Development of Gelatin/Polyvinyl Alcohol Films Incorporated with Blueberry Extracts for Freshness Detection of Shrimp
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Aqueous Concentrate
2.2.2. Purification of Aqueous Concentrated Blueberry Extracts
2.2.3. Quantification of Phenolic Compounds
2.2.4. Quantification of Total Anthocyanins
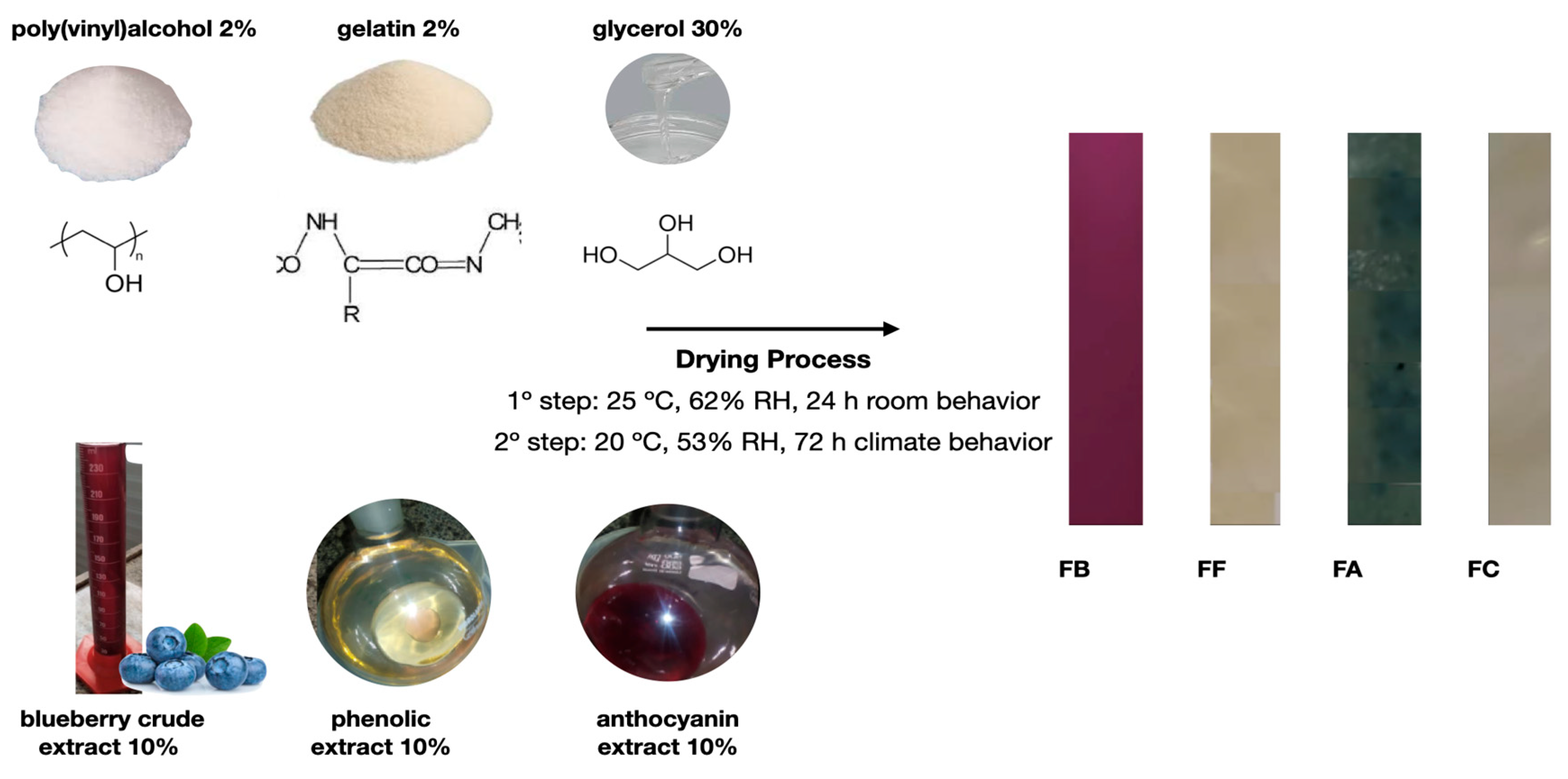
2.2.5. Production of Colorimetric Indicators (Polymer Blend Based on PVA and GL with Blueberry Extract)
2.2.6. Experimental Design
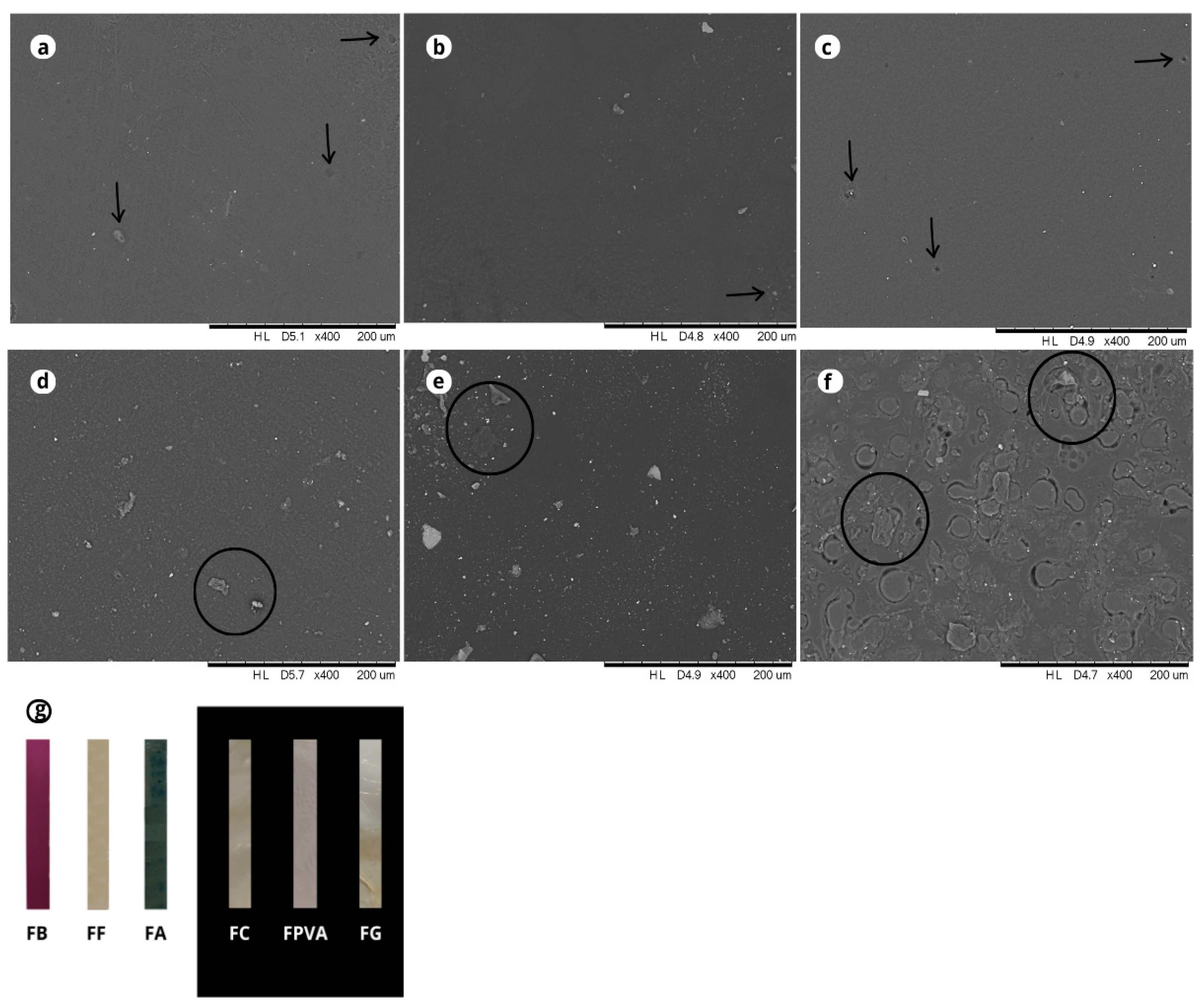
2.2.7. Microstructural Characteristics
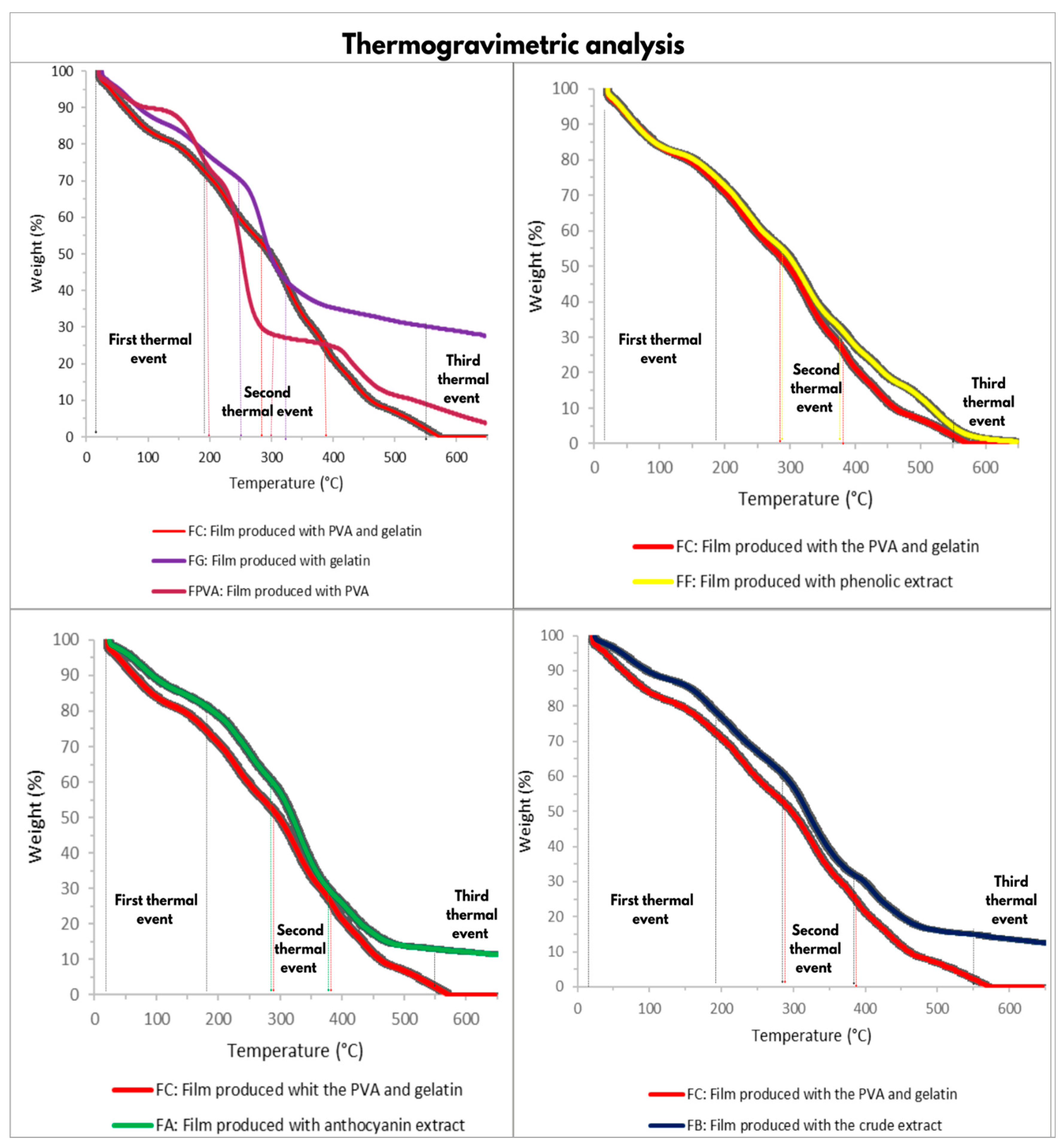
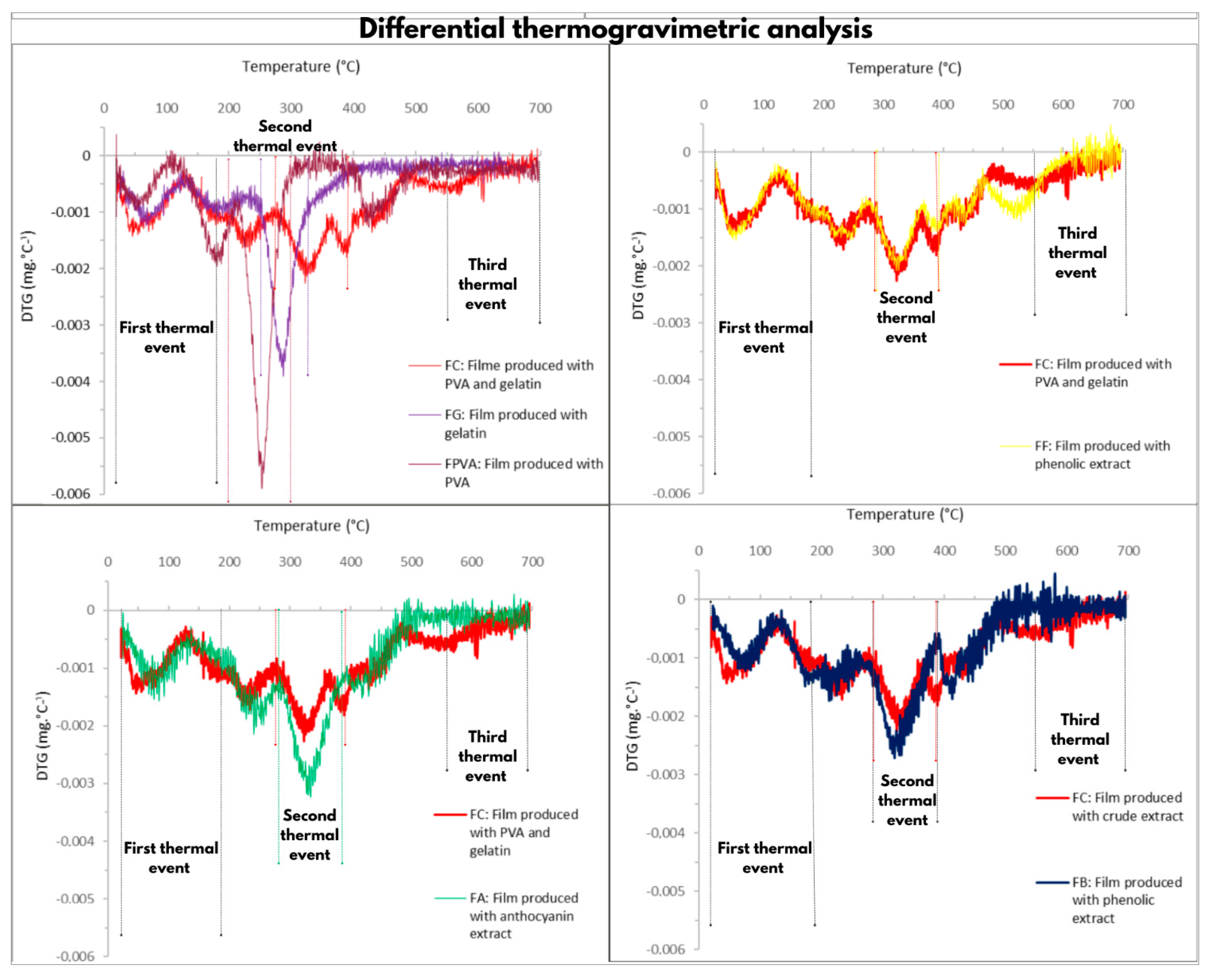
2.2.8. Thermogravimetric Analysis
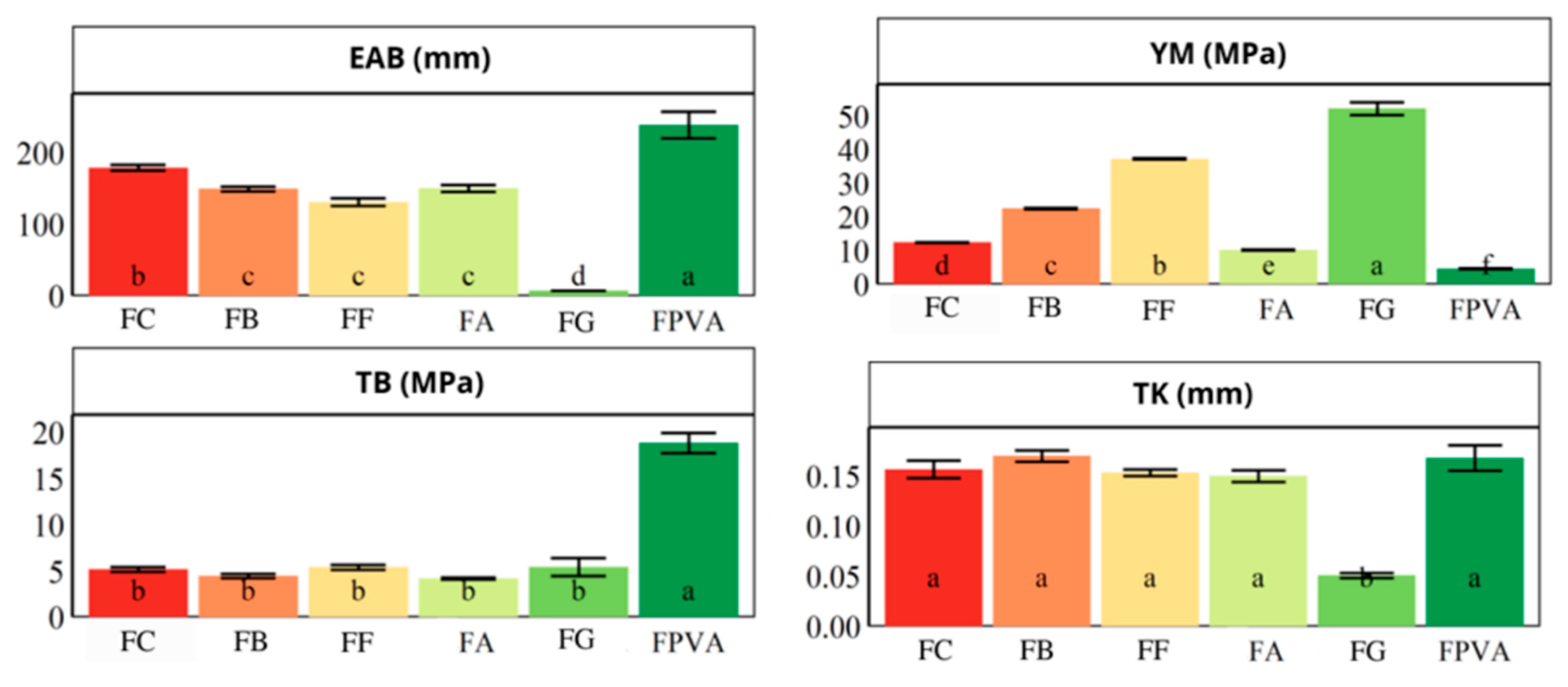
2.2.9. Thickness and Mechanical Properties
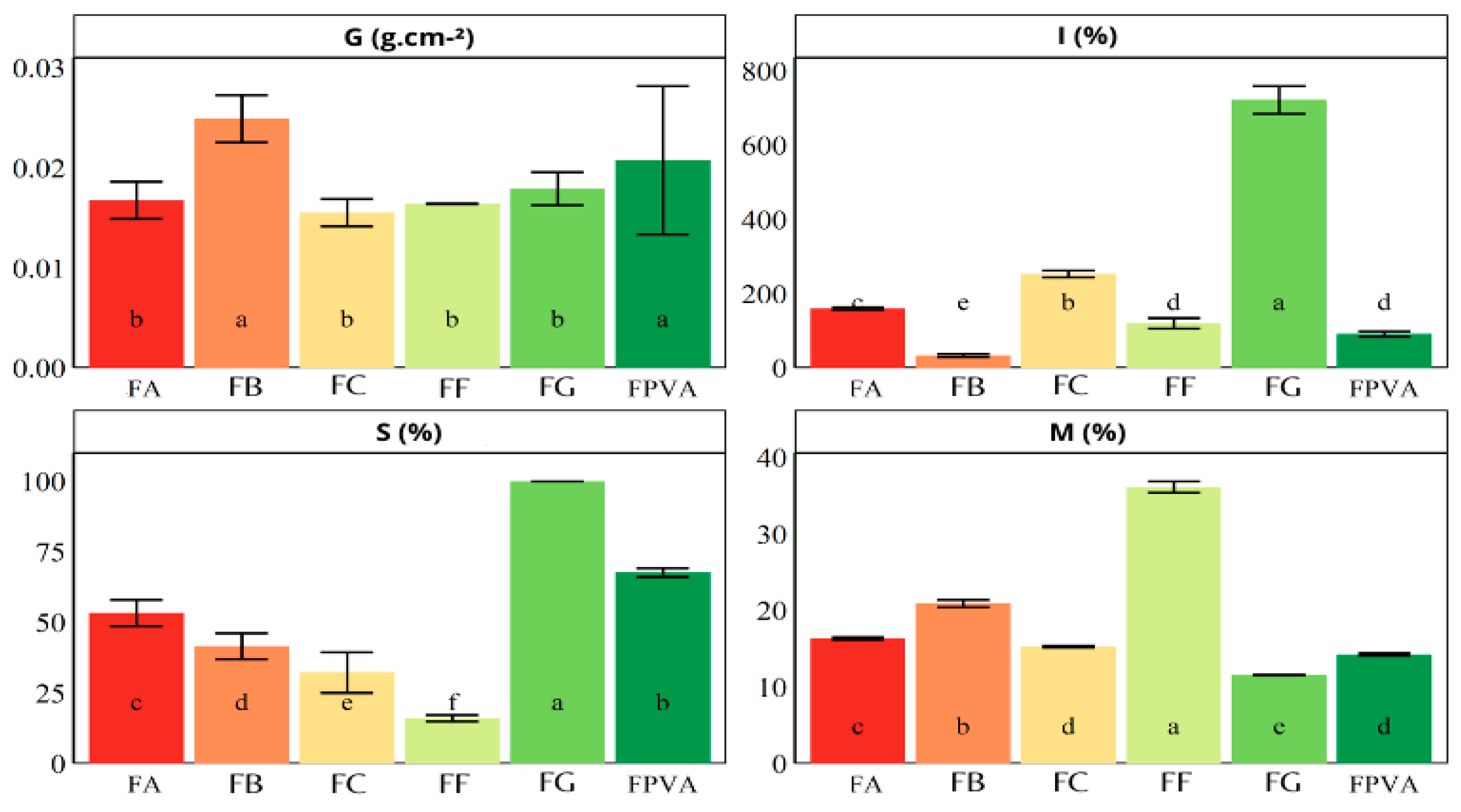
2.2.10. Grammage
2.2.11. Moisture, Swelling Index, and Solubility
2.2.12. Application and Evaluation of the Colorimetric Sensitivity of Indicators in Shrimp
2.2.13. pH
2.2.14. Monitoring Shrimp Degradation
2.2.15. Statistical Analysis
3. Results and Discussion
3.1. Bioactive Compounds from Blueberry Extracts
3.2. Scanning Electron Microscopy
3.3. Thermogravimetric Analyses
3.4. Thicknesses and Mechanical Properties
3.5. Physical and Chemical Properties
3.6. Evaluation of the Colourimetric Sensitivity of Indicators in Shrimp
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| EB | Elongation at break |
| EDS | Energy dispersive spectroscopy |
| FA film | FPVA:GL incorporated with blueberry anthocyanin extract |
| FC film | Film produced with GLY, PVA and GL |
| FB film | FPVA:GL incorporated with crude blueberry extract |
| FG film | Film produced with GLY and GL |
| FF film | FPVA:GL incorporated with phenolic blueberry extract |
| FPVA film | Film produced with GLY and PVA. |
| G | Grammage |
| GL | Gelatin |
| GLY | Glycerol |
| I | Swelling index |
| M | Moisture |
| PVA | Polyvinyl alcohol |
| RH | Relative humidity |
| SEM | Scanning electron microscope |
| S | Solubility |
| TGA | Thermogravimetric analysis |
| TK | Thickness |
| TS | Tensile strength |
| YM | Young’s modulus |
References
- Bai, X.; Zhou, L.; Zhou, L.; Cang, S.; Liu, Y.; Liu, R.; Liu, J.; Feng, X.; Fan, R. The Research Progress of Extraction, Purification and Analysis Methods of Phenolic Compounds from Blueberry: A Comprehensive Review. Molecules 2023, 28, 3610. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; Santisteban, A.; Gordillo, B.; Hernanz, D.; Heredia, F.J.; Escudero-Gilete, M.L. Comparative Study of Red Berry Pomaces (Blueberry, Red Raspberry, Red Currant and Blackberry) as Source of Antioxidants and Pigments. Eur. Food Res. Technol. 2019, 245, 1–9. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Vaccinium myrtillus, L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications-a Review. Curr. Pharm. Des. 2020, 26, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.T.; Borges, L.L.R.; Costa, N.M.E.P.d.L.d.; Arruda, T.R.; Ribeiro, A.R.C.; Marques, C.S.; Stringheta, P.C.; de Oliveira, T.V.; Soares, N.d.F.F. Gelatin/Polyvinyl Alcohol Films Incorporated with Different Blueberry Extracts as Potential Colorimetric Indicators to Detect Acidic and Basic Vapors. Food Control 2024, 165, 110648. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, Isolation, and Purification of Anthocyanins. Curr. Protoc. Food Anal. Chem. 2001. [Google Scholar] [CrossRef]
- Coklar, H.; Akbulut, M. Anthocyanins and Phenolic Compounds of Mahonia Aquifolium Berries and Their Contributions to Antioxidant Activity. J. Funct. Foods 2017, 35, 166–174. [Google Scholar] [CrossRef]
- Ma, Q.; Du, L.; Wang, L. Tara Gum/Polyvinyl Alcohol-Based Colorimetric NH3 Indicator Films Incorporating Curcumin for Intelligent Packaging. Sens. Actuators B Chem. 2017, 244, 759–766. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of Antioxidant and Intelligent PH-Sensing Packaging Films by Incorporating Purple-Fleshed Sweet Potato Extract into Chitosan Matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, T.; Cao, L.; Wang, L. Intelligent Poly (Vinyl Alcohol)-Chitosan Nanoparticles-Mulberry Extracts Films Capable of Monitoring PH Variations. Int. J. Biol. Macromol. 2018, 108, 576–584. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, X.; Qin, Y.-R.; Zhang, Y.-H.; Wang, X.-P.; Wang, J.-Y.; Ning, Z.-X.; Ruan, Q.-J.; Zhang, Y.-S. Preparation and Characterization of a Novel Colorimetric Indicator Film Based on Gelatin/Polyvinyl Alcohol Incorporating Mulberry Anthocyanin Extracts for Monitoring Fish Freshness. Food Res. Int. 2019, 126, 108604. [Google Scholar] [CrossRef]
- Djagny, K.B.; Wang, Z.; Xu, S. Gelatin: A Valuable Protein for Food and Pharmaceutical Industries. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Estevez, I.; Cruz, L.; Oliveira, J.; Mateus, N.; de Freitas, V.; Soares, S. First Evidences of Interaction between Pyranoanthocyanins and Salivary Proline-Rich Proteins. Food Chem. 2017, 228, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.D.; Bertoldi, M.C.; Krenek, K.; Talcott, S.T.; Stringheta, P.C.; Mertens-Talcott, S.U. Anticarcinogenic Effects of Polyphenolics from Mango (Mangifera indica) Varieties. J. Agric. Food Chem. 2010, 58, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. I. Extraction and determination of total anthocyanin in cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Vieira, M.E.M.; e Silva, M.L.S.; de Oliveira, L.F.C.; Perrone, Í.T.; Stephani, R. Espectroscopia de Energia Dispersiva de Raios-X (EDS) Acoplada Ao Microscópio Eletrônico de Varredura (MEV): Fundamentos e Aplicações Em Produtos Lácteos. Res. Soc. Dev. 2021, 10, e262101018622. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: West Conshohocken, PA, USA, 1995.
- Tian, B.; Li, W.; Wang, J.; Liu, Y. Functional Polysaccharide-Based Film Prepared from Chitosan and β-Acids: Structural, Physicochemical, and Bioactive Properties. Int. J. Biol. Macromol. 2021, 181, 966–977. [Google Scholar] [CrossRef]
- Erfiza, N.M.; Purba, N.R.; Ahda, K.; Sulaiman, I.; Rohaya, S.; Razi, F. Characterization of Tannin Based Colorimetric Indicator and Its Application on Fish Packaging. Proc. IOP Conf. Ser. Earth Environ. Sci. 2021, 922, 012057. [Google Scholar] [CrossRef]
- Jebel, F.S.; Roufegarinejad, L.; Alizadeh, A.; Amjadi, S. Development and Characterization of a Double-Layer Smart Packaging System Consisting of Polyvinyl Alcohol Electrospun Nanofibers and Gelatin Film for Fish Fillet. Food Chem. 2025, 462, 140985. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz. Métodos Físicos-Quimicos Para Análise de Alimentos, 4th ed.; Secretaria de Estado da Saúde: São Paulo, SP, Brazil, 2008.
- Teixeira, S.C.; Silva, R.R.A.; de Oliveira, T.V.; Stringheta, P.C.; Pinto, M.; Soares, N.d.F.F. Glycerol and Triethyl Citrate Plasticizer Effects on Molecular, Thermal, Mechanical, and Barrier Properties of Cellulose Acetate Films. Food Biosci. 2021, 42, 101202. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking Color and Pigment Changes in Anthocyanin Products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a Colorimetric PH Indicator Based on Bacterial Cellulose Nanofibers and Red Cabbage (Brassica oleraceae) Extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.; Khalid, S.H.; Ullah Khan, I.; Chauhdary, Z.; Mahmood, H.; Saleem, A.; Umair, M.; Asghar, S. Curcumin-Loaded Bioactive Polymer Composite Film of Pva/Gelatin/Tannic Acid Downregulates the pro-Inflammatory Cytokines to Expedite Healing of Full-Thickness Wounds. ACS Omega 2023, 8, 7575–7586. [Google Scholar] [CrossRef] [PubMed]
- Tymczewska, A.; Furtado, B.U.; Nowaczyk, J.; Hrynkiewicz, K.; Szydłowska-Czerniak, A. Functional Properties of Gelatin/Polyvinyl Alcohol Films Containing Black Cumin Cake Extract and Zinc Oxide Nanoparticles Produced via Casting Technique. Int. J. Mol. Sci. 2022, 23, 2734. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Roy, S.; Rhim, J.-W. Gelatin/Agar-Based Color-Indicator Film Integrated with Clitoria Ternatea Flower Anthocyanin and Zinc Oxide Nanoparticles for Monitoring Freshness of Shrimp. Food Hydrocoll. 2022, 124, 107294. [Google Scholar] [CrossRef]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and Degradability Properties of Polyvinyl Alcohol/Gelatin Nanocomposite Films Filled Water Hyacinth Cellulose Nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-Starch Films with Natural Extracts: Physical, Chemical, Morphological and Thermal Properties. Materials 2018, 11, 120. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Silva, R.R.A.; de Oliveira, T.V.; Soares, R.R.A.; Junior, N.S.; Moraes, A.R.F.; Pires, A.C.d.S.; Soares, N.F.F. Development and Characterization of Intelligent Cellulose Acetate-Based Films Using Red Cabbage Extract for Visual Detection of Volatile Bases. Lebensm.-Wiss. Technol. 2020, 132, 109780. [Google Scholar] [CrossRef]
- Qiao, D.; Huang, Y.; Hou, X.; Ye, F.; Wu, K.; Jiang, F.; Zhao, G.; Zhang, B.; Xie, F. Enhancing Thermal Stability and Mechanical Resilience in Gelatin/Starch Composites through Polyvinyl Alcohol Integration. Carbohydr. Polym. 2024, 344, 122528. [Google Scholar] [CrossRef]
- Basumatary, K.; Daimary, P.; Das, S.K.; Thapa, M.; Singh, M.; Mukherjee, A.; Kumar, S. Lagerstroemia Speciosa Fruit-Mediated Synthesis of Silver Nanoparticles and Its Application as Filler in Agar Based Nanocomposite Films for Antimicrobial Food Packaging. Food Packag. Shelf Life 2018, 17, 99–106. [Google Scholar] [CrossRef]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.-T.; Dias, A.R.G.; da Rosa Zavareze, E. PH-Sensitive Films Containing Anthocyanins Extracted from Black Bean Seed Coat and Red Cabbage. Lebensm.-Wiss. Technol. 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Oliveira, T.V.d.; Batista, L.F.; Silva, R.R.A.; Lopes, M.d.P.; Ribeiro, A.R.C.; Rigolon, T.C.B.; Stringheta, P.C.; Soares, N.d.F.F. Anthocyanins of Açaí Applied as a Colorimetric Indicator of Milk Spoilage: A Study Using Agar-Agar and Cellulose Acetate as Solid Support to Be Applied in Packaging. Polysaccharides 2022, 3, 715–727. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Braga, A.R.C.; de Oliveira, B.R.; Gomes, F.P.; Moreira, V.L.; Pereira, V.A.C.; Egea, M.B. The Potential of Anthocyanins in Smart, Active, and Bioactive Eco-Friendly Polymer-Based Films: A Review. Food Res. Int. 2021, 142, 110202. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Chathuranga, K.; Lee, J.S.; Park, W.H. Effects of Polyphenols on the Thermal Decomposition, Antioxidative, and Antimicrobial Properties of Poly (Vinyl Alcohol) and Poly (Vinyl Pyrrolidone). Polym. Test. 2022, 116, 107786. [Google Scholar] [CrossRef]
- Haghighi, H.; Gullo, M.; La China, S.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Characterization of Bio-Nanocomposite Films Based on Gelatin/Polyvinyl Alcohol Blend Reinforced with Bacterial Cellulose Nanowhiskers for Food Packaging Applications. Food Hydrocoll. 2021, 113, 106454. [Google Scholar] [CrossRef]
- Dong, F.; Dong, Z.; Mao, L.; Yao, J.; Wang, C. Development of Crosslinked Gelatin Films through Maillard Reaction and Reinforced with Poly(Vinyl Alcohol) for Active Food Packaging. Int. J. Biol. Macromol. 2024, 277, 134095. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Biomedical Evaluation of Polyvinyl Alcohol–Gelatin Esterified Hydrogel for Wound Dressing. J. Mater. Sci. Mater. Med. 2007, 18, 1889–1894. [Google Scholar] [CrossRef]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel Colorimetric Films Based on Starch/Polyvinyl Alcohol Incorporated with Roselle Anthocyanins for Fish Freshness Monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Bras, J.; Siqueira, G.; Chappey, C.; Lebrun, L.; Khelifi, B.; Marais, S.; Dufresne, A. Water Sorption Behavior and Gas Barrier Properties of Cellulose Whiskers and Microfibrils Films. Carbohydr. Polym. 2011, 83, 1740–1748. [Google Scholar] [CrossRef]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology; Springer Science & Business: Berlin/Heidelberg, Germany, 2008; ISBN 0387234136. [Google Scholar]
- Ministério da Agricultura e Pecuária. Instrução Normativa No 23, de 20 de Agosto de 2019. Diário Oficial da União Edição: 166|Seção: 1|Página: 1 Órgão; Ministério da Agricultura e Pecuária: Brasília, Brazil, 2019; pp. 1–5.
- Liu, J.; Huang, J.; Ying, Y.; Hu, L.; Hu, Y. PH-Sensitive and Antibacterial Films Developed by Incorporating Anthocyanins Extracted from Purple Potato or Roselle into Chitosan/Polyvinyl Alcohol/Nano-ZnO Matrix: Comparative Study. Int. J. Biol. Macromol. 2021, 178, 104–112. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tafaghodi, M.; Bazzaz, B.S.F.; Mohammad Aboutorabzade, S.; Fathi, M. PH-sensitive Soluble Soybean Polysaccharide/SiO2 Incorporated with Curcumin for Intelligent Packaging Applications. Food Sci. Nutr. 2021, 9, 2169–2179. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. PH-Responsive Pectin-Based Multifunctional Films Incorporated with Curcumin and Sulfur Nanoparticles. Carbohydr. Polym. 2020, 230, 115638. [Google Scholar] [CrossRef]
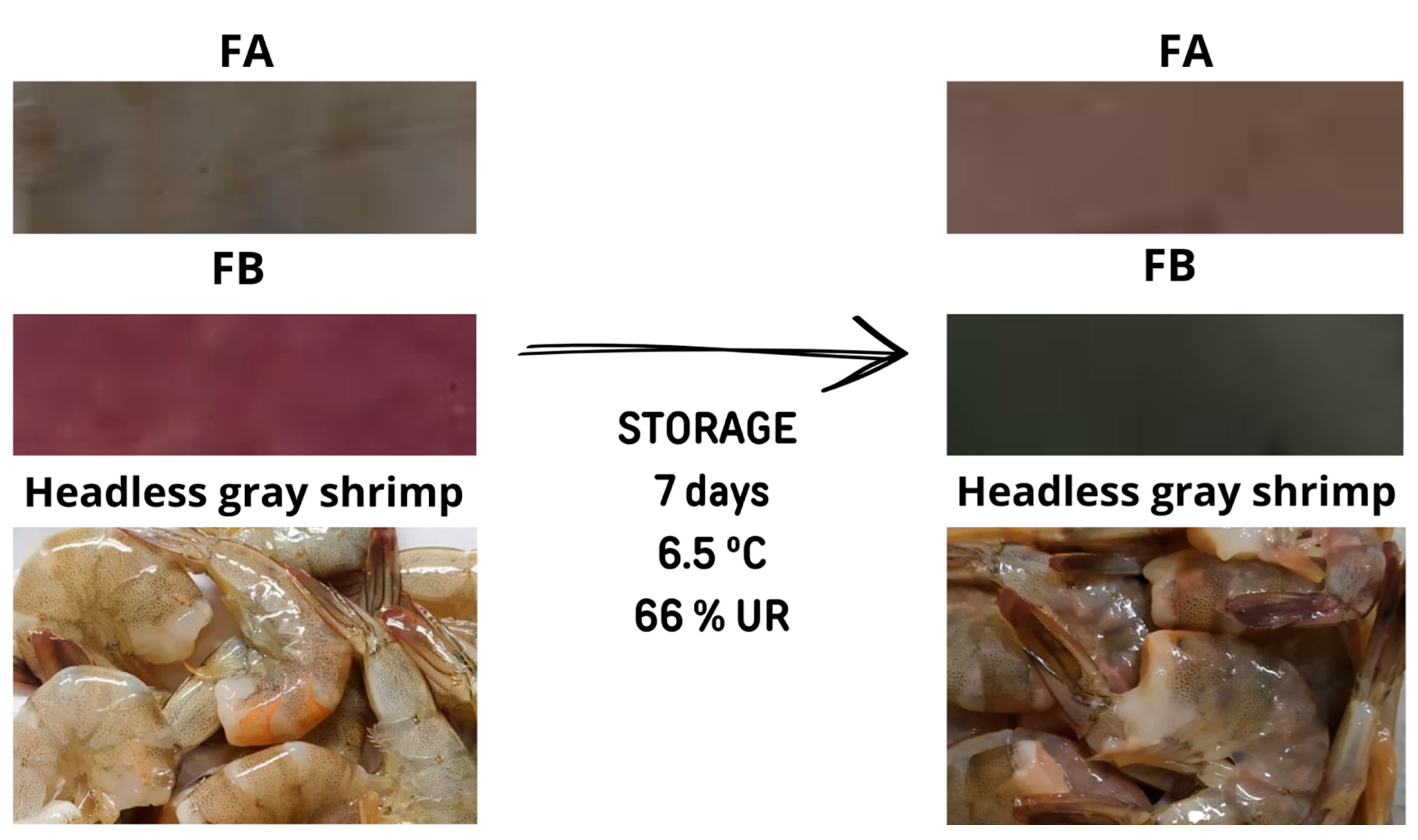
| Color Coordinates Condition | L* | a* | b* | h° | C* | ΔE |
|---|---|---|---|---|---|---|
| FB before storage | 44.64 ± 5.95 a | 9.24 ± 1.48 a | −3.12 ± 2.47 b | 342.94 ± 11.52 a | 9.88 ± 2.12 a | 0.00 ± 0.00 b |
| FB after storage | 50.26 ± 6.81 a | −6.51 ± 2.95 b | 6.87 ± 1.56 a | 131.81 ± 8.73 b | 9.54 ± 3.02 a | 20.47 ± 2.87 a |
| FA before storage | 64.70 ± 4.51 a | −2.49 ± 0.91 b | 7.73 ± 1.69 a | 108.52 ± 9.01 a | 8.19 ± 1.49 a | 0.00 ± 0.00 b |
| FA after storage | 68.98 ± 0.79 a | 0.76 ± 0.25 a | 8.59 ± 0.97 a | 84.78 ± 2.12 b | 8.62 ± 0.95 a | 6.38 ± 2.59 a |
| Shrimp before storage | 46.48 ± 4.29 a | 3.98 ± 3.40 a | 3.69 ± 1.47 a | 49.64 ± 16.79 a | 5.56 ± 3.38 a | 0.00 ± 0.00 a |
| Shrimp after storage | 44.66 ± 0.61 a | 0.21 ± 0.13 a | 2.01 ± 0.65 a | 83.65 ± 3.75 a | 2.03 ± 0.66 a | 5.99 ± 3.53 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, B.T.; Jesus, M.; Santos, J.; Marques, C.S.; Costa, N.M.E.P.d.L.d.; Mata, F.; Stringheta, P.C.; Oliveira, T.V.d.; Soares, N.d.F.F. Development of Gelatin/Polyvinyl Alcohol Films Incorporated with Blueberry Extracts for Freshness Detection of Shrimp. Polymers 2025, 17, 2188. https://doi.org/10.3390/polym17162188
Gomes BT, Jesus M, Santos J, Marques CS, Costa NMEPdLd, Mata F, Stringheta PC, Oliveira TVd, Soares NdFF. Development of Gelatin/Polyvinyl Alcohol Films Incorporated with Blueberry Extracts for Freshness Detection of Shrimp. Polymers. 2025; 17(16):2188. https://doi.org/10.3390/polym17162188
Chicago/Turabian StyleGomes, Bárbara Teixeira, Meirielly Jesus, Joana Santos, Clara Suprani Marques, Noé Mitterhofer Eiterer Ponce de Leon da Costa, Fernando Mata, Paulo Cesar Stringheta, Taila Veloso de Oliveira, and Nilda de Fatima Ferreira Soares. 2025. "Development of Gelatin/Polyvinyl Alcohol Films Incorporated with Blueberry Extracts for Freshness Detection of Shrimp" Polymers 17, no. 16: 2188. https://doi.org/10.3390/polym17162188
APA StyleGomes, B. T., Jesus, M., Santos, J., Marques, C. S., Costa, N. M. E. P. d. L. d., Mata, F., Stringheta, P. C., Oliveira, T. V. d., & Soares, N. d. F. F. (2025). Development of Gelatin/Polyvinyl Alcohol Films Incorporated with Blueberry Extracts for Freshness Detection of Shrimp. Polymers, 17(16), 2188. https://doi.org/10.3390/polym17162188








