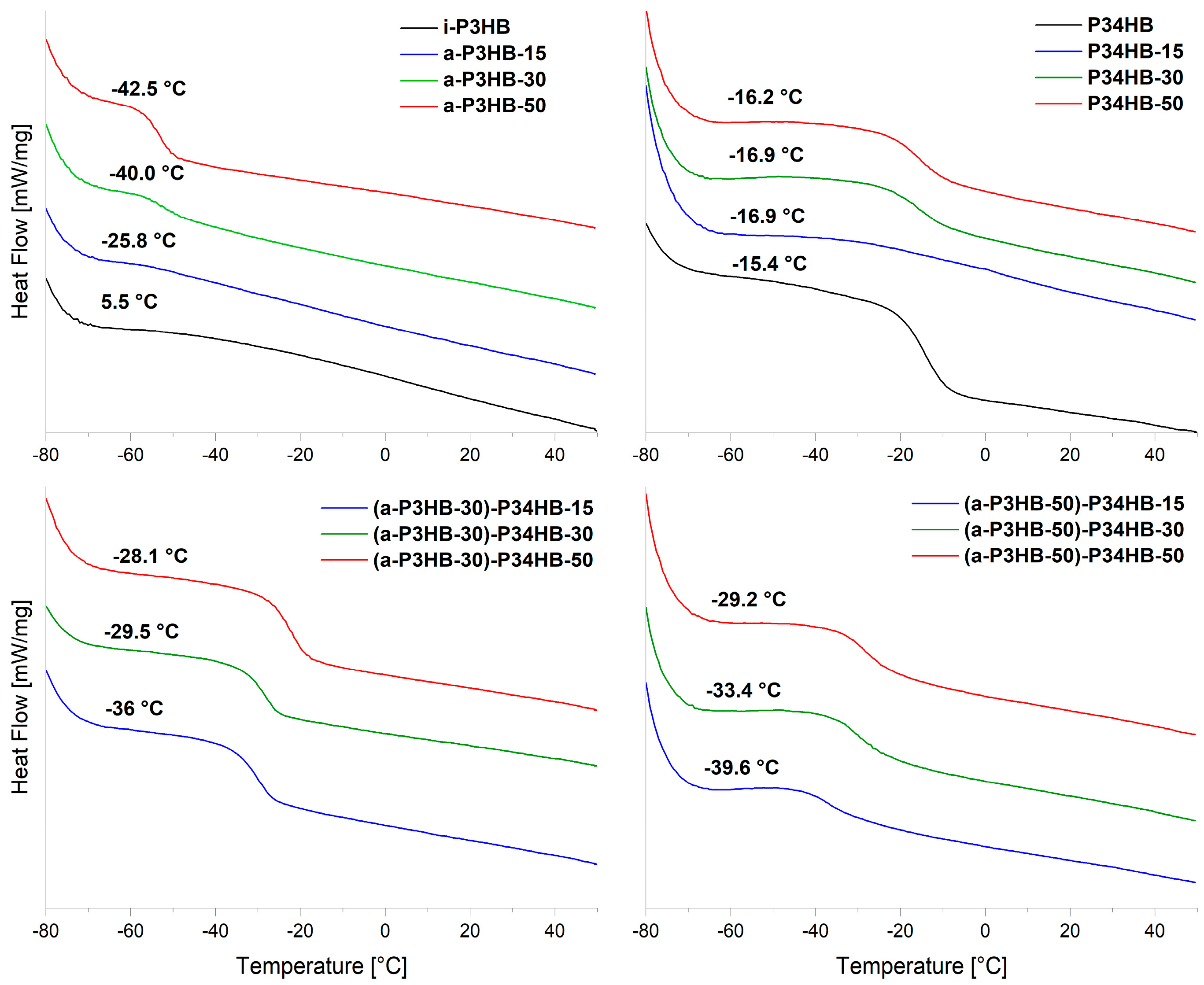
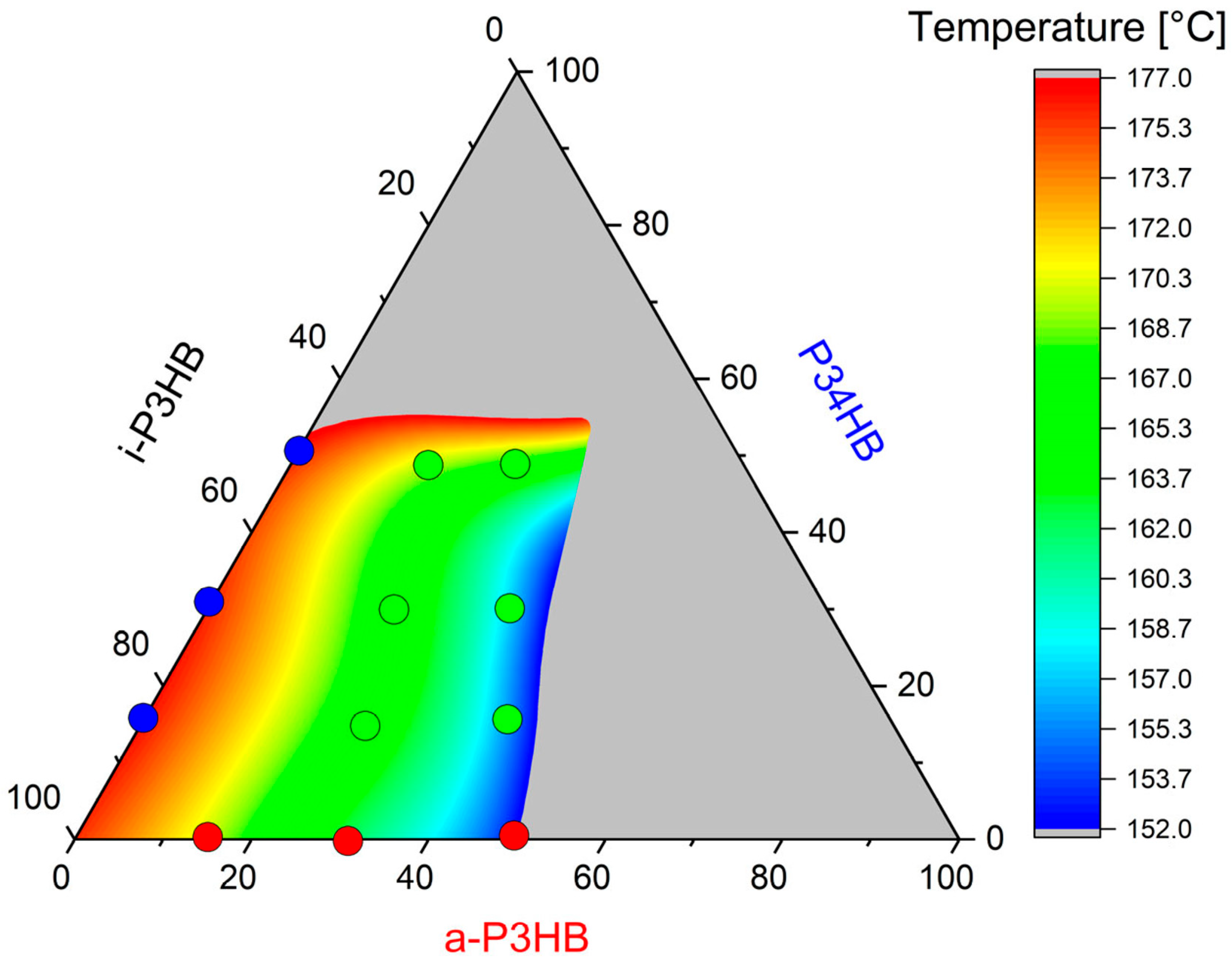
| Sample | Tg [°C] | Tm1 [°C] | Tm2 [°C] | Tc [°C] | Xc (%) |
|---|---|---|---|---|---|
| P34HB | −15.4 ± 0.4 | ||||
| i-P3HB | 5.5 ± 0.3 | 175.5 ± 1.6 | 91.7 ± 3.34 | 68 ± 1.2 | |
| a-P3HB | −42.4 ± 1.2 | ||||
| a-P3HB-15 | −25.8 ± 0.9 | 166.3 ± 0.4 | 171 ± 0.1 | 101.2 ± 1.3 | 55 ± 0.2 |
| a-P3HB-30 | −42.1 ± 0.4 | 153.8 ± 0.2 | 165.5 ± 0.1 | 90.2 ± 0.2 | 48 ± 0.6 |
| a-P3HB-50 | −42.5 ± 1.3 | 130.8 ± 1.4 | 153 ± 0.6 | 71.4 ± 1.4 | 32 ± 0.1 |
| P34HB-15 | −16.9 ± 0.5 | 165.6 ± 0.7 | 175.4 ± 0.4 | 82 ± 0.6 | 54 ± 0.6 |
| P34HB-30 | −16.9 ± 0.3 | 173.9 ± 0.1 | 71.5 ± 1.2 | 46 ± 0.8 | |
| P34HB-50 | −16.2 ± 0.1 | 173.7 ± 0.2 | 70.4 ± 3.6 | 32 ± 3.9 | |
| a-P3HB-50-P34HB-15 | −39.6 ± 1.8 | 138.4 ± 0.2 | 159.4 ± 0.1 | 61.7 ± 0.1 | 29 ± 0.2 |
| a-P3HB-50-P34HB-30 | −33.4 ± 2.1 | 140.1 ± 1.1 | 162.1 ± 0.6 | 66.7 ± 0.2 | 25 ± 1.8 |
| a-P3HB-50-P34HB-50 | −29.2 ± 1.7 | 165.3 ± 0.5 | 58.5 ± 0.9 | 21 ± 0.4 | |
| a-P3HB-30-P34HB-15 | −36.0 ± 1.6 | 154.4 ± 0.1 | 167.6 ± 0.1 | 87.6 ± 0.1 | 40 ± 0.8 |
| a-P3HB-30-P34HB-30 | −29.5 ± 1.4 | 151.7 ± 0.4 | 167.5 ± 0.2 | 84.1 ± 0.3 | 38 ± 0.1 |
| a-P3HB-30-P34HB-50 | −28.1 ± 0.4 | 167.7 ± 0.5 | 78.6 ± 3.3 | 31 ± 2.4 |
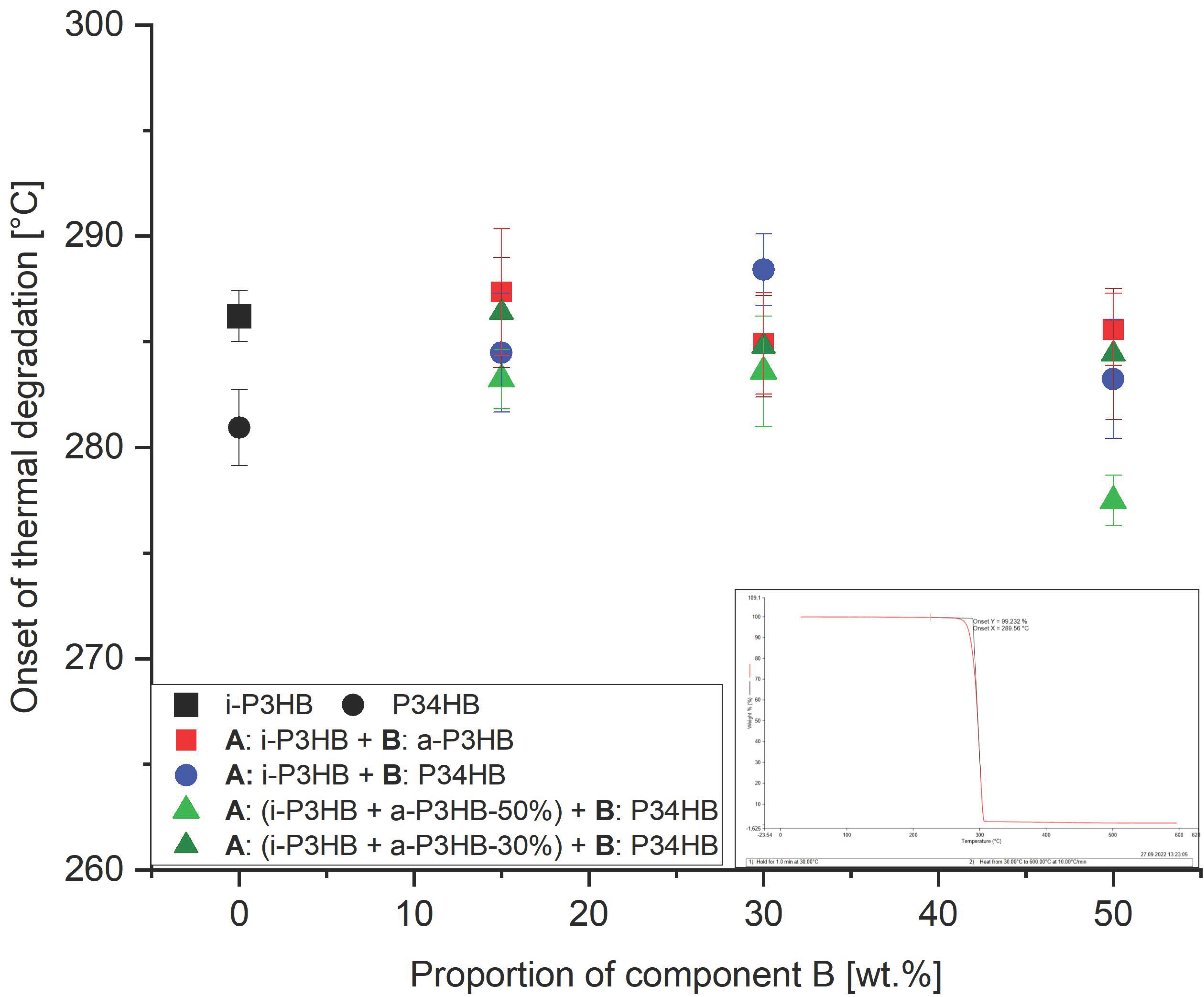
| Sample | Mw [g∙mol−1] | Td [°C] |
|---|---|---|
| i-P3HB 1 | 262,000 ± 20,000 | 285.6 ± 1.2 |
| P34HB 2 | 196,000 ± 26,000 | 280.9 ± 1.8 |
| a-P3HB-15 | 270,000 ± 10,000 | 287.4 ± 3.0 |
| a-P3HB-30 | 272,000 ± 18,000 | 284.9 ± 2.4 |
| a-P3HB-50 | 255,000 ± 24,000 | 285.6 ± 1.7 |
| P34HB-15 | 205,000 ± 35,000 | 284.5 ± 2.8 |
| P34HB-30 | 216,000 ± 41,000 | 288.4 ± 1.7 |
| P34HB-50 | 215,000 ± 16,000 | 283.2 ± 3.5 |
| a-P3HB-50-P34HB-15 | 201,000 ± 25,000 | 283.2 ± 1.4 |
| a-P3HB-50-P34HB-30 | 200,000 ± 13,000 | 283.6 ± 2.6 |
| a-P3HB-50-P34HB-50 | 215,000 ± 29,000 | 277.5 ± 1.2 |
| a-P3HB-30-P34HB-15 | 203,000 ± 30,000 | 286.4 ± 2.6 |
| a-P3HB-30-P34HB-30 | 200,000 ± 38,000 | 284.8 ± 2.4 |
| a-P3HB-30-P34HB-50 | 209,000 ± 25,000 | 284.4 ± 3.1 |
| After Extrusion | After 30 Days | |||
|---|---|---|---|---|
| Sample | σB * [MPa] | εB ** [%] | σB [MPa] | εB [%] |
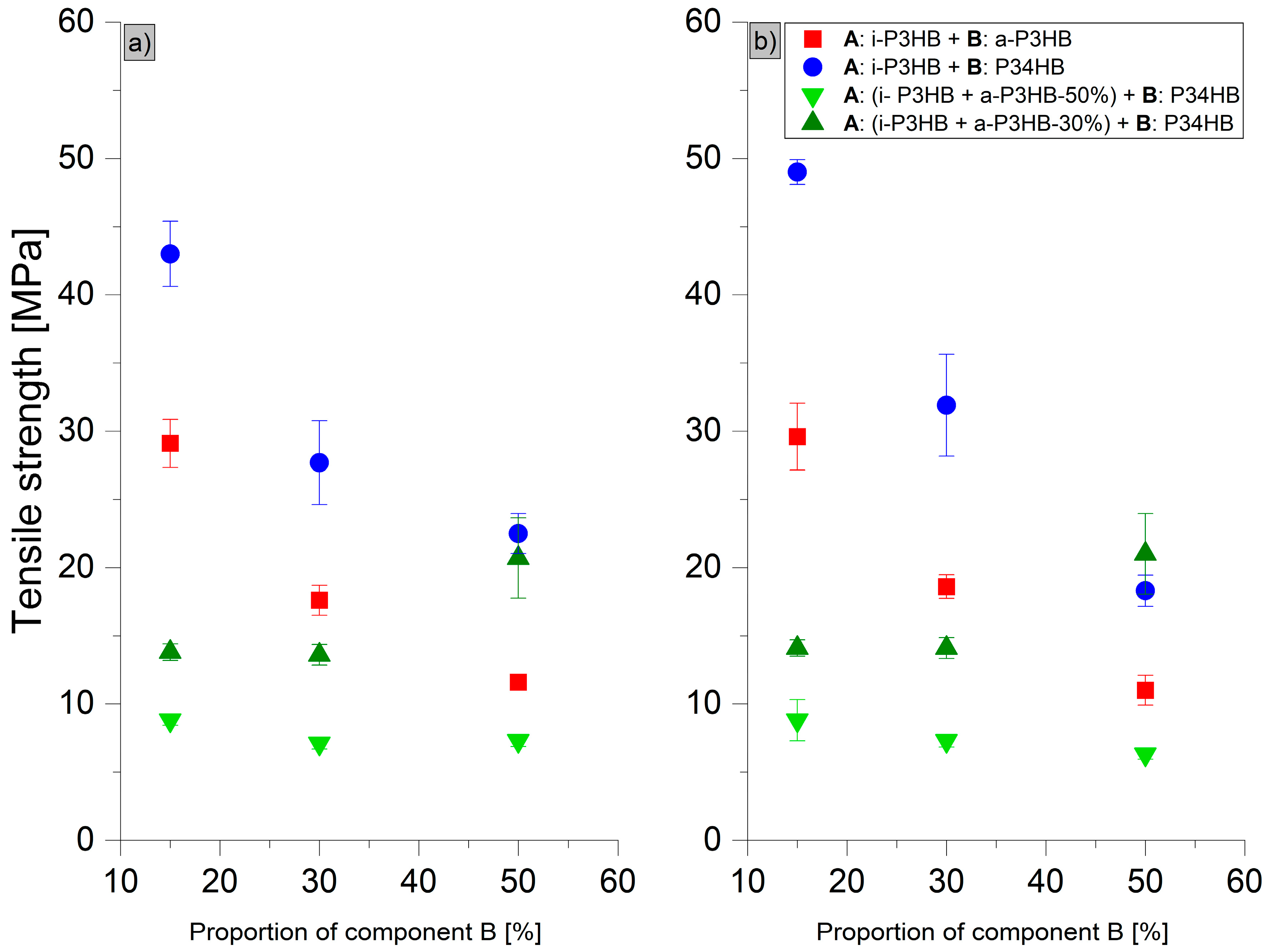
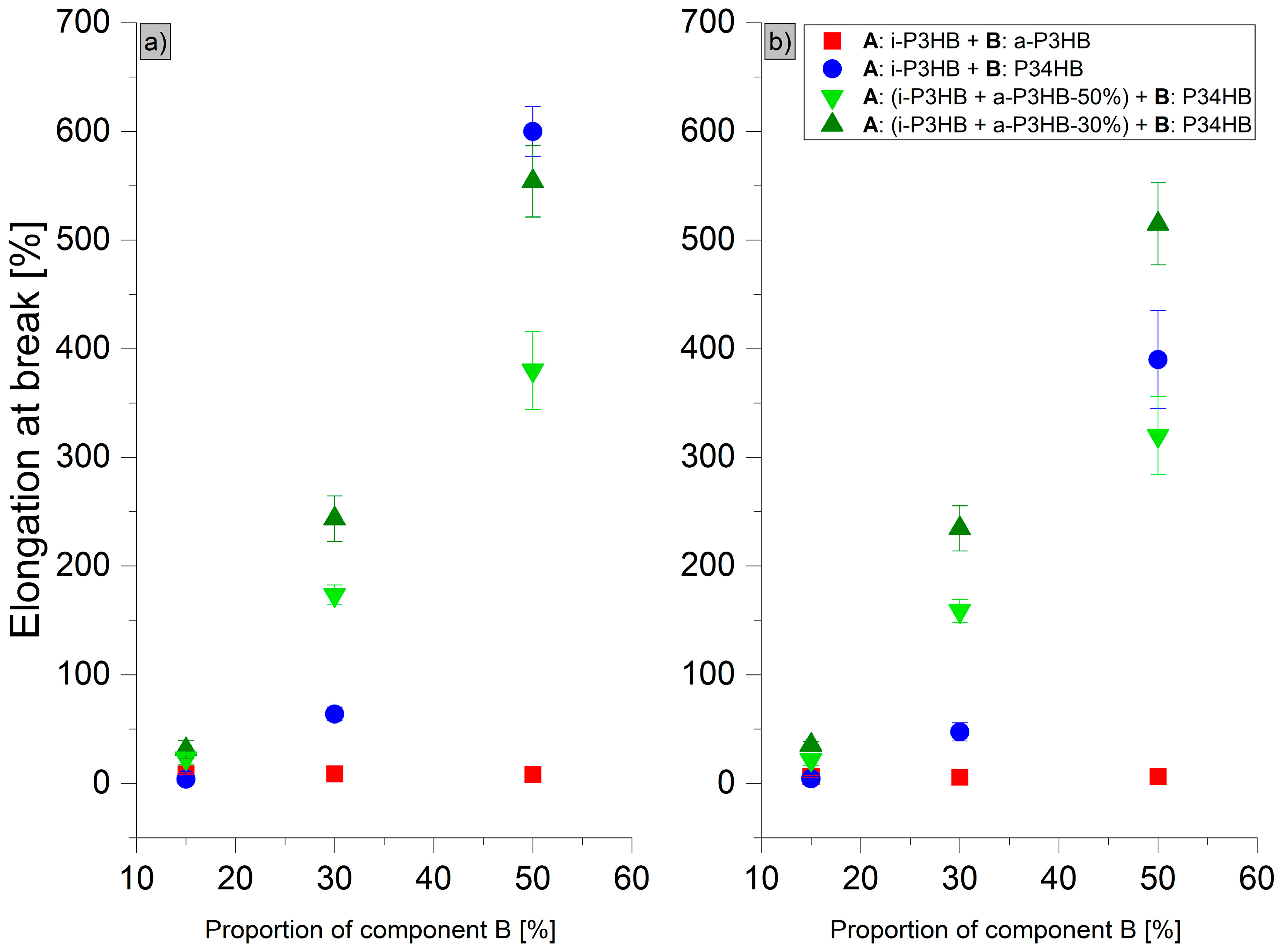
| i-P3HB | 40 1 | 5 1 | - | - |
| a-P3HB-15 | 29.1 ± 1.8 | 8.8 ± 0.8 | 29.6 ± 2.4 | 6.4 ± 1.1 |
| a-P3HB-30 | 17.6 ± 1.1 | 8.8 ± 2.3 | 18.6 ± 0.9 | 5.9 ± 0.5 |
| a-P3HB-50 | 11.6 ± 0.2 | 8.2 ± 0.6 | 11.0 ± 1.1 | 6.6 ± 1.0 |
| P34HB-15 | 43.0 ± 2.4 | 4.1 ± 1.0 | 49.0 ± 0.9 | 4.3 ± 0.5 |
| P34HB-30 | 28.7 ± 3.6 | 23.0 ± 13 | 32.2 ± 3.4 | 17.0 ± 6.4 |
| P34HB-50 | 22.5 ± 1.5 | 600.0 ± 23 | 18.3 ± 1.2 | 390.0 ± 45 |
| a-P3HB-50-P34HB-15 | 8.8 ± 0.4 | 23.0 ± 5.6 | 8.8 ± 1.7 | 8.0 ± 4.5 |
| a-P3HB-50-P34HB-30 | 7.2 ± 0.3 | 180.0 ± 68 | 6.9 ± 0.1 | 120.0 ± 18 |
| a-P3HB-50-P34HB-50 | 7.3 ± 0.4 | 380.0 ± 36 | 6.3 ± 0.4 | 320.0 ± 36 |
| a-P3HB-30-P34HB-15 | 13.0 ± 0.5 | 26.0 ± 4.2 | 17.1 ± 0.4 | 7.9 ± 0.9 |
| a-P3HB-30-P34HB-30 | 12.1 ± 0.7 | 41.4 ± 20.4 | 14.2 ± 0.6 | 15.0 ± 3.2 |
| a-P3HB-30-P34HB-50 | 12.3 ± 0.5 | 528.8 ± 69.3 | 11.1 ± 0.6 | 490.0 ± 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almustafa, W.; Grishchuk, S.; Redel, M.; Schubert, D.W.; Grun, G. Solvent-Free Processing of i-P3HB Blends: Enhancing Processability and Mechanical Properties for Sustainable Applications. Polymers 2025, 17, 2231. https://doi.org/10.3390/polym17162231
Almustafa W, Grishchuk S, Redel M, Schubert DW, Grun G. Solvent-Free Processing of i-P3HB Blends: Enhancing Processability and Mechanical Properties for Sustainable Applications. Polymers. 2025; 17(16):2231. https://doi.org/10.3390/polym17162231
Chicago/Turabian StyleAlmustafa, Wael, Sergiy Grishchuk, Michael Redel, Dirk W. Schubert, and Gregor Grun. 2025. "Solvent-Free Processing of i-P3HB Blends: Enhancing Processability and Mechanical Properties for Sustainable Applications" Polymers 17, no. 16: 2231. https://doi.org/10.3390/polym17162231
APA StyleAlmustafa, W., Grishchuk, S., Redel, M., Schubert, D. W., & Grun, G. (2025). Solvent-Free Processing of i-P3HB Blends: Enhancing Processability and Mechanical Properties for Sustainable Applications. Polymers, 17(16), 2231. https://doi.org/10.3390/polym17162231








