Exploring Biodegradable Polymeric Nanocomposite Films for Sustainable Food Packaging Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CAS and STA Dispersions
2.3. Preparation of BENT Suspension
2.4. Design of Biodegradable Film System
2.5. Determination of Final Polymer Viscosity
2.6. Bio-Nanocomposite Film Characterization
2.6.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6.2. Surface Microstructure
2.6.3. Mechanical Properties
2.6.4. Color Analysis
2.6.5. Water Vapor Permeability (WVP) Determination
2.6.6. Microbiological Assessment
2.6.7. Biodegradation Rate
3. Results and Discussion
3.1. Viscosity of the Final Solution
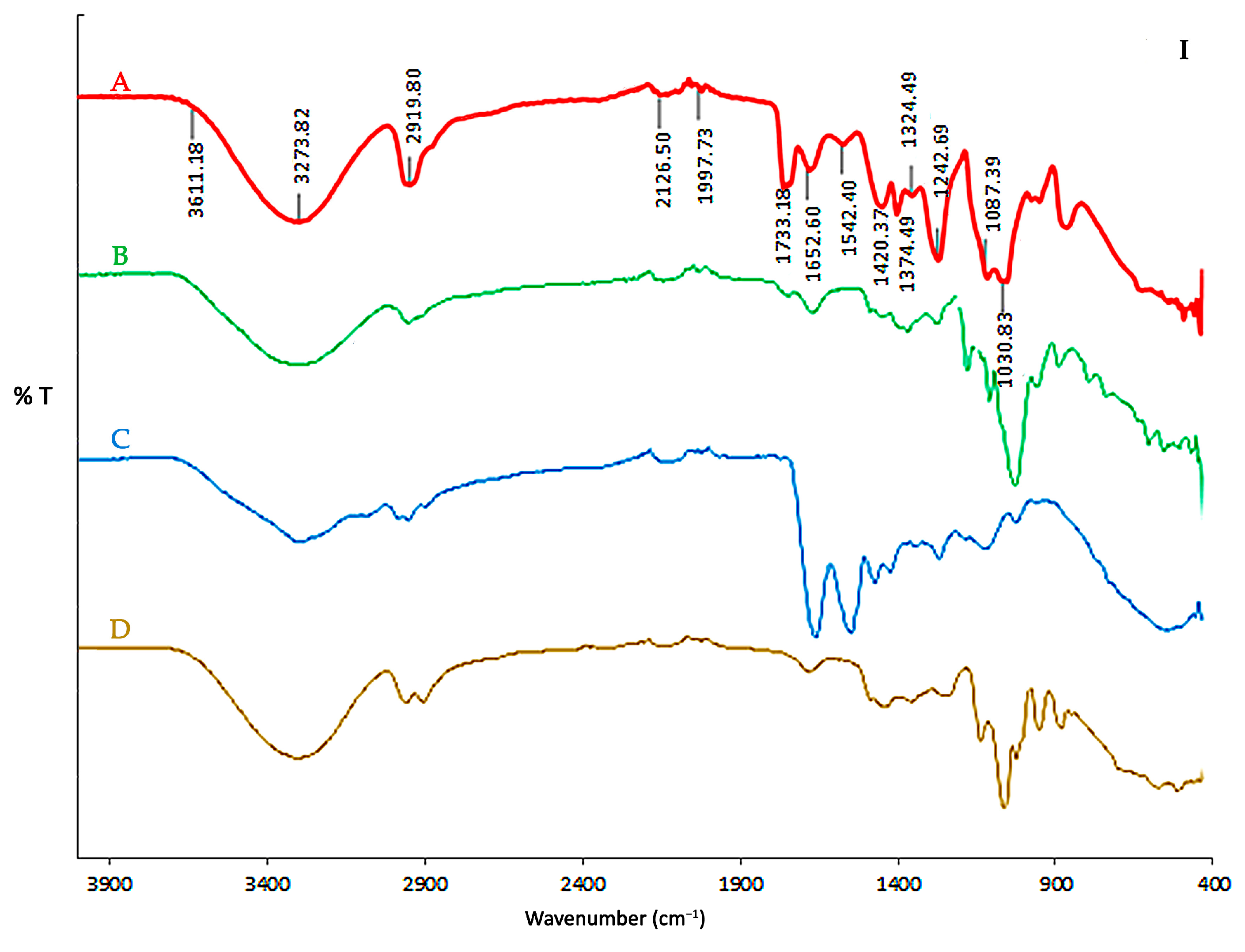
3.2. FTIR Spectra
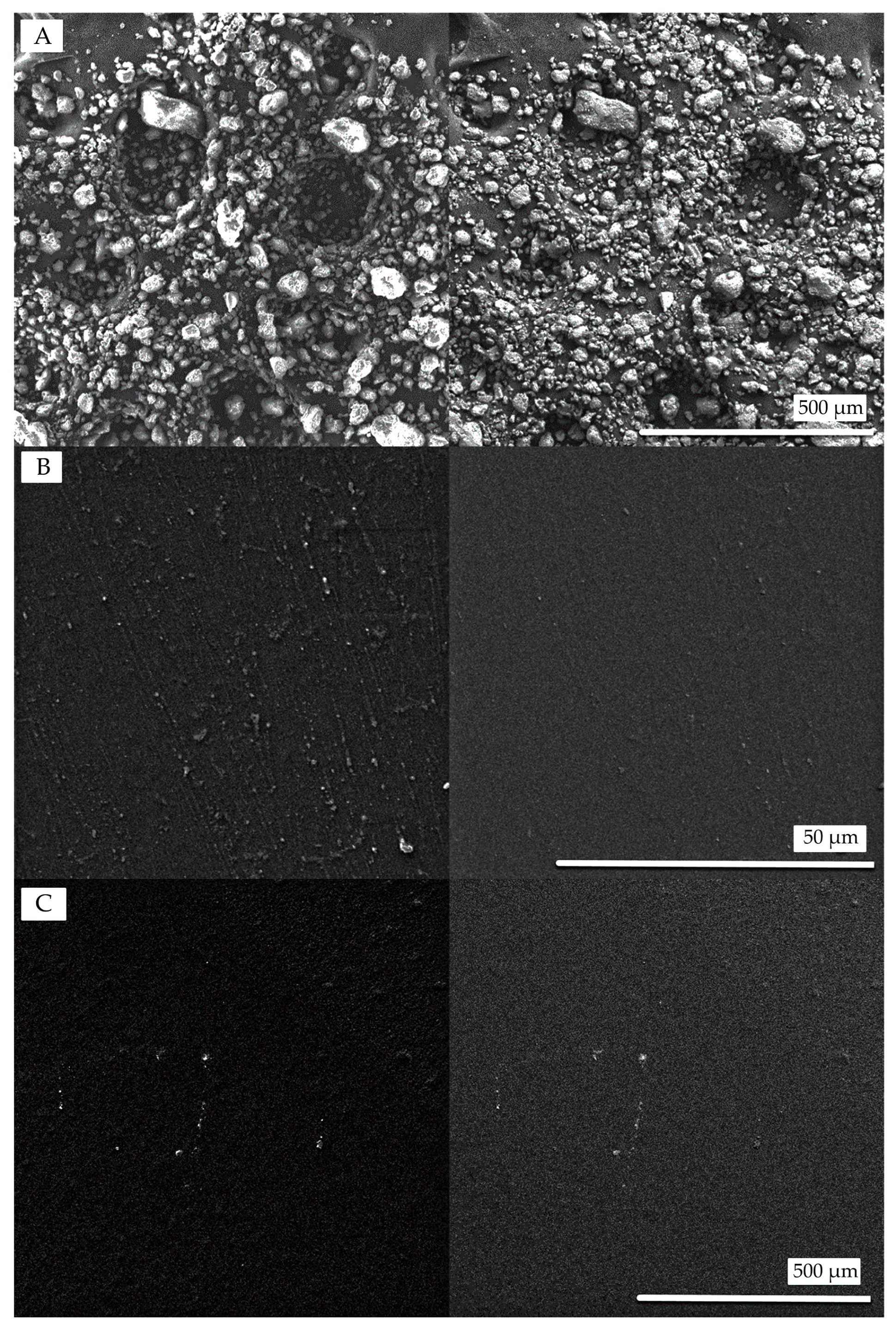
3.3. Surface Microstructure
3.4. Mechanical Properties
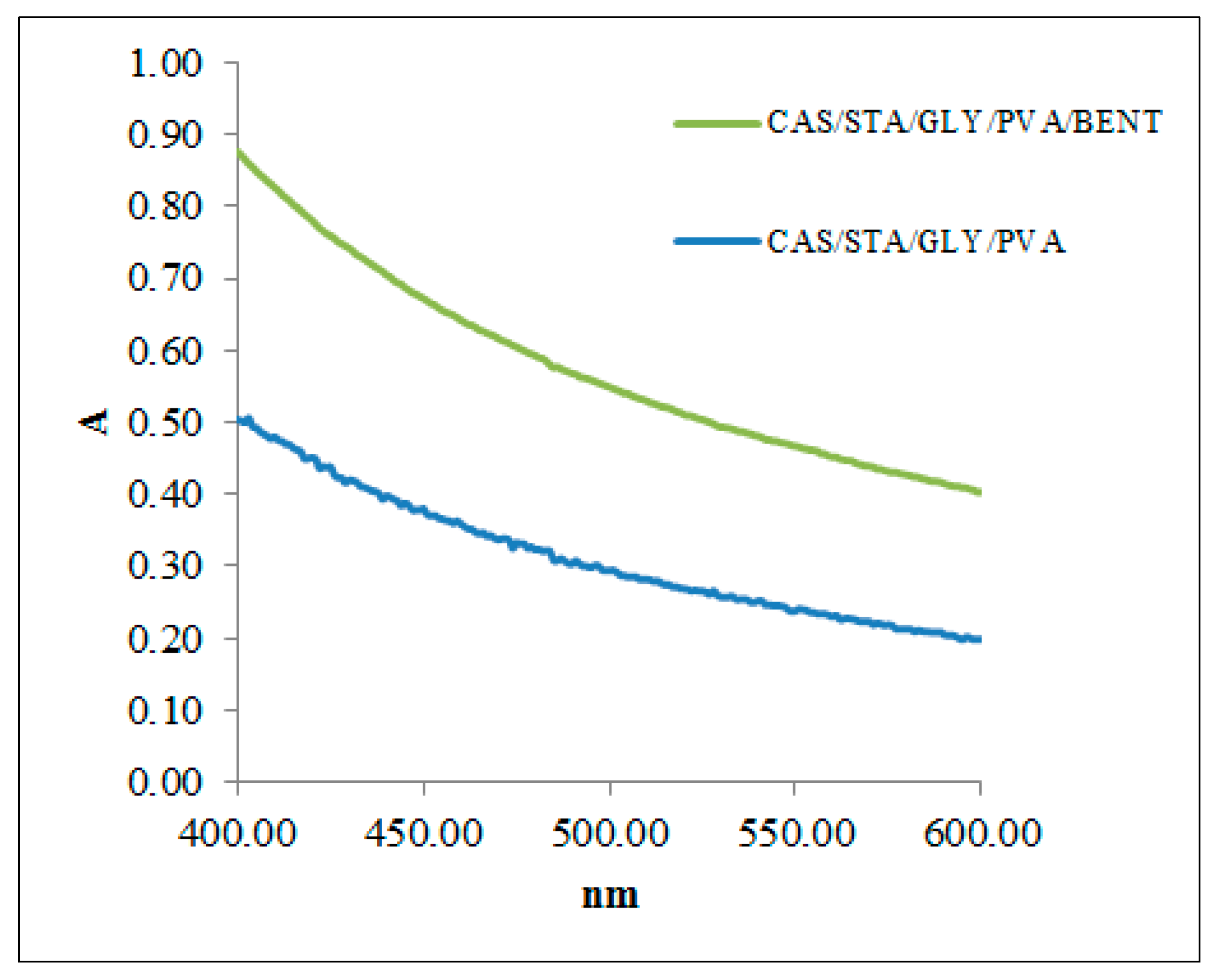
3.5. Optical Characterization
3.6. Water Vapor Permeability (WVP) Assay
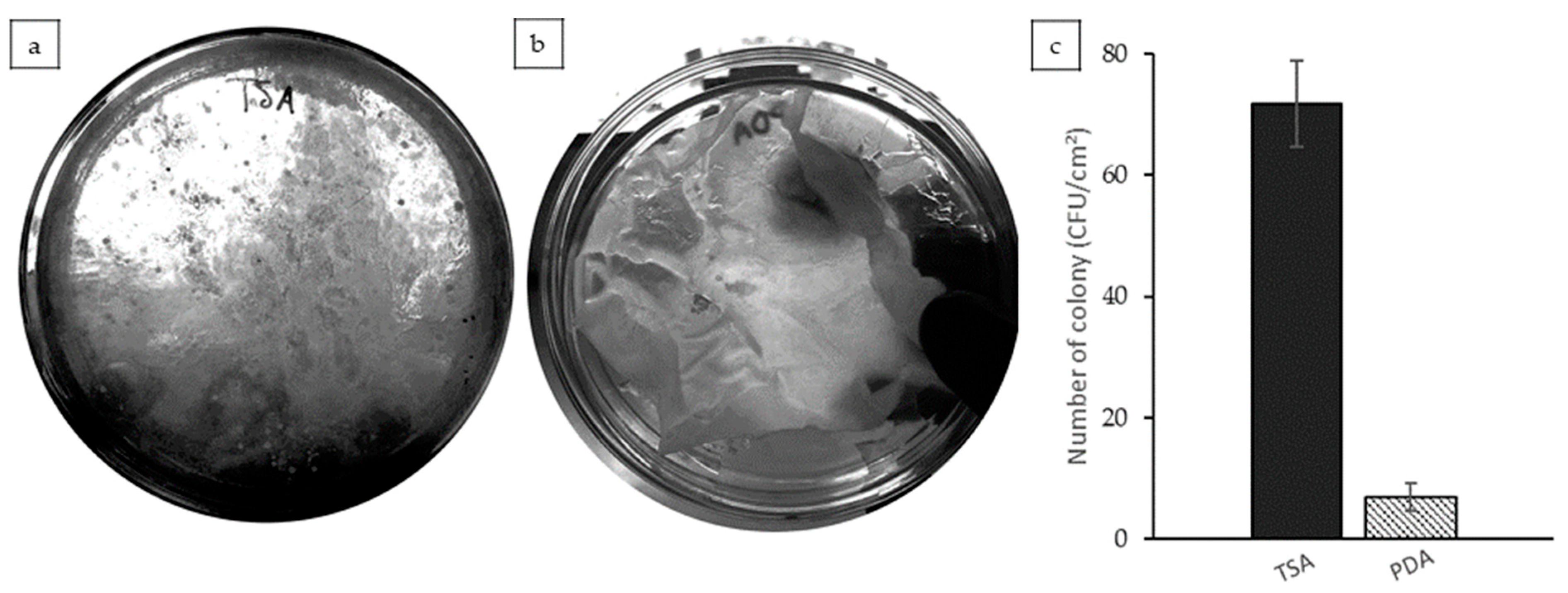
3.7. Microbiology Test
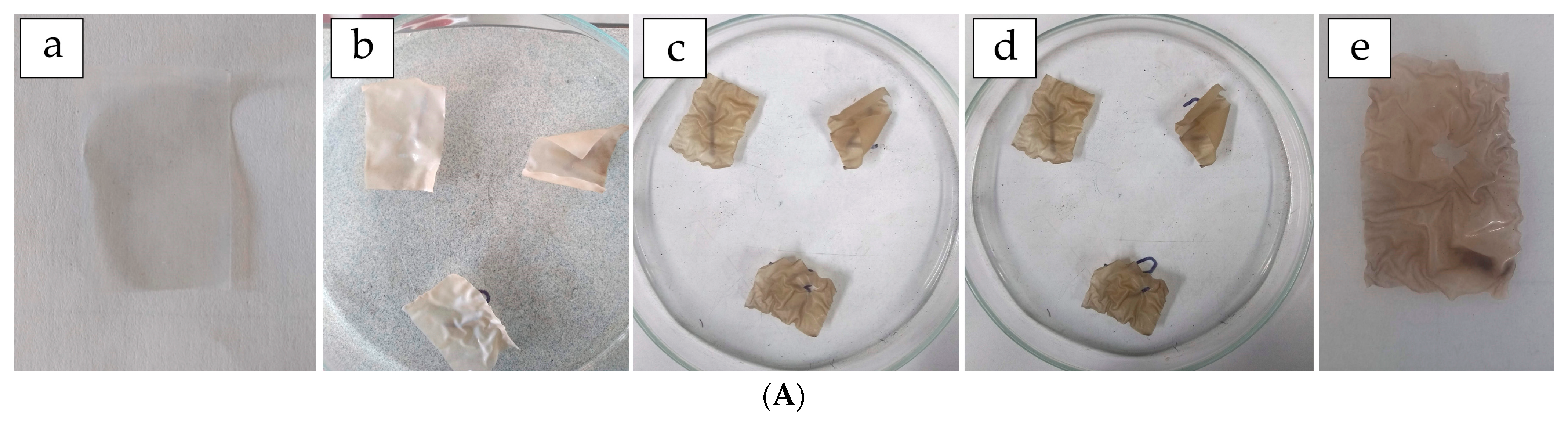
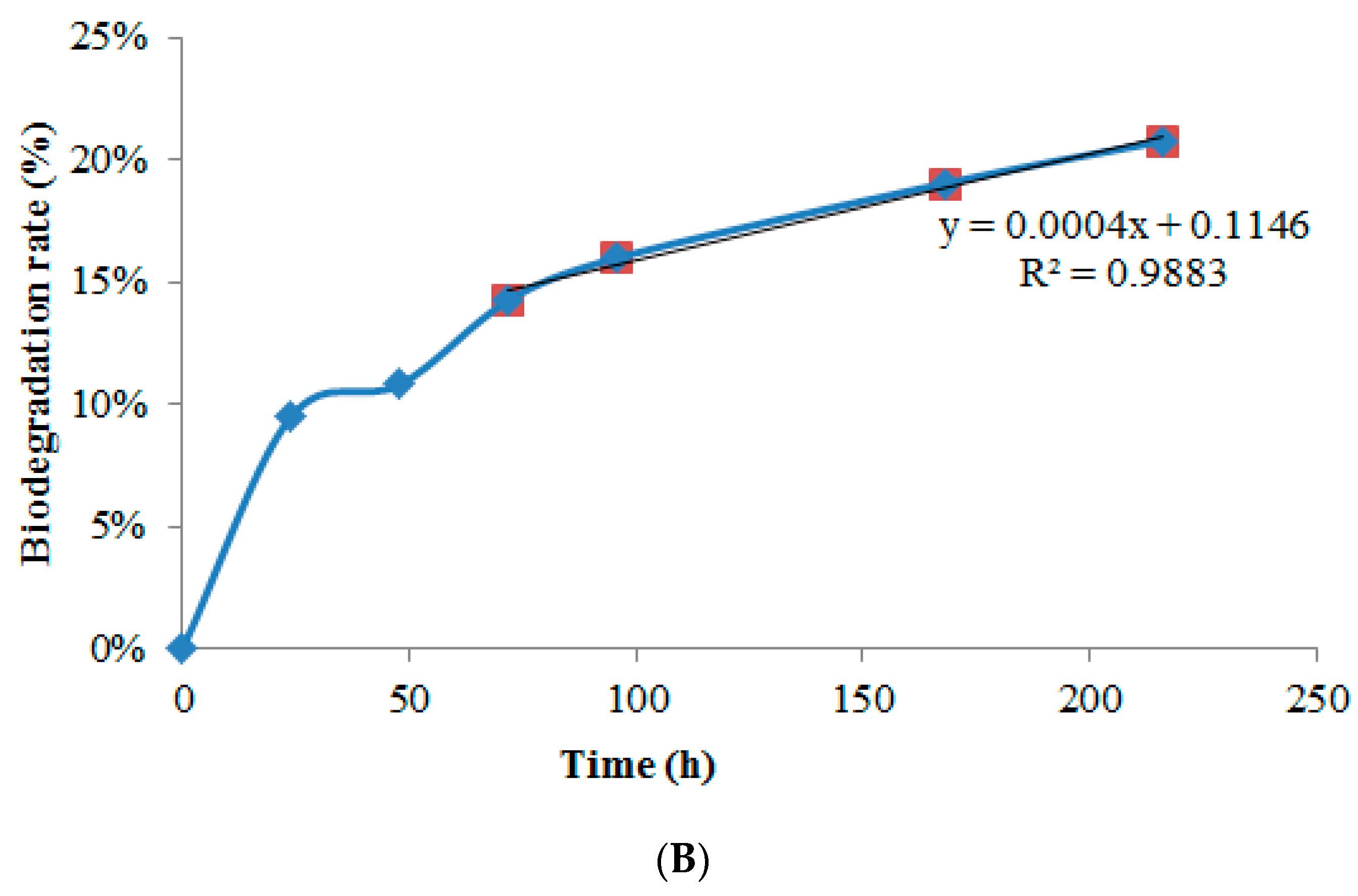
3.8. Biodegradation Rate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wicochea-Rodríguez, J.D.; Chalier, P.; Ruiz, T.; Gastaldi, E. Active Food Packaging Based on Biopolymers and Aroma Compounds: How to Design and Control the Release. Front. Chem. 2019, 7, 398. [Google Scholar] [CrossRef]
- Otitoju, M.A.; Olawoye, T.; Abiola, S.S.; Ahmed, S.; Okoma, O. Plastic waste management and recycling: A review. J. Glob. Soc. Sci. 2023, 4, 60–71. [Google Scholar] [CrossRef]
- Khoaele, K.K.; Gbadeyan, O.J.; Chunilall, V.; Sithole, B. The Devastation of Waste Plastic on the Environment and Remediation Processes: A Critical Review. Sustainability 2023, 15, 5233. [Google Scholar] [CrossRef]
- Cai, Z.; Li, M.; Zhu, Z.; Wang, X.; Huang, Y.; Li, T.; Gong, H.; Yan, M. Biological Degradation of Plastics and Microplastics: A Recent Perspective on Associated Mechanisms and Influencing Factors. Microorganisms 2023, 11, 1661. [Google Scholar] [CrossRef]
- Eze, W.U.; Umunakwe, R.; Obasi, H.C.; Ugbaja, M.I.; Uche, C.C.; Madufor, I.C. Plastics waste management: A review of pyrolysis technology. Clean Technol. Recycl. 2021, 1, 50–69. [Google Scholar] [CrossRef]
- Nayanathara Thathsarani Pilapitiya, P.G.C.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Rajmohan, K.V.S.; Ramya, C.; Raja Viswanathan, M.; Varjani, S. Plastic pollutants: Effective waste management for pollution control and abatement. Curr. Opin. Environ. Sci. Health 2019, 12, 72–84. [Google Scholar] [CrossRef]
- Babaremu, K.; Oladijo, O.P.; Akinlabi, E. Biopolymers: A suitable replacement for plastics in product packaging. Adv. Ind. Eng. Polym. Res. 2023, 6, 333–340. [Google Scholar] [CrossRef]
- Gnanasekaran, D. Green Biopolymers and Its Nanocomposites in Various Applications: State of the Art. In Green Biopolymers and Their Nanocomposites; Gnanasekaran, D., Ed.; Springer: Singapore, 2019; pp. 1–27. [Google Scholar]
- Ucpinar Durmaz, B.; Aytac, A. Poly (vinyl alcohol) and casein films: The effects of glycerol amount on the properties of films. Res. Eng. Struct. Mater. 2019, 5, 155–165. [Google Scholar] [CrossRef]
- Getino, L.; Martin, J.L.; Chamizo-Ampudia, A. A Review of Polyhydroxyalkanoates: Characterization, Production, and Application from Waste. Microorganisms 2024, 12, 2028. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, A.J.; Chen, C.; Arif, M.; Mubarak, N.M. Bio-based plastics, biodegradable plastics, and compostable plastics: Biodegradation mechanism, biodegradability standards and environmental stratagem. Int. Biodeterior. Biodegrad. 2024, 195, 105887. [Google Scholar] [CrossRef]
- Bhawani, S.A.; Hussain, H.; Bojo, O.; Fong, S.S. Proteins as Agricultural Polymers for Packaging Production. In Bionanocomposites for Packaging Applications; Springer International Publishing: Cham, Switzerland, 2018; pp. 243–267. [Google Scholar]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Alvarez Igarzabal, C.I. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Banerjee, A.; Saurabh, C.K.; Tye, Y.Y.; Suriani, A.B.; Mohamed, A.; Karim, A.A.; Rizal, S.; Paridah, M.T. Biodegradable Films for Fruits and Vegetables Packaging Application: Preparation and Properties. Food Eng. Rev. 2018, 10, 139–153. [Google Scholar] [CrossRef]
- Picchio, M.L.; Ronco, L.I.; Passeggi, M.C.G.; Minari, R.J.; Gugliotta, L.M. Poly(n-butyl acrylate)–Casein Nanocomposites as Promising Candidates for Packaging Films. J. Polym. Environ. 2018, 26, 2579–2587. [Google Scholar] [CrossRef]
- Domene-Lopez, D.; Delgado-Marin, J.J.; Martin-Gullon, I.; Garcia-Quesada, J.C.; Montalban, M.G. Comparative study on properties of starch films obtained from potato, corn and wheat using 1-ethyl-3-methylimidazolium acetate as plasticizer. Int. J. Biol. Macromol. 2019, 135, 845–854. [Google Scholar] [CrossRef]
- Shendurse, A.M. Milk protein based edible films and coatings–preparation, properties and food applications. J. Nutr. Health Food Eng. 2018, 8, 219–226. [Google Scholar] [CrossRef]
- Murrieta-Martínez, C.L.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; Márquez Ríos, E. Edible protein films: Sources and behavior. Packag. Technol. Sci. 2018, 31, 113–122. [Google Scholar] [CrossRef]
- Fox, P.F.; Brodkorb, A. The casein micelle: Historical aspects, current concepts and significance. Int. Dairy J. 2008, 18, 677–684. [Google Scholar] [CrossRef]
- Augustin, M.A.; Oliver, C.M.; Hemar, Y. Casein, Caseinates, and Milk Protein Concentrates. In Dairy Ingredients for Food Processing, 1st ed.; Wiley-Blackwell: Ames, IA, USA, 2011; pp. 161–178. [Google Scholar]
- Picchio, M.L.; Paredes, A.J.; Palma, S.D.; Passeggi, M.C.G.; Gugliotta, L.M.; Minari, R.J.; Igarzabal, C.I.A. pH-responsive casein-based films and their application as functional coatings in solid dosage formulations. Colloids Surf. Physicochem. Eng. Asp. 2018, 541, 1–9. [Google Scholar] [CrossRef]
- Sadeghi, M.; Madadlou, A.; Khosrowshahi, A.; Mohammadifar, M. Acid-induced gelation behavior of casein/whey protein solutions assessed by oscillatory rheology. J. Food Sci. Technol. 2014, 51, 2113–2119. [Google Scholar] [CrossRef]
- Nicolai, T.; Chassenieux, C. Heat-induced gelation of casein micelles. Food Hydrocoll. 2021, 118, 106755. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kajla, P.; Kumari, P.; Bangar, S.P.; Rusu, A.; Trif, M.; Lorenzo, J.M. Milk protein-based active edible packaging for food applications: An eco-friendly approach. Front. Nutr. 2022, 9, 942524. [Google Scholar] [CrossRef]
- Khan, M.R.; Volpe, S.; Valentino, M.; Miele, N.A.; Cavella, S.; Torrieri, E. Active Casein Coatings and Films for Perishable Foods: Structural Properties and Shelf-Life Extension. Coatings 2021, 11, 899. [Google Scholar] [CrossRef]
- Kolahreez, D.; Ghasemi-Mobarakeh, L.; Quartinello, F.; Liebner, F.W.; Guebitz, G.M.; Ribitsch, D. Multifunctional Casein-Based Wound Dressing Capable of Monitoring and Moderating the Proteolytic Activity of Chronic Wounds. Biomacromolecules 2024, 25, 700–714. [Google Scholar] [CrossRef]
- Abu Diak, O.; Bani-Jaber, A.; Amro, B.; Jones, D.; Andrews, G.P. The manufacture and characterization of casein films as novel tablet coatings. Food Bioprod. Process. 2007, 85, 284–290. [Google Scholar] [CrossRef]
- Pella, M.C.G.; Silva, O.A.; Pella, M.G.; Beneton, A.G.; Caetano, J.; Simoes, M.R.; Dragunski, D.C. Effect of gelatin and casein additions on starch edible biodegradable films for fruit surface coating. Food Chem. 2020, 309, 125764. [Google Scholar] [CrossRef]
- Wagh, Y.R.; Pushpadass, H.A.; Emerald, F.M.; Nath, B.S. Preparation and characterization of milk protein films and their application for packaging of Cheddar cheese. J. Food Sci. Technol. 2014, 51, 3767–3775. [Google Scholar] [CrossRef]
- Daniloski, D.; Petkoska, A.T.; Lee, N.A.; Bekhit, A.E.-D.; Carne, A.; Vaskoska, R.; Vasiljevic, T. Active edible packaging based on milk proteins: A route to carry and deliver nutraceuticals. Trends Food Sci. Technol. 2021, 111, 688–705. [Google Scholar] [CrossRef]
- Bonnaillie, L.; Zhang, H.; Akkurt, S.; Yam, K.; Tomasula, P. Casein Films: The Effects of Formulation, Environmental Conditions and the Addition of Citric Pectin on the Structure and Mechanical Properties. Polymers 2014, 6, 2018–2036. [Google Scholar] [CrossRef]
- Hu, G.; Zheng, Y.; Liu, Z.; Xiao, Y.; Deng, Y.; Zhao, Y. Effects of high hydrostatic pressure, ultraviolet light-C, and far-infrared treatments on the digestibility, antioxidant and antihypertensive activity of α-casein. Food Chem. 2017, 221, 1860–1866. [Google Scholar] [CrossRef]
- Nayik, G.A.; Gull, A.; Masoodi, L.; Navaf, M.; Sunooj, K.V.; Ucak, İ.; Afreen, M.; Kaur, P.; Rehal, J.; Jagdale, Y.D.; et al. Milk proteins: Chemistry, functionality and diverse industrial applications. Cogent Food Agric. 2024, 10, 2377686. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutierrez, M.C.; Rangel-Vargas, E.; Castro-Rosas, J.; Aguirre-Loredo, R.Y. Effect of polyvinyl alcohol on the physicochemical properties of biodegradable starch films. Mater. Chem. Phys. 2020, 239, 122027. [Google Scholar] [CrossRef]
- Huang, L.; Xu, H.; Zhao, H.; Xu, M.; Qi, M.; Yi, T.; An, S.; Zhang, X.; Li, C.; Huang, C.; et al. Properties of thermoplastic starch films reinforced with modified cellulose nanocrystals obtained from cassava residues. New J. Chem. 2019, 43, 14883–14891. [Google Scholar] [CrossRef]
- Sadeghizadeh-Yazdi, J.; Habibi, M.; Kamali, A.; Banaei, M. Application of Edible and Biodegradable Starch-Based Films in Food Packaging: A Systematic Review and Meta-Analysis. Curr. Res. Nutr. Food Sci. 2019, 7, 3. [Google Scholar] [CrossRef]
- Parvin, F.; Rahman, M.A.; Islam, J.M.M.; Khan, M.A.; Saadat, A.H.M. Preparation and Characterization of Starch/PVA Blend for Biodegradable Packaging Material. Adv. Mater. Res. 2010, 123–125, 351–354. [Google Scholar] [CrossRef]
- Karaogul, E.; Altuntas, E.; Salan, T.; Hakki Alma, M. The Effects of Novel Additives Used in PVA/Starch Biohybrid Films. In Fillers, Syntheses, Characterizations and Industrial Applications; El-Ghanam, A.H.A., El-Maghraby, E.M.M., Al-Najar, A., Eds.; IntechOpen: Rijeka, Croatia, 2019; pp. 173–195. [Google Scholar] [CrossRef]
- Tian, H.; Yan, J.; Rajulu, A.V.; Xiang, A.; Luo, X. Fabrication and properties of polyvinyl alcohol/starch blend films: Effect of composition and humidity. Int. J. Biol. Macromol. 2017, 96, 518–523. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Cerisuelo, J.P.; Domínguez, I.; López-Carballo, G.; Catalá, R.; Gavara, R. Nanotechnology in Food Packaging. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 205–232. [Google Scholar]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Ilyas, R.A.; Chowdhury, A.; Navaf, M.; Sunooj, K.V.; Siroha, A.K. Bentonite clay as a nanofiller for food packaging applications. Trends Food Sci. Technol. 2023, 142, 104242. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Avella, M.; De Vlieger, J.J.; Errico, M.E.; Fischer, S.; Vacca, P.; Volpe, M.G. Biodegradable starch/clay nanocomposite films for food packaging applications. Food Chem. 2005, 93, 467–474. [Google Scholar] [CrossRef]
- Naderizadeh, S.; Shakeri, A.; Mahdavi, H.; Nikfarjam, N.; Qazvini, N.T. Hybrid Nanocomposite Films of Starch, Poly(vinyl alcohol) (PVA), Starch Nanocrystals (SNCs), and Montmorillonite (Na-MMT): Structure–Properties Relationship. Starch-Stärke 2018, 71, 1800027. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Biodegradable and Water Resistant Poly(vinyl) Alcohol (PVA)/Starch (ST)/Glycerol (GL)/Halloysite Nanotube (HNT) Nanocomposite Films for Sustainable Food Packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef]
- Pojanavaraphan, T.; Magaraphan, R.; Chiou, B.S.; Schiraldi, D.A. Development of biodegradable foamlike materials based on casein and sodium montmorillonite clay. Biomacromolecules 2010, 11, 2640–2646. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Panjagari, N.R.; Kanade, P.P.; Singh, A.K.; Badola, R.; Ganguly, S.; Behare, P.V.; Sharma, R.; Alam, T. Sodium caseinate-starch-modified montmorillonite based biodegradable film: Laboratory food extruder assisted exfoliation and characterization. Food Packag. Shelf Life 2018, 15, 17–27. [Google Scholar] [CrossRef]
- Bier, M.C.; Kohn, S.; Stierand, A.; Grimmelsmann, N.; Homburg, S.V.; Rattenholl, A.; Ehrmann, A. Investigation of eco-friendly casein fibre production methods. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 192004. [Google Scholar] [CrossRef]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.; Almajano Pablos, M.P. Gelatine-Based Antioxidant Packaging Containing Caesalpinia decapetala and Tara as a Coating for Ground Beef Patties. Antioxidants 2016, 5, 10. [Google Scholar] [CrossRef]
- Cazon, P.; Vazquez, M.; Velazquez, G. Cellulose-glycerol-polyvinyl alcohol composite films for food packaging: Evaluation of water adsorption, mechanical properties, light-barrier properties and transparency. Carbohydr. Polym. 2018, 195, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Slavutsky, A.M.; Bertuzzi, M.A.; Armada, M. Water barrier properties of starch-clay nanocomposite films. Braz. J. Food Technol. 2012, 15, 208–218. [Google Scholar] [CrossRef]
- ASTM D1003-21; Standard Test Method for Haze and Luminous Transmittance of Transparent Plastics. ASTM: West Conshohocken, PA, USA, 2003. [CrossRef]
- ASTM E96-00e1; Standard Test Methods for Water Vapor Transmission of Materials. ASTM: West Conshohocken, PA, USA, 2017.
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation of three-layer laminate films based on gelatin under indoor soil conditions. Polym. Degrad. Stab. 2009, 94, 1307–1313. [Google Scholar] [CrossRef]
- Torres-Medina, C.T.; Murillo, E.A. Rheological Behavior of Polyvinyl Alcohol/Starch Blends: Influence of the Sorbitol Citrate Content. J. Polym. Environ. 2023, 32, 1233–1243. [Google Scholar] [CrossRef]
- Chen, C.; Zong, L.; Wang, J.; Xie, J. Microfibrillated cellulose reinforced starch/polyvinyl alcohol antimicrobial active films with controlled release behavior of cinnamaldehyde. Carbohydr. Polym. 2021, 272, 118448. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, I.; Villa, C.; Cong, R.; Karjala, T. Viscosity Modeling of Polymer Solutions. Macromol. Symp. 2018, 377, 1600179. [Google Scholar] [CrossRef]
- Vlachopoulos, J.; Strutt, D. The Role of Rheology in Polymer Extrusion. In Proceedings of the New Technology for Extrusion Conference, Milan, Italy, 20–21 November 2003. [Google Scholar]
- Babaie, A.; Madadkhani, S.; Stoeber, B. Evaporation-driven low Reynolds number vortices in a cavity. Phys. Fluids 2014, 26, 33102. [Google Scholar] [CrossRef]
- Ucpinar Durmaz, B.; Aytac, A. Effects of Polyol-Based Plasticizer Types and Concentration on the Properties of Polyvinyl Alcohol and Casein Blend Films. J. Polym. Environ. 2020, 29, 313–322. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Wu, J.; Chen, N.; Wang, Q. Preparation of novel thermoplastic poly(vinyl alcohol) with improved processability for fused deposition modeling. Polym. Adv. Technol. 2018, 29, 1447–1455. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Jia, R.; Dai, Y.; Dong, H.; Hou, H.; Guo, Q. High performance extrusion blown starch/polyvinyl alcohol/clay nanocomposite films. Food Hydrocoll. 2018, 79, 534–543. [Google Scholar] [CrossRef]
- Mohammad Ali Zadeh, M.; Keyanpour-Rad, M.; Ebadzadeh, T. Effect of viscosity of polyvinyl alcohol solution on morphology of the electrospun mullite nanofibres. Ceram. Int. 2014, 40, 5461–5466. [Google Scholar] [CrossRef]
- Fan, M.; Dai, D.; Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres. In Fourier Transform—Materials Analysis; InTechOpen: London, UK, 2012. [Google Scholar]
- Siročić, A.P. Characterization of Casein Fractions—Comparison of Commercial Casein and Casein Extracted from Cow’s Milk. Chem. Biochem. Eng. Q. J. 2017, 30, 501–509. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Rácz, I.; Marcovich, N.E. Structure and properties of nanocomposite films based on sodium caseinate and nanocellulose fibers. J. Food Eng. 2011, 103, 76–83. [Google Scholar] [CrossRef]
- Marques, P.T.; Lima, A.M.F.; Bianco, G.; Laurindo, J.B.; Borsali, R.; Le Meins, J.F.; Soldi, V. Thermal properties and stability of cassava starch films cross-linked with tetraethylene glycol diacrylate. Polym. Degrad. Stab. 2006, 91, 726–732. [Google Scholar] [CrossRef]
- Lopez, M.; Osorio, G.; Arellano, S.; Gallardo, T.; Flores, S.; Lopez, M.S. Preparation of Starch/Clay/Glycerol Nanocomposite Films and Their Ftir, Xrd, Sem and Mechanical Characterizations. Rev. Mex. Ing. Química 2017, 16, 793–804. [Google Scholar]
- de Oliveira, C.I.R.; Rocha, M.C.G.; da Silva, A.L.N.; Bertolino, L.C. Characterization of bentonite clays from Cubati, Paraíba (Northeast of Brazil). Cerâmica 2016, 62, 272–277. [Google Scholar] [CrossRef]
- Santos, R.A.L.; Muller, C.M.O.; Grossmann, M.V.E.; Mali, S.; Yamashita, F. Starch/Poly (Butylene Adipate-Co-Terephthalate)/Montmorillonite Films Produced by Blow Extrusion. Química Nova 2014, 37, 937–942. [Google Scholar] [CrossRef]
- Rai, S.; Poonia, A. Formulation and characterization of edible films from pea starch and casein. J. Pharmacogn. Phytochem. 2019, 8, 317–321. [Google Scholar]
- Sapalidis, A.A.; Katsaros, F.K.; Steriotis, T.A.; Kanellopoulos, N.K. Properties of poly(vinyl alcohol)—Bentonite clay nanocomposite films in relation to polymer–clay interactions. J. Appl. Polym. Sci. 2011, 123, 1812–1821. [Google Scholar] [CrossRef]
- Khodaeimehr, R.; Peighambardoust, S.J.; Peighambardoust, S.H. Preparation and Characterization of Corn Starch/Clay Nanocomposite Films: Effect of Clay Content and Surface Modification. Starch-Stärke 2018, 70, 1700251. [Google Scholar] [CrossRef]
- Sapalidis, A.; Katsaros, F.; Kanellopoulos, N. PVA/Montmorillonite Nanocomposites: Development and Properties. In Nanocomposites and Polymers with Analytical Methods; Cuppoletti, J., Ed.; InTechOpen: London, UK, 2011; pp. 21–44. [Google Scholar]
- Rhim, J.W.; Lee, S.B.; Hong, S.I. Preparation and characterization of agar/clay nanocomposite films: The effect of clay type. J. Food Sci. 2011, 76, N40–N48. [Google Scholar] [CrossRef]
- Folorunso, D.O.; Olubambi, P.; Borode, J.O. Characterization and Qualitative Analysis of Some Nigerian Clay Deposits for Rafractory Applications. IOSR J. Appl. Chem. 2014, 7, 40–47. [Google Scholar] [CrossRef]
- Gonzaga, V.d.A.M.; Chrisostomo, B.A.; Poli, A.L.; Schmitt, C.C. Preparation, Characterization and Photostability of Nanocomposite Films Based on Poly(acrylic acid) and Montmorillonite. Mater. Res. 2018, 21, e20171024. [Google Scholar] [CrossRef]
- Faria, K.C.P.; Holanda, J.N.F. Using SEM / EDS for characterization of clay ceramic bearing sugarcane bagasse ash waste. In Current Microscopy Contributions to Advances in Science and Technology; Méndez-Vilas, A., Ed.; Formatex Research Center: Norristown, PA, USA, 2012; pp. 1085–1092. [Google Scholar]
- Tang, X.; Alavi, S.; Herald, T.J. Barrier and Mechanical Properties of Starch-Clay Nanocomposite Films. Cereal Chem. 2008, 85, 433–439. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. Characterization of the new biodegradable WPI/clay nanocomposite films based on kefiran exopolysaccharide. J. Food Sci. Technol. 2015, 52, 3485–3493. [Google Scholar] [CrossRef][Green Version]
- Krupskaya, V.; Zakusin, S.; Tyupina, E.; Dorzhieva, O.; Zhukhlistov, A.; Belousov, P.; Timofeeva, M. Experimental Study of Montmorillonite Structure and Transformation of Its Properties under Treatment with Inorganic Acid Solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Cyras, V.P.; Manfredi, L.B.; Ton-That, M.-T.; Vázquez, A. Physical and mechanical properties of thermoplastic starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2008, 73, 55–63. [Google Scholar] [CrossRef]
- Wahyuningsih, K.; Iriani, E.S.; Fahma, F. Utilization of Cellulose from Pineapple Leaf Fibers as Nanofiller in Polyvinyl Alcohol-Based Film. Indones. J. Chem. 2018, 16, 181–189. [Google Scholar] [CrossRef]
- Su, J.; Wang, S. Influence of food packaging color and foods type on consumer purchase intention: The mediating role of perceived fluency. Front. Nutr. 2023, 10, 1344237. [Google Scholar] [CrossRef]
- Stiller, B. The Effect of Montmorillonite Nanoclay on Mechanical and Barrier Properties of Mung Bean Starch Films. Master’s Thesis, Clemson University, Clemson, SC, USA, 2008. [Google Scholar]
- Abdollahi, M.; Alboofetileh, M.; Rezaei, M.; Behrooz, R. Comparing physico-mechanical and thermal properties of alginate nanocomposite films reinforced with organic and/or inorganic nanofillers. Food Hydrocoll. 2013, 32, 416–424. [Google Scholar] [CrossRef]
- Koosha, M.; Hamedi, S. Intelligent Chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog. Org. Coat. 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Tammina, S.K.; Rhim, J.-W. Biopolymer-based UV protection functional films for food packaging. Food Hydrocoll. 2023, 142, 108771. [Google Scholar] [CrossRef]
- Aadil, K.R.; Prajapati, D.; Jha, H. Improvement of physcio-chemical and functional properties of alginate film by Acacia lignin. Food Packag. Shelf Life 2016, 10, 25–33. [Google Scholar] [CrossRef]
- Naidu, D.S.; John, M.J. Effect of Clay Nanofillers on the Mechanical and Water Vapor Permeability Properties of Xylan-Alginate Films. Polymers 2020, 12, 2279. [Google Scholar] [CrossRef] [PubMed]
- Choudalakis, G.; Gotsis, A.D. Permeability of polymer/clay nanocomposites: A review. Eur. Polym. J. 2009, 45, 967–984. [Google Scholar] [CrossRef]
- Rhim, J.-W. Effect of PLA lamination on performance characteristics of agar/κ-carrageenan/clay bio-nanocomposite film. Food Res. Int. 2013, 51, 714–722. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Laurindo, J.B.; Yamashita, F. Effect of nanoclay incorporation method on mechanical and water vapor barrier properties of starch-based films. Ind. Crops Prod. 2011, 33, 605–610. [Google Scholar] [CrossRef]
- Park, H.-M.; Lee, W.-K.; Park, C.-Y.; Cho, W.-J.; Ha, C.-S. Environmentally friendly polymer hybrids Part I Mechanical, thermal, and barrier properties of thermoplastic starch/clay nanocomposites. J. Mater. Sci. 2003, 38, 909–915. [Google Scholar] [CrossRef]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony count at 30 °C by the pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater Than 0.95. ISO: Geneva, Switzerland, 2008.
- Cesur, S.; Köroğlu, C.; Yalçın, H.T. Antimicrobial and biodegradable food packaging applications of polycaprolactone/organo nanoclay/chitosan polymeric composite films. J. Vinyl Addit. Technol. 2017, 24, 376–387. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Jayakumar, A.; Radoor, S.; C Nair, I.; Siengchin, S.; Parameswaranpillai, J.; E.K, R. Polyvinyl alcohol -nanocomposite films incorporated with clay nanoparticles and lipopeptides as active food wraps against food spoilage microbes. Food Packag. Shelf Life 2021, 30, 100727. [Google Scholar] [CrossRef]
- González, A.; Strumia, M.C.; Alvarez Igarzabal, C.I. Cross-linked soy protein as material for biodegradable films: Synthesis, characterization and biodegradation. J. Food Eng. 2011, 106, 331–338. [Google Scholar] [CrossRef]
- Dey Sadhu, S.; Soni, A.; Varmani, S.G.; Garg, M. Preparation of Starch-Poly Vinyl Alcohol (PVA) Blend Using Potato and Study of Its Mechanical Properties. Int. J. Pharm. Sci. Invent. ISSN 2014, 3, 33–37. [Google Scholar]




| Casein [g] | Starch [g] | Casein/Starch Ratio |
|---|---|---|
| 0.9 | 0 | 1:0 |
| 1.8 | 0.9 | 2:1 |
| 0.9 | 0.9 | 1:1 |
| 0.9 | 1.8 | 1:2 |
| 0 | 0.9 | 0:1 |
| Sample | Thickness (mm) | Tensile Strength, (MPa) | Tensile Strain, % | Young’s Modulus (MPa) | Reference |
|---|---|---|---|---|---|
| CAS/STA/GLY/PVA/BENT * | 0.09 ± 0.01 | 13.08 ± 2.11 | 109.30 ± 0.08 | 11.73 ± 0.96 | This work |
| CAS/GLY/PVA | - | 19 | 275 | - | [10] |
| CAS/STA/GLY/CLAY | 0.13 ± 0.003 | 9.92 ± 1.27 | 92.33 ± 19.21 | - | [50] |
| STA/GLY/PVA/CLAY | - | 12.41 ± 4.19 | 3.20 ± 0.81 | - | [83] |
| CAS/GLY/WPI | 0.19 ± 0.05 | 3.40 ± 0.59 | 55.30 ± 1.83 | 5.50 ± 1.13 | [84] |
| Sample | L* | a* | b* | C* | h° |
|---|---|---|---|---|---|
| CAS/STA/GLY/PVA | 39.74 ± 0.44 | 0.40 ± 0.10 | −2.76 ± 0.09 | 1.21 ± 1.77 | 282.932 ± 2.95 |
| CAS/STA/GLY/PVA/BENT | 38.94 ± 0.77 | 0.42 ± 0.18 | −1.12 ± 1.55 | 2.8 ± 0.08 | 278.65 ± 3.77 |
| Sample | Absorbance | %T | Thickness (mm) | Transparency | Opacity (AU nm) |
|---|---|---|---|---|---|
| CAS/STA/GLY/PVA | 0.25 | 60.206 | 0.1016 | 17.52 ± 0.77 | 134.48 ± 0.527 |
| CAS/STA/GLY/PVA/BENT | 0.36 | 44.370 | 0.1016 | 16.21 ± 0.434 | 181 ± 2.0 |
| SLOPE (g/s) | i (m) | A (m2) | ∆P (Pa) | WVP (g/m·s·Pa) |
|---|---|---|---|---|
| 6.00 × 10−4 | 1.02 × 10−4 | 3.14 × 10−4 | 2.49 × 103 | 8.23 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez Mesa, N.E.; Pataquiva-Mateus, A.Y.; Tang, Y. Exploring Biodegradable Polymeric Nanocomposite Films for Sustainable Food Packaging Application. Polymers 2025, 17, 2207. https://doi.org/10.3390/polym17162207
Gomez Mesa NE, Pataquiva-Mateus AY, Tang Y. Exploring Biodegradable Polymeric Nanocomposite Films for Sustainable Food Packaging Application. Polymers. 2025; 17(16):2207. https://doi.org/10.3390/polym17162207
Chicago/Turabian StyleGomez Mesa, Nikolay Estiven, Alis Yovana Pataquiva-Mateus, and Youhong Tang. 2025. "Exploring Biodegradable Polymeric Nanocomposite Films for Sustainable Food Packaging Application" Polymers 17, no. 16: 2207. https://doi.org/10.3390/polym17162207
APA StyleGomez Mesa, N. E., Pataquiva-Mateus, A. Y., & Tang, Y. (2025). Exploring Biodegradable Polymeric Nanocomposite Films for Sustainable Food Packaging Application. Polymers, 17(16), 2207. https://doi.org/10.3390/polym17162207







