Development and Characterization of Polymer Blends Based on Polyvinyl Alcohol for Application as Pharmaceutical Dosage Form
Abstract
1. Introduction
2. Materials and Methods
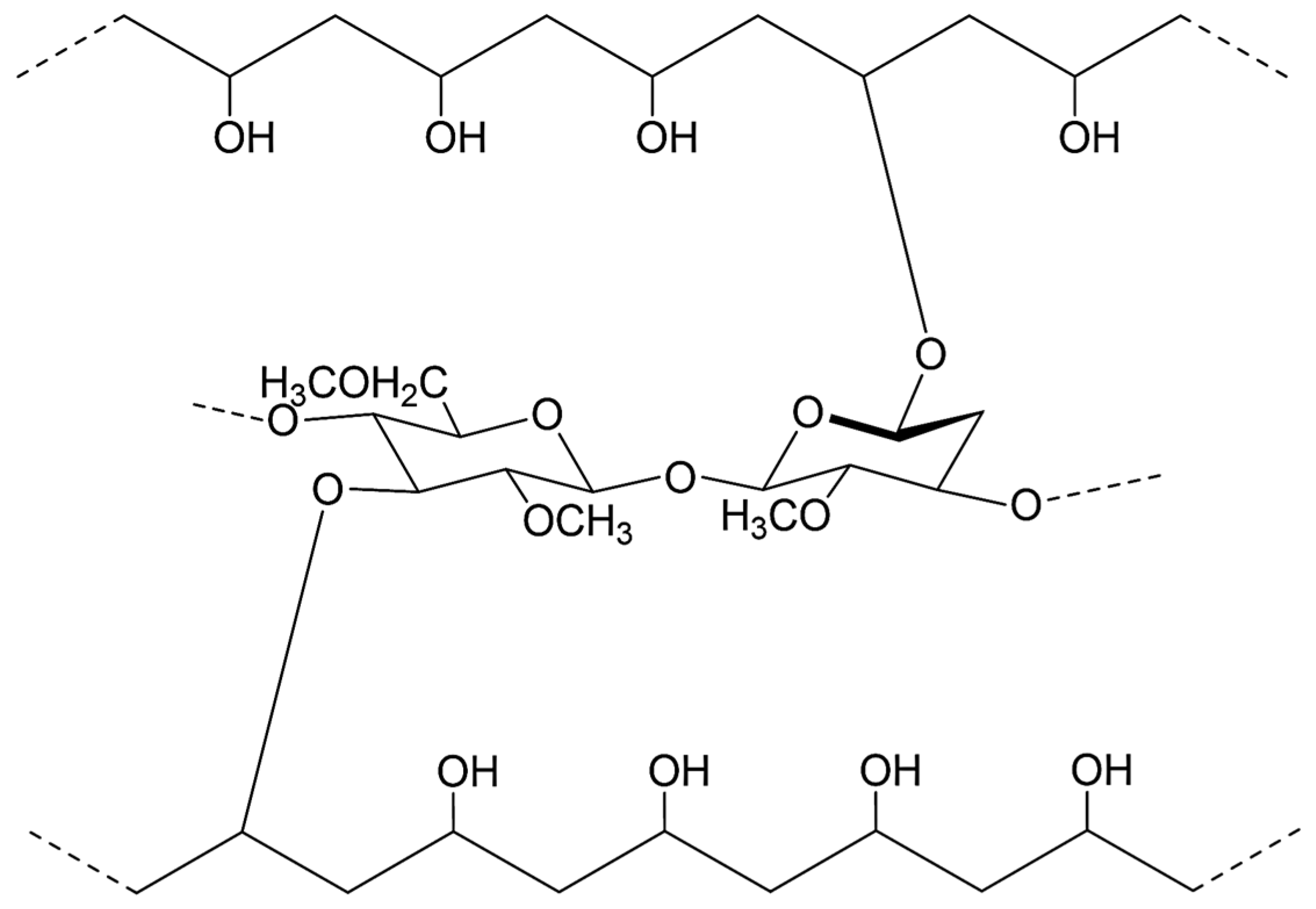
2.1. Characteristics of the Starting Materials
2.2. Preparation of PVA-MC-Based Mixtures for the Production of Radiation Cross-Linked Hydrogels
2.3. Physicochemical Research Methods
3. Results and Discussion
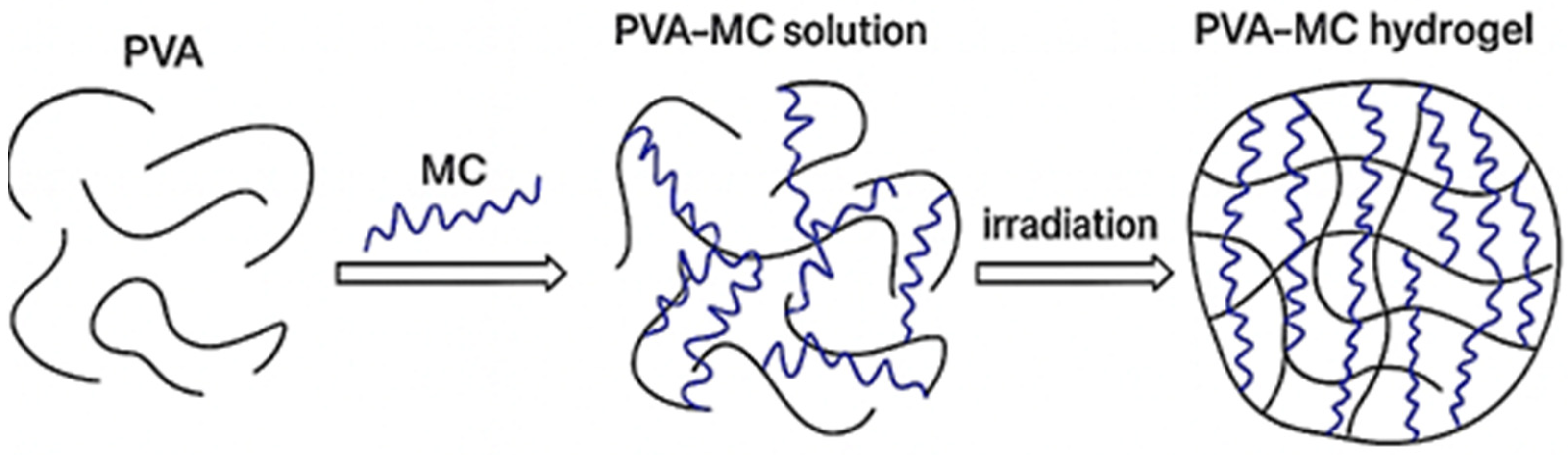
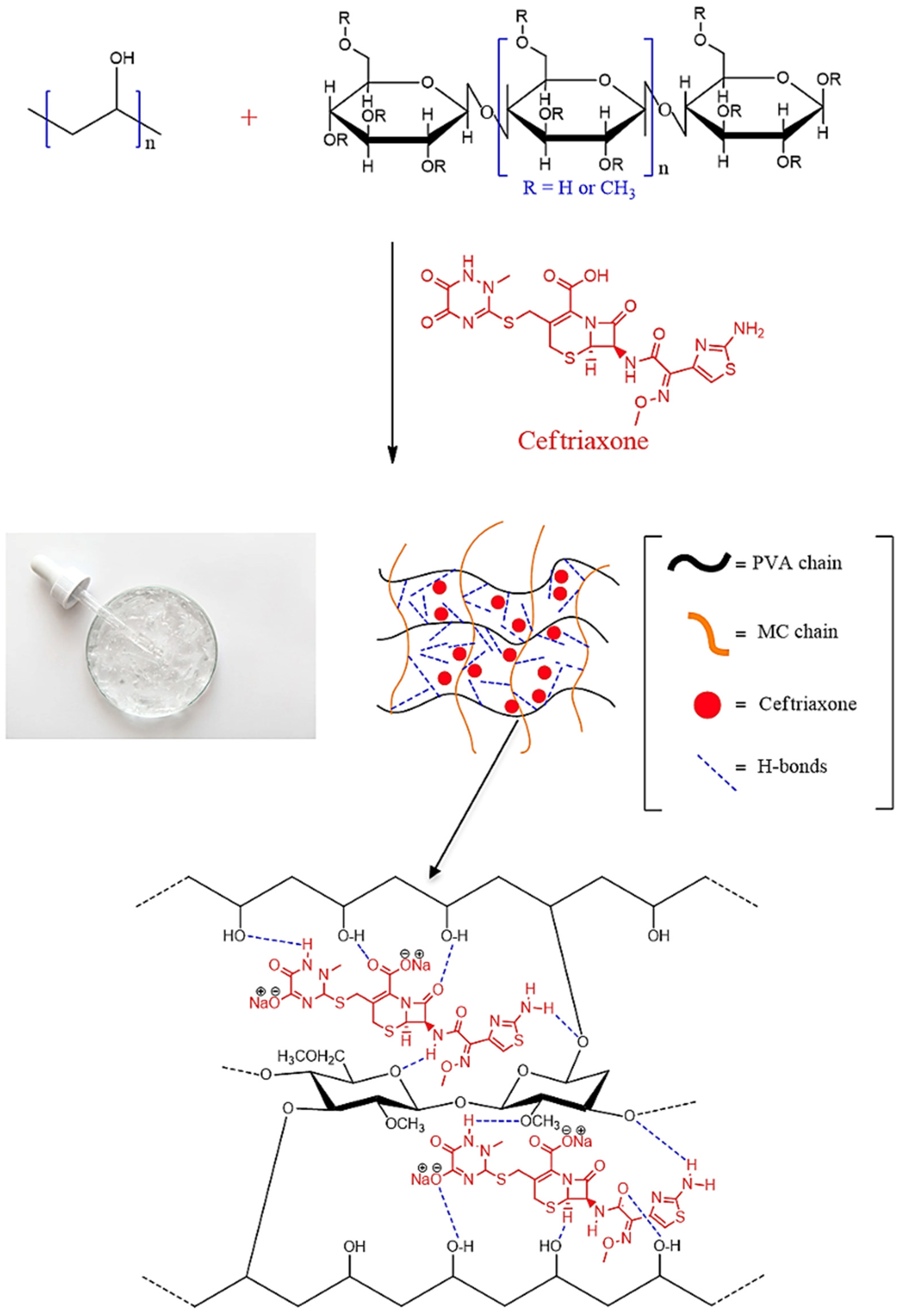
3.1. Preparation of Mixtures Based on PVA-MC
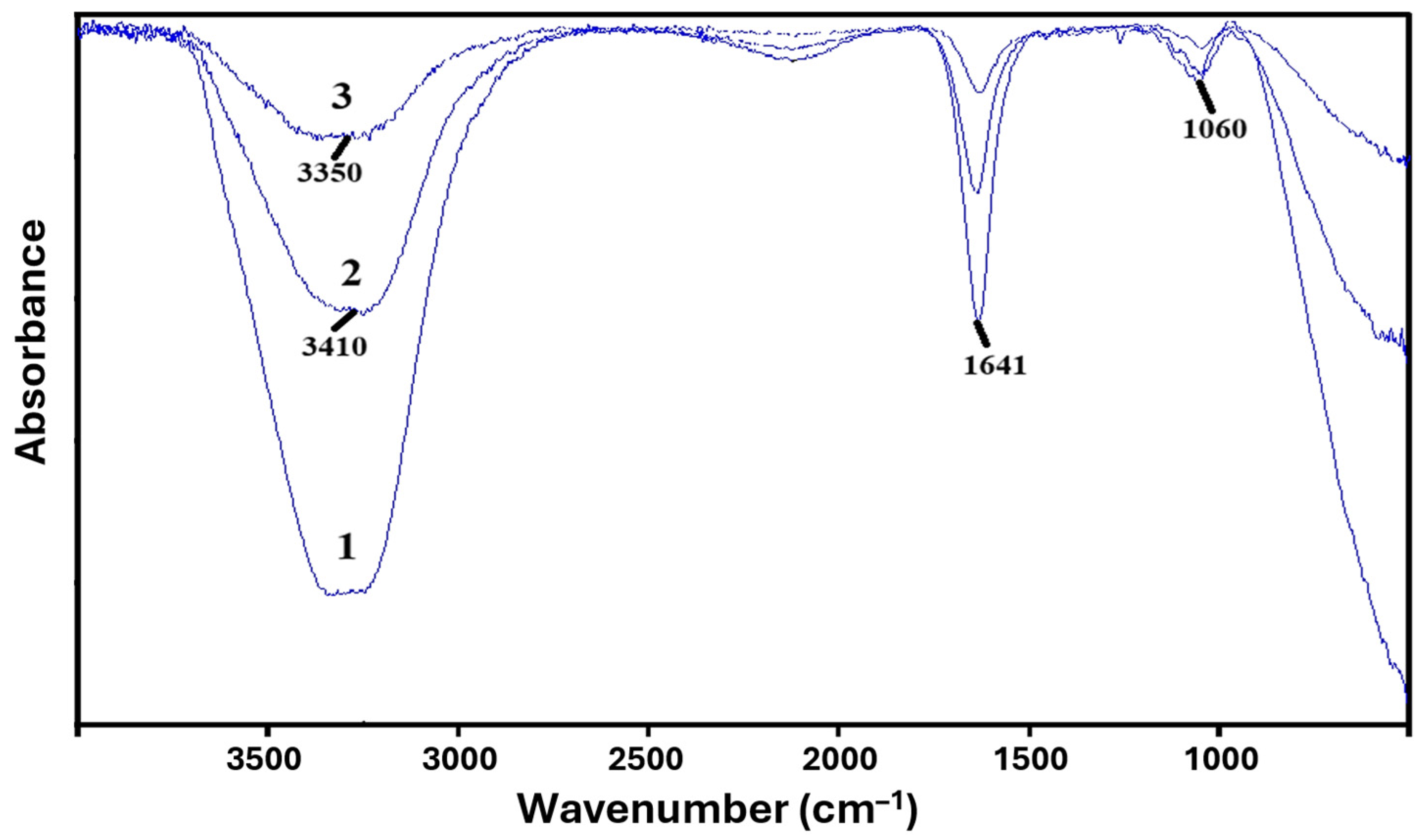
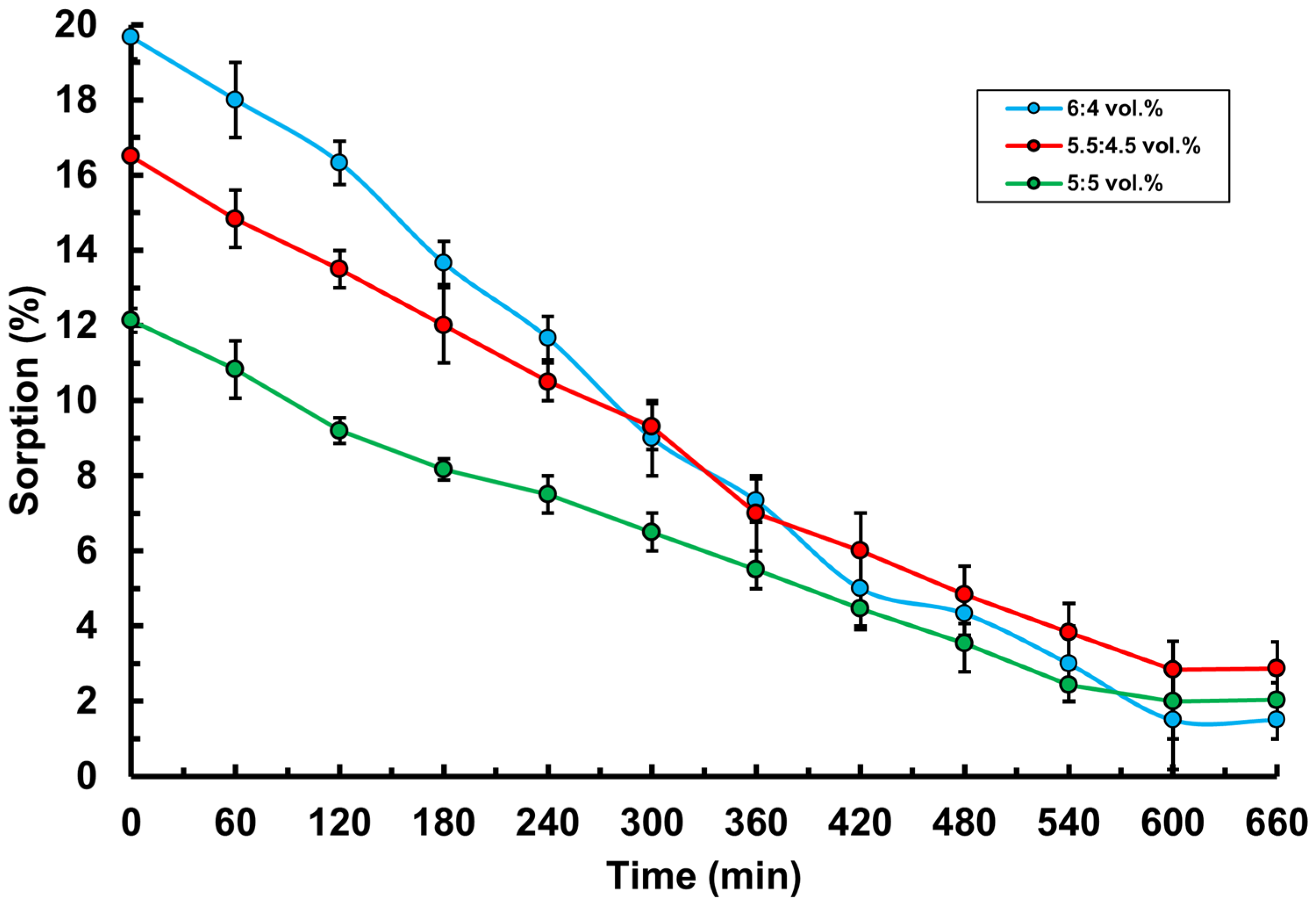
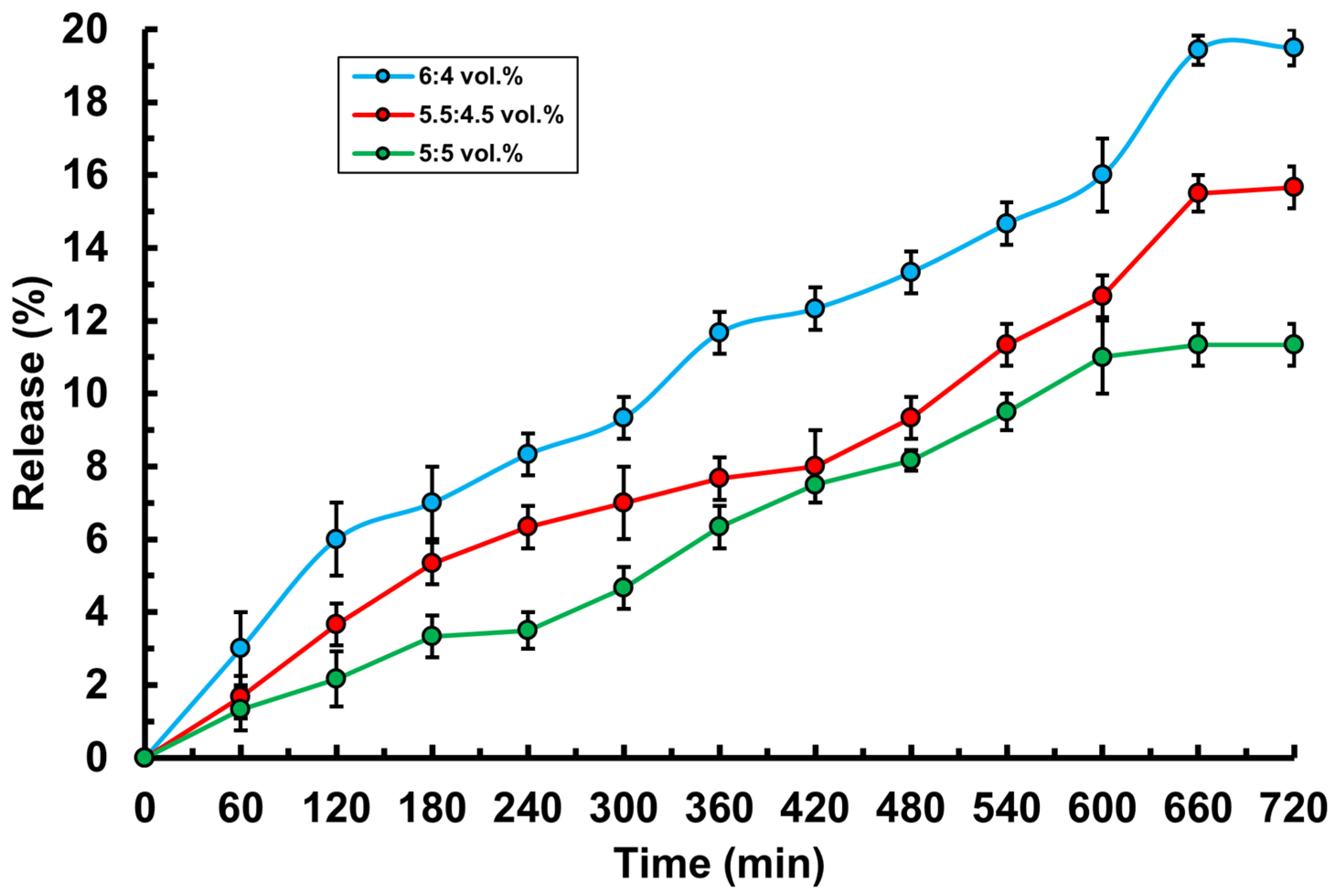
3.2. Study of Basic Physico-Chemical Properties of PVA-MC-Based Hydrogels
- Hydrogen bonding: Hydroxyl groups (–OH) in the structure of PVA and MC can form hydrogen bonds with the carboxyl (–COOH) and amide (–CONH2) groups of ceftriaxone.
- Van der Waals forces: Non-covalent intermolecular interactions arising from the interaction of dipole (or multipole) moments of molecules and the polarization of their electron clouds.
- Hydrophobic interactions: Between the non-polar parts of the ceftriaxone molecules and the polymer matrix.
3.3. Microbiological Testing of PVA-MC-Based Hydrogels Containing Ceftriaxone
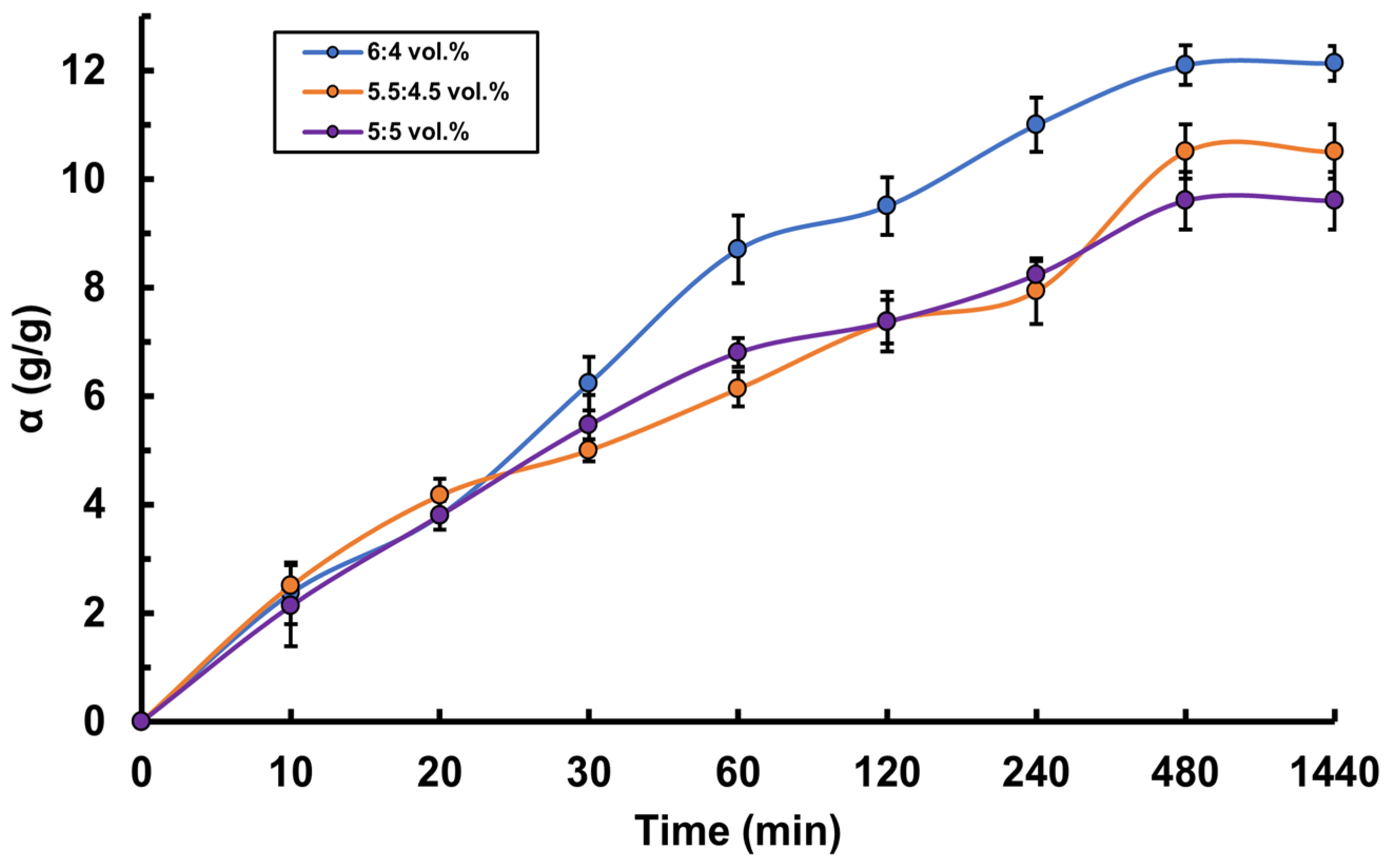
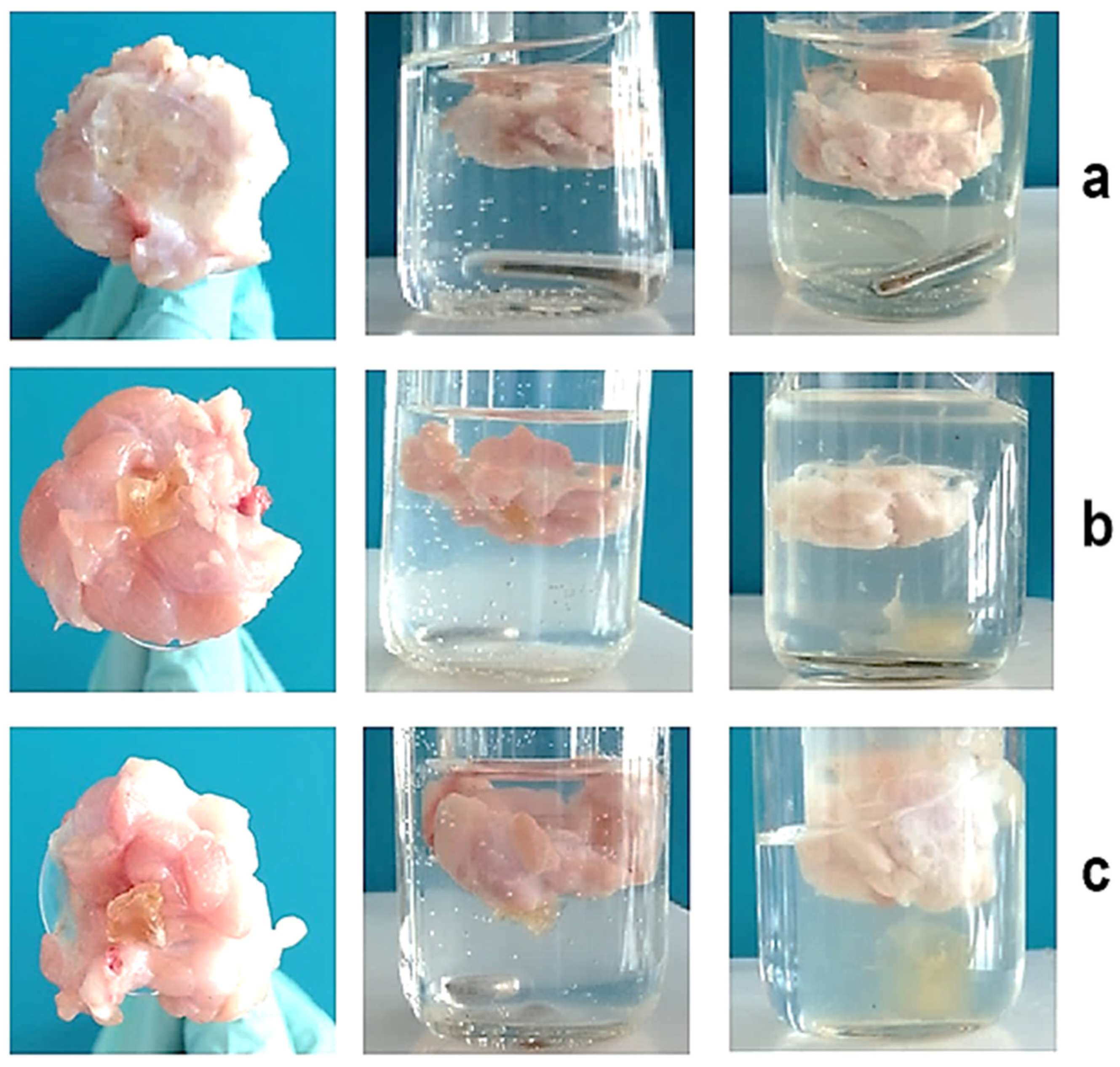
3.4. Determination of Mucoadhesive Properties of PVA-MC-Based Hydrogels Containing Ceftriaxone
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rosiak, J.M.; Yoshii, F. Hydrogels and their medical applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1999, 151, 56–64. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Li, M.; Yin, Z.; Butt, H.A. The synthesis, mechanisms, and additives for bio-compatible polyvinyl alcohol hydrogels: A review on current advances, trends, and future outlook. J. Vinyl Addit. Technol. 2022, 29, 939–959. [Google Scholar] [CrossRef]
- Razzak, M.T.; Darwis, D.; Zainuddin; Sukirno. Irradiation of polyvinyl alcohol and polyvinyl pyrrolidone blended hydrogel for wound dressing. Radiat. Phys. Chem. 2001, 62, 107–113. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 1, 2225426. [Google Scholar] [CrossRef]
- Sun, Z.; Song, C.; Wang, C.; Hu, Y.; Wu, J. Hydrogel-based controlled drug delivery for cancer treatment: A review. Mol. Pharm. 2020, 17, 373–391. [Google Scholar] [CrossRef]
- Husain, M.S.B.; Gupta, A.; Alashwal, B.Y.; Sharma, S. Synthesis of PVA/PVP based hydrogel for biomedical applications: A review. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 2388–2393. [Google Scholar] [CrossRef]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and characterization of PVA/starch hydrogel membranes incorporating essential oils aimed to be used in wound dressing applications. J. Polym. Environ. 2020, 29, 156–174. [Google Scholar] [CrossRef]
- Chowdhury, M.N.K.; Alam, A.K.M.M.; Dafader, N.C.; Haque, M.E.; Akhtar, F.; Ahmed, M.U.; Rashid, H.; Begum, R. Radiation processed hydrogel of poly(vinyl alcohol) with biodegradable polysaccharides. Biomed. Mater. Eng. 2006, 16, 223–228. Available online: https://pubmed.ncbi.nlm.nih.gov/16518021/ (accessed on 7 August 2025).
- Wang, Z.; Han, X.; Wang, Y.; Men, K.; Cui, L.; Wu, J.; Meng, G.; Liu, Z.; Guo, X. Facile preparation of low swelling, high strength, self-healing and pH-responsive hydrogels based on the triple-network structure. Front. Mater. Sci. 2019, 13, 54–63. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Nho, Y.C.; Park, K.R. Preparation and properties of PVA/PVP hydrogels containing chitosan by radiation. J. Appl. Polym. Sci. 2002, 85, 1787–1794. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Mallaiah, S.H. Swelling and mechanical properties of radiation crosslinked Au/PVA hydrogel nanocomposites. Radiat. Eff. Defects Solids 2016, 171, 869–878. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. In Biopolymers · PVA Hydrogels, Anionic Polymerisation Nanocomposites; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2000; Volume 153, pp. 37–65. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Kopeček, J. Smart and genetically engineered biomaterials and drug delivery systems. Eur. J. Pharm. Sci. 2003, 20, 1–16. [Google Scholar] [CrossRef]
- Das, S.; Subuddhi, U. Controlled delivery of ibuprofen from poly(vinyl alcohol)-poly(ethylene glycol) interpenetrating polymeric network hydrogels. J. Pharm. Anal. 2019, 9, 108–116. [Google Scholar] [CrossRef]
- Yoshimatsu, G.; Sakata, N.; Tsuchiya, H.; Ishida, M.; Motoi, F.; Egawa, S.; Sumi, S.; Goto, M.; Unno, M. Development of polyvinyl alcohol bioartificial pancreas with rat islets and mesenchymal stem cells. Transplant. Proc. 2013, 45, 1875–1880. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hyu, H.S. Development and evaluation of polyvinyl alcohol-hydrogels as an artificial atrticular cartilage for orthopedic implants. Materials 2010, 3, 2753. [Google Scholar] [CrossRef]
- de Oliveira, P.P.M.; Bavaresco, V.P.; Silveira-Filho, L.M.; Schenka, A.A.; Vilarinho, K.A.d.S.; Severino, E.S.B.d.O.; Petrucci, O. Use of a novel polyvinyl alcohol membrane as a pericardial substitute reduces adhesion formation and inflammatory response after cardiac reoperation. J. Thorac. Cardiovasc. Surg. 2014, 147, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Sumi, S.; Yanai, G.; Qi, M.; Sakata, N.; Qi, Z.; Yang, K.; Shirouzu, Y.; Hiura, A.; Gu, Y.; Inoue, K. Review: Macro-encapsulation of islets in polyvinyl alcohol hydrogel. J. Med. Biol. Eng. 2014, 34, 204–210. [Google Scholar] [CrossRef]
- Lungu, R.; Paun, M.-A.; Peptanariu, D.; Ailincai, D.; Marin, L.; Nichita, M.-V.; Paun, V.-A.; Paun, V.-P. Biocompatible chitosan-based hydrogels for bioabsorbable wound dressings. Gels 2022, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, J.; Zhou, H.; Jiang, X.; Yang, C.; Wang, F.; Pan, Y.; Li, N.; Li, X.; Shi, L.; et al. Morphological and swelling behavior of cellulose nanofiber (CNF)/poly(vinyl alcohol) (PVA) hydrogels: Poly(ethylene glycol) (PEG) as porogen. RSC Adv. 2016, 6, 43626–43633. [Google Scholar] [CrossRef]
- Popescu, I.; Constantin, M.; Solcan, G.; Ichim, D.L.; Rata, D.M.; Horodincu, L.; Solcan, C. Composite hydrogels with embedded silver nanoparticles and ibuprofen as wound dressing. Gels 2023, 9, 654. [Google Scholar] [CrossRef]
- Santos, A.M.N.; Moreira, A.P.D.; Carvalho, C.W.P.; Luchese, R.; Ribeiro, E.; McGuinness, G.B.; Mendes, M.F.; Oliveira, R.N. Physically cross-linked gels of PVA with natural polymers as matrices for manuka honey release in wound-care applications. Materials 2019, 12, 559. [Google Scholar] [CrossRef]
- Islam, A.; Yasin, T.; Bano, I.; Riaz, M. Controlled release of aspirin from pH-sensitive chitosan/poly(vinyl alcohol) hydrogel. J. Appl. Polym. Sci. 2012, 124, 4184–4192. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, X.-L.; Li, Z.-H.; Zhu, Z.-G.; Qian, S.-H.; Flewitt, A.J. Current and emerging technology for continuous glucose monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef]
- Cai, Y.; Che, J.; Yuan, M.; Shi, X.; Chen, W.; Yuan, W.-E. Effect of glycerol on sustained insulin release from PVA hydrogels and its application in diabetes therapy. Exp. Ther. Med. 2016, 12, 2039–2044. [Google Scholar] [CrossRef][Green Version]
- Chen, G.; Tang, W.; Wang, X.; Zhao, X.; Chen, C.; Zhu, Z. Applications of hydrogels with special physical properties in biomedicine. Polymers 2019, 11, 1420. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, Z.; Jin, Y.; Zhu, B.; Xing, J.; Ma, G.; Xiang, X.; Cai, W. Novel in vitro dynamic metabolic system for predicting the human pharmacokinetics of tolbutamide. Acta Pharmacol. Sin. 2018, 39, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Sullad, A.G.; Manjeshwar, L.S.; Aminabhavi, T.M.; Naik, P.N. Microspheres of poly(vinyl alcohol) and methyl cellulose for the controlled release of losartan potassium and clopidogrel bisulphate. Am. J. Adv. Drug Deliv. 2014, 2, 407–423. [Google Scholar]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A.P. Characterization of poly(vinyl alcohol)/poly(ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Mansur, H.S.; Oréfice, R.; Pereira, M.M.; Lobato, Z.; Vasconcelos, W.L.; Machado, L. FTIR and UV-vis study of chemically engineered biomaterial surfaces for protein immobilization. Spectroscopy 2002, 16, 351–360. [Google Scholar] [CrossRef]
- Călina, I.; Demeter, M.; Scărișoreanu, A.; Sătulu, V.; Mitu, B. One step e-beam radiation cross-linking of quaternary hydrogels dressings based on chitosan-poly(vinyl-pyrrolidone)-poly(ethylene glycol)-poly(acrylic acid). Int. J. Mol. Sci. 2020, 21, 9236. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Bock, N.; Dargaville, B.L.; Hutmacher, D.W. Deciphering the molecular mechanism of water interaction with gelatin methacryloyl hydrogels: Role of ionic strength, pH, drug loading and hydrogel network characteristics. Biomedicines 2021, 9, 574. [Google Scholar] [CrossRef]
- Bayat, M.R.; Baghani, M. A review on swelling theories of pH-sensitive hydrogels. J. Intell. Mater. Syst. Struct. 2021, 32, 2349–2365. [Google Scholar] [CrossRef]
- FitzSimons, T.M.; Anslyn, E.V.; Rosales, A.M. Effect of pH on the properties of hydrogels cross-linked via dynamic thia-Michael addition bonds. ACS Polym. Au 2022, 2, 129–136. [Google Scholar] [CrossRef]



| Sample Designation | Inhibition Zones, mm | |
|---|---|---|
| S. aureus | E. carotovora | |
| φPVA:φMC/ceftriaxone = 6:4 vol.% | 19 | 25 |
| φPVA:φMC/ceftriaxone = 5.5:4.5 vol.% | 15 | 20 |
| φPVA:φMC/ceftriaxone = 5:5 vol.% | 4 | 5 |
| φPVA:φMC = 6:4 vol.% (without drug) | 0 | 0 |
| Metrogyl Denta 1% (control) | 5 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenessova, Z.A.; Mun, G.A.; Urkimbayeva, P.I.; Toktabayeva, A.K.; Rakhmetullayeva, R.K.; Yermukhambetova, B.B.; Kenzhebai, Z.; Kurmanova, Z.T.; Yermaganbetov, M.; Alikulov, A.Z. Development and Characterization of Polymer Blends Based on Polyvinyl Alcohol for Application as Pharmaceutical Dosage Form. Polymers 2025, 17, 2203. https://doi.org/10.3390/polym17162203
Kenessova ZA, Mun GA, Urkimbayeva PI, Toktabayeva AK, Rakhmetullayeva RK, Yermukhambetova BB, Kenzhebai Z, Kurmanova ZT, Yermaganbetov M, Alikulov AZ. Development and Characterization of Polymer Blends Based on Polyvinyl Alcohol for Application as Pharmaceutical Dosage Form. Polymers. 2025; 17(16):2203. https://doi.org/10.3390/polym17162203
Chicago/Turabian StyleKenessova, Zarina A., Grigoriy A. Mun, Perizat I. Urkimbayeva, Assel K. Toktabayeva, Raikhan K. Rakhmetullayeva, Bayana B. Yermukhambetova, Zhazira Kenzhebai, Zhuldyzay T. Kurmanova, Mubarak Yermaganbetov, and Adilet Zh. Alikulov. 2025. "Development and Characterization of Polymer Blends Based on Polyvinyl Alcohol for Application as Pharmaceutical Dosage Form" Polymers 17, no. 16: 2203. https://doi.org/10.3390/polym17162203
APA StyleKenessova, Z. A., Mun, G. A., Urkimbayeva, P. I., Toktabayeva, A. K., Rakhmetullayeva, R. K., Yermukhambetova, B. B., Kenzhebai, Z., Kurmanova, Z. T., Yermaganbetov, M., & Alikulov, A. Z. (2025). Development and Characterization of Polymer Blends Based on Polyvinyl Alcohol for Application as Pharmaceutical Dosage Form. Polymers, 17(16), 2203. https://doi.org/10.3390/polym17162203







