Edible Films Based on Fish Gelatin and Soluble Soybean Polysaccharide Enriched with Tea Polyphenol for Active Food Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Film Characterization
2.4. Packaging Application
2.5. Statistical Analysis
3. Results and Discussion
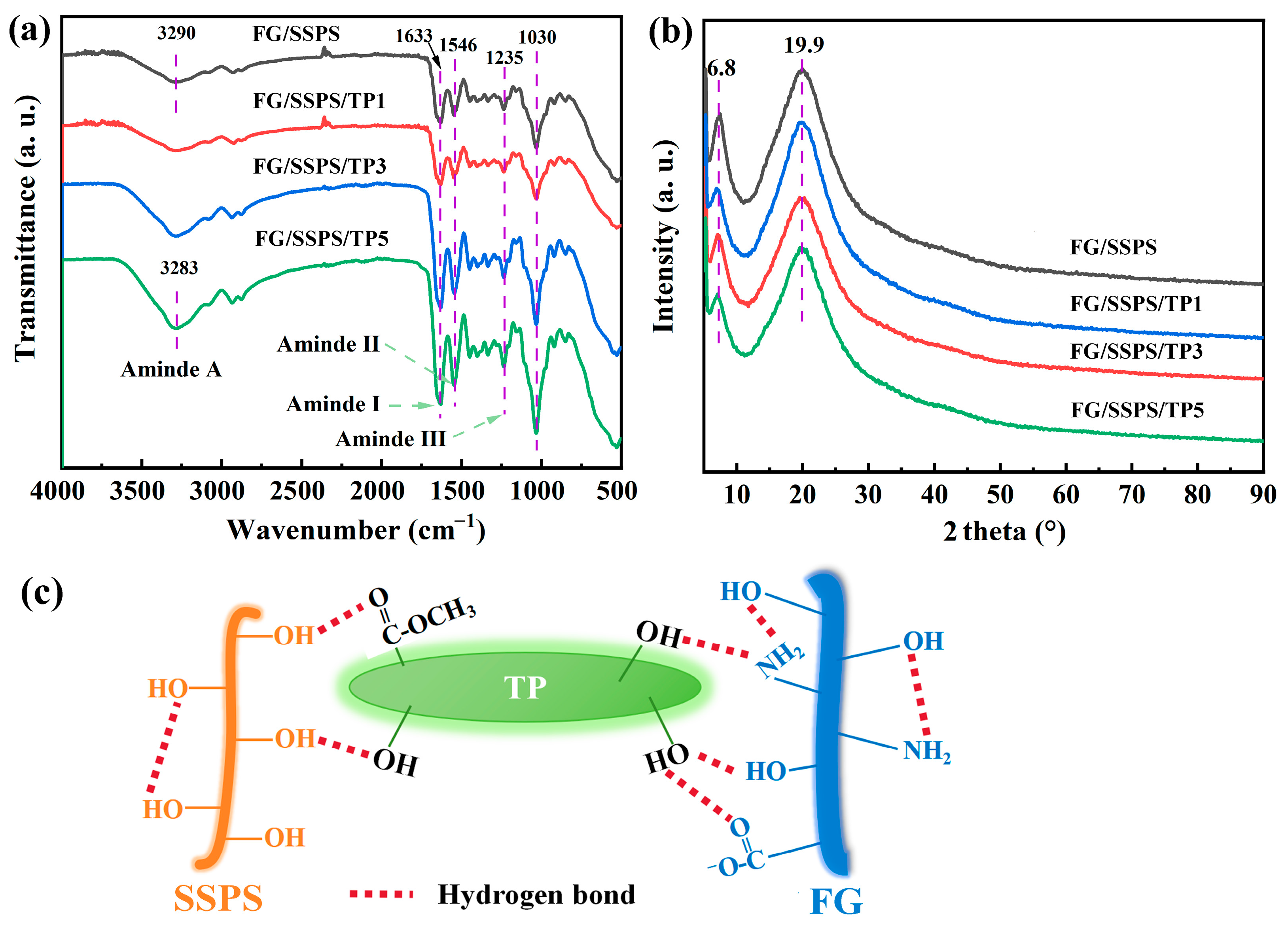
3.1. FTIR and XRD Analyses
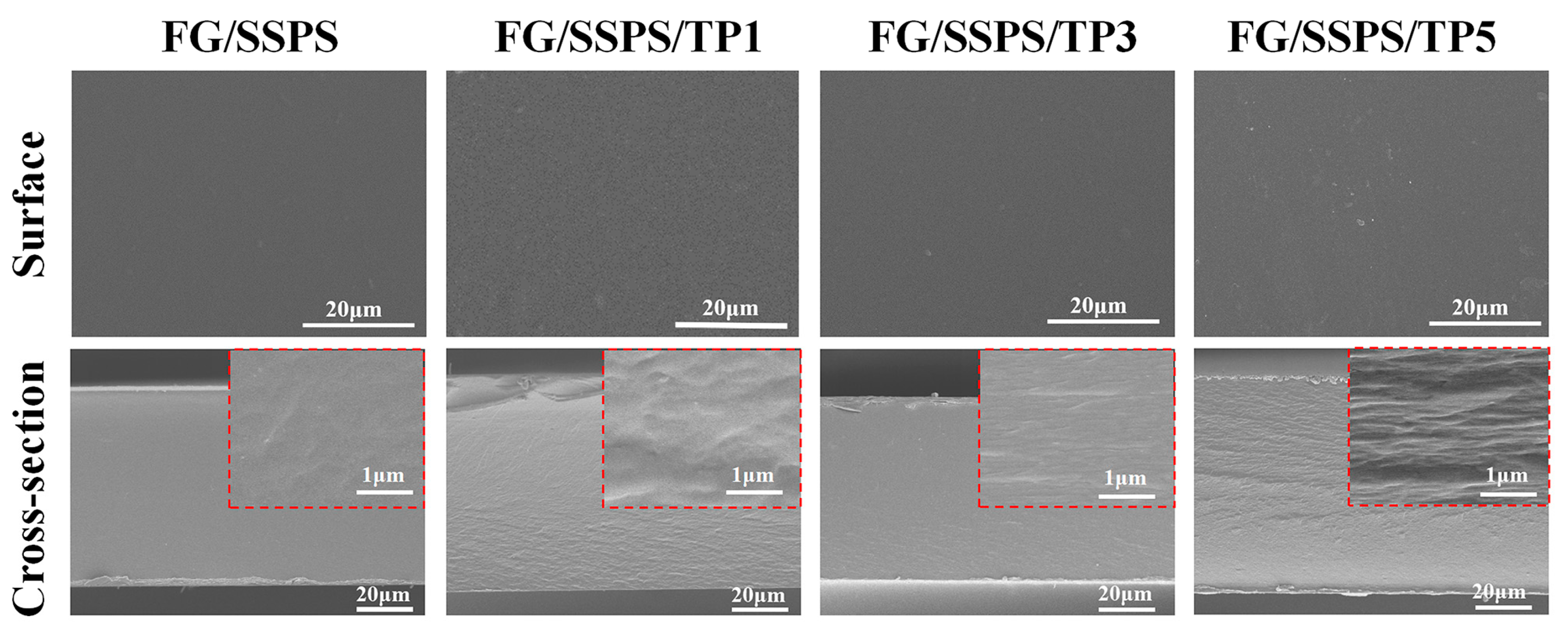
3.2. SEM Analysis
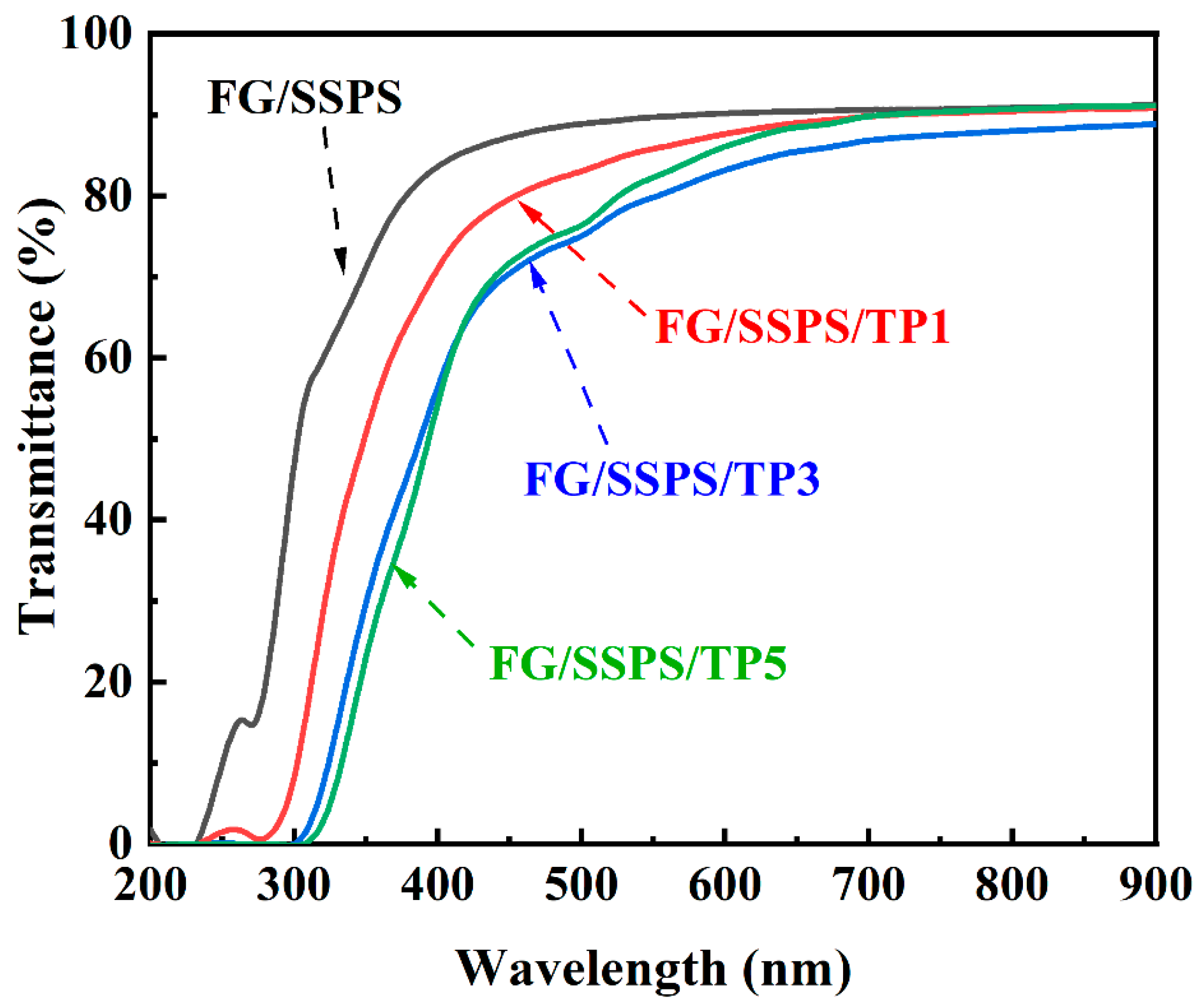
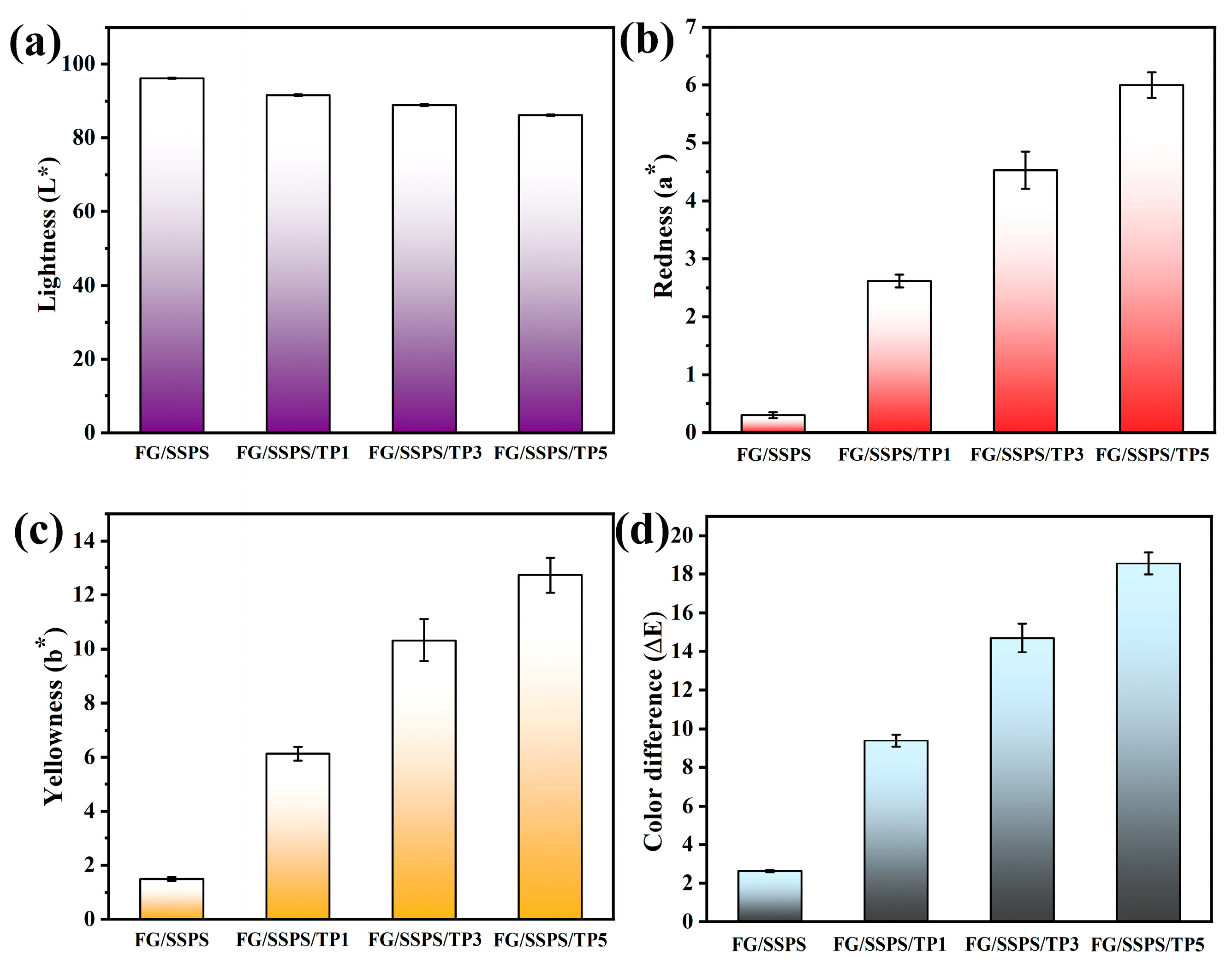
3.3. Optical Properties
3.4. Thickness and Mechanical Properties
3.5. Water Content and Water Solubility
3.6. Water Contact Angle and Water Vapor Permeability
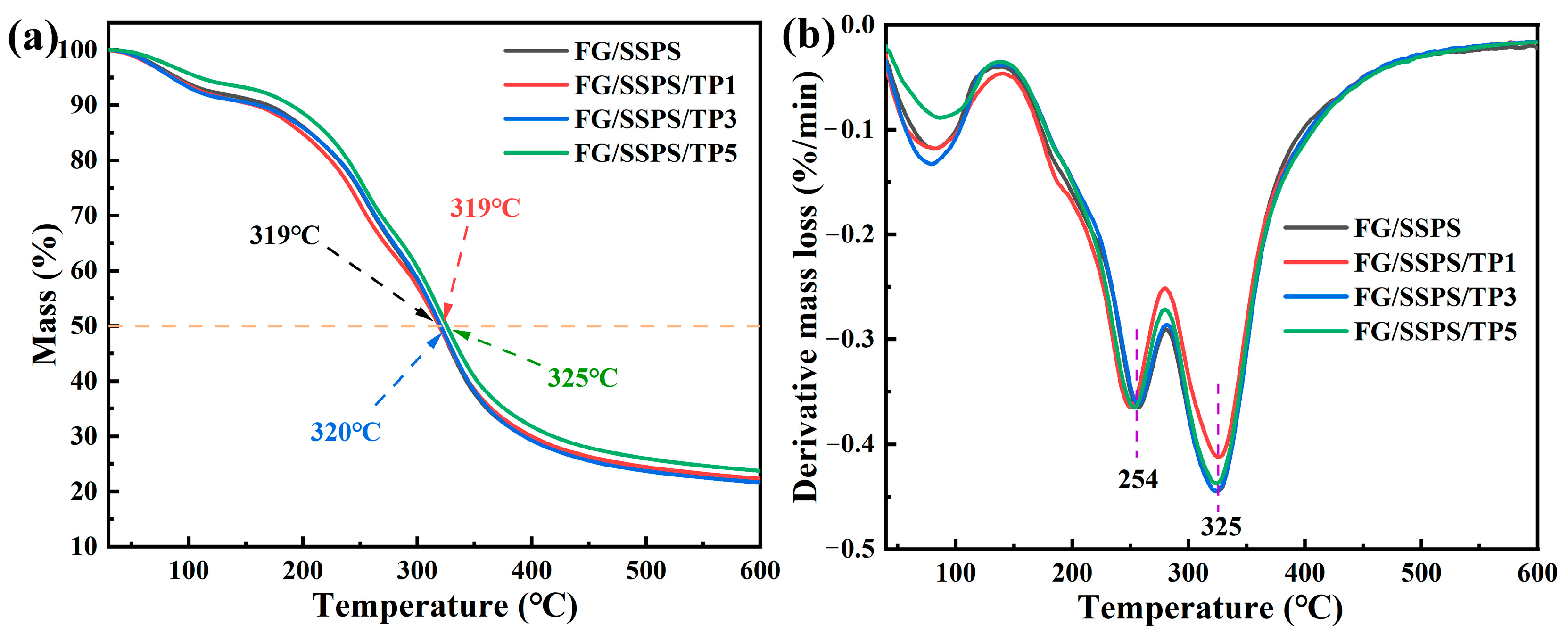
3.7. Thermal Properties
3.8. Antioxidant Activity
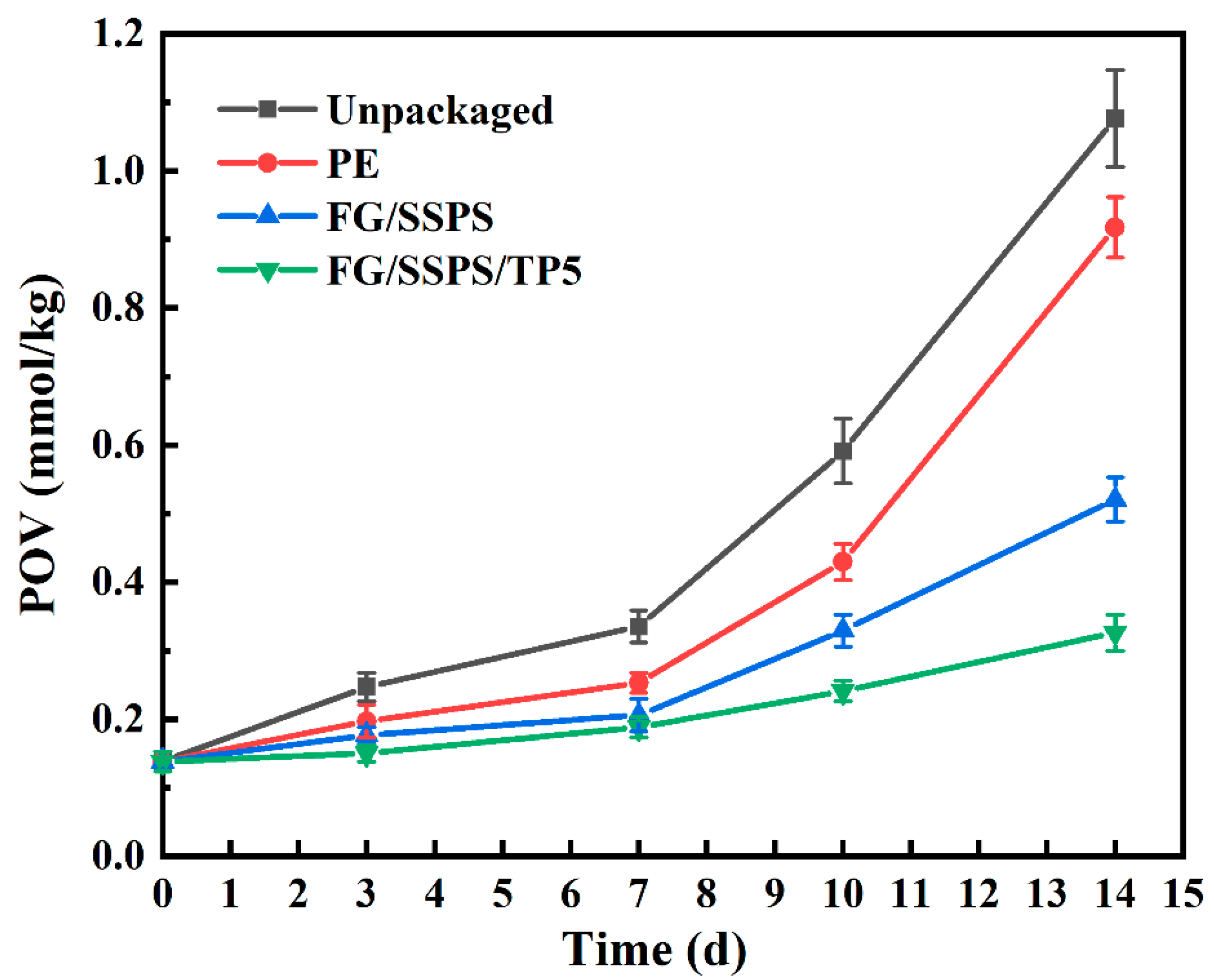
3.9. Packaging Application in Beef Tallow Preservation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Hu, L. Recent advances of proteins, polysaccharides and lipids-based edible films/coatings for food packaging applications: A review. Food Biophys. 2024, 19, 29–45. [Google Scholar] [CrossRef]
- Dirpan, A.; Ainani, A.F.; Djalal, M. A review on biopolymer-based biodegradable film for food packaging: Trends over the last decade and future research. Polymers 2023, 15, 2781. [Google Scholar] [CrossRef]
- Eze, C.G.; Nwankwo, C.E.; Dey, S.; Sundaramurthy, S.; Okeke, E.S. Food chain microplastics contamination and impact on human health: A review. Environ. Chem. Lett. 2024, 22, 1889–1927. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, D.; Yin, L.; Wang, R.; Jin, Z.; Xu, H.; Xia, G. Physicochemical and functional properties of yanbian cattle bone gelatin extracted using acid, alkaline, and enzymatic hydrolysis methods. Gels 2025, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Nitsuwat, S.; Zhang, P.; Ng, K.; Fang, Z. Fish gelatin as an alternative to mammalian gelatin for food industry: A meta-analysis. LWT-Food Sci. Technol. 2021, 141, 110899. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S. Modified fish gelatin as an alternative to mammalian gelatin in modern food technologies. Polymers 2020, 12, 3051. [Google Scholar] [CrossRef]
- Shi, X.D.; Huang, J.J.; Wu, J.L.; Cai, X.X.; Tian, Y.Q.; Rao, P.F.; Huang, J.L.; Wang, S.Y. Fabrication, interaction mechanism, functional properties, and applications of fish gelatin-polysaccharide composites: A review. Food Hydrocoll. 2022, 122, 107106. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin-chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Al-Hassan, A.A.; Norziah, M.H. Starch-gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Chen, H.; Lan, X.; Guan, X.; Luo, R.; Zhang, Q.; Ren, H.; Xu, Z.; Tang, J. Comparative study on the effects of chitosan, carrageenan, and sodium alginate on the film-forming properties of fish skin gelatin. LWT-Food Sci. Technol. 2024, 199, 116111. [Google Scholar] [CrossRef]
- Tabari, M. Investigation of carboxymethyl cellulose (CMC) on mechanical properties of cold water fish gelatin biodegradable edible films. Foods 2017, 6, 41. [Google Scholar] [CrossRef]
- Tagrida, M.; Nilsuwan, K.; Gulzar, S.; Prodpran, T.; Benjakul, S. Fish gelatin/chitosan blend films incorporated with betel (Piper betle L.) leaf ethanolic extracts: Characteristics, antioxidant and antimicrobial properties. Food Hydrocoll. 2023, 137, 108316. [Google Scholar] [CrossRef]
- Azizah, F.; Nursakti, H.; Ningrum, A.; Supriyadi. Development of edible composite film from fish gelatin-pectin incorporated with lemongrass essential oil and its application in chicken meat. Polymers 2023, 15, 2075. [Google Scholar] [CrossRef]
- Feng, X.; Ng, V.K.; Mikš-Krajnik, M.; Yang, H. Effects of fish gelatin and tea polyphenol coating on the spoilage and degradation of myofibril in fish fillet during cold storage. Food Bioprocess Technol. 2017, 10, 89–102. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Jiménez-Escrig, A.; Rupérez, P. Multifunctional antioxidant activity of polysaccharide fractions from the soybean byproduct okara. Carbohydr. Polym. 2010, 82, 245–250. [Google Scholar] [CrossRef]
- Salarbashi, D.; Bazeli, J.; Tafaghodi, M. Environment-friendly green composites based on soluble soybean polysaccharide: A review. Int. J. Biol. Macromol. 2019, 122, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, X.; Nakamura, A.; Burchard, W.; Hallett, F.R. Molecular characterisation of soybean polysaccharides: An approach by size exclusion chromatography, dynamic and static light scattering methods. Carbohydr. Res. 2005, 340, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Ding, Y.; Zheng, X.; Jiang, Y.; Tang, K. Fabrication and characterization of fish gelatin/soluble soybean polysaccharide edible blend films through complex coacervation. Food Hydrocoll. 2024, 155, 110226. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tafaghodi, M.; Bazzaz, B.S.F.; Aboutorabzade, S.M.; Fathi, M. pH-sensitive soluble soybean polysaccharide/SiO2 incorporated with curcumin for intelligent packaging applications. Food Sci. Nutr. 2021, 9, 2169–2179. [Google Scholar] [CrossRef]
- Dong, Y.; Li, Y.; Ma, Z.; Rao, Z.; Zheng, X.; Tang, K.; Liu, J. Effect of polyol plasticizers on properties and microstructure of soluble soybean polysaccharide edible films. Food Packag. Shelf Life 2023, 35, 101023. [Google Scholar] [CrossRef]
- Cao, L.; Li, J.; Song, Y.; Shao, P.; Wang, Y.; Song, H.; Zhang, R.; Liu, J.; Meng, Y.; Wu, L.; et al. Fortification of soluble soybean polysaccharide edible films with licorice root extract for nut preservation. Int. J. Biol. Macromol. 2025, 304, 140986. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Zheng, X.; Liu, S.; Lu, K.; Tang, K.; Liu, J. Heat sealable soluble soybean polysaccharide/gelatin blend edible films for food packaging applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- Dong, Y.; Rao, Z.; Liu, Y.; Zheng, X.; Tang, K.; Liu, J. Soluble soybean polysaccharide/gelatin active edible films incorporated with curcumin for oil packaging. Food Packag. Shelf Life 2023, 35, 101039. [Google Scholar] [CrossRef]
- Feng, M.; Yu, L.; Zhu, P.; Zhou, X.; Liu, H.; Yang, Y.; Zhou, J.; Gao, C.; Bao, X.; Chen, P. Development and preparation of active starch films carrying tea polyphenol. Carbohydr. Polym. 2018, 196, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Wan, X.; Zhan, J.; Ho, C.T. Focusing on the recent progress of tea polyphenol chemistry and perspectives. Food Sci. Hum. Wellness 2022, 11, 437–444. [Google Scholar] [CrossRef]
- GB 5009.227-2016; National Standard for Food Safety—Determination of Peroxide Value in Food. National Health and Family Planning Commission of the People’s Republic of China; China Quality Inspection Press: Beijing, China, 2016.
- Núñez-Flores, R.; Giménez, B.; Fernández-Martín, F.; López-Caballero, M.E.; Montero, M.P.; Gómez-Guillén, M.C. Physical and functional characterization of active fish gelatin films incorporated with lignin. Food Hydrocoll. 2013, 30, 163–172. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Zheng, X.; Pei, Y.; Tang, K. Citric acid crosslinked soluble soybean polysaccharide films for active food packaging applications. Food Chem. 2024, 438, 138009. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Zhang, J.; Yao, J. Molecular interactions in gelatin/chitosan composite films. Food Chem. 2017, 235, 45–50. [Google Scholar] [CrossRef]
- Mao, L.; Ma, L.; Fu, Y.; Chen, H.; Dai, H.; Zhu, H.; Wang, H.; Yu, Y.; Zhang, Y. Transglutaminase modified type a gelatin gel: The influence of intra-molecular and inter-molecular cross-linking on structure-properties. Food Chem. 2022, 395, 133578. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yang, Z.; Xu, X.; Wang, X.; Du, X. Effects of tea polyphenols on the structural and physicochemical properties of high-hydrostatic-pressure-gelatinized rice starch. Food Hydrocoll. 2019, 91, 256–262. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, L.; Deshmukh, R.K.; Gaikwad, K.K. Ultraviolet blocking films for food packaging applications. Food Bioprocess Technol. 2024, 17, 1563–1582. [Google Scholar] [CrossRef]
- Kumar, P.V.S.; Basheer, S.; Ravi, R.; Thakur, M.S. Comparative assessment of tea quality by various analytical and sensory methods with emphasis on tea polyphenols. J. Food Sci. Technol. 2011, 48, 440–446. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential oils. J. Food Eng. 2013, 117, 350–360. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Liu, J.; Zheng, X.; Tang, K. Effect of gelatin type on the structure and properties of microfibrillated cellulose reinforced gelatin edible films. J. Appl. Polym. Sci. 2022, 139, 52119. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, C.; Gu, Y.; Jérôme, F. Glycerol in energy transportation: A state-of-the-art review. Green Chem. 2021, 23, 7865–7889. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, T.; Peng, L.; Wang, J.; Lei, Y.; Li, S.; Li, Q.; Yuan, X.; Zhou, M.; Zhang, Z. Development and characterization of antioxidant composite films based on starch and gelatin incorporating resveratrol fabricated by extrusion compression moulding. Food Hydrocoll. 2023, 139, 108509. [Google Scholar] [CrossRef]
| Films | FG (wt%) | SSPS (wt%) | TP (wt%) | Glycerol (wt%) |
|---|---|---|---|---|
| FG/SSPS | 75.0 | 25.0 | 0.0 | 30 |
| FG/SSPS/TP1 | 1.0 | |||
| FG/SSPS/TP3 | 3.0 | |||
| FG/SSPS/TP5 | 5.0 |
| Films | Thickness (μm) | TS (MPa) | EB (%) | YM (MPa) |
|---|---|---|---|---|
| FG/SSPS | 85 ± 3 a | 33.11 ± 1.53 a | 11.00 ± 2.29 b | 868.26 ± 58.90 a |
| FG/SSPS/TP1 | 89 ± 4 a | 28.44 ± 1.87 b | 21.17 ± 1.39 a | 676.64 ± 74.28 b |
| FG/SSPS/TP3 | 85 ± 4 a | 28.40 ± 1.91 b | 19.76 ± 0.50 a | 683.75 ± 52.33 b |
| FG/SSPS/TP5 | 89 ± 3 a | 26.16 ± 1.43 b | 20.72 ± 0.74 a | 866.08 ± 35.60 a |
| Films | WC (%) | WS (%) | WCA (°) | WVP × 10−10 (g·m−1·s−1·Pa−1) |
|---|---|---|---|---|
| FG/SSPS | 6.26 ± 1.40 a | 31.52 ± 1.17 b | 108.7 ± 4.2 a | 2.47 ± 0.12 a |
| FG/SSPS/TP1 | 7.51 ± 0.47 a | 29.83 ± 0.63 b | 107.7 ± 3.7 a | 2.53 ± 0.09 a |
| FG/SSPS/TP3 | 6.76 ± 0.70 a | 30.86 ± 0.71 b | 64.9 ± 1.9 b | 2.37 ± 0.06 a |
| FG/SSPS/TP5 | 6.01 ± 0.18 a | 33.31 ± 0.89 a | 61.7 ± 5.6 b | 2.43 ± 0.05 a |
| Films | ABTS (%) | DPPH (%) |
|---|---|---|
| FG/SSPS | 7.4 ± 2.2 a | 1.1 ± 0.3 b |
| FG/SSPS/TP1 | 23.3 ± 2.5 a | 16.9 ± 3.5 b |
| FG/SSPS/TP3 | 70.3 ± 5.1 a | 50.8 ± 5.2 b |
| FG/SSPS/TP5 | 90.9 ± 2.6 a | 67.4 ± 4.8 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Song, Z.; Wang, Y.; Pei, Y.; Tang, K. Edible Films Based on Fish Gelatin and Soluble Soybean Polysaccharide Enriched with Tea Polyphenol for Active Food Packaging. Polymers 2025, 17, 2174. https://doi.org/10.3390/polym17162174
Liu J, Song Z, Wang Y, Pei Y, Tang K. Edible Films Based on Fish Gelatin and Soluble Soybean Polysaccharide Enriched with Tea Polyphenol for Active Food Packaging. Polymers. 2025; 17(16):2174. https://doi.org/10.3390/polym17162174
Chicago/Turabian StyleLiu, Jie, Zhongfeng Song, Yiwei Wang, Ying Pei, and Keyong Tang. 2025. "Edible Films Based on Fish Gelatin and Soluble Soybean Polysaccharide Enriched with Tea Polyphenol for Active Food Packaging" Polymers 17, no. 16: 2174. https://doi.org/10.3390/polym17162174
APA StyleLiu, J., Song, Z., Wang, Y., Pei, Y., & Tang, K. (2025). Edible Films Based on Fish Gelatin and Soluble Soybean Polysaccharide Enriched with Tea Polyphenol for Active Food Packaging. Polymers, 17(16), 2174. https://doi.org/10.3390/polym17162174








